Minoxidil
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Loniten, Rogaine, others |
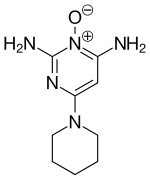
| Other names | 2,4-Diamino-6-piperidinopyrimidine 3-oxide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682608 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Primarily liver |
| Elimination half-life | 4.2 h |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.959 |
| Chemical and physical data | |
| Formula | C9H15N5O |
| Molar mass | 209.253 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 248 °C (478 °F) |
| Solubility in water | <1 |
| |
| |
| | |
Minoxidil is a medication used for the treatment of high blood pressure and pattern hair loss.[5][6][7] It is an antihypertensive and a vasodilator.[10] It is available as a generic medication by prescription in oral tablet form and over the counter as a topical liquid or foam.[8][9][11][12]
Medical uses[edit]
Minoxidil, when used for hypertension, is generally reserved for use in severe hypertension patients who do not respond to at least two agents and a diuretic.[13] Minoxidil is also generally administered with a loop diuretic to prevent sodium and potassium retention.[13] It may also cause a reflex tachycardia and thus is prescribed with a beta blocker.[13]
Hair loss[edit]
Minoxidil, applied topically, is used for the treatment of hair loss.[14] It is effective in helping promote hair growth in people with androgenic alopecia regardless of sex.[14] Minoxidil must be used indefinitely for continued support of existing hair follicles and the maintenance of any experienced hair regrowth.[6][7]
Low-dose oral minoxidil (LDOM) is used off-label against hair loss and to promote hair regrowth.[15] Oral minoxidil has been found to be an effective and well-tolerated treatment alternative for patients having difficulty with topical formulations.[16][17][18]
Side effects[edit]

Topically applied minoxidil is generally well tolerated, but common side effects include itching of the eyes, general itching, irritation at the treated area, and unwanted hair growth elsewhere on the body.[20]
Alcohol and propylene glycol present in some topical preparations may dry the scalp, resulting in dandruff and contact dermatitis.[21]
Side effects of oral minoxidil may include swelling of the face and extremities, rapid heartbeat, or lightheadedness. Cardiac lesions, such as focal necrosis of the papillary muscle and subendocardial areas of the left ventricle, have been observed in laboratory animals treated with minoxidil.[8] Pseudoacromegaly is an extremely rare side effect reported with large doses of oral minoxidil.[22]
In 2013 or 2014, a seven-year-old girl was admitted to a children's hospital in Toulouse in France after accidentally ingesting a teaspoon of Alopexy (a brand name for minoxidil in France). The child vomited constantly after ingestion and showed hypotension and tachycardia for 40 hours.[23] The authors of the report on the incident stressed that the product should be kept out of reach of children, and urged manufacturers to consider more secure child-resistant packaging.[24]
Pharmacology[edit]
Mechanism of action[edit]
The mechanism by which minoxidil promotes hair growth is not fully understood. Minoxidil is an adenosine 5'-triphosphate-sensitive potassium channel opener,[25] causing hyperpolarization of cell membranes. Theoretically, by widening blood vessels and opening potassium channels, it allows more oxygen, blood, and nutrients to the follicles. Moreover, minoxidil contains a nitric oxide moiety and may act as a nitric oxide agonist. This may cause follicles in the telogen phase to shed, which are then replaced by thicker hairs in a new anagen phase. Minoxidil is a prodrug that is converted by sulfation via the sulfotransferase enzyme SULT1A1 to its active form, minoxidil sulfate. The effect of minoxidil is mediated by adenosine, which triggers intracellular signal transduction via both adenosine A1 receptors and two sub-types of adenosine A2 receptors (A2A and A2B receptors).[26] Minoxidil acts as an activator of the Kir6/SUR2 channel upon selective binding to SUR2.[27] The expression of SUR2B in dermal papilla cells might play a role in the production of adenosine.[26] Minoxidil induces cell growth factors such as VEGF, HGF, IGF-1 and potentiates HGF and IGF-1 actions by the activation of uncoupled sulfonylurea receptor on the plasma membrane of dermal papilla cells.[28]
