List of COVID-19 vaccine authorizations: Difference between revisions
m →Pfizer–BioNTech: cite repair; |
Tags: Mobile edit Mobile web edit |
||
| Line 217: | Line 217: | ||
|caption= |
|caption= |
||
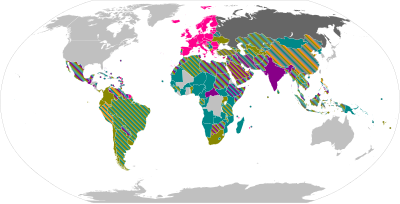
{{leftlegend|blueviolet|Full authorization}} |
{{leftlegend|blueviolet|Full authorization}} |
||
<!-- The color of Turkey on the map should be changed to reflect its approved status --> |
|||
{{leftlegend|dodgerblue|Emergency authorization}} |
{{leftlegend|dodgerblue|Emergency authorization}} |
||
{{leftlegend|limegreen|Eligible [[COVAX]] recipient}}}} |
{{leftlegend|limegreen|Eligible [[COVAX]] recipient}}}} |
||
Revision as of 15:33, 30 March 2021
National regulatory authorities have granted emergency use authorizations for twelve COVID-19 vaccines. Six of those have been approved for emergency or full use by at least one WHO-recognized stringent regulatory authorities.
Overview maps
|
|
Pfizer–BioNTech
| |||
The Pfizer–BioNTech COVID-19 vaccine, also known as Comirnaty, is an RNA vaccine[1] produced by the German company BioNTech and the American company Pfizer.[1][2][3]
Full (10)
- Australia[4][5]
- Brazil[6]
- European Union[a][8][9][10]
- Faroe Islands[11]
- Greenland[12][13]
- Japan[14]
- Malaysia[15]
- Saudi Arabia[16][17]
- Serbia[18][19]
- South Korea[20][21]
Emergency (38)
- Albania[22]
- Andorra[23]
- Argentina[24]
- Aruba[25]
- Bahrain[26]
- Canada[1][27]
- Caribbean Public Health Agency[b][28]
- Chile[29]
- Colombia[30]
- Costa Rica[31]
- Ecuador[32]
- Hong Kong[33]
- Iraq[34]
- Israel[35]
- Jordan[36]
- Kuwait[37]
- Lebanon[38]
- Mexico[39][40]
- Monaco[41]
- Mongolia[42]
- New Zealand[43]
- North Macedonia[44]
- Oman[45]
- Panama[46]
- Philippines[47]
- Qatar[48]
- Saint Vincent and the Grenadines[49]
- Singapore[50]
- South Africa[51][52]
- Suriname[53]
- Switzerland[54][55]
- Turkey[56]
- UAE[57]
- Ukraine[58]
- United Kingdom[59][60][61]
- United States[c][64][65]
- Vatican City[66][67]
- World Health Organization (WHO)[68][69]
Sputnik V
| |||
The Sputnik V COVID-19 vaccine is a viral vector vaccine[70] produced by the Russian Gamaleya Research Institute of Epidemiology and Microbiology.
- Full (12)
- Angola[71]
- Antigua and Barbuda[72]
- Azerbaijan[73]
- Cameroon[74]
- Congo Republic[71]
- Djibouti[71]
- Kyrgyzstan[75]
- Mali [76]
- Mauritius[77]
- Syria[78]
- Turkmenistan[79][80]
- Uzbekistan[81]
- Emergency (47)
- Albania[82]
- Algeria[83]
- Argentina[84]
- Armenia[85]
- Bahrain[86]
- Belarus[87]
- Bolivia[88]
- Egypt[89]
- Gabon[90]
- Ghana[91]
- Guatemala[92]
- Guinea[93]
- Guyana[94]
- Honduras[95]
- Hungary[96][80][97]
- Iran[98]
- Iraq[99]
- Jordan[100]
- Kazakhstan[101]
- Kenya[102]
- Laos[103]
- Lebanon[104]
- Mexico[105]
- Mongolia[106]
- Montenegro[107]
- Morocco[108]
- Myanmar[109]
- Nicaragua[110]
- North Macedonia[111]
- Pakistan[112]
- Palestine[113]
- Paraguay[114]
- Philippines[115]
- Republika Srpska[80]
- Russia[116]
- Saint Vincent and the Grenadines[49]
- San Marino[117]
- Serbia[118]
- Seychelles[119]
- Slovakia[120]
- Sri Lanka[121]
- Tunisia[122]
- UAE[123]
- Uruguay[124]
- Venezuela[125]
- Vietnam[126]
- Zimbabwe[127]
Oxford–AstraZeneca
| |||
The Oxford–AstraZeneca COVID-19 vaccine, sold under the brand names Vaxzevria[128] and Covishield, is a viral vector vaccine[129] produced by the British University of Oxford, British-Swedish company AstraZeneca, and the Coalition for Epidemic Preparedness Innovations
- Full (6)
- Australia[132][133]
- Brazil[134]
- Canada[135][27]
- European Union[a][136][137][138]
- Malaysia[139]
- South Korea[140][141]
- Emergency (47)
- Afghanistan[142][143]
- Albania[82]
- Andorra[144]
- Argentina[145]
- Bahrain[146]
- Bangladesh[147][148]
- Colombia[149]
- Dominican Republic[150]
- Ecuador[151]
- Egypt[89]
- El Salvador[152]
- Ethiopia[153][154][155]
- Gambia[156]
- Georgia[157]
- Guyana[158][159]
- India[160]
- Indonesia[161]
- Iraq[162]
- Ivory Coast[163]
- Kenya[164]
- Lesotho[165]
- Malawi[166][167]
- Maldives[168]
- Mauritius[169]
- Mexico[170]
- Morocco[171]
- Myanmar[172]
- Nepal[173]
- Nigeria[174]
- Pakistan[175]
- Philippines[176]
- Rwanda[177]
- Saint Vincent and the Grenadines[49]
- Saudi Arabia[178]
- Serbia[179]
- Sierra Leone[180]
- Sri Lanka[181]
- Sudan[182][183]
- Suriname[184]
- Taiwan[185]
- Thailand[186]
- Togo[187]
- Uganda[188]
- Ukraine[189]
- United Kingdom[190][191][192]
- Vietnam[193]
- World Health Organization (WHO)[68][194][195]
Sinopharm
Full authorization
Emergency authorization
Received donated doses
Eligible COVAX recipient (assessment in progress) | |||
BBIBP-CorV is an inactivated virus vaccine[196] produced by the China National Pharmaceutical Group (Sinopharm).[196]
- Full (4)
- Emergency (36)
- Afghanistan[202]
- Algeria[203]
- Argentina[204]
- Belarus[205]
- Bolivia[206]
- Brunei[207]
- Cambodia[208]
- Dominica[209]
- Egypt[210]
- Equatorial Guinea[211]
- Gabon[212]
- Guyana[213]
- Hungary[214][97]
- Iran[215]
- Iraq[162]
- Jordan[216]
- Kyrgyzstan[217]
- Laos[103]
- Lebanon[218]
- Macau[219]
- Maldives[220]
- Mauritania[221]
- Moldova[222]
- Mongolia[223]
- Montenegro[224]
- Morocco[225]
- Mozambique[226]
- Namibia[227]
- Nepal[228]
- Niger[229]
- Pakistan[230]
- Peru[231]
- Senegal[232]
- Serbia[233]
- Sierra Leone[234]
- Sri Lanka[235]
- Venezuela[236]
- Zimbabwe[237]
CoronaVac a/k/a SinoVac
| |||
CoronaVac is an inactivated virus vaccine[238] produced by the Chinese company Sinovac Biotech.[238][239][240]
- Full (2)
- Emergency (23)
- Albania[82]
- Azerbaijan[242]
- Bolivia[243]
- Brazil[244]
- Cambodia[245]
- Chile[246]
- Colombia[247]
- Dominican Republic[248]
- Ecuador[249]
- El Salvador[250]
- Fiji[251]
- Hong Kong[252]
- Indonesia[253][254]
- Laos[241]
- Mexico[255]
- Paraguay[256]
- Philippines[257]
- Thailand[258]
- Tunisia[259]
- Turkey[260]
- Ukraine[261]
- Uruguay[241]
- Zimbabwe[127]
Moderna
| |||
The Moderna COVID-19 vaccine is an RNA vaccine[262] produced by the American company Moderna, the U.S. National Institute of Allergy and Infectious Diseases, the U.S. Biomedical Advanced Research and Development Authority, and the Coalition for Epidemic Preparedness Innovations.[263][264]
- Full (1)
- Emergency (10)
- Andorra[144]
- Canada[269][27]
- Israel[270]
- Saint Vincent and the Grenadines[49]
- Saudi Arabia[178]
- Singapore[271]
- Switzerland[272]
- United Kingdom[273][274]
- United States[c][275][276]
- Vietnam[126]
Johnson & Johnson
| |||
The Johnson & Johnson COVID-19 vaccine is a viral vector vaccine[277] produced by Janssen Pharmaceutica (a subsidiary of Johnson & Johnson) and Beth Israel Deaconess Medical Center.[278][279]
- Full (1)
- Emergency (10)
- Andorra[144]
- Bahrain[282][283]
- Canada[284]
- Colombia[285]
- Saint Vincent and the Grenadines[49]
- South Africa[286]
- Switzerland[287]
- Thailand[288]
- United States[c][289][290]
- World Health Organization (WHO)[291]
Convidecia
| |||
Convidecia is a viral vector vaccine[292] produced by the Chinese company CanSino Biologics and the Beijing Institute of Biotechnology of the Academy of Military Medical Sciences.
- Full (1)
- China[293]
- Emergency (4)
Covaxin
Full authorization
Emergency authorization | |||
Covaxin is an inactivated virus vaccine[297] produced by the Indian company Bharat Biotech and the Indian Council of Medical Research.
- Full (0)
- None
- Emergency (6)
EpiVacCorona
EpiVacCorona is a peptide vaccine produced by the Russian State Research Center of Virology and Biotechnology VECTOR
- Full (1)
- Emergency (1)
RBD-Dimer
RBD-Dimer is a subunit vaccine produced by the Chinese company Anhui Zhifei Longcom Biopharmaceutical.[309]
- Full (0)
- None
- Emergency (2)
CoviVac
CoviVac is an inactivated virus vaccine[313] produced by the Chumakov Centre at the Russian Academy of Sciences.[314]
- Full (0)
- None
- Emergency (1)
- Russia[315]
Notes
- ^ a b c d The EU authorization covers all European Union member states (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden) and also Iceland, Norway and Liechtenstein[7]
- ^ The Caribbean Public Health Agency has 24 full members: Anguilla, Antigua and Barbuda, Aruba, Bahamas, Barbados, Belize, Bermuda, Caribbean Netherlands, British Virgin Islands, Cayman Islands, Curaçao, Dominica, Grenada, Haiti, Guyana, Jamaica, Montserrat, Saint Kitts and Nevis, Saint Lucia, Sint Maarten, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, and Turks and Caicos Islands.
- ^ a b c U.S. authorization also includes the three sovereign nations in the Compact of Free Association: Palau, the Marshall Islands, and Micronesia.[62][63]
References
- ^ a b c "Regulatory Decision Summary – Pfizer-BioNTech COVID-19 Vaccine". Health Canada, Government of Canada. 9 December 2020. Retrieved 9 December 2020.
- ^ "Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Adults". ClinicalTrials.gov. United States National Library of Medicine. 30 April 2020. NCT04368728. Archived from the original on 11 October 2020. Retrieved 14 July 2020.
- ^ "A Multi-site Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-19 Using Different Dosing Regimens in Healthy Adults". EU Clinical Trials Register. European Union. 14 April 2020. EudraCT 2020-001038-36. Archived from the original on 22 April 2020. Retrieved 22 April 2020.
- ^ "Comirnaty". Therapeutic Goods Administration. 25 January 2021. Retrieved 25 January 2021.
- ^ Australian Public Assessment Report for BNT162b2 (mRNA) (PDF) (Report). Therapeutic Goods Administration (TGA). Retrieved 25 January 2021.
- ^ "Vacina da Pfizer é a 1ª a obter registro definitivo no Brasil" (in Brazilian Portuguese). G1. 23 February 2021. Retrieved 23 February 2021.
- ^ "Comirnaty EPAR". European Medicines Agency (EMA). Retrieved 23 December 2020.
- ^ "Questions and Answers: COVID-19 vaccination in the EU". European Commission. 21 December 2020. Retrieved 21 December 2020.
- ^ "Comirnaty". Union Register of medicinal products. Retrieved 8 January 2021.
- ^ Nielsdóttir, Alda (2020-12-30). "First Covid-19 vaccines administered in the Faroe Islands". Local.fo. Retrieved 2021-03-29.
{{cite web}}: CS1 maint: url-status (link) - ^ Sevunts, Levon; International, Radio Canada (4 January 2021). "Greenland launches COVID-19 vaccination campaign". Eye on the Arctic. Retrieved 21 March 2021.
- ^ McGwin, Kevin (2020-12-30). "Greenland to begin new year with COVID vaccinations". ArcticToday. Retrieved 21 March 2021.
