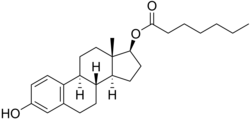
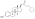Estradiol enantate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Perlutal, Topasel, Unalmes, Yectames, others |
| Other names | EEn; E2-EN; EE; E2E; Estradiol enanthate; Estradiol heptanoate; SQ-16150 |
| Routes of administration | Intramuscular injection[1][2] |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High |
| Protein binding | Estradiol: ~98% (to albumin and SHBG)[3][4] |
| Metabolism | Cleavage via esterases in the liver, blood, and tissues[5][6] |
| Metabolites | Estradiol, heptanoic acid, and metabolites of estradiol[5][6] |
| Elimination half-life | IM: 5.6–7.5 days[7][1][8][9] |
| Duration of action | IM (10 mg): ~20–30 days[10][5] |
| Excretion | Urine[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.023.272 |
| Chemical and physical data | |
| Formula | C25H36O3 |
| Molar mass | 384.560 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Estradiol enantate (EEn or E2-EN), also spelled estradiol enanthate and sold under the brand names Perlutal and Topasel among others, is an estrogen medication which is used in hormonal birth control for women.[1][2][11] It is formulated in combination with dihydroxyprogesterone acetophenide (DHPA; algestone acetophenide), a progestin, and is used specifically as a combined injectable contraceptive.[1][2] Estradiol enantate is not available for medical use alone.[12][13][14][15] The medication, in combination with DHPA, is given by injection into muscle once a month.[1][2]
Side effects of estradiol enantate include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[16] Estradiol enantate is an estrogen and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[6][5] It is an estrogen ester and a long-lasting prodrug of estradiol in the body.[5][6] Because of this, it is considered to be a natural and bioidentical form of estrogen.[5][17]
Estradiol enantate was first described by 1954,[18] and was first studied in combination with DHPA as a combined injectable contraceptive in 1964.[19][20] The combination was introduced for clinical use by the mid-1970s.[21][22][23] Estradiol enantate is not available as a standalone medication (i.e., by itself without DHPA).[15] The combination is available in Latin America and Hong Kong, and was also previously marketed in Spain and Portugal.[15][2][13]
Medical uses
[edit]Estradiol enantate is used in combination with the progestin DHPA as a once-monthly combined injectable contraceptive for women in Latin America and Hong Kong.[1][2][24][15] Estradiol enantate has been studied in feminizing hormone therapy for transgender women as well.[25] The combination of estradiol enantate and DHPA has likewise been used by transgender women for such purposes.[26] Since at least the 2020s, it has grown in popularity among the transfeminine community as a mean of DIY hormone therapy (without DHPA).[27]
Available forms
[edit]The following forms of estradiol enantate are or have been available for use:[11][28][29][23][2]
- Estradiol enantate 10 mg and DHPA 150 mg (brand names Perlutal, Topasel, many others)
- Estradiol enantate 5 mg and DHPA 75 mg (brand names Anafertin, Patector NF, Yectames)
- Estradiol enantate 10 mg and DHPA 120 mg (brand names Unalmes, Yectuna)
- Estradiol enantate 10 mg and DHPA 75 mg (brand name Ova Repos; discontinued)
A 6 mg estradiol enantate and 90 mg DHPA formulation was also studied, but was never marketed.[30][31][32] The combination of estradiol enantate and DHPA has also been studied at other doses ranging from 5 to 50 mg estradiol enantate and 75 to 200 mg DHPA.[33]
The combination of estradiol enantate and DHPA is provided in ampoules at estradiol enantate concentrations of 5 mg/mL and 10 mg/mL.