A number of in vitro effects of minoxidil have been described in monocultures of various skin and hair follicle cell types including stimulation of cell proliferation, inhibition of collagen synthesis, and stimulation of vascular endothelial growth factor, prostaglandin synthesis and leukotriene B4 expression.[29]
Minoxidil causes a redistribution of cellular iron through its apparent capacity to bind this metal ion. By binding iron in a Fenton-reactive form, intracellular hydroxyl radical production would ensue, but hydroxyl would be immediately trapped and scavenged by the minoxidil to generate a nitroxyl radical. It is presumed that this nitroxyl radical will be capable of reduction by glutathione to reform minoxidil. Such a process would cycle until the minoxidil is otherwise metabolized and would result in rapid glutathione depletion with glutathione disulphide formation and therefore with concomitant consumption of NADPH/ NADH and other reducing equivalents.[30] Minoxidil inhibited PHD by interfering with the normal function of ascorbate, a cofactor of the enzyme, leading to a stabilization of HIF-1α protein and a subsequent activation of HIF-1. In an in vivo angiogenesis assay, millimolar minoxidil increased blood vessel formation in a VEGF-dependent manner. Minoxidil inhibition of PHD occurs via interrupting ascorbate binding to iron.[31] The structural feature of positioning amines adjacent to nitric oxide may confer the ability of millimolar minoxidil to chelate iron, thereby inhibiting PHD. Minoxidil is capable of tetrahydrobiopterin inhibition as a cofactor for nitric oxide synthase.[32]
Minoxidil stimulates prostaglandin E2 production by activating COX-1[33] and prostaglandin endoperoxide synthase-1 but inhibits prostacyclin production. Additionally, expression of the prostaglandin E2 receptor, the most upregulated target gene in the β-catenin pathway of DP cells, was enhanced by minoxidil, which may enable hair follicles to grow continuously and maintain the anagen phase.[34]
Due to anti-fibrotic activity of minoxidil inhibition of enzyme lysyl hydroxylase present in fibroblast may result in synthesis of a hydroxylysine-deficient collagen. Minoxidil can also potentially stimulate elastogenesis in aortic smooth muscle cells, and in skin fibroblasts in a dose-dependent manner. In hypertensive rats, minoxidil increases elastin level in the mesenteric, abdominal, and renal arteries by a decrease in "elastase" enzyme activity in these tissues. In rats, potassium channel openers decrease calcium influx which inhibits elastin gene transcription through extracellular signal-regulated kinase 1/2 (ERK 1/2)-activator protein 1 signaling pathway. ERK 1/2 increases, through elastin gene transcription, adequately cross-linked elastic fiber content synthesized by smooth muscle cells, and decreases the number of cells in the aorta.[35]
Minoxidil possesses alpha 2-adrenoceptor agonist activity,[36] stimulates the peripheral sympathetic nervous system (SNS) by way of carotid and aortic baroreceptor reflexes. Minoxidil administration also brings an increase in plasma renin activity, largely due to the aforementioned activation of the SNS. This activation of the renin-angiotensin axis further prompts increased biosynthesis of aldosterone; whereas plasma and urinary aldosterone levels are increased early in the course of treatment with minoxidil, over time these values tend to normalize presumably because of accelerated metabolic clearance of aldosterone in association with hepatic vasodilation.[13]
Minoxidil may be involved in the inhibition of serotonergic (5-HT2) receptors.[37]
Minoxidil might increase blood-tumor barrier permeability in a time-dependent manner by down-regulating tight junction protein expression and this effect could be related to ROS/RhoA/PI3K/PKB signal pathway.[38] Minoxidil significantly increases ROS concentration when compared to untreated cells.