- ^ "Japan approves Pfizer's COVID-19 vaccine, 1st for domestic use". Nikkei Asia. 14 February 2021. Retrieved 14 February 2021.
- ^ Lim I (8 January 2021). "Khairy: Malaysia can use Pfizer's Covid-19 vaccine now as conditional registration granted". The Malay Mail. Retrieved 8 January 2021.
- ^ "Coronavirus: Saudi Arabia approves Pfizer COVID-19 vaccine for use". Al Arabiya English. 10 December 2020. Retrieved 10 December 2020.
- ^ Zimmer C, Corum J, Wee SL (10 June 2020). "Coronavirus Vaccine Tracker". The New York Times. Retrieved 12 December 2020.
- ^ "Serbia leads region in expecting COVID-19 vaccines within days". BalkanInsight. 21 December 2020. Retrieved 26 December 2020.
- ^ "First shipment of Pfizer-BioNTech vaccine arrives in Serbia". Serbian government website. 22 December 2020. Retrieved 26 December 2020.
- ^ Lee, Kyung-eun (27 February 2021). "S. Korea begins rolling out Pfizer vaccines on second day of national vaccination program". Arirang News. Retrieved 28 February 2021.
- ^ Kim, Han-joo (5 March 2021). "S. Korea approves Pfizer's COVID-19 vaccine amid immunization push". Yonhap News Agency. Retrieved 5 March 2021.
- ^ "Albania to start COVID-19 immunisation with Pfizer vaccine in January – report". Seenews.
- ^ "ANDORRA COVID-19". govern.ad. Retrieved 19 February 2021.
- ^ "Coronavirus en la Argentina: La ANMAT aprobo el uso de emergencia de la vacuna Pfizer". La Nación (in Spanish). Retrieved 23 December 2020.
- ^ Overheid, Aruba (7 January 2021). "COVID-19 vaccine update". government.aw. Retrieved 19 February 2021.
- ^ "Bahrain becomes second country to approve Pfizer COVID-19 vaccine". Al Jazeera. Retrieved 5 December 2020.
- ^ a b c "Drug and vaccine authorizations for COVID-19: List of applications received". Health Canada, Government of Canada. 9 December 2020. Retrieved 9 December 2020.
- ^ "Pfizer vaccine approved for region but Health Ministry wants more info". Trinidad and Tobago Newsday. 6 January 2021. Retrieved 24 February 2021.
- ^ "Chile approves Pfizer-BioNTech Covid-19 vaccine for emergency use". The Straits Times. 17 December 2020. Retrieved 17 December 2020.
- ^ "Colombia regulator approves Pfizer-BioNTech vaccine for emergency use". Reuters. 6 January 2021. Retrieved 6 January 2021.
- ^ "Costa Rica authorizes Pfizer-BioNTech coronavirus vaccine". The Tico Times. 16 December 2020. Retrieved 16 December 2020.
- ^ "Arcsa autoriza ingreso al país de vacuna Pfizer-BioNTech para el Covid-19 – Agencia Nacional de Regulación, Control y Vigilancia Sanitaria" (in Spanish). Retrieved 17 December 2020.
- ^ "Use of vaccine approved". Government of Hong Kong. 25 January 2021. Retrieved 15 February 2021.
- ^ "Iraq grants emergency approval for Pfizer COVID-19 vaccine". MSN. Retrieved 27 December 2020.
- ^ "Israeli Health Minister 'pleased' as FDA approves Pfizer COVID-19 vaccine". The Jerusalem Post. Retrieved 28 December 2020.
- ^ "Jordan approves Pfizer-BioNTech Covid vaccine". France 24. 15 December 2020. Retrieved 15 December 2020.
- ^ "Kuwait authorizes emergency use of Pfizer-BioNTech COVID-19 vaccine". Arab News. 13 December 2020. Retrieved 15 December 2020.
- ^ "Lebanon starts Covid-19 vaccination campaign". France 24. 14 February 2021. Retrieved 16 March 2021.
- ^ "Mexico Approves Pfizer Vaccine for Emergency Use as Covid Surges". Bloomberg. 12 December 2020. Retrieved 12 December 2020.
- ^ "Mexico authorizes emergency use of Pfizer-BioNTech coronavirus vaccine". The Economic Times. 12 December 2020. Retrieved 14 January 2021.
- ^ "FIRST VACCINES ARRIVE IN MONACO – COVID-19 vaccination campaign begins tomorrow / Actualités / Coronavirus (Covid-19) / Policy & Practice / Portail du Gouvernement – Monaco". en.gouv.mc. Retrieved 19 February 2021.
- ^ "Mongolia will receive 300,000 doses of COVID-19 vaccine developed by China". akipress.com. Retrieved 19 March 2021.
- ^ "Robust assessment ahead of Medsafe approval of vaccine". Ministry of Health. 3 February 2021. Retrieved 6 February 2021.
- ^ "North Macedonia Kicks Off COVID-19 Vaccinations As Serbia Looks To Highlight Regional Efforts". RadioFreeEurope/RadioLiberty. Retrieved 19 February 2021.
- ^ "Oman issues licence to import Pfizer BioNTech Covid vaccine – TV". Reuters. 15 December 2020. Retrieved 16 December 2020.
- ^ "Panama approves Pfizer's COVID-19 vaccine – health ministry". Yahoo! Finance. Retrieved 16 December 2020.
- ^ "PH authorizes Pfizer's COVID-19 vaccine for emergency use". CNN Philippines. 14 January 2021. Retrieved 14 January 2021.
- ^ "Qatar, Oman to receive Pfizer-BioNTech COVID-19 vaccine this week". Reuters. Retrieved 24 December 2020.
- ^ a b c d e "Public Health (Emergency Authorisation of COVID-19 Vaccine) Rules, 2021" (PDF). Government of Saint Vincent and the Grenadines. 11 February 2021. Retrieved 12 February 2021.
- ^ "Singapore approves use of Pfizer's COVID-19 vaccine". Associated Press. 14 December 2020. Retrieved 15 December 2020.
- ^ "South Africa approves Pfizer Covid-19 vaccine for emergency use". Retrieved 18 March 2021.
- ^ https://www.timeslive.co.za/authors/unathi-nkanjeni. "Pfizer-BioNTec approved for emergency use in SA: here is what you need to know about the vaccine". TimesLIVE. Retrieved 19 March 2021.
{{cite web}}: External link in|last= - ^ "Suriname begins coronavirus vaccination campaign with donated doses". Reuters. 23 February 2021. Retrieved 28 February 2021.