Contraindications
[edit]Contraindications of estrogens include coagulation problems, cardiovascular diseases, liver disease, and certain hormone-sensitive cancers such as breast cancer and endometrial cancer, among others.[34][35][36][37]
Side effects
[edit]The side effects of estradiol enantate are the same as those of estradiol. Examples of such side effects include breast tenderness and enlargement, nausea, bloating, edema, headache, and melasma.[16] The combination of estradiol enantate and DHPA as a combined injectable contraceptive has shown no adverse effects on liver function, lipid metabolism, or coagulation.[38][2]
A Brazilian case report of a prolactinoma in a transgender woman treated with 10 mg estradiol enantate every 2 weeks exists.[39][40] While DHPA was not mentioned in this instance,[39][40] estradiol enantate is normally formulated in combination with DHPA including in Brazil.[12][14]
Overdose
[edit]Symptoms of estrogen overdosage may include nausea, vomiting, bloating, increased weight, water retention, breast tenderness, vaginal discharge, heavy legs, and leg cramps.[34] These side effects can be diminished by reducing the estrogen dosage.[34]
Interactions
[edit]Inhibitors and inducers of cytochrome P450 may influence the metabolism of estradiol and by extension circulating estradiol levels.[41]
Pharmacology
[edit]
Pharmacodynamics
[edit]Estradiol enantate is an estradiol ester, or a prodrug of estradiol.[5][6] As such, it is an estrogen, or an agonist of the estrogen receptors.[5][6] Estradiol enantate is of about 41% higher molecular weight than estradiol due to the presence of its C17β enantate ester.[42][15] Because estradiol enantate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[5][17]
The combination of 10 mg estradiol enantate and 150 mg DHPA as a once-monthly combined injectable contraceptive (which achieves levels of estradiol of around 350 pg/mL)[10][43][44] has been found to have little to no effect on many markers of estrogen-modulated liver protein synthesis, including circulating levels of HDL and LDL cholesterol, copper, ceruloplasmin, total and free cortisol, corticosteroid-binding globulin, and sex hormone-binding globulin.[45][46] However, it was found to significantly increase levels of triglycerides and to significantly decrease levels of total and free testosterone.[46] In contrast to the estradiol enantate-containing combined injectable contraceptive, low-dose ethinylestradiol-containing birth control pills produce highly significant changes in all of the preceding parameters.[45][46]
Studies in women and female capuchin monkeys have found that injections of estradiol enantate and DHPA significantly alter levels of coagulation factors.[47][48]
The clinical estrogenic effects of estradiol enantate and ethinylestradiol have been compared in other studies as well.[49]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Pharmacokinetics
[edit]When estradiol enantate is administered in an oil solution by intramuscular injection, a depot effect occurs, and this results in it having a long duration of action.[10][6][50] The duration of action of estradiol enantate is considerably longer than that of various other estradiol esters, such as estradiol benzoate and estradiol valerate, whereas its duration is shorter than that of estradiol undecylate.[10][51][52] In general, the longer the fatty acid ester chain, the more lipophilic the estradiol ester, the more slowly it is released from the depot and absorbed into the circulation, and the longer its duration of action.[6][50]
The pharmacokinetics of estradiol enantate have been assessed in a number of studies.[10][53][43][7][44][54] It has usually been studied in combination with DHPA.[10][53][43][44] Following an intramuscular injection of estradiol enantate, levels of estradiol have been found to peak after 3 to 8 days.[10][44][7] Maximal levels of estradiol after a 5 mg injection of estradiol enantate have been found to be about 163 to 209 pg/mL and after a 10 mg injection of estradiol enantate have been found to be about 283 to 445 pg/mL.[10][43][44] However, one outlying study reported peak estradiol levels of 850 pg/mL after an intramuscular injection of 10 mg estradiol enantate in three postmenopausal women.[7] It used radioimmunoassay for the determinations, with no mention of chromatographic separation.[7] Estradiol levels following an intramuscular injection of 10 mg estradiol enantate have been found to return to baseline levels of around 50 pg/mL after about 20 to 30 days.[43][7][5][54][10] However, a metabolic study found that traces of radiolabeled estradiol enantate remained detectable in blood for at least 30 to 40 days and for as long as 60 days.[53] Studies have reported that the elimination half-life of estradiol enantate after a single 10 mg intramuscular injection was 5.6 to 7.5 days.[7][1][8] The volume of distribution of estradiol enantate has been reported to be 5.087 L.[9] Estradiol enantate is excreted preferentially in urine.[22]
