Solriamfetol: Difference between revisions
trim unneeded EL and remove ref parameter that is deprecated |
revise with more refs. move clutter out of first sentence |
||
| Line 56: | Line 56: | ||
}} |
}} |
||
'''Solriamfetol''' is a [[norepinephrine–dopamine reuptake inhibitor]] (NDRI) under development by Jazz Pharmaceuticals for the treatment of [[narcolepsy]] and [[sleep apnea]]. |
|||
'''Solriamfetol''' (developmental code names '''JZP-110''', '''ADX-N05''', others) is a [[norepinephrine–dopamine reuptake inhibitor]] (NDRI) which is under development by Jazz Pharmaceuticals for the treatment of [[narcolepsy]], [[sleep apnea]], [[hypersomnia]], and [[renal failure]].<ref name=AdisInsight>{{cite web|title=Solriamfetol - Jazz Pharmaceuticals/SK Biopharmaceuticals|url=https://adisinsight.springer.com/drugs/800030225|publisher=AdisInsight|accessdate=15 April 2018|language=en}}</ref> It is a low-[[potency (pharmacology)|potency]] NDRI, with respective [[IC50|IC<sub>50</sub>]] values for the [[dopamine transporter|dopamine]] and [[norepinephrine transporter]]s of 2.9 and 4.4 μM, and shows a low [[abuse potential|abuse liability]]. As of July 2017, it is in [[Phases of clinical research#Phase III|phase III]] [[clinical trial]]s for narcolepsy and sleep apnea, [[Phases of clinical research#Phase II|phase II]] clinical trials for [[hypersomnia]], and [[Phases of clinical research#Phase I|phase I]] clinical trials for renal failure.<ref name=AdisInsight/> |
|||
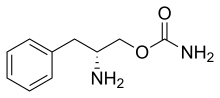
It is derived from [[phenylalanine]] and is chemical name is [R]-2-amino-3-phenylpropylcarbamate hydrochloride.<ref name=Abad2017>{{cite journal|last1=Abad|first1=VC|last2=Guilleminault|first2=C|title=New developments in the management of narcolepsy.|journal=Nature and science of sleep|date=2017|volume=9|pages=39-57|doi=10.2147/NSS.S103467|pmid=28424564|pmc=5344488}}</ref> |
|||
Its originator, Aerial BioPharma, ran two Phase II trials of the drug in narcolepsy<ref>{{cite journal|last1=Sullivan|first1=SS|last2=Guilleminault|first2=C|title=Emerging drugs for common conditions of sleepiness: obstructive sleep apnea and narcolepsy.|journal=Expert opinion on emerging drugs|date=2015|volume=20|issue=4|pages=571-82|doi=10.1517/14728214.2015.1115480|pmid=26558298}}</ref> before licensing solriamfetol to Jazz in 2014 for $125 million up front and up to $272 million in milestone payments.<ref>{{cite news|last1=Garde|first1=Damian|title=Jazz bets up to $397M on Aerial's narcolepsy drug|url=https://www.fiercebiotech.com/financials/jazz-bets-up-to-397m-on-aerial-s-narcolepsy-drug|work=FierceBiotech|date=January 14, 2014|language=en}}</ref> Aerial had also tested in animal models of depression, but did not advance that work to clinical trials.<ref>{{cite journal|last1=de Biase|first1=S|last2=Nilo|first2=A|last3=Gigli|first3=GL|last4=Valente|first4=M|title=Investigational therapies for the treatment of narcolepsy.|journal=Expert opinion on investigational drugs|date=August 2017|volume=26|issue=8|pages=953-963|doi=10.1080/13543784.2017.1356819|pmid=28726523}}</ref> During development it has been called ADX-N05 and JZP-110.<ref name=AdisInsight/> |
|||
In March 2018 the FDA accepted Jazz' NDA for use of solriamfetol in narcolepsy and sleep apnea.<ref name=AdisInsight>{{cite web|title=Solriamfetol - Jazz Pharmaceuticals/SK Biopharmaceuticals|url=https://adisinsight.springer.com/drugs/800030225|publisher=AdisInsight|accessdate=15 April 2018|language=en}}</ref> |
|||
==See also== |
==See also== |
||
Revision as of 08:48, 16 April 2018
 | |
| Clinical data | |
|---|---|
| Other names | JZP-110; R-228060; ADX-N05; YKP-10A; SKL-N05; O-Carbamoyl-D-phenylalaninol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H14N2O2 |
| Molar mass | 194.234 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Solriamfetol is a norepinephrine–dopamine reuptake inhibitor (NDRI) under development by Jazz Pharmaceuticals for the treatment of narcolepsy and sleep apnea.
It is derived from phenylalanine and is chemical name is [R]-2-amino-3-phenylpropylcarbamate hydrochloride.[1]
Its originator, Aerial BioPharma, ran two Phase II trials of the drug in narcolepsy[2] before licensing solriamfetol to Jazz in 2014 for $125 million up front and up to $272 million in milestone payments.[3] Aerial had also tested in animal models of depression, but did not advance that work to clinical trials.[4] During development it has been called ADX-N05 and JZP-110.[5]
In March 2018 the FDA accepted Jazz' NDA for use of solriamfetol in narcolepsy and sleep apnea.[5]
See also
References
- ^ Abad, VC; Guilleminault, C (2017). "New developments in the management of narcolepsy". Nature and science of sleep. 9: 39–57. doi:10.2147/NSS.S103467. PMC 5344488. PMID 28424564.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sullivan, SS; Guilleminault, C (2015). "Emerging drugs for common conditions of sleepiness: obstructive sleep apnea and narcolepsy". Expert opinion on emerging drugs. 20 (4): 571–82. doi:10.1517/14728214.2015.1115480. PMID 26558298.
- ^ Garde, Damian (January 14, 2014). "Jazz bets up to $397M on Aerial's narcolepsy drug". FierceBiotech.
- ^ de Biase, S; Nilo, A; Gigli, GL; Valente, M (August 2017). "Investigational therapies for the treatment of narcolepsy". Expert opinion on investigational drugs. 26 (8): 953–963. doi:10.1080/13543784.2017.1356819. PMID 28726523.
- ^ a b "Solriamfetol - Jazz Pharmaceuticals/SK Biopharmaceuticals". AdisInsight. Retrieved 15 April 2018.