- ^ "Comirnaty Product information" (in German). Retrieved 31 January 2021. [Due to incomplete clinical data at the time of the assessment of the authorization application, the Comirnaty medicinal product is authorized for a limited period (Art. 9a Medicinal Products Act).]
- ^ Gözde Bayar (2021-03-18). "Turkey to receive 4.5M doses of Pfizer vaccine this month". Anadolu Agency.
- ^ "Dubai approves the Pfizer-BioNTech vaccine which will be free of charge". Emirates Woman. 23 December 2020. Retrieved 28 December 2020.
- ^ "Наказ МОЗ України від 22.02.2021 № 308 "Про державну реєстрацію лікарського засобу (медичного імунобіологічного препарату) для екстреного медичного застосування"". moz.gov.ua. Retrieved 19 March 2021.
- ^ "UK medicines regulator gives approval for first UK COVID-19 vaccine". Medicines and Healthcare Products Regulatory Agency, Government of the UK. 2 December 2020. Retrieved 2 December 2020.
- ^ "Information for Healthcare Professionals on Pfizer/BioNTech COVID-19 vaccine". Medicines & Healthcare products Regulatory Agency (MHRA). 8 December 2020. Retrieved 13 December 2020.
- ^ "Conditions of Authorisation for Pfizer/BioNTech COVID-19 vaccine". Medicines & Healthcare products Regulatory Agency (MHRA). 3 December 2020. Retrieved 13 December 2020.
- ^ "Interior Applauds Inclusion of Insular Areas through Operation Warp Speed to Receive COVID-19 Vaccines" (Press release). United States Department of the Interior (DOI). 12 December 2020. Retrieved 13 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Dorman B (6 January 2021). "Asia Minute: Palau Administers Vaccines to Keep Country Free of COVID". Hawaii Public Radio. Retrieved 13 January 2021.
- ^ "FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine" (Press release). U.S. Food and Drug Administration (FDA). 11 December 2020. Retrieved 11 December 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Pfizer-BioNTech COVID-19 Vaccine EUA Fact Sheet for Healthcare Providers" (PDF). Pfizer. 25 February 2021. Retrieved 25 February 2021.
- ^ McElwee, Joshua J. (11 December 2020). "Vatican to offer coronavirus vaccine to residents and staff in early 2021". National Catholic Reporter. Retrieved 9 February 2021.
- ^ Menichetti, Massimiliano (11 December 2020). "In Vaticano parte il piano vaccinale anticovid". vatican.va (Press release). Retrieved 9 February 2021.
- ^ a b "Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process" (PDF). World Health Organization (WHO).
- ^ "WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access". World Health Organization (WHO) (Press release). 31 December 2020. Retrieved 6 January 2021.
- ^ "An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19". ClinicalTrials.gov. United States National Library of Medicine. 17 June 2020. NCT04436471. Retrieved 5 March 2021.
- ^ a b c "Angola, Congo Republic and Djibouti approve Russia's Sputnik V vaccine". Reuters. 3 March 2021.
- ^ "Antigua and Barbuda authorizes Sputnik V". sputnikvaccine.com (Press release). 26 March 2021.
- ^ "Azerbaijan approves Russia's Sputnik V vaccine for use against COVID-19 -Azeri health ministry". Reuters. 12 March 2021.
- ^ "Cameroon approves Russia's Sputnik V coronavirus vaccine for use - RDIF". Reuters. 19 March 2021.
- ^ "Sputnik V registered in Kyrgyzstan". Gamaleya Center (Press release). 23 February 2021.
- ^ "Mali approves Russia's Sputnik V vaccine - Russian sovereign wealth fund". Reuters. 30 March 2021.
- ^ "Mauritius approves Russia's Sputnik V coronavirus vaccine - Russian RDIF fund". Reuters. 22 March 2021.
- ^ "Syria authorizes use of Sputnik-V". Roya. 22 February 2021.
- ^ "Turkmenistan is the first in Central Asia to have registered "Sputnik V" vaccine". Orient. 18 January 2021.
- ^ a b c "Sputnik V vaccine registered in Bosnia and Herzegovina's Republika Srpska". TASS. 5 February 2021. Retrieved 8 February 2021.
- ^ "Uzbekistan Certifies Russia's Sputnik Vaccine For Mass Use". Agence France-Presse (Barron's). 17 February 2021.
- ^ a b c Semini, Llazar. "Albania starts mass COVID vaccinations before tourist season". ABC News. Retrieved 2021-03-29.
{{cite web}}: CS1 maint: url-status (link) - ^ "Covid19: National Pharmaceuticals Agency registers Sputnik V vaccine". Algeria Press service. 10 January 2021.
- ^ "Argentina has registered the Sputnik V vaccine based on Russian clinical trial data" (Press release). Gamaleya Center. Retrieved 1 January 2021.
- ^ "Armenia approves Russia's Sputnik V coronavirus vaccine -Russia's RDIF". Reuters. 1 February 2021. Retrieved 1 February 2021.
- ^ "Bahrain authorises Sputnik V COVID-19 vaccine for emergency use – Bahrain TV". Reuters. 10 February 2021. Retrieved 19 February 2021.
- ^ "Belarus registers Sputnik V vaccine, in first outside Russia – RDIF". Reuters. 21 December 2020. Retrieved 22 December 2020.
- ^ "Ministerio de Salud de Bolivia – Bolivia y Rusia firman contrato para adquirir 5,2 millones de dosis de la vacuna Sputnik-V contra la COVID-19". minsalud.gob.bo. Retrieved 1 January 2021.
- ^ a b "COVID-19: Egypt authorises Sputnik V, AstraZeneca virus jabs". Gulf News. Retrieved 24 February 2021.
- ^ "Sputnik V authorised in Gabon" (Press release). Gamaleya Center. Retrieved 17 February 2021.
- ^ "Ghana approves Russia's Sputnik V vaccine for emergency use – RDIF". Reuters. 20 February 2021.
- ^ https://www.pharmiweb.com/press-release/2021-02-26/guatemala-authorizes-sputnik-v
- ^ "Guinea Begins Administering Russia's Sputnik V Covid-19 Vaccine". Africa news. 31 December 2020.
- ^ "Russia's Sputnik V vaccine expands its reach in Latin America". CNN. 3 March 2021.
- ^ "Honduras approves use of Sputnik V vaccine against COVID-19". Xinhua News Agency. 25 February 2021.
- ^ "Hungarian drug regulator approves Sputnik V vaccine: website". The Moscow Times. 7 February 2021.
- ^ a b c Spike J (22 March 2021). "Hungary approves 2 more vaccines from outside EU amid spike". The Washington Post. Retrieved 22 March 2021.
