From Wikipedia, the free encyclopedia
Etynodiol diacetate Routes of Oral ATC code Legal status
In general: ℞ (Prescription only)
(3β,17β)-17-ethynylestr-4-ene-3,17-diyl diacetate
CAS Number PubChem CID DrugBank ChemSpider CompTox Dashboard (EPA ) ECHA InfoCard 100.005.496 Formula C 24 H 32 O 4 Molar mass 384.509 g/mol g·mol−1 3D model (JSmol )
O=C(O[C@@H]4/C=C3\[C@@H]([C@H]2CC[C@]1([C@@H](CC[C@]1(C#C)OC(=O)C)[C@@H]2CC3)C)CC4)C
InChI=1S/C24H32O4/c1-5-24(28-16(3)26)13-11-22-21-8-6-17-14-18(27-15(2)25)7-9-19(17)20(21)10-12-23(22,24)4/h1,14,18-22H,6-13H2,2-4H3/t18-,19-,20+,21+,22-,23-,24-/m0/s1
Key:ONKUMRGIYFNPJW-KIEAKMPYSA-N
Etynodiol diacetate (INN ) (sold as Continuin , Femulen , Luteonorm , Luto-Metrodiol , and Metrodiol ), or ethynodiol diacetate (USAN , BAN ), also known as norethindrol diacetate ,[ 1] steroidal progestin which is used as a hormonal contraceptive .[ 2] [ 3] 19-nortestosterone derivatives, it has relatively little or no potency as an androgen ,[ 4] [ 5] noretynodrel ,[ 6] estrogenic effects.[ 7] [ 4]
Synthesis
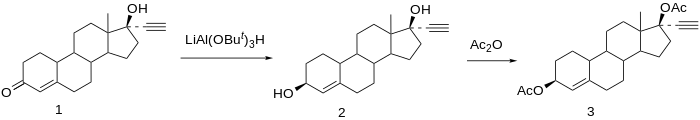
Ethynodiol diacetate synthesis:[ 8] U.S. patent 2,843,609 Searle ). Prepn of the 3-acetate, 17-acetate, and diacetate: P. D. Klimstra, U.S. patent 3,176,013 Searle ); see also:[ 9] Reduction of norethisterone (1 ) affords the 3,17-diol. The 3β-hydroxy compound is the desired product; since reactions at C-3 do not show nearly the stereoselectivity as those at C-17 by virtue of the relative lack of stereo-directing proximate substituents, the formation of the desired isomer is engendered by use of a bulky reducing agent, Lithium tri-tert-butoxyaluminum hydride . Acetylation of the 3β,17β-diol affords Etynodiol diacetate (3 ), one of the most potent oral progestins.[ 8]
See also
References
Estrogens
ER Tooltip Estrogen receptor agonists
Steroidal: Alfatradiol Certain androgens /anabolic steroids (e.g., testosterone , testosterone esters , methyltestosterone , metandienone , nandrolone esters ) (via estrogenic metabolites)
Certain progestins (e.g., norethisterone , noretynodrel , etynodiol diacetate , tibolone )
Clomestrone Cloxestradiol acetate Conjugated estriol Conjugated estrogens Epiestriol Epimestrol Esterified estrogens Estetrol † Estradiol Estradiol esters (e.g., estradiol acetate , estradiol benzoate , estradiol cypionate , estradiol enanthate , estradiol undecylate , estradiol valerate , polyestradiol phosphate , estradiol ester mixtures (Climacteron ))Estramustine phosphate Estriol Estriol esters (e.g., estriol succinate , polyestriol phosphate )Estrogenic substances Estrone Estrone esters
Ethinylestradiol #
Hydroxyestrone diacetate Mestranol Methylestradiol Moxestrol Nilestriol Prasterone (dehydroepiandrosterone; DHEA)
Promestriene Quinestradol Quinestrol Progonadotropins
Antiestrogens
ER Tooltip Estrogen receptor antagonistsSERMs Tooltip selective estrogen receptor modulators /SERDs Tooltip selective estrogen receptor downregulators )Aromatase inhibitors Antigonadotropins
Androgens /anabolic steroids (e.g., testosterone , testosterone esters , nandrolone esters , oxandrolone , fluoxymesterone )D2 receptor antagonists (prolactin releasers) (e.g., domperidone , metoclopramide , risperidone , haloperidol , chlorpromazine , sulpiride )GnRH agonistsleuprorelin , goserelin )GnRH antagonistscetrorelix , elagolix )Progestogens (e.g., chlormadinone acetate , cyproterone acetate , gestonorone caproate , hydroxyprogesterone caproate , medroxyprogesterone acetate , megestrol acetate ) Others
PR Tooltip Progesterone receptor
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone 6,6-Difluoronorethisterone acetate 17α-Allyl-19-nortestosterone Allylestrenol Altrenogest Chloroethynylnorgestrel Cingestol Danazol Desogestrel Dienogest Ethinylandrostenediol
Ethisterone Ethynerone Etonogestrel Etynodiol Etynodiol diacetate Gestodene Gestrinone Levonorgestrel Levonorgestrel esters (e.g., levonorgestrel butanoate )Lynestrenol Lynestrenol phenylpropionate Metynodiol Metynodiol diacetate Norelgestromin Norethisterone (norethindrone) Norethisterone esters (e.g., norethisterone acetate , norethisterone enanthate )Noretynodrel Norgesterone Norgestimate Norgestrel Norgestrienone Norvinisterone Oxendolone Quingestanol Quingestanol acetate Tibolone Tigestol Tosagestin ; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone 11β-Methyl-19-nortestosterone dodecylcarbonate 19-Nor-5-androstenediol 19-Nor-5-androstenedione 19-Nordehydroepiandrosterone Bolandiol Bolandiol dipropionate Bolandione Dimethisterone Dienedione Dienolone Dimethandrolone Dimethandrolone buciclate Dimethandrolone dodecylcarbonate Dimethandrolone undecanoate Dimethyldienolone Dimethyltrienolone Ethyldienolone Ethylestrenol (ethylnandrol) Methyldienolone Metribolone (R-1881) Methoxydienone (methoxygonadiene) Mibolerone Nandrolone Nandrolone esters (e.g., nandrolone decanoate , nandrolone phenylpropionate )Norethandrolone Normethandrone (methylestrenolone, normethandrolone, normethisterone) RU-2309 Tetrahydrogestrinone Trenbolone (trienolone) Trenbolone esters (e.g., trenbolone acetate , trenbolone enanthate )Trendione Trestolone Trestolone acetate MixedSPRMs Tooltip Selective progesterone receptor modulators ) Antagonists
mPR Tooltip Membrane progesterone receptor PAQR Tooltip Progestin and adipoQ receptor )
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown