Halazepam
Appearance
 | |
 | |
| Clinical data | |
|---|---|
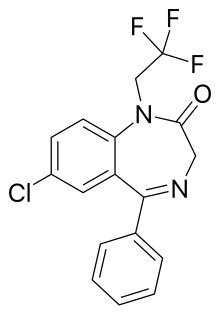
| Other names | 9-chloro- 6-phenyl- 2-(2,2,2-trifluoroethyl)- 2,5-diazabicyclo[5.4.0] undeca- 5,8,10,12-tetraen -3-one |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a684001 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | 14 hours (drug), 50-100 hours (metabolites). |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.281 |
| Chemical and physical data | |
| Formula | C17H12ClF3N2O |
| Molar mass | 352.7 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Halazepam[1] is a benzodiazepine derivative and is marketed under the brand names Alapryl and Pacinone It is no longer marketed in the United States. It had been marketed under the name Paxipam, but was withdrawn by its manufacturer, Schering Plough, for poor sales. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. It is a trifluoroethyl derivative of nordazepam.[2] While its structure may be similar to chlordiazepoxide and diazepam, it has both less toxicity and less tendency to cause paradoxical hostility and aggression than either of them.[3] Halazepam has active benzodiazepine metabolites.[4]
Indications
Halazepam is indicated for the treatment of anxiety.[5]
See also
References
- ^ FR Patent 1518382
- ^ Greenblatt, D. J. (June 12, 1982). "Halazepam, another precursor of desmethyldiazepam". Lancet. 1 (8285): 1358–9. doi:10.1016/s0140-6736(82)92424-2. PMID 6123659.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fann, W. E. (Mar–Apr 1982). "Pharmacology, efficacy, and adverse effects of halazepam, a new benzodiazepine". Pharmacotherapy. 2 (2): 72–9. PMID 6152591.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jochemsen R, Breimer DD (1984). "Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles". Curr Med Res Opin. 8. Suppl 4: 60–79. doi:10.1185/03007998409109545. PMID 6144464.
- ^ Lozano, Lozano, Lozano; et al. (1990). "[Open clinical study of the efficacy and safety of Halazepam in anxiety disorders]". Actas Luso Esp Neurol Psiquiatr Cienc Afines (in Spanish; Castilian). 18 (4): 205–11. PMID 1981637.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unrecognized language (link)
External links