- ^ "Iran approves Russian coronavirus vaccine Sputnik V". Reuters. 26 January 2021.
- ^ "Sputnik V authorized in Iraq" (Press release). PharmiWeb.com. 4 March 2021.
- ^ "Jordan approves Russia's Sputnik V vaccine for use against COVID-19" (Press release). Reuters. 10 March 2021.
- ^ "Kazakhstan begins mass vaccination by Russian Sputnik V". 1 February 2021. Retrieved 19 February 2021.
- ^ "Morocco, Kenya approve Russian coronavirus vaccine for use - RDIF". 10 March 2021. Retrieved 12 March 2021.
- ^ a b "Laos declares Covid-19 vaccinations safe, more to be inoculated next week". The Star. Malaysia. Retrieved 19 February 2021.
- ^ "Lebanon authorises emergency use of Russia's Sputnik V vaccine". Reuters. 5 February 2021.
- ^ "Mexico, Germany warm to Russia's Sputnik V virus vaccine". The Jakarta Post. 3 February 2021.
- ^ "Mongolia Approves Russia's Sputnik V Coronavirus Vaccine – RDIF". Urdu Point. 9 February 2021.
- ^ "Montenegro and St. Vincent approve Russia's Sputnik V vaccine – RDIF". Reuters. 12 February 2021.
- ^ "Morocco orders one million doses of Russia's Sputnik V vaccine". Yabiladi. 11 March 2021.
- ^ "Myanmar registers Russia's Sputnik V COVID-19 vaccine". TASS. Retrieved 19 February 2021.
- ^ "Nicaragua approves Russian COVID-19 vaccine". wsoctv. 3 February 2021.
- ^ "NRussia's Sputnik V COVID 19 vaccine registered in North Macedonia". TASS. 7 March 2021.
- ^ "Govt okays Russian vaccine for 'emergency use'". Dawn. 24 January 2021.
- ^ "Palestine has become the first country in the Middle East to register Sputnik V vaccine". RFID. 11 January 2021.
- ^ "Paraguay approves Russia's Sputnik V vaccine: RDIF". Reuters. 15 January 2021. Retrieved 15 January 2021.
- ^ "Russia's Sputnik V approved for emergency use in PH". CNN Philippines. 19 March 2021. Retrieved 19 March 2021.
- ^ Burki TK (November 2020). "The Russian vaccine for COVID-19". The Lancet. Respiratory Medicine. 8 (11): e85–e86. doi:10.1016/S2213-2600(20)30402-1. PMC 7837053. PMID 32896274.
- ^ "San Marino buys the Sputnik vaccine: "First doses already in the next few days"". Unioneonline. 20 February 2021.
- ^ "Agencija odobrila uvoz ruske vakcine Sputnjik V u Srbiju". N1 (in Serbian). 31 December 2020.
- ^ "Sputnik V vaccine approved in Seychelles". TASS. 19 March 2021.
- ^ "Sputnik V approved for use in Slovakia". rdif.ru. Retrieved 1 March 2021.
- ^ "Sri Lanka approves Russia's Sputnik V vaccine". The Hindu. 4 March 2021.
- ^ "Sputnik V vaccine authorized in Tunisia" (Press release). Gamaleya Center. Retrieved 30 January 2021.
- ^ "UAE approves Russia's Sputnik vaccine for emergency use". Khaleej Times. 21 January 2021. Retrieved 21 January 2021.
- ^ "Uruguay to start covid 19 Vaccination in April 2021". India Blooms.
- ^ "Venezuela firma contrato para la adquisición de la vacuna rusa Sputnik V" (in Spanish). Reuters. 29 December 2020.
- ^ a b "Vietnam approves US, Russia Covid-19 vaccines for emergency use". VnExpress. Retrieved 26 February 2021.
- ^ a b "Covid-19: Zimbabwe authorises Sputnik V, Sinovac vaccines for emergency use". news24.com. 9 March 2021.
- ^ "Vaxzevria (previously COVID-19 Vaccine AstraZeneca) EPAR". European Medicines Agency (EMA).
- ^ a b "Investigating a Vaccine Against COVID-19". ClinicalTrials.gov. United States National Library of Medicine. 26 May 2020. NCT04400838. Archived from the original on 11 October 2020. Retrieved 14 July 2020.
- ^ "A Phase 2/3 study to determine the efficacy, safety and immunogenicity of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19". EU Clinical Trials Register. European Union. 21 April 2020. EudraCT 2020-001228-32. Archived from the original on 5 October 2020. Retrieved 3 August 2020.
- ^ O'Reilly P (26 May 2020). "A Phase III study to investigate a vaccine against COVID-19". ISRCTN. doi:10.1186/ISRCTN89951424. ISRCTN89951424.
- ^ "AstraZeneca coronavirus vaccine approved for use in Australia by TGA". ABC News. 16 February 2021. Retrieved 16 February 2021.
- ^ "TGA provisionally approves AstraZeneca's COVID-19 vaccine". Therapeutic Goods Administration (TGA). 16 February 2021. Retrieved 16 February 2021.
- ^ "Brazil grants full approval to Oxford vaccine, orders Sputnik". France 24. Brasilia. Agence France-Presse. 12 March 2021. Retrieved 13 March 2021.
- ^ "Regulatory Decision Summary – AstraZeneca COVID-19 Vaccine". Health Canada. 26 February 2021. Retrieved 26 February 2021.
- ^ "COVID-19 Vaccine AstraZeneca: Pending EC decision". European Medicines Agency (EMA). 28 January 2021. Retrieved 29 January 2021.
- ^ "EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU". European Medicines Agency (EMA) (Press release). 29 January 2021. Retrieved 29 January 2021.
- ^ "European Commission authorises third safe and effective vaccine against COVID-19". European Commission (Press release). 29 January 2021. Retrieved 29 January 2021.
- ^ a b Sipalan, Joseph; Donovan, Kirsten (3 March 2021). "Malaysia approves Sinovac, AstraZeneca COVID-19 vaccines for use". Reuters. Retrieved 7 March 2021.
- ^ Kim, Han-joo (10 February 2021). "S. Korea approves AstraZeneca's COVID-19 vaccine for all adults". Yonhap News Agency. Retrieved 10 February 2021.
- ^ Maresca, Thomas (10 February 2021). "South Korea approves AstraZeneca COVID-19 vaccine". United Press International. Retrieved 10 February 2021.
- ^ Sediqi, Abdul Qadir (7 February 2021). "First doses of COVID-19 vaccine arrive in Afghanistan from India". Reuters. Retrieved 24 February 2021.
- ^ "India donates 500,000 COVID vaccines to Afghanistan". Al Jazeera. Retrieved 24 February 2021.