In vitro Minoxidil treatment resulted in a 0.22 fold change for 5α-R2 (p < 0.0001). This antiandrogenic effect of minoxidil, shown by significant downregulation of 5α-R2 gene expression in HaCaT cells, may be one of its mechanisms of action in alopecia.[39]
Minoxidil is less effective when the area of hair loss is large. In addition, its effectiveness has largely been demonstrated in younger men who have experienced hair loss for less than 5 years. Minoxidil use is indicated for central (vertex) hair loss only.[40] Two clinical studies are being conducted in the US for a medical device that may allow patients to determine if they are likely to benefit from minoxidil therapy.[41]
Conditions such as Cantú syndrome have been shown to mimic the pharmacological properties of minoxidil.[42]
Chemistry[edit]
Minoxidil is an odorless, white to off-white, crystalline powder (crystals from methanol-acetonitrile). When heated to decomposition it emits toxic fumes of nitrogen oxides. It decomposes at 259-261 °C.[43]
Solubility (mg/ml): propylene glycol 75, methanol 44, ethanol 29, 2-propanol 6.7, dimethylsulfoxide 6.5, water 2.2, chloroform 0.5, acetone <0.5, ethyl acetate <0.5, diethyl ether <0.5, benzene <0.5, acetonitrile <0.5.
Commercially available minoxidil topical solution should be stored at a temperature of 20-25 °C. Extemporaneous formulations of minoxidil have been reported to have variable stability, depending on the vehicle and method of preparation, and the FDA requests that physicians and pharmacists refrain from preparing extemporaneous topical formulations using commercially available minoxidil tablets. Minoxidil tablets should be stored in well-closed containers at 15-30 °C.
Minoxidil, 6-amino-1,2-dihydro-1-hydroxy-2-imino-4-piperidinopyrimidine, is synthesized from barbituric acid, the reaction of which with phosphorus oxychloride gives 2,4,6-trichloropyrimidine. Upon reaction with ammonium, this turns into 2,4-diamino-6-chloropyrimidine. Next, the resulting 2,4-diamino-6-chloropyrimidine undergoes reaction with 2,4-dichlorophenol in the presence of potassium hydroxide, giving 2,4-diamino-6-(2,4-dichlorophenoxy)-pyrimidine. Oxidation of this product with 3-chloroperbenzoic acid gives 2,4-diamino-6-(2,4-dichlorophenoxy)pyrimidine-3-oxide, the 2,4-dichlorophenoxyl group of which is replaced with a piperidine group at high temperature, giving minoxidil.[44]
Another synthesis approach is depicted here:

Compounds related to minoxidil include kopexil (diaminopyrimidine oxide).
History[edit]
Initial application[edit]
Minoxidil was developed in the late 1950s by the Upjohn Company (later became part of Pfizer) to treat ulcers. In trials using dogs, the compound did not cure ulcers, but proved to be a powerful vasodilator. Upjohn synthesized over 200 variations of the compound, including the one it developed in 1963 and named minoxidil.[45] These studies resulted in the U.S. Food and Drug Administration (FDA) approving minoxidil (with the brand name 'Loniten') in the form of oral tablets to treat high blood pressure in 1979.[46][47]
Hair growth[edit]
When Upjohn received permission from the U.S. Food and Drug Administration (FDA) to test the new drug as medicine for hypertension they approached Charles A. Chidsey, at the University of Colorado School of Medicine.[45] He conducted two studies,[48][49] the second study showing unexpected hair growth. Puzzled by this side-effect, Chidsey consulted Guinter Kahn (who while a dermatology resident at the University of Miami had been the first to observe and report hair development on patients using the minoxidil patch) and discussed the possibility of using minoxidil for treating hair loss.