There were concerns about possible accumulation of estradiol enantate and consequent estrogenic overexposure with once-monthly combined injectable contraceptives containing the medication due its long duration, and this may have limited the use of such combined injectable contraceptives.[8][10] Subsequent clinical studies have found that there is very limited or no accumulation of estradiol enantate when it is used in once-a-month injectable contraceptives.[8][38][2]
-
Estradiol levels after the most recent intramuscular injection during once-monthly 5 or 10 mg estradiol enanthate and 75 or 150 mg dihydroxyprogesterone acetophenide contraception in one premenopausal woman each.[43] Assays were performed using radioimmunoassay.[43] Source was Recio et al. (1986).[43]
-
Estradiol levels after a single intramuscular injection of 10 mg estradiol enanthate in three postmenopausal women.[7] Assays were performed using radioimmunoassay.[7] Source was Wiemeyer et al. (1986).[7]
-
Estradiol and prolactin levels after the most recent intramuscular injection during once-monthly 10 mg estradiol enanthate and 150 mg dihydroxyprogesterone acetophenide contraception in 10 premenopausal women.[54] Only four determinations were made: days 0, 10, 20, and 30.[54] Assays were performed using radioimmunoassay.[54] Source was Garza-Flores et al. (1989).[54]
Chemistry
[edit]Estradiol enantate, also known as estradiol 17β-enantate or estra-1,3,5(10)-triene-3,17β-diol 17β-heptanoate, is a synthetic estrane steroid and the C17β enantate (heptanoate) fatty acid ester of estradiol.[42][15] Other common esters of estradiol used clinically include estradiol benzoate, estradiol cypionate, estradiol undecylate, and estradiol valerate.[15] Estradiol dienantate (component of Climacteron), or estradiol 3,17β-dienantate, has also been used.[42][55][56][57]
The experimental octanol/water partition coefficient (logP) of estradiol enanthate is 6.7.[58]
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
[edit]Estradiol enantate was first described, along with a variety of other estradiol esters, by Karl Junkmann of Schering AG in 1953.[59][18][60][61][52][62][63] The first clinical study of estradiol enantate and DHPA as a combined injectable contraceptive was conducted in 1964.[19][20] The combination was marketed by the mid-1970s.[21][22][23]
Society and culture
[edit]Generic names
[edit]Estradiol enantate is the British English generic name of the medication and its INNM and BANM, while estradiol enanthate is its USAN and American English generic name.[42][15][12][64] Its generic names in other languages are as follows:[13][12]
- French: enantate d'estradiol and estradiol enantate
- German: estradiol enantat
- Italian: estradiolo enantato
- Portuguese and Spanish: enantato de estradiol and estradiol enantato
Estradiol enantate is also known by its former developmental code name SQ-16150.[65] It has been referred to as estradiol heptanoate.[15][42][14][12][13]
Brand names
[edit]Estradiol enantate has been marketed under a wide variety of brand names.[13][12][66][67][11][68][29][69][23][2][10] It has been marketed in a few different preparations, with varying doses of estradiol enantate and DHPA.[29][11][68][28][23][2][10] These formulations all have different brand names, which include the following († = discontinued):[13][12][66][67][28][29][11][68][2][70]
- EEn 10 mg / DHPA 150 mg: Acefil, Agurin†, Atrimon†, Ciclomes, Ciclovar, Ciclovular, Cicnor†, Clinomin, Cycloven, Daiva, Damix, Deprans, Deproxone, Exuna, Ginestest, Ginoplan†, Gynomes, Horprotal, Listen, Luvonal, Neogestar, Neolutin, Nomagest, Nonestrol, Normagest, Normensil, Novular, Oterol, Ovoginal, Patector, Patectro, Perludil, Perlumes, Perlutal, Perlutale, Perlutan, Perlutin, Perlutin-Unifarma, Permisil, Preg-Less, Pregnolan, Progestrol†, Protegin, Proter, Seguralmes, Synovular, Topasel, Unigalen, Uno-Ciclo, and Vagital.
- EEn 10 mg / DHPA 120 mg: Anafertin†, Patector NF, and Yectames.
- EEn 5 mg / DHPA 75 mg: Unalmes and Yectuna.
- EEn 10 mg / DHPA 75 mg: Ova Repos†.
- Unsorted: Evitas†, Femineo†, and Primyfar†.