- ^ a b c "Informació en relació amb la vacunació contra la COVID-19" (PDF) (in Catalan). Govern d'Andorra. Retrieved 14 March 2021.
- ^ "Argentine regulator approves AstraZeneca/Oxford COVID-19 vaccine". Reuters. 30 December 2020. Retrieved 30 December 2020.
- ^ "Bahrain approves Oxford/AstraZeneca coronavirus vaccine produced in India". Saudigazette. 25 January 2021. Retrieved 30 January 2021.
- ^ "Oxford University-Astrazeneca vaccine: Bangladesh okays it for emergency use". The Daily Star. Retrieved 6 January 2021.
- ^ "Bangladesh approves Oxford-AstraZeneca COVID-19 vaccine". aa.com.tr. Retrieved 6 January 2021.
- ^ Reuters Staff (23 February 2021). "Colombia approves emergency use of AstraZeneca coronavirus vaccine". Reuters. Bogotá. Retrieved 22 March 2021.
{{cite web}}:|author=has generic name (help) - ^ "La República Dominicana aprueba la vacuna de AstraZeneca contra la covid-19". Agencia EFE (in Spanish). 31 December 2020.
- ^ "Ecuador approves use of AstraZeneca vaccine for COVID-19". Reuters. 24 January 2021. Retrieved 30 January 2021.
- ^ "El Salvador greenlights AstraZeneca, Oxford University COVID-19 vaccine". Reuters. 30 December 2020.
- ^ "Ethiopia begins COVID-19 vaccine rollout". www.aa.com.tr. Retrieved 19 March 2021.
- ^ "Ethiopia introduces COVID-19 vaccine in a national launching ceremony". World Health Organization (WHO). Retrieved 19 March 2021.
- ^ "Ethiopia says it has secured 9 million doses of COVID-19 vaccines till April". Reuters. 9 February 2021. Retrieved 19 March 2021.
- ^ "The Arrival of COVAX vaccines Raises Hope in The Gambia". World Health Organization (WHO). Retrieved 19 March 2021.
- ^ "Georgia to receive first doses of COVID-19 vaccine in early March instead of February". Agenda.ge. Retrieved 22 March 2021.
- ^ "Guyana gets first batch of COVID-19 vaccines; CARICOM Secretariat to get 100 doses". 10 February 2021. Retrieved 19 February 2021.
- ^ "Nurse administers first shot of AstraZeneca ( ChAdOx1 nCoV-19, ) vaccine to Brinnet Bernarai at Georgetown Public Hospital Corporation – PAHO/WHO | Pan American Health Organization". paho.org. Retrieved 19 February 2021.
- ^ a b Schmall E, Yasir S (3 January 2021). "India Approves Oxford-AstraZeneca Covid-19 Vaccine and 1 Other". The New York Times. Retrieved 3 January 2021.
- ^ "BPOM Terbitkan Izin Penggunaan Darurat Vaksin Covid-19 AstraZeneca". Kompas.com. Retrieved 10 February 2021.
- ^ a b "Iraq approves Sinopharm, AstraZeneca vaccines". Big News Network.com. Retrieved 30 January 2021.
- ^ "Covax: Ivory Coast and Ghana begin mass Covid vaccination rollouts". BBC News. 1 March 2021. Retrieved 19 March 2021.
- ^ "Kenya Receives 1M Vaccine Doses, Will Distribute to Health Workers First". Voice of America. Retrieved 19 March 2021.
- ^ "Lesotho receives 1st batch of COVID-19 vaccines". www.aa.com.tr. Retrieved 19 March 2021.
- ^ "Malawi to receive COVID-19 vaccines in late February - Xinhua". www.xinhuanet.com. Retrieved 19 March 2021.
- ^ "Malawi Sticks to AstraZeneca Despite Concerns Over Efficacy". Voice of America. Retrieved 19 March 2021.
- ^ "Maldives starts training healthcare workers on COVID-19 vaccine distribution – Xinhua". Xinhua News Agency. Retrieved 19 February 2021.
- ^ AfricaNews (26 January 2021). "Mauritius begins vaccinating frontline health workers against covid-19". Africanews. Retrieved 24 February 2021.
- ^ "Mexico approves AstraZeneca COVID-19 vaccine, minister says". Reuters. 5 January 2021. Retrieved 14 January 2021.
- ^ "Morocco approves AstraZeneca/Oxford COVID-19 vaccine- Minister". Reuters. 7 January 2021. Retrieved 22 March 2021.
- ^ "Myanmar launches nationwide COVID-19 vaccination program". Xinhua News. 27 January 2021.
- ^ "Nepal approves AstraZeneca COVID vaccine for emergency use – government statement". Reuters. 15 January 2021.
- ^ "Wetin we sabi about NAFDAC approval of Oxford AstraZeneca vaccine use for Nigeria". BBC News Pidgin. Retrieved 24 February 2021.
- ^ "Pakistan approves AstraZeneca COVID-19 vaccine for emergency use". 16 January 2021.
- ^ "Philippine regulator approves emergency use of AstraZeneca vaccine". Reuters. 28 January 2021. Retrieved 28 January 2021.
- ^ "Rwanda receives COVID-19 Vaccines through COVAX". World Health Organization (WHO). Retrieved 19 March 2021.
- ^ a b "AstraZeneca and Moderna vaccines to be administered in Saudi Arabia". Gulf News. Retrieved 19 January 2021.
- ^ "Serbia receives first shipment of AstraZeneca COVID-19 vaccine". Reuters. 21 February 2021. Retrieved 19 March 2021.
- ^ "COVID-19 vaccines shipped by COVAX arrive in Sierra Leone". World Health Organization (WHO). Retrieved 19 March 2021.
- ^ "Sri Lanka approves vaccine amid warnings of virus spread". Associated Press. 22 January 2021. Retrieved 22 January 2021.
- ^ "COVID-19 vaccination in Sudan". UNICEF. Retrieved 19 March 2021.
- ^ "Sudan receives first delivery of COVID-19 vaccines with over 800,000 doses". UNICEF. Retrieved 19 March 2021.
- ^ "Suriname begins coronavirus vaccination campaign with donated doses". Reuters. 23 February 2021. Retrieved 28 February 2021.
- ^ "Taiwan grants emergency authorisation for AstraZeneca COVID-19 vaccine". Reuters. 20 February 2021. Retrieved 13 March 2021.
- ^ "Thai Food and Drug registers COVID-19 vaccine developed by AstraZeneca". Pattaya Mail. 23 January 2021.
- ^ First, Togo. "Covid-19: First vaccination campaign to soon begin". www.togofirst.com. Retrieved 19 March 2021.