Kahn, along with his colleague Paul J. Grant, had obtained a certain amount of the drug and conducted their own research, since they were first to make the side effect observation. Neither Upjohn or Chidsey at the time were aware of the side effect of hair growth.[50] The two doctors had been experimenting with a 1% solution of minoxidil mixed with several alcohol-based liquids.[51] Both parties filed patents to use the drug for hair loss prevention, which resulted in a decade-long trial between Kahn and Upjohn, which ended with Kahn's name included in a consolidated patent (U.S. #4,596,812 Charles A Chidsey, III and Guinter Kahn) in 1986 and royalties from the company to both Kahn and Grant.[50]
Meanwhile, the effect of minoxidil on hair loss prevention was so clear that in the 1980s physicians were prescribing Loniten off-label to their balding patients.[47]
In August 1988, the FDA approved the drug for treating baldness in men[47][51] under the brand name "Rogaine" (FDA rejected Upjohn's first choice, Regain, as misleading[52]). The agency concluded that although "the product will not work for everyone", 39% of the men studied had "moderate to dense hair growth on the crown of the head".[52] "Men's Rogaine", marketed by Johnson & Johnson went off-patent on 20 January 2006.[53]
In 1991, Upjohn made the product available for women.[51] "Women's Rogaine", marketed by Johnson & Johnson, went off-patent on 14 February 2014.[53]
Society and culture[edit]
Economics[edit]
In February 1996, the FDA approved both the over-the-counter sale of the medication and the production of generic formulations of minoxidil.[47] Upjohn replied to that by lowering prices to half the price of the prescription drug[51] and by releasing a prescription 5% formula of Rogaine in 1997.[47][54] In 1998, a 5% formulation of minoxidil was approved for nonprescription sale by the FDA.[55] The 5% aerosol foam formula was approved for medical use in the US in 2006.[56][57] The generic versions of the 5% aerosol foam formula were approved in 2017.[58][59]
In 2017, JAMA published a study of pharmacy prices in four states for 41 over-the-counter minoxidil products which were "gender-specified". The authors found that the mean price for minoxidil solutions was the same for women and men even though the women's formulations were 2% and the men's were 5%, while the mean price for minoxidil foams, which were all 5%, was 40% higher for women. The authors noted this was the first time gender-based pricing had been shown for a medication.[60]
Brand names[edit]
As of June 2017[update], Minoxidil was marketed under many trade names worldwide: Alomax, Alopek, Alopexy, Alorexyl, Alostil, Aloxid, Aloxidil, Anagen, Apo-Gain, Axelan, Belohair, Boots Hair Loss Treatment, Botafex, Capillus, Carexidil, Coverit, Da Fei Xin, Dilaine, Dinaxcinco, Dinaxil, Ebersedin, Eminox, Folcare, Follixil, Guayaten, Hair Grow, Hair-Treat, Hairgain, Hairgaine, Hairgrow, Hairway, Headway, Inoxi, Ivix, Keranique, Lacovin, Locemix, Loniten, Lonnoten, Lonolox, Lonoten, Loxon, M E Medic, Maev-Medic, Mandi, Manoxidil, Mantai, Men's Rogaine, Minodil, Minodril, Minostyl, Minovital, Minox, Minoxi, Minoxidil, Minoxidilum, Minoximen, Minoxiten, Minscalp, Mintop, Modil, Morr, Moxidil, Neo-Pruristam, Neocapil, Neoxidil, Nherea, Nioxin, Noxidil, Oxofenil, Pilfud, Pilogro, Pilomin, Piloxidil,Re-Stim, Re-Stim+, Recrea, Regain, Regaine, Regaxidil, Regro, Regroe, Regrou, Regrowth, Relive, Renobell Locion, Reten, Rexidil, Rogaine, Rogan, Si Bi Shen, Splendora, Superminox, Trefostil, Tricolocion, Tricoplus, Tricovivax, Tricoxane, Trugain, Tugain, Unipexil, Vaxdil, Vius, Women's Regaine, Xenogrow, Xtreme Boost, Xtreme Boost+, Xue Rui, Ylox, and Zeldilon.[61] It was also marketed as combination drug with amifampridine under the brand names Gainehair and Hair 4 U, and as a combination with tretinoin and clobetasol under the brand name Sistema GB.[61]
Research[edit]
Minoxidil is being investigated as a potential treatment for treating ovarian cancer.[62]
Toxicity to animals[edit]
Minoxidil is highly toxic to dogs and cats, even in doses as small as a drop or lick.[63] There are reported cases of cats dying shortly after coming in contact with minimal amounts of the substance.[64]
There is no specific antidote, but lipid rescue has been used successfully.[65][66]
References[edit]
- ^ a b "Loniten Product Information". Therapeutic Goods Administration (TGA). Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved October 22, 2023.
- ^ "59445 WOMEN'S REGAINE EXTRA STRENGTH Minoxidil 5% application bottle". Therapeutic Goods Administration (TGA) / Australian Government Department of Health and Aged Care. Retrieved February 15, 2022.
- ^ "Search the TGA website". Tga-search.clients.funnelback.com. Archived from the original on August 16, 2021.
- ^ a b "Loniten Tablets 5mg - Summary of Product Characteristics (SmPC)". Medicines.org.uk. November 24, 2020. Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ a b c "Regaine for Men Extra Strength Scalp Foam 5% w/w Cutaneous Foam (GSL) - Summary of Product Characteristics (SmPC)". (emc). April 27, 2021. Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ a b c "Regaine for Women Once a Day Scalp Foam 5% w/w Cutaneous Foam - Summary of Product Characteristics (SmPC)". Medicines.org.uk. October 28, 2020. Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ a b c "Minoxidil tablet". DailyMed. Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ a b "Minoxidil aerosol, foam". DailyMed. Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ "Vasodilators". mayoclinic.com. Archived from the original on March 9, 2011.
- ^ "Mens Rogaine Extra Strength Unscented- minoxidil solution". DailyMed. Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ "Womens Rogaine Unscented- minoxidil solution". DailyMed. Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ a b c d Sica DA (May 2004). "Minoxidil: an underused vasodilator for resistant or severe hypertension". Journal of Clinical Hypertension. 6 (5): 283–287. doi:10.1111/j.1524-6175.2004.03585.x. PMC 8109604. PMID 15133413. S2CID 21366804.
- ^ a b Varothai S, Bergfeld WF (July 2014). "Androgenetic alopecia: an evidence-based treatment update". American Journal of Clinical Dermatology. 15 (3): 217–230. doi:10.1007/s40257-014-0077-5. PMID 24848508. S2CID 31245042.
- ^ Kolata G (August 18, 2022). "An Old Medicine Grows New Hair for Pennies a Day, Doctors Say". The New York Times. Archived from the original on August 19, 2022.
- ^ Randolph M, Tosti A (March 2021). "Oral minoxidil treatment for hair loss: A review of efficacy and safety". Journal of the American Academy of Dermatology. 84 (3): 737–746. doi:10.1016/j.jaad.2020.06.1009. PMID 32622136. S2CID 220348503.
- ^ Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, Moreno-Arrones OM, Ortega-Quijano D, Fernandez-Nieto D, et al. (November 2020). "Safety of low-dose oral minoxidil treatment for hair loss. A systematic review and pooled-analysis of individual patient data". Dermatologic Therapy. 33 (6): e14106. doi:10.1111/dth.14106. PMID 32757405. S2CID 221017080.
- ^ Sharma AN, Michelle L, Juhasz M, Muller Ramos P, Atanaskova Mesinkovska N (August 2020). "Low-dose oral minoxidil as treatment for non-scarring alopecia: a systematic review". International Journal of Dermatology. 59 (8): 1013–1019. doi:10.1111/ijd.14933. PMID 32516434. S2CID 219561573.
- ^ Wu M, Yu Q, Li Q (December 2016). "Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases". Oncotarget. 7 (50): 82074–82084. doi:10.18632/oncotarget.12617. PMC 5347675. PMID 27738338.
- ^ "Rogaine Side Effects". Drugs.com. Archived from the original on September 22, 2017. Retrieved December 8, 2020.
- ^ Pray WS (2001). "Dandruff and Seborrheic Dermatitis". US Pharmacist. 26 (4): 16–24. Archived from the original on October 28, 2010.
- ^ Nguyen KH, Marks JG (June 2003). "Pseudoacromegaly induced by the long-term use of minoxidil". Journal of the American Academy of Dermatology. 48 (6): 962–965. doi:10.1067/mjd.2003.325. PMID 12789195.
