Ethyl loflazepate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Victan, Meilax, Ronlax |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | 51-103 h |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.976 |
| Chemical and physical data | |
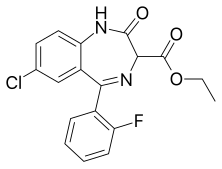
| Formula | C18H14ClFN2O3 |
| Molar mass | 360.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ethyl loflazepate[2] (marketed under the brand names Meilax, Ronlax and Victan)[3][4][5] is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties.[6] In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses.[7] Its elimination half-life is 51–103 hours.[8] Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound.[9] Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate.[10] The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate.[11] Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment.[12] The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children.[13] Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity.[14] High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.[15]
Ethyl loflazeplate is commercialized in Mexico, under the trade name Victan. It is officially approved for the following conditions:[16][17]
- Anxiety
- Post-trauma anxiety
- Anxiety associated with severe neuropathic pain
- Generalized anxiety disorder (GAD)
- Obsessive–compulsive disorder
- Panic attack
- Delirium tremens
See also
[edit]References
[edit]- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ DE 2012190
- ^ Ba BB, Iliadis A, Durand A, Berger Y, Cano JP (October 1988). "New approach in bioavailability study of two formulations of ethyl loflazepate". Arzneimittel-Forschung. 38 (10): 1486–1489. PMID 2904268.
- ^ Ba BB, Iliadis A, Cano JP (1989). "Pharmacokinetic modeling of ethyl loflazepate (Victan) and its main active metabolites". Annals of Biomedical Engineering. 17 (6): 633–646. doi:10.1007/BF02367467. PMID 2574017. S2CID 31310535.
- ^ "Benzodiazepine Names". non-benzodiazepines.org.uk. Archived from the original on 2008-12-08. Retrieved 2009-04-05.
- ^ Ueki S, Watanabe S, Yamamoto T, Shibata S, Shibata K, Ohta H, et al. (November 1983). "[Behavioral effects of ethyl loflazepate and its metabolites]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 82 (5): 395–409. doi:10.1254/fpj.82.395. PMID 6142848.
- ^ Mazue G, Berthe J, Newmann AJ, Brunaud M (October 1981). "A toxicologic evaluation of ethyl fluclozepate (CM 6912)". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 19 (10): 453–472. PMID 6116677.
- ^ Cautreels W, Jeanniot JP (November 1980). "Quantitative analysis of CM 6912 (ethyl loflazepate) and its metabolites in plasma and urine by chemical ionization gas chromatography mass spectrometry. Application to pharmacokinetic studies in man". Biomedical Mass Spectrometry. 7 (11–12): 565–571. doi:10.1002/bms.1200071124. PMID 6112027.
- ^ Sakai Y, Namima M (April 1985). "Inhibitory effect on 3H-diazepam binding and potentiating action on GABA of ethyl loflazepate, a new minor tranquilizer". Japanese Journal of Pharmacology. 37 (4): 373–379. doi:10.1254/jjp.37.373. PMID 2861304.
- ^ Chambon JP, Perio A, Demarne H, Hallot A, Dantzer R, Roncucci R, Bizière K (1985). "Ethyl loflazepate: a prodrug from the benzodiazepine series designed to dissociate anxiolytic and sedative activities". Arzneimittel-Forschung. 35 (10): 1573–1577. PMID 2866771.
- ^ Davi H, Guyonnet J, Sales Y, Cautreels W (1985). "Metabolism of ethyl loflazepate in the rat, the dog, the baboon and in man". Arzneimittel-Forschung. 35 (7): 1061–1065. PMID 2864933.
- ^ Ba BB, Iliadis A, Durand A, Berger Y, Necciari J, Cano JP (1989). "Effect of renal failure on the pharmacokinetics of ethyl loflazepate (Victan) in man". Fundamental & Clinical Pharmacology. 3 (1): 11–17. doi:10.1111/j.1472-8206.1989.tb00025.x. PMID 2714728. S2CID 2850707.
- ^ Pulce C, Mollon P, Pham E, Frantz P, Descotes J (April 1992). "Acute poisonings with ethyle loflazepate, flunitrazepam, prazepam and triazolam in children". Veterinary and Human Toxicology. 34 (2): 141–143. PMID 1354907.
- ^ Kamijo Y, Hayashi I, Nishikawa T, Yoshimura K, Soma K (March 2005). "Pharmacokinetics of the active metabolites of ethyl loflazepate in elderly patients who died of asphyxia associated with benzodiazepine-related toxicity". Journal of Analytical Toxicology. 29 (2): 140–144. doi:10.1093/jat/29.2.140. PMID 15902983.
- ^ Imanishi T, Onozawa K, Hayashi A, Baba J (December 2001). "[Effects of fluvoxamine on both the desired anxiolytic effect and the adverse motor incoordination and amnesia induced by benzodiazepines]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 118 (6): 403–410. doi:10.1254/fpj.118.403. PMID 11778459.
- ^ "Victan". saludzac.gob.mx/. Archived from the original on 2009-05-26. Retrieved 2009-12-25.
- ^ "VICTAN". Farmacia Guerra. Retrieved 2009-12-25.
External links
[edit]- "Meilax Fine Granules" (PDF) (in Japanese).