The combination of EEn 10 mg and DHPA 150 mg was developed under the developmental brand name Deladroxate, but this brand name was never used commercially.[23][2]
Availability
[edit]
Estradiol enantate has been marketed in combination with DHPA as a combined injectable contraceptive in at least 19 countries, mostly in Latin America.[11][68][29][69][13][12][66][67] A few different preparations, with varying doses of EEn and DHPA and varying availability, have been introduced.[29][11][68][28][23][2][10] These formulations have the following approval and availability († = discontinued in this country):[13][12][66][67][28][29][11][68][2]
- EEn 10 mg / DHPA 150 mg: at least 19 countries, including Argentina, Belize, Brazil, Chile, Colombia, Costa Rica, the Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Hong Kong, Mexico, Nicaragua, Panama, Paraguay, Peru, Portugal†, and Spain†.
- EEn 10 mg / DHPA 120 mg: at least 3 countries, including Brazil†, Chile, and Paraguay.
- EEn 5 mg / DHPA 75 mg: at least 9 countries, including Costa Rica, the Dominican Republic, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, and Spain†.
EEn is also available in Canada in combination with estradiol benzoate and testosterone enantate for veterinary use as Uni-Bol.[71]
Usage
[edit]EEn/DHPA is the most widely used combined injectable contraceptive in Latin America.[72] It was estimated in 1995 that EEn/DHPA was used as a combined injectable contraceptive in Latin America by at least 1 million women.[29] However, combined injectable contraceptives like EEn/DHPA are unlikely to constitute a large proportion of total contraceptive use in the countries in which they are available.[29]
See also
[edit]References
[edit]- ^ a b c d e f g h Jarquín González JD, Elda de Aguirre L, Rodríguez C, Abrego de Aguilar M, Carrillo F, León DA, et al. (September 1996). "Dihydroxyprogesterone acetophenide 150 mg + estradiol enantate 10 mg as monthly injectable contraceptives". Advances in Contraception. 12 (3): 213–225. doi:10.1007/BF01849664. PMID 8910663. S2CID 2522426.
- ^ a b c d e f g h i j k l m n o Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". Journal of Obstetrics and Gynaecology. 4 (Suppl 1): S1-34. doi:10.3109/01443619409027641. PMID 12290848.
- ^ Stanczyk FZ, Archer DF, Bhavnani BR (June 2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
- ^ Falcone T, Hurd WW (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 978-0-323-03309-1.
- ^ a b c d e f g h i j Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 261, 271. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens. [...] Wiemeyer et al. (1986) measured elevated estradiol levels up to 31 days after an intramuscular dose of 10mg estradiol enanthate.
- ^ a b c d e f g h Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b c d e f g h i j Wiemeyer JC, Fernandez M, Moguilevsky JA, Sagasta CL (November 1986). "Pharmacokinetic studies of estradiol enantate in menopausic women". Arzneimittel-Forschung. 36 (11): 1674–1677. PMID 3814225.
- ^ a b c d Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–385. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ a b "Bula do Algestona Acetofenida + Enantato de Estradiol". Consulta Remédios. Archived from the original on 18 September 2018. Retrieved 18 September 2018.
- ^ a b c d e f g h i j k l m n o p q Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–359. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ^ a b c d e f g h Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- ^ a b c d e f g h i Sweetman SC, ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- ^ a b c d e f g h "Micromedex Products: Please Login".
- ^ a b c "Estradiol: Uses, Dosage & Side Effects".
- ^ a b c d e f g h i Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 405. ISBN 978-3-88763-075-1. Retrieved 20 May 2012.
- ^ a b Ghosh AK (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- ^ a b Arun N, Narendra M, Shikha S (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 419–. ISBN 978-93-5090-575-3.
- ^ a b International Neurochemical Symposium Proceedings. Academic Press. 1954. p. 453.
- ^ a b Rutherford RN, Banks AL, Coburn WA (1964). "Deladroxate for the Prevention of Ovulation". Fertility and Sterility. 15 (6): 648–652. doi:10.1016/s0015-0282(16)35410-3. PMID 14236841.
- ^ a b Taymor ML, Planck S, Yahia C (1964). "Ovulation Inhibition with a Long-Acting Parenteral Progestogen-Estrogen Combination". Fertility and Sterility. 15 (6): 653–660. doi:10.1016/s0015-0282(16)35411-5. PMID 14236842.
- ^ a b Bringer J, Hedon B (15 September 1995). Fertility and Sterility: A Current Overview. CRC Press. pp. 47–. ISBN 978-1-85070-694-6.