- ^ Claudet I, Cortey C, Honorat R, Franchitto N (January 1, 2015). "Minoxidil Topical Solution: An Unsafe Product for Children". Pediatric Emergency Care. 31 (1): 44–46. doi:10.1097/PEC.0000000000000301. ISSN 0749-5161.
- ^ Neumann J (December 10, 2014). "Hair loss treatment may be dangerous to kids". Reuters. Archived from the original on May 17, 2021. Retrieved March 23, 2021.
- ^ Wang T (February 2003). "The effects of the potassium channel opener minoxidil on renal electrolytes transport in the loop of henle". The Journal of Pharmacology and Experimental Therapeutics. 304 (2): 833–840. doi:10.1124/jpet.102.043380. PMID 12538840. S2CID 6948410.
- ^ a b Li M, Marubayashi A, Nakaya Y, Fukui K, Arase S (December 2001). "Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: possible involvement of sulfonylurea receptor 2B as a target of minoxidil". The Journal of Investigative Dermatology. 117 (6): 1594–1600. doi:10.1046/j.0022-202x.2001.01570.x. PMID 11886528.
The expression of sulfonylurea receptor 2B and of the adenosine A1, A2A, and A2B receptors was detected in dermal papilla cells by means of reverse transcription polymerase chain reaction analysis.
- ^ Fukushiro-Lopes D, Hegel AD, Russo A, Senyuk V, Liotta M, Beeson GC, et al. (May 8, 2020). "Repurposing Kir6/SUR2 Channel Activator Minoxidil to Arrests Growth of Gynecologic Cancers". Frontiers in Pharmacology. 11: 577. doi:10.3389/fphar.2020.00577. PMC 7227431. PMID 32457608.
- ^ Otomo S (March 2002). "[Hair growth effect of minoxidil]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 119 (3): 167–174. doi:10.1254/fpj.119.167. PMID 11915519.
- ^ Messenger AG, Rundegren J (February 2004). "Minoxidil: mechanisms of action on hair growth". The British Journal of Dermatology. 150 (2): 186–194. doi:10.1111/j.1365-2133.2004.05785.x. PMID 14996087. S2CID 19308112.
- ^ Chung LY, Andrews AM, Schmidt RJ, Turner TD (1992). "Effects of minoxidil on cell proliferation and intracellular glutathione status of murine (L929) fibroblasts". In Harding KG, Leaper DL, Turner TD (eds.). Proceedings of the 1st European Conference on Advances in Wound Management, Cardiff, 4-6 September 1991. Macmillan Magazines. pp. 122–128. ISBN 978-0-333-58645-7.
- ^ Yum S, Jeong S, Kim D, Lee S, Kim W, Yoo JW, et al. (December 2017). "Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase". International Journal of Molecular Sciences. 19 (1): 53. doi:10.3390/ijms19010053. PMC 5796003. PMID 29295567.
- ^ US 5874433, Gross SS, "Blocking utilization of tetrahydrobiopterin to block induction of nitric oxide synthesis", published 1999-02-23
- ^ Hwang JH, Chu H, Ahn Y, Kim J, Kim DY (April 2019). "HMGB1 promotes hair growth via the modulation of prostaglandin metabolism". Scientific Reports. 9 (1): 6660. Bibcode:2019NatSR...9.6660H. doi:10.1038/s41598-019-43242-2. PMC 6491442. PMID 31040377.
- ^ Suchonwanit P, Thammarucha S, Leerunyakul K (2019). "Minoxidil and its use in hair disorders: a review". Drug Design, Development and Therapy. 13: 2777–2786. doi:10.2147/DDDT.S214907. PMC 6691938. PMID 31496654.
- ^ Kassai B, Bouyé P, Gilbert-Dussardier B, Godart F, Thambo JB, Rossi M, et al. (May 2019). "Minoxidil versus placebo in the treatment of arterial wall hypertrophy in children with Williams Beuren Syndrome: a randomized controlled trial". BMC Pediatrics. 19 (1): 170. doi:10.1186/s12887-019-1544-1. PMC 6537216. PMID 31138170.