- ^ AfricaNews (10 March 2021). "Uganda starts COVID-19 vaccinations". Africanews. Retrieved 19 March 2021.
- ^ "COVID-19: AstraZeneca vaccine certified for use in Ukraine". covid.unian.info. Retrieved 10 March 2021.
- ^ "Information for Healthcare Professionals on COVID-19 Vaccine AstraZeneca". Medicines and Healthcare products Regulatory Agency (MHRA). 30 December 2020. Retrieved 4 January 2021.
- ^ "Oxford University/AstraZeneca vaccine authorised by UK medicines regulator" (Press release). Department of Health and Social Care. 30 December 2020. Retrieved 30 December 2020.
- ^ "Conditions of Authorisation for COVID-19 Vaccine AstraZeneca". Medicines and Healthcare products Regulatory Agency (MHRA). 30 December 2020. Retrieved 4 January 2021.
- ^ "Vietnam approves AstraZeneca COVID-19 vaccine, cuts short Communist Party congress". CNA. Retrieved 5 February 2021.
- ^ "Interim recommendations for use of the AZD1222 (ChAdOx1-S (recombinant)) vaccine against COVID-19 developed by Oxford University and AstraZeneca". World Health Organization (WHO). Retrieved 27 February 2021.
- ^ "AstraZeneca/Oxford Covid-19 Vaccine Gets Emergency Approval From WHO". Forbes. Retrieved 16 February 2021.
- ^ a b Chen W, Al Kaabi N (18 July 2020). "A Phase III clinical trial for inactivated novel coronavirus pneumonia (COVID-19) vaccine (Vero cells)". Chinese Clinical Trial Registry. Retrieved 15 August 2020.
- ^ "Bahrain approves China's Sinopharm coronavirus vaccine". Arabian Business Industries. 13 December 2020.
- ^ Kuo L. "China approves Sinopharm coronavirus vaccine, the country's first for general use". The Washington Post.
- ^ "President Ramkalawan and First Lady receives second dose SinoPharm Vaccine". statehouse.gov.sc. Retrieved 5 February 2021.
- ^ Wee SL (9 December 2020). "Chinese Covid-19 Vaccine Gets Key Push, but Doubts Swirl". The New York Times. Retrieved 12 December 2020.
- ^ "Coronavirus: UAE authorises emergency use of vaccine for frontline workers". The National. Retrieved 24 November 2020.
- ^ "China 'to provide 400,000 COVID vaccine doses' to Afghanistan". www.aljazeera.com. Retrieved 22 March 2021.
- ^ Presse, AFP-Agence France. "Algeria Receives 200,000 Coronavirus Jabs From China". Barrons. Retrieved 22 March 2021.
- ^ Biannchi, Walter (21 February 2021). "Argentina approves Sinopharm COVID-19 vaccine for emergency use". Reuters. Retrieved 23 February 2021.
- ^ "Belarus begins COVID-19 vaccinations with Chinese shots". Belta. 15 March 2021. Retrieved 16 March 2021.
- ^ "Bolivia begins inoculation with Sinopharm jabs". The Star. Malaysia. Retrieved 28 February 2021.
- ^ "Vaccine donation from China arrives". The Star. Malaysia. Retrieved 22 March 2021.
- ^ Rinith T (4 February 2021). "Health Ministry grants Emergency Use Authorization to China's Sinopharm vaccine". Khmer Times. Retrieved 4 February 2021.
- ^ "Dominica: Melissa Skerrit receives the Sinopharm COVID-19 vaccine". WIC News. 4 March 2021. Retrieved 7 March 2021.
- ^ "Egypt licenses China's Sinopharm COVID-19 vaccine for emergency use: health minister – Xinhua". Xinhua News Agency.
- ^ "StackPath". dailynewsegypt.com. Retrieved 22 March 2021.
- ^ "Gabon receives 100,000 doses of Sinopharm's vaccine from China". Gabon 24. 12 March 2021. Retrieved 15 March 2021.
- ^ "Sinopharm vaccine rollout starts this weekend". Stabroek News. 6 March 2021. Retrieved 7 March 2021.
- ^ "Hungary signs deal for Chinese Sinopharm's COVID-19 vaccine, first in EU". National Post.
- ^ "Iran Launches Phase Two of Mass Inoculation Campaign". Financial Tribune. 22 February 2021. Retrieved 23 February 2021.
- ^ "First batch of Chinese Sinopharm vaccine arrives in Jordan". Roya News. Retrieved 9 January 2021.
- ^ KHARIZOV, Ruslan (19 March 2021). "150,000 doses of Sinopharm coronavirus vaccine delivered to Kyrgyzstan". 24.kg. Retrieved 22 March 2021.
- ^ Crean, Rosabel. "China donates 50,000 doses of Sinopharm vaccine to Lebanon". The Daily Star. Retrieved 2 March 2021.
- ^ "Macau receives first batch of COVID-19 vaccines". IAG. 7 February 2021. Retrieved 24 February 2021.
- ^ Rasheed, Aishath Hanaan Hussain. "MFDA approves Pfizer, Sinopharm Covid-19 vaccines for emergency use". raajje.mv. Retrieved 16 March 2021.
- ^ "Mauritania begins COVID-19 vaccination campaign". www.aa.com.tr. Retrieved 2021-03-29.
- ^ Cristina (19 March 2021). "O mie de studenți și medici-rezidenți din cadrul USMF vor fi imunizați anti-COVID cu vaccinul BBIBP-CorV, produs de către Sinopharm Beijing Institute of Biological Products". Ziarul de Gardă (in Romanian). Retrieved 19 March 2021.
- ^ "Deputy PM and City Governor get the first dose of Sinopharm vaccine". MONTSAME News Agency. Retrieved 15 March 2021.
- ^ "Montenegro receives 30,000 doses of China's COVID-19 vaccine". seenews.com. Retrieved 22 March 2021.
- ^ "Covid-19: Morocco authorizes use of the Sinopharm vaccine". en.yabiladi.com.
- ^ Reuters Staff (6 March 2021). "Mozambique expects to vaccinate 16 million against coronavirus by 2022". Reuters. Retrieved 7 March 2021.
{{cite web}}:|author=has generic name (help) - ^ Namibian, The. "Khomas, Erongo first to get vaccinated". The Namibian. Retrieved 17 March 2021.
- ^ Poudel, Arjun. "China's Shinopharm vaccine gets emergency use authorisation in Nepal". Kathmandu Post. Retrieved 19 February 2021.
- ^ https://www.channelnewsasia.com/news/world/china-donates-400-000-doses-of-sinopharm-covid-19-vaccine-to-14461352
- ^ Shahzad A (19 January 2021). "Pakistan approves Chinese Sinopharm COVID-19 vaccine for emergency use". Reuters.