- ^ a b c Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstetrical & Gynecological Survey. 32 (6): 335–347. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ^ a b c d e f g Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ Zutshi V, Rathore AM, Sharma K (1 January 2005). Hormones in Obstetrics and Gynaecology. New Delhi: Jaypee Brothers Publishers. p. 138. ISBN 978-81-8061-427-9. Retrieved 20 May 2012.[permanent dead link]
- ^ Becerra Fernández A, de Luis Román DA, Piédrola Maroto G (October 1999). "[Morbidity in transsexual patients with cross-gender hormone self-treatment]" [Morbidity in transsexual patients with cross-gender hormone self-treatment] (PDF). Medicina Clinica (in Spanish). 113 (13): 484–487. PMID 10604171.
- ^ Kulick D (12 January 2009). Travesti: Sex, Gender, and Culture among Brazilian Transgendered Prostitutes. University of Chicago Press. pp. 64–66. ISBN 978-0-226-46101-4.
- ^ Aly (16 July 2021). "An Informal Meta-Analysis of Estradiol Curves with Injectable Estradiol Preparations". Transfeminine Science.
- ^ a b c d e IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 431–433, 467. ISBN 978-92-832-1291-1.
- ^ a b c d e f g h i IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0. Archived from the original (PDF) on 28 August 2021. Retrieved 19 September 2018.
- ^ d'Arcangues C, Snow RC (1999). "Injectable Contraceptives.". In Rabe T, Runnebaum B (eds.). Fertility Control — Update and Trends. pp. 121–149. doi:10.1007/978-3-642-86696-8_6. ISBN 978-3-642-86698-2.
- ^ Coutinho EM, Spinola P, Barbosa I, Gatto M, Tomaz G, Morais K, et al. (March 1997). "Multicenter, double-blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate". Contraception. 55 (3): 175–181. doi:10.1016/S0010-7824(97)00018-8. PMID 9115007.
- ^ Coutinho EM, Spinola P, Tomaz G, Morais K, Nassar de Souza R, Sabino Pinho Neto J, et al. (April 2000). "Efficacy, acceptability, and clinical effects of a low-dose injectable contraceptive combination of dihydroxyprogesterone acetophenide and estradiol enanthate". Contraception. 61 (4): 277–280. doi:10.1016/S0010-7824(00)00099-8. PMID 10899484.
- ^ Koetsawang S (April 1994). "Once-a-month injectable contraceptives: efficacy and reasons for discontinuation". Contraception. 49 (4): 387–398. doi:10.1016/0010-7824(94)90034-5. PMID 8013221.
- ^ a b c Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ Lauritzen C, Studd JW (22 June 2005). Current Management of the Menopause. CRC Press. pp. 95–98, 488. ISBN 978-0-203-48612-2.
- ^ Laurtizen C (2001). "Hormone Substitution Before, During and After Menopause". In Fisch FH (ed.). Menopause – Andropause: Hormone Replacement Therapy Through the Ages (PDF). Krause & Pachernegg: Gablitz. pp. 67–88. ISBN 978-3-901299-34-6.
- ^ Midwinter A (1976). "Contraindications to estrogen therapy and management of the menopausal syndrome in these cases". In Campbell S (ed.). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. MTP Press Limited. pp. 377–382. doi:10.1007/978-94-011-6165-7_33. ISBN 978-94-011-6167-1.
- ^ a b De Aguilar MA, Altamirano L, Leon DA, De Fung RC, Grillo AE, Gonzalez JD, et al. (December 1997). "Current status of injectable hormonal contraception, with special reference to the monthly method". Advances in Contraception. 13 (4): 405–417. doi:10.1023/A:1006501526018. PMID 9404550. S2CID 19603384.
- ^ a b Camara VL, Zanardi UV, Glezer A, Paraiba DB, Bronstein MD, Mendonca BB, Costa EM (June 2010). "Estrogen as a Presumed Risk Factor for Prolactinoma in a Male-to-Female Transsexual Patient" (PDF). Endocrine Reviews. 31 (3, Supplement 1): S347.
- ^ a b Camara VL (July 2010). "Estradiol enantate First report of prolactinoma, in a transsexual". Reactions. 24 (1311): 24. doi:10.2165/00128415-201013110-00077. S2CID 195175382.