- ^ Sharma N, Mehta AA, Santani DD, Goyal RK (September 1997). "Evidence for alpha 2-adrenoceptor agonist activity of minoxidil". The Journal of Pharmacy and Pharmacology. 49 (9): 935–937. doi:10.1111/j.2042-7158.1997.tb06139.x. PMID 9306265. S2CID 84233773.
- ^ Wasiński R, Godlewski G, Wróbel B, Buckzko W (May 1995). "Interactions of minoxidil with vasoconstrictive agents in isolated rat tail artery". Acta Poloniae Pharmaceutica. 52 (3): 253–256. PMID 8960256.
- ^ Gu YT, Xue YX, Wang YF, Wang JH, Chen X, ShangGuan QR, et al. (December 2013). "Minoxidil sulfate induced the increase in blood-brain tumor barrier permeability through ROS/RhoA/PI3K/PKB signaling pathway". Neuropharmacology. 75: 407–415. doi:10.1016/j.neuropharm.2013.08.004. PMID 23973310. S2CID 20676988.
- ^ Pekmezci E, Türkoğlu M (December 2017). "Minoxidil Acts as an Antiandrogen: A Study of 5α-reductase Type 2 Gene Expression in a Human Keratinocyte Cell Line" (PDF). Acta Dermatovenerologica Croatica. 25 (4): 271–275. PMID 30064598. Archived (PDF) from the original on September 3, 2021. Retrieved September 3, 2021.
- ^ Scow DT, Nolte RS, Shaughnessy AF (April 1999). "Medical treatments for balding in men". American Family Physician. 59 (8): 2189–94, 2196. PMID 10221304. Archived from the original on September 28, 2012.
- ^ Clinical trial number NCT02198261 for "Minoxidil Response Testing in Males With Androgenetic Alopecia" at ClinicalTrials.gov
- ^ Ohko K, Nakajima K, Nakajima H, Hiraki Y, Kubota K, Fukao T, et al. (March 2020). "Skin and hair abnormalities of Cantu syndrome: A congenital hypertrichosis due to a genetic alteration mimicking the pharmacological effect of minoxidil". The Journal of Dermatology. 47 (3): 306–310. doi:10.1111/1346-8138.15216. PMID 31907964. S2CID 210042523.
- ^ "COMPOUND SUMMARY Minoxidil". PubChem - National Center for Biotechnology Information. Archived from the original on September 1, 2022. Retrieved September 25, 2022.
- ^ "Antihypertensive Drugs, R.S. Vardanyan, V.J. Hruby, in Synthesis of Essential Drugs, 2006". Elsevier B.V., ScienceDirect. Archived from the original on September 1, 2022. Retrieved September 1, 2022.
- ^ a b Martin D (September 19, 2014). "Guinter Kahn, Inventor of Baldness Remedy, Dies at 80". The New York Times. Archived from the original on October 11, 2014. Retrieved May 11, 2015.
- ^ "Loniten: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on December 28, 2021. Retrieved August 15, 2021.
- ^ a b c d e Conrad P (2008). "Extension". The Medicalization of Society: On the Transformation of Human Conditions into Treatable Disorders. JHU Press. p. 37. ISBN 978-0-8018-9234-9. Retrieved May 11, 2015. (Google Books)
- ^ Gilmore E, Weil J, Chidsey C (March 1970). "Treatment of essential hypertension with a new vasodilator in combination with beta-adrenergic blockade". The New England Journal of Medicine. 282 (10): 521–527. doi:10.1056/NEJM197003052821001. PMID 4391708.
- ^ Gottlieb TB, Katz FH, Chidsey CA (March 1972). "Combined therapy with vasodilator drugs and beta-adrenergic blockade in hypertension. A comparative study of minoxidil and hydralazine". Circulation. 45 (3): 571–582. doi:10.1161/01.CIR.45.3.571. PMID 4401051.