- ^ "Peru grants 'exceptional' approval for Sinopharm COVID-19 vaccine – government sources". Reuters. 27 January 2021.
- ^ Asala, Kizzi. "Senegal Kicks Off COVID-19 Vaccination Campaign with China's Sinopharm". Africanews. Retrieved 23 February 2021.
- ^ "Serbia Becomes First European Nation To Use China's Sinopharm Vaccine". RadioFreeEurope/RadioLiberty.
- ^ Thomas, Abdul Rashid (15 March 2021). "Sierra Leone's President Bio leads the way in taking COVID-19 Vaccine". Sierra Leone Telegraph. Retrieved 16 March 2021.
- ^ "NMRA approves sinopharm vaccine for emergency use". Colombo Gazette. 2021-03-19. Retrieved 2021-03-29.
{{cite web}}: CS1 maint: url-status (link) - ^ Sequera, Vivian (1 March 2021). "Venezuela approves use of China's Sinopharm coronavirus vaccine". Reuters. Retrieved 2 March 2021.
- ^ Mutsaka, Farai (18 February 2021). "Zimbabwe starts administering China's Sinopharm vaccines". Toronto Star. Retrieved 20 February 2021.
- ^ a b "Safety and Immunogenicity Study of Inactivated Vaccine for Prevention of SARS-CoV-2 Infection (COVID-19) (Renqiu)". ClinicalTrials.gov. United States National Library of Medicine. 12 May 2020. NCT04383574. Archived from the original on 11 October 2020. Retrieved 14 July 2020.
- ^ "Clinical Trial of Efficacy and Safety of Sinovac's Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals (PROFISCOV)". ClinicalTrials.gov. United States National Library of Medicine. 2 July 2020. NCT04456595. Archived from the original on 11 October 2020. Retrieved 3 August 2020.
- ^ PT. Bio Farma (10 August 2020). "A Phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-COV-2 inactivated vaccine in healthy adults aged 18–59 years in Indonesia". Registri Penyakit Indonesia. Retrieved 15 August 2020.
- ^ a b c Liu R (6 February 2021). "China approves Sinovac Biotech COVID-19 vaccine for general public use". Reuters. Retrieved 7 February 2021.
- ^ Aliyev J. "Azerbaijan kicks off COVID-19 vaccination". Anadolu Agency. Retrieved 7 February 2021.
- ^ "Bolívia autoriza uso de vacinas Sputnik V e CoronaVac contra covid-19". noticias.uol.com.br (in Brazilian Portuguese). Retrieved 6 January 2021.
- ^ McGeever J, Fonseca P (17 January 2021). "Brazil clears emergency use of Sinovac, AstraZeneca vaccines, shots begin". Reuters. Retrieved 17 January 2021.
- ^ Chanritheara, Torn. "Cambodia Approves AstraZeneca and Sinovac Vaccines for COVID-19 Emergency Use". Cambodianess. Retrieved 12 February 2021.
- ^ "Chile aprueba el uso de emergencia de la vacuna china de Sinovac contra covid-19". France 24. 20 January 2021. Retrieved 30 January 2021.
- ^ Aliyev J. "Colombia approves emergency use of CoronaVac vaccine". Anadolu Agency. Retrieved 7 February 2021.
- ^ "Anticovid vaccines run out as Dominican Republic awaits arrival of more doses". DominicanToday. Retrieved 10 March 2021.
- ^ "Ecuador signs agreement with Sinovac for 2 million COVID-19 vaccine: minister". National Post. Retrieved 26 February 2021.
- ^ "Llegan a El Salvador un millón de dosis de la vacuna china CoronaVac contra el covid-19 de la farmacéutica Sinovac". Noticias de El Salvador - La Prensa Gráfica | Informate con la verdad (in European Spanish). Retrieved 2021-03-29.
- ^ "China to donate Sinovac Vaccine to Fiji". Fiji Broadcasting Corporation. Retrieved 22 March 2021.
- ^ "Use of Sinovac vaccine authorised". Government of Hong Kong. 18 February 2021. Retrieved 19 February 2021.
- ^ Soeriaatmadja W (11 January 2021). "Indonesia grants emergency use approval to Sinovac's vaccine, local trials show 65% efficacy". The Straits Times. Retrieved 11 January 2021.
- ^ "BPOM Grants Emergency Use Authorization for Sinovac Vaccine". Tempo. 11 January 2021. Retrieved 11 January 2021.
- ^ a b Barrera, Adriana (11 February 2021). "Mexico approves China's CanSino and Sinovac COVID-19 vaccines". Reuters. Retrieved 11 February 2021.
- ^ "CoronaVac, vacuna de alta eficacia". Ministerio de Salud Publica Y Bienestar Social.
- ^ "Philippines approves Sinovac's COVID-19 vaccine for emergency use". Reuters. 22 February 2021.
- ^ Thepgumpanat, Panarat (22 February 2021). "Thailand allows emergency use of Sinovac's COVID-19 vaccine". Reuters. Retrieved 23 February 2021.
- ^ "Tunisia approva vaccino cinese Sinovac" (in Italian). Agenzia Nazionale Stampa Associata (in Italian). 5 March 2021. Retrieved 7 March 2021.
- ^ "Turkey to begin COVID-19 vaccine jabs by this weekend". Anadolu. 11 January 2021. Retrieved 11 January 2021.
- ^ Zinets, Natalia (9 March 2021). "Ukraine approves China's Sinovac COVID-19 vaccine". Reuters. Retrieved 10 March 2021.
- ^ "Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)". ClinicalTrials.gov. United States National Library of Medicine. 16 March 2020. NCT04283461. Retrieved 5 March 2021.
- ^ "A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19". ClinicalTrials.gov. United States National Library of Medicine. 14 July 2020. NCT04470427. Archived from the original on 11 October 2020. Retrieved 27 July 2020.
- ^ Palca J (27 July 2020). "COVID-19 vaccine candidate heads to widespread testing in U.S." NPR. Archived from the original on 11 October 2020. Retrieved 27 July 2020.
- ^ "COVID-19 Vaccine Moderna EPAR". European Medicines Agency (EMA). Retrieved 20 January 2021.
- ^ "European Commission authorises second safe and effective vaccine against COVID-19". European Commission (Press release). Retrieved 6 January 2021.
- ^ "EMA recommends COVID-19 Vaccine Moderna for authorisation in the EU" (Press release). European Medicines Agency. 6 January 2021. Retrieved 6 January 2021.
- ^ "COVID-19 Vaccine Moderna". Union Register of medicinal