- ^ Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (February 2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacologica Sinica. 22 (2): 148–154. PMID 11741520.
- ^ a b c d e Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 898–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g h Recio R, Garza-Flores J, Schiavon R, Reyes A, Diaz-Sanchez V, Valles V, et al. (June 1986). "Pharmacodynamic assessment of dihydroxyprogesterone acetophenide plus estradiol enanthate as a monthly injectable contraceptive". Contraception. 33 (6): 579–589. doi:10.1016/0010-7824(86)90046-6. PMID 3769482.
- ^ a b c d e f Schiavon R, Benavides S, Oropeza G, Garza-Flores J, Recio R, Díaz-Sanchez V, Pérez-Palacios G (June 1988). "Serum estrogens and ovulation return in chronic users of a once-a-month injectable contraceptive". Contraception. 37 (6): 591–598. doi:10.1016/0010-7824(88)90005-4. PMID 3396358.
- ^ a b Wiemeyer JC, Vidal M, Gallardo, E (March 1995). "IX International Congress. Session 22 Long-Acting Contraception II. Abstracts. Experiences with dihydroxyprogesterone acetophenide (DHPA) 150 mg plus estradiol enanthate (E2EN) 10 mg as a once a month injectable contraceptive in Latin America". Advances in Contraception. 11: 54–60. doi:10.1007/BF02436103. S2CID 75854488.
- ^ a b c Wiemeyer JC, Sagasta CL, Roncales Mateo JM, Lavarello AC, Angel de Toro LA, Salas Diaz R (July 1990). "Multicentred clinical study of the metabolic effect of the monthly injectable contraceptive containing dihydroxyprogesterone acetophenide 150 mg + estradiol enanthate 10 mg". Contraception. 42 (1): 13–28. doi:10.1016/0010-7824(90)90088-D. PMID 2117515.
- ^ Oliva Filho, W. M., & Santos, N. da C. (1992). Efeitos na coagulação sanguinea em usuárias da associação acetofenido de dihidroxiprogesterona 150mg e enantato de estradiol 10mg como metodo anticoncepcional injetavel. Universidade de São Paulo, São Paulo. https://bdpi.usp.br/item/000736190
- ^ Duarte RC, Belham FS, Tavares MC (2018). "Risco de doenca tromboliticas apos o uso de algestona acetofenida e enantato de estradiol" [Risk of thrombolytic disease after the use of algestone acetophenide and estradiol enanthate]. Revista de Patologia do Tocantins [Journal of Pathology of Tocantins] (in Portuguese). 5 (1): 17. doi:10.20873/uft.2446-6492.2018v5n1p17. ISSN 2446-6492.
- ^ Moguilevsky JA, Wiemeyer JC, Sagasta CL, Leiderman S (November 1986). "Estrogenic activities of estradiol enantate and ethinylestradiol compared at a clinical level". Arzneimittel-Forschung. 36 (11): 1671–1674. PMID 3101711.
- ^ a b Vermeulen A (1975). "Longacting steroid preparations". Acta Clinica Belgica. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- ^ Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–424. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
- ^ a b Wilde PR, Coombs CF, Short AJ (1959). The Medical Annual: A Year Book of Treatment and Practitioner's Index ... Publishing Science Group.
As in the case of progestogens the esters of oestradiol vary in the duration of their effect. Oestradiol benzoate is short-acting (three days to a week). Oestradiol valerianate is somewhat longer-acting, and oestradiol enanthate and undecylate have considerably more prolonged duration of effectiveness. The undecylate may remain effective for some months, and should not be employed, [...]
- ^ a b c Gual C, Pérez-Palacios G, Perez AE, Ruiz MR, Solis J, Cervantes A, et al. (1973). "Metabolic fate of a long-acting injectable estrogen-progestogen contraceptive 1,2". Contraception. 7 (4): 271–287. doi:10.1016/0010-7824(73)90145-5. ISSN 0010-7824.
- ^ a b c d e f Garza-Flores J, Alba VM, Cravioto MC, Hernandez L, Perez-Palacios G, Alvarado G, et al. (May 1989). "Estrogen-progestogen once-a-month injectable contraceptives and serum prolactin". Contraception. 39 (5): 519–529. doi:10.1016/0010-7824(89)90107-8. PMID 2524362.