- ^ a b Goldfarb NM (March 2006). "When Patents Became Interesting in Clinical Research" (PDF). The Journal of Clinical Research Best Practices. 2 (3). Archived from the original (PDF) on May 18, 2015. Retrieved May 11, 2015.
- ^ a b c d Lester W (May 13, 1996). "Hair-raising tale: no fame for men who discovered Rogaine". The Daily Gazette. Archived from the original on July 4, 2022. Retrieved May 11, 2015.
- ^ a b Kuntzman G (2001). Hair!: Mankind's Historic Quest to End Baldness. Random House Publishing Group. p. 172. ISBN 978-0-679-64709-6. Retrieved May 11, 2015. (Google Books)
- ^ a b "Generic MINOXIDIL INN equivalents, pharmaceutical patent expiry and freedom to operate". Deep knowledge on small-molecule drugs and the global patents covering them. Archived from the original on February 23, 2023. Retrieved February 23, 2023.
- ^ "Drug Approval Package: Rogaine NDA# 020834". U.S. Food and Drug Administration (FDA). August 8, 2003. Archived from the original on September 27, 2021. Retrieved August 15, 2021.
- ^ Pray SW (2006). Nonprescription Product Therapeutics. Lippincott Williams & Wilkins. p. 663. ISBN 978-0-7817-3498-1. Retrieved May 11, 2015. (Google Books)
- ^ "Drug Approval Package: Men's Rogaine (5% Minoxidil) NDA #021812". U.S. Food and Drug Administration (FDA). May 6, 2008. Archived from the original on August 6, 2021. Retrieved August 15, 2021.
- ^ "Rogaine: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ "Minoxidil: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on August 16, 2021. Retrieved August 15, 2021.
- ^ "CDER 2017 First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). February 20, 2018. Archived from the original on April 26, 2020. Retrieved August 15, 2021.
- ^ Wehner MR, Nead KT, Lipoff JB (August 2017). "Association Between Gender and Drug Cost for Over-the-Counter Minoxidil". JAMA Dermatology. 153 (8): 825–826. doi:10.1001/jamadermatol.2017.1394. PMC 5817599. PMID 28593214.
- ^ a b "International brand names for minoxidil". Drugs.com. Archived from the original on August 8, 2017. Retrieved June 26, 2017.
- ^ Liotta M (October 3, 2023). "Oral Minoxidil for the Treatment of Recurrent Platinum Resistant Epithelial Ovarian Cancer". clinicaltrials.gov. Archived from the original on January 1, 2024. Retrieved January 1, 2024.
- ^ Tater KC, Gwaltney-Brant S, Wismer T (September 2021). "Topical Minoxidil Exposures and Toxicoses in Dogs and Cats: 211 Cases (2001-2019)". J Am Anim Hosp Assoc. 57 (5): 225–231. doi:10.5326/JAAHA-MS-7154. PMID 34370845.
- ^ DeClementi C, Bailey KL, Goldstein SC, Orser MS (November 2004). "Suspected toxicosis after topical administration of minoxidil in 2 cats". Journal of Veterinary Emergency and Critical Care. 14 (4): 287–292. doi:10.1111/j.1476-4431.2004.04014.x.
- ^ Johnson TE, Fick ME, Haraschak JL, Vernier ME, Kadotani S (2023). "Successful management of minoxidil 5% toxicosis in 2 cats from the same household". J Vet Emerg Crit Care (San Antonio). 33 (4): 454–459. doi:10.1111/vec.13296. PMID 37222073.
- ^ Jordan TJ, Yaxley PE, Culler CA, Balakrishnan A (January 2018). "Successful management of minoxidil toxicosis in a dog". J Am Vet Med Assoc. 252 (2): 222–226. doi:10.2460/javma.252.2.222. PMID 29319439.
External links[edit]
- "Minoxidil". Drug Information Portal. U.S. National Library of Medicine.
- "Minoxidil Topical". MedlinePlus.

