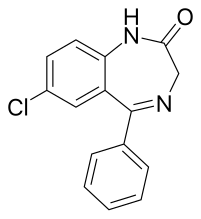
Nordazepam
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Nordiazepam, desoxydemoxepam, desmethyldiazepam |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | 36-200 hours[2] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.840 |
| Chemical and physical data | |
| Formula | C15H11ClN2O |
| Molar mass | 270.72 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nordazepam (INN; marketed under brand names Nordaz, Stilny, Madar, Vegesan, and Calmday; also known as nordiazepam, desoxydemoxepam, and desmethyldiazepam) is a 1,4-benzodiazepine derivative. Like other benzodiazepine derivatives, it has amnesic, anticonvulsant, anxiolytic, muscle relaxant, and sedative properties. However, it is used primarily in the treatment of anxiety disorders. It is an active metabolite of diazepam, chlordiazepoxide, clorazepate, prazepam, pinazepam, and medazepam.[3]
Nordazepam is among the longest lasting (longest half-life) benzodiazepines, and its occurrence as a metabolite is responsible for most cumulative side-effects of its myriad of pro-drugs when they are used repeatedly at moderate-high doses; the nordazepam metabolite oxazepam is also active (and is a more potent, full BZD-site agonist), which contributes to nordazepam cumulative side-effects but occur too minutely to contribute to the cumulative side-effects of nordazepam pro-drugs (except when they are abused chronically in extremely supra-therapeutic doses).[citation needed]
Side effects
[edit]Common side effects of nordazepam include somnolence, which is more common in elderly patients and/or people on high-dose regimens. Hypotonia, which is much less common, is also associated with high doses and/or old age.
Contraindications and special caution
[edit]Benzodiazepines require special precaution if used in the elderly, during pregnancy, in children, alcohol- or drug-dependent individuals, and individuals with comorbid psychiatric disorders.[4] As with many other drugs, changes in liver function associated with aging or diseases such as cirrhosis, may lead to impaired clearance of nordazepam.[5]
Pharmacology
[edit]Nordazepam is a partial agonist at the GABAA receptor, which makes it less potent than other benzodiazepines, particularly in its amnesic and muscle-relaxing effects.[6] Its elimination half life is between 36 and 200 hours, with wide variation among individuals; factors such as age and sex are known to impact it.[2] The variation of reported half-lives are attributed to differences in nordazepam metabolism and that of its metabolites as nordazepam is hydroxylated to active metabolites such as oxazepam, before finally being glucuronidated and excreted in the urine.[7] This can be attributed to extremely variable hepatic and renal metabolic functions among individuals depending upon a number of factors (including age, ethnicity, disease, and current or previous use/abuse of other drugs/medicines).
Chemistry
[edit]Nordazepam is similar to diazepam, except that the methyl group at the R1 position has been replaced with a hydrogen. Nordazepam can be synthesized with 2-amino-5-chlorobenzophenone and chloroacetyl chloride.[8] Nordazepam itself can also be used in the synthesis of diazepam by methylating the R1 position using dimethyl sulfate.[8]
Pregnancy and nursing mothers
[edit]Nordazepam, like other benzodiazepines, easily crosses the placental barrier, so the drug should not be administered during the first trimester of pregnancy.[9] In case of serious medical reasons, nordazepam can be given in late pregnancy, but the fetus, due to the pharmacological action of the drug, may experience side effects such as hypothermia, hypotonia, and sometimes mild respiratory depression. Since nordazepam and other benzodiazepines are excreted in breast milk, the substance should not be administered to mothers who are breastfeeding. Discontinuing of breast-feeding is indicated for regular intake by the mother.[10]
Recreational use
[edit]Nordazepam and other sedative-hypnotic drugs are detected frequently in cases of people suspected of driving under the influence of drugs. Many drivers have blood levels far exceeding the therapeutic dose range, suggesting benzodiazepines are commonly used in doses higher than the recommended doses.[11]
See also
[edit]- Benzodiazepine
- Benzodiazepine dependence
- Benzodiazepine withdrawal syndrome
- Long-term effects of benzodiazepines
References
[edit]- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b Ashton CH (March 2007). "Benzodiazepine Equivalence Table". benzo.org.uk. Retrieved 2009-04-05.
- ^ Ator NA, Griffiths RR (September 1997). "Selectivity in the generalization profile in baboons trained to discriminate lorazepam: benzodiazepines, barbiturates and other sedative/anxiolytics". The Journal of Pharmacology and Experimental Therapeutics. 282 (3): 1442–1457. PMID 9316858.
- ^ Authier N, Balayssac D, Sautereau M, Zangarelli A, Courty P, Somogyi AA, et al. (November 2009). "Benzodiazepine dependence: focus on withdrawal syndrome". Annales Pharmaceutiques Françaises. 67 (6): 408–413. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ^ Klotz U, Müller-Seydlitz P (January 1979). "Altered elimination of desmethyldiazepam in the elderly". British Journal of Clinical Pharmacology. 7 (1): 119–120. doi:10.1111/j.1365-2125.1979.tb00908.x. PMC 1429605. PMID 367407.
- ^ Gobbi M, Barone D, Mennini T, Garattini S (May 1987). "Diazepam and desmethyldiazepam differ in their affinities and efficacies at 'central' and 'peripheral' benzodiazepine receptors". The Journal of Pharmacy and Pharmacology. 39 (5): 388–391. doi:10.1111/j.2042-7158.1987.tb03404.x. PMID 2886589. S2CID 24858867.
- ^ Marland A, Sarkar P, Leavitt R (Jan–Feb 1999). "The urinary elimination profiles of diazepam and its metabolites, nordiazepam, temazepam, and oxazepam, in the equine after a 10-mg intramuscular dose". Journal of Analytical Toxicology. 23 (1): 29–34. doi:10.1093/jat/23.1.29. PMID 10022206.
- ^ a b Massah AR, Gharaghani S, Lordejani HA, Asakere N (August 2016). "New and mild method for the synthesis of alprazolam and diazepam and computational study of their binding mode to GABAA receptor". Medicinal Chemistry Research. 25 (8): 1538–1550. doi:10.1007/s00044-016-1585-z. ISSN 1054-2523.
- ^ Olive G, Rey E (1983). "[Benzodiazepines and pregnancy. Transplacental passage, labor and lactation]". L'Encéphale (in French). 9 (4 Suppl 2): 87B–96B. PMID 6144535.
- ^ Dusci LJ, Good SM, Hall RW, Ilett KF (January 1990). "Excretion of diazepam and its metabolites in human milk during withdrawal from combination high dose diazepam and oxazepam". British Journal of Clinical Pharmacology. 29 (1): 123–126. doi:10.1111/j.1365-2125.1990.tb03612.x. PMC 1380071. PMID 2105100.
- ^ Jones AW, Holmgren A, Kugelberg FC (April 2007). "Concentrations of scheduled prescription drugs in blood of impaired drivers: considerations for interpreting the results". Therapeutic Drug Monitoring. 29 (2): 248–260. doi:10.1097/FTD.0b013e31803d3c04. PMID 17417081. S2CID 25511804.
