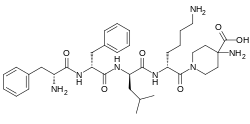
Difelikefalin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Intravenous |
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV)[1] |
| Metabolism | Not metabolized[1] |
| Elimination half-life | 2 hours[1] |
| Excretion | Excreted as unchanged drug via bile and urine[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C36H53N7O6 |
| Molar mass | 679.863 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Difelikefalin (INN) (developmental code names CR845, FE-202845), also known as D-Phe-D-Phe-D-Leu-D-Lys-[γ-(4-N-piperidinyl)amino carboxylic acid] (as the acetate salt), is an analgesic opioid peptide[2] acting as a peripherally specific, highly selective agonist of the κ-opioid receptor (KOR).[1][3][4][5] It is under development by Cara Therapeutics as an intravenous agent for the treatment of postoperative pain.[1][3][5] An oral formulation has also been developed.[5] Due to its peripheral selectivity, difelikefalin lacks the central side effects like sedation, dysphoria, and hallucinations of previous KOR-acting analgesics such as pentazocine and phenazocine.[1][3] In addition to use as an analgesic, difelikefalin is also being investigated for the treatment of pruritus (itching).[1][3][4] Difelikefalin has completed phase II clinical trials for postoperative pain and has demonstrated significant and "robust" clinical efficacy, along with being safe and well tolerated.[3][5] It has also completed a phase III clinical trial for uremic pruritus in hemodialysis patients.[6]
Difelikefalin acts as an analgesic by activating KORs on peripheral nerve terminals and KORs expressed by certain immune system cells.[1] Activation of KORs on peripheral nerve terminals results in the inhibition of ion channels responsible for afferent nerve activity, causing reduced transmission of pain signals, while activation of KORs expressed by immune system cells results in reduced release of proinflammatory, nerve-sensitizing mediators (e.g., prostaglandins).[1]
See also
References
- ^ a b c d e f g h i j Raymond S. Sinatra; Jonathan S. Jahr; J. Michael Watkins-Pitchford (14 October 2010). The Essence of Analgesia and Analgesics. Cambridge University Press. pp. 490–491. ISBN 978-1-139-49198-3.
- ^ Janecka A, Perlikowska R, Gach K, Wyrebska A, Fichna J (2010). "Development of opioid peptide analogs for pain relief". Curr. Pharm. Des. 16 (9): 1126–35. doi:10.2174/138161210790963869. PMID 20030621.
- ^ a b c d e Jeffrey Apfelbaum (8 September 2014). Ambulatory Anesthesia, An Issue of Anesthesiology Clinics. Elsevier Health Sciences. pp. 190–. ISBN 978-0-323-29934-3.
- ^ a b Alan Cowan; Gil Yosipovitch (10 April 2015). Pharmacology of Itch. Springer. pp. 307–. ISBN 978-3-662-44605-8.
- ^ a b c d Charlotte Allerton (2013). Pain Therapeutics: Current and Future Treatment Paradigms. Royal Society of Chemistry. pp. 56–. ISBN 978-1-84973-645-9.
- ^ Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F (2020). "A phase 3 trial of difelikefalin in hemodialysis patients with pruritus". N Engl J Med. 382 (3): 222–232. doi:10.1056/NEJMoa1912770. PMID 31702883.
External links
