Reboxetine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 94.5%[1] |
| Protein binding | 98% |
| Metabolism | Hepatic, CYP3A4-mediated |
| Elimination half-life | 13 hours[2] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H23NO3 |
| Molar mass | 313.391 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Reboxetine is a drug of the Norepinephrine Reuptake Inhibitor class (or NRI)(marketed as an antidepressant for use in the treatment of clinical depression, anxiety, panic disorder and ADD/ADHD, developed by Pharmacia (now Pfizer). Its mesylate (i.e. methanesulfonate) salt is sold under tradenames including Edronax, Norebox, Prolift, Solvex, Davedax or Vestra. It is approved for use in many countries worldwide, but has not yet been approved for use in the United States. Although its efficacy as an antidepressant has been challenged in multiple published reports, its popularity has continued to increase.[3]
Mode of action
This article contains content that is written like an advertisement. (June 2013) |
Unlike most antidepressants on the market, reboxetine is a norepinephrine reuptake inhibitor (NRI); it does not inhibit the reuptake of serotonin. This means it does not obtain the unwanted mood blunting and sexual side effects common to the SSRI's. Reboxetine's selective norepinephrine mode of action shows most benefit in treating apathy, fatigue, concentration, self confidence and anxiety related symptoms. As a selective norepinephrine reuptake inhibitor, reboxetine has been found to be an effective and safe anti-depressant. Furthermore reboxetine restores a patients' social functioning, producing a better quality of remission than fluoxetine.[4]
Efficacy
According to a meta-analysis of 12 new-generation antidepressants, reboxetine was no more effective than placebo, was "significantly less" effective, and was less acceptable, than the other drugs in treating the acute-phase treatment of adults with unipolar major depression.[5][6][7]
The British MHRA said in September 2011 that the study had several limitations, and that "Overall the balance of benefits and risks for reboxetine remains positive in its authorised indication."[8]
A UK and Europe-wide review of available efficacy and safety data has confirmed that reboxetine has benefit over placebo in its authorised indication. Efficacy was clearly shown in patients with severe or very severe depression.[9]
There's a fair bit of evidence that chronically depressive people have dysfunctional and atypical noradrenergic systems - particularly their alpha 2- and beta-adrenoceptors. Reboxetine itself typically doesn't have the disruptive effects on cognitive function or psychomotor performance common to older, tricyclic antidepressants - though alas antimuscarinic effects are still not completely absent. Indeed for one sub-population of depressives, the new Norepinephrine Reuptake Inhibitors (NRIs) are possibly under-used.[10] Unfortunately, catecholaminergic strategies to combat depression were eclipsed in the late 1980s and 1990s by the marketing hype surrounding selective serotonin reuptake inhibitors (SSRIs).[11]
Norepinephrine Reuptake Inhibitors (NRIs) like reboxetine may be especially useful in drive-deficient "anergic" states where the capacity for sustained motivation is lacking; and for melancholic depressives with a poor ability to cope with stress.[12]
Side effects
Common side effects of reboxetine include: dry mouth, constipation, headache, drowsiness, dizziness, excessive sweating and insomnia[citation needed]. Hypertension has been infrequently seen[citation needed].