- ^ Ginsburg ES (1999). "Androgen Replacement in Postmenopausal Women". In Seifer DB, Kennard EA (eds.). Menopause: Endocrinology and Management. Vol. 18. pp. 209–219. doi:10.1007/978-1-59259-246-3_13. ISBN 978-1-61737-129-5.
- ^ Greenblatt RB, Barfield WE, Jungck EC (January 1962). "The treatment of the menopause". Canadian Medical Association Journal. 86 (3): 113–114. PMC 1848811. PMID 13901504.
- ^ Harlow BL, Abraham ME (27 July 1999). "Depression in Menopause". In Seifer DB, Kennard EA (eds.). Menopause: Endocrinology and Management. Springer Science & Business Media. pp. 183–. doi:10.1007/978-1-59259-246-3_7. ISBN 978-1-59259-246-3.
- ^ "Estradiol enanthate | C25H36O3". ChemSpider.
- ^ Junkmann K (1953). "Über protrahiert wirksame Östrogene" [Over protracted effective estrogens]. Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie. 220 (5). doi:10.1007/BF00246561. ISSN 0028-1298. S2CID 20753905.
- ^ Waelsch H (1955). Biochemistry of the Developing Nervous System: Proceedings. Academic Press. p. 453.
- ^ Acta Cytologica. International Academy of Cytology. 1958. p. 378.
- ^ Gauthier B, Le Dreff L, Aubry R (1958). "Hormone derivatives of long-lasting action. I. Esters of estradiol". Annales Pharmaceutiques Françaises. 16: 757–66. ISSN 0003-4509.
Treating 10 g. estradiol benzoate in 30 cc.dry C5H5N dropwise with 4.3 g. n-C6H13COCl (b20 71-2°), heating 1 hr. at 50-60°, pouring into 100 cc. 10% H2SO4, sepg. the oil after its solidification, washing with petr. ether, heating with 50 cc. MeOH, and cooling gave 10 g. 17-heptoyl-3β-benzoylestradiol, m. 95-8°. Dissolving 10 g. of this in 210 cc. 0.1N NaOH in MeOH and 40 cc. Me2CO with stirring, adding HCl to pH 7, filtering, evapg. in vacuo, and stirring the residue with petr. ether gave 7.9 g. 17-heptoyl-β-estradiol, m. 94-6° (iso-Pr2O). Adding to 5 g. estradiol stirred in 10 cc. anhyd. pyridine 8 g. n-C10H21COCl (b20 135-6°), keeping 1 hr. at 100°, cooling, adding 50 cc. 10% H2SO4, dissolving the sepd. ester in 50 cc. iso-Pr2O, washing with satd. NaHCO3 soln. and H2O, drying, and evapg. at room temp. gave 10.7 g. 3,17-diundecanoylestradiol, m. 48-9° (MeOH-Me2CO, then Me2O-Et2O), λmax. (0.005% in MeOH contg. 4% iso-Pr2O) 268 mμ, λmin. 282 and 250 mμ, inflexion 215 mμ. Stirring 8.8 g. estradiol divalerate in 90 cc. MeOH and 0.4 g. NaOH under N 210 min. to soln., adding 20% HCl to pH 7, evapg. in vacuo to 10 cc., keeping overnight at a low temp., and washing with H2O, MeOH, and petr. ether gave 4.4 g. 17-valeryl-β-estradiol, m. 145-6°, λmax. (0.005% in EtOH) 282 mμ, λmin. 248 mμ, inflexion 215 mμ. A single dose of 25 mg. of the diundecanate gave a therapeutic effect lasting 3 weeks.
- ^ ES 241206A1, Alter SA, "Esters of cortical hormones, androgens, or esterogens by transesterification and alcoholysis", published 1958-07-16
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 206–. ISBN 978-94-011-4439-1.
- ^ Milne GW (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 1404–. ISBN 978-1-351-78989-9.
- ^ a b c d "Archived copy". Archived from the original on 18 September 2018. Retrieved 19 September 2018.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ a b c d "Progestin Oral, Parenteral, Vaginal Advanced Patient Information".
- ^ a b c d e f Senanayake P, Potts M (14 April 2008). Atlas of Contraception, Second Edition. CRC Press. pp. 50–. ISBN 978-0-203-34732-4.
