Diphenpipenol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H28N2O2 |
| Molar mass | 388.511 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
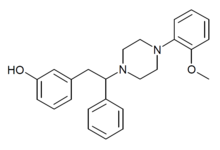
Diphenpipenol is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co.[1] It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative related to compounds such as MT-45 and AD-1211, but diphenpipenol is the most potent compound in the series, with the more active (S) enantiomer being around 105 times the potency of morphine in animal studies.[2] This makes it a similar strength to fentanyl and its analogues, and consequently diphenpipenol can be expected to pose a significant risk of producing life-threatening respiratory depression, as well as other typical opioid side effects such as sedation, itching, nausea and vomiting.
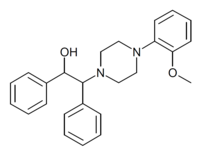
Diphenpipenol has been offered for sale online as a designer drug, though analysis of a sample of supposed diphenpipenol found it to instead contain a structural isomer with much weaker opioid activity, and it is unclear if genuine diphenpipenol has actually been sold.[3]
See also
References
- ^ US 4080453, Nishimura H, Uno H, Natsuka, Shimokawa N, Shimizu M, Nakamura H, "1-Substituted-4-(1,2-diphenylethyl)piperazine derivatives and compositions containing the same.", issued 21 March 1978, assigned to Dainippon Pharmaceutical Co Ltd
- ^ Natsuka K, Nakamura H, Nishikawa Y, Negoro T, Uno H, Nishimura H (October 1987). "Synthesis and structure-activity relationships of 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives having narcotic agonist and antagonist activity". Journal of Medicinal Chemistry. 30 (10): 1779–87. doi:10.1021/jm00393a017. PMID 3656354.
- ^ Cannaert A, Hulpia F, Risseeuw M, Van Uytfanghe K, Deconinck E, Van Calenbergh S, et al. (June 2020). "Report on a new opioid NPS: chemical and in vitro functional characterization of a structural isomer of the MT-45 derivative diphenpipenol". Journal of Analytical Toxicology. doi:10.1093/jat/bkaa066. PMID 32514558.