In 4 to 8% of all patients treated, the medication has to be discontinued for the following reasons (percentages represent mean values):
- insomnia 1.3%
- excessive sweating 1.1%
- vertigo/hypotension and paraesthesia 0.8%
- dizziness, impotence, and other urological problems 0.5% each
Some other rare side effects include anxiety, loss of appetite, loss of or increased libido, urinary retention in men, pain on ejaculation, increased orgasm intensity, and premature/quickened ejaculation.[citation needed]
Reboxetine is normally well tolerated[citation needed]. So far no attributable fatalities have been noted.[citation needed]
Dosage
Treatment is usually started with a dose of 2 mg/day and slowly increased to the recommended therapeutic dose of between 4 – 8 mg/day administered orally. After 3 weeks, the dose can be increased to a maximum of 12 mg/day in cases of incomplete clinical response. Lower doses of between 2 – 4 mg/day have often been reported effective.[13]
Metabolism
Both the (R,R)-(–) and (S,S)-(+)-enantiomers of reboxetine are predominantly metabolized by the CYP3A4 isoenzyme.[14] The primary metabolite of reboxetine is O-desethylreboxetine, and there are also three minor metabolites—Phenol A, Phenol B, and UK1, Phenol B being the most minor.[14]
Interactions with other medications
Because of its reliance on CYP3A4, reboxetine O-desethylation is markedly inhibited by papaverine and ketoconazole.[14]
According to Weiss et al., reboxetine is an intermediate-level inhibitor of P-glycoprotein, which gives it the potential to interact with ciclosporin, tacrolimus, paroxetine, sertraline, quinidine, fluoxetine, fluvoxamine.[15]
The potency and duration of the effects of benzodiazepines can be increased because reboxetine interferes with their excretion.[citation needed]
History
By mid-2007, reboxetine was licensed worldwide in over 50 countries, including Italy, Germany, Australia and the United Kingdom. In May 2007, however, the Food and Drug Administration declined Pharmacia's license application for the United States market. To date, it is unclear why the further development of reboxetine as an antidepressant in the US has been halted. Despite this setback, reboxetine has been a valuable pharmacological tool to assess the role of the noradrenergic system in preclinical studies of depressive disorder.[10]
Chemistry
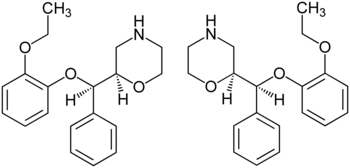
Reboxetine has two chiral centers. Thus, four stereoisomers may exist, the (R,R)-, (S,S)-, (R,S)-, and (S,R)-isomers. The active ingredient of reboxetine is a racemic mixture of two enantiomers, the (R,R)-(–)- and (S,S)-(+)-isomer.[16]
Notes and references
- ^ Fleishaker JC (2000). "Clinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression". Clinical Pharmacokinetics. 39 (6): 413–27. doi:10.2165/00003088-200039060-00003. PMID 11192474.
- ^ Edwards DM, Pellizzoni C, Breuel HP, Berardi A, Castelli MG, Frigerio E, Poggesi I, Rocchetti M, Dubini A, Strolin Benedetti M (1995). "Pharmacokinetics of reboxetine in healthy volunteers. Single oral doses, linearity and plasma protein binding". Biopharmaceutics & Drug Disposition. 16 (6): 443–60. doi:10.1002/bdd.2510160603. PMID 7579027.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ name=review>Eyding, Dirk, et al (2010). "Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials", British Medical Journal, 12 October 2010.
- Goldacre, Ben (2012). Bad Pharma: How drug companies mislead doctors and harm patients. Fourth Estate, pp. 5–7.
- ^ longecity.org
- ^ Analysis shows sertraline and escitalopram are the best of 12 new-generation antidepressants Lancet Public release date: 28-Jan-2009
- ^ Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis, Andrea Cipriani, Toshiaki A Furukawa, Georgia Salanti, John R Geddes, et al. The Lancet, Published Online, January 29, 2009, doi:10.1016/S0140-6736(09)60046-5
- ^ Zoloft, Lexapro the Best of Newer Antidepressants, HealthDay News, Washington Post, January 29, 2009
- ^ "Reboxetine: benefit-risk balance reviewed", MHRA, September 2011.
- ^ http://www.mhra.gov.uk
- ^ a b www.reboxetine.com
- ^ http://www.reboxetine.com/
- ^ www.reboxitine.com
- ^ www.medicines.org.uk
- ^ a b c Wienkers LC, Allievi C, Hauer MJ, Wynalda MA. (1999). "Cytochrome P-450-Mediated Metabolism of the Individual Enantiomers of the Antidepressant Agent Reboxetine in Human Liver Microsomes". Drug Metabolism & Disposition. 27 (11): 1334–1340. PMID 10534319.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weiss J, Dormann SM, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, Haefeli WE (2003). "Inhibition of P-glycoprotein by newer antidepressants". Journal of Pharmacology & Experimental Therapeutics. 305 (1): 197–204. doi:10.1124/jpet.102.046532. PMID 12649369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Melloni P, Della Torre A, Lazzari E, Mazzini G and Meroni M (1985). "Configuration studies on 2-[alpha -(2-ethoxyphenoxy)benzyl]-morpholine FCE 20124". Tetrahedron. 41 (1): 1393–1399. doi:10.1016/S0040-4020(01)96541-X.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brenner, Eric; Baldwin, Ronald M.; Tamagnan, Gilles (2005). "Asymmetric Synthesis of (+)-(S,S)-Reboxetine via a New (S)-2-(Hydroxymethyl)morpholine Preparation". Organic Letters. 7 (5): 937–9. doi:10.1021/ol050059g. PMID 15727479.