- ^ a b Lähteenmäki P (6 December 2012). "Intrauterine Hormone-Releasing Systems". In Rabe T, Runnebaum B (eds.). Fertility Control — Update and Trends: Update and Trends. Springer Science & Business Media. pp. 173-184 (183). ISBN 978-3-642-86696-8.
Two additional monthly, combined injectable methods warrant mention. Deladroxate (commercially labelled as Perlutan, Topasel, Agurin, Horprotal and Uno-Ciclo in various countries), is a combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate, and is available in many Latin American countries and Spain. The method is highly effective, without a single pregnancy reported in large clinical trials (Koetsawang 1994). Although available since the 1960s, the method has not been studied as extensively as Cyclofem or Mesigyna. The original manufacturer withdrew support due to toxicological concerns with dihydroxyprogesterone acetophenide, and clinical evaluations continue to be published. A recent dose-finding trial compared the standard available dose of 150/10 with a lower dose of 90/6, and concluded the lower dose was equally effective (Coutinho et al., 1997).
- ^ Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C (2013). "Combination injectable contraceptives for contraception". The Cochrane Database of Systematic Reviews. 3: CD004568. doi:10.1002/14651858.CD004568.pub3. PMC 6513542. PMID 23641480.
- ^ "Drug Product Database Online Query". 25 April 2012.
- ^ Speroff L, Fritz MA (2005). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 969–. ISBN 978-0-7817-4795-0.

![Estradiol levels after the most recent intramuscular injection during once-monthly 5 or 10 mg estradiol enanthate and 75 or 150 mg dihydroxyprogesterone acetophenide contraception in one premenopausal woman each.[43] Assays were performed using radioimmunoassay.[43] Source was Recio et al. (1986).[43]](http://upload.wikimedia.org/wikipedia/commons/thumb/1/19/Estradiol_levels_after_an_intramuscular_injection_of_different_doses_of_estradiol_enanthate_in_premenopausal_women.png/288px-Estradiol_levels_after_an_intramuscular_injection_of_different_doses_of_estradiol_enanthate_in_premenopausal_women.png)
![Estradiol levels after a single intramuscular injection of 10 mg estradiol enanthate in three postmenopausal women.[7] Assays were performed using radioimmunoassay.[7] Source was Wiemeyer et al. (1986).[7]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c7/Estradiol_levels_after_a_single_intramuscular_injection_of_10_mg_estradiol_enantate_in_postmenopausal_women.png/292px-Estradiol_levels_after_a_single_intramuscular_injection_of_10_mg_estradiol_enantate_in_postmenopausal_women.png)
![Estradiol and prolactin levels after the most recent intramuscular injection during once-monthly 10 mg estradiol enanthate and 150 mg dihydroxyprogesterone acetophenide contraception in 10 premenopausal women.[54] Only four determinations were made: days 0, 10, 20, and 30.[54] Assays were performed using radioimmunoassay.[54] Source was Garza-Flores et al. (1989).[54]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Estradiol_and_prolactin_levels_after_the_last_injection_during_therapy_with_estradiol_enantate_and_dihydroxyprogesterone_acetophenide_in_women.png/300px-Estradiol_and_prolactin_levels_after_the_last_injection_during_therapy_with_estradiol_enantate_and_dihydroxyprogesterone_acetophenide_in_women.png)
![Simplified curves of estradiol levels after an intramuscular injection of 10 mg estradiol enanthate (E2-EN) and 150 mg dihydroxyprogesterone acetophenide (DHPA) in oil solution with single or continuous once-monthly use in women.[44][10] Source was Garza-Flores (1994).[10]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/49/Estradiol_levels_with_estradiol_enanthate_and_dihydroxyprogesterone_acetophenide_after_single_or_repeated_injections_in_premenopausal_women.png/280px-Estradiol_levels_with_estradiol_enanthate_and_dihydroxyprogesterone_acetophenide_after_single_or_repeated_injections_in_premenopausal_women.png)
![Simplified curves of estradiol levels after injection of different estradiol esters in women.[10] Source was Garza-Flores (1994).[10]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8c/Idealized_curves_of_estradiol_levels_after_injection_of_different_estradiol_esters_in_women.png/258px-Idealized_curves_of_estradiol_levels_after_injection_of_different_estradiol_esters_in_women.png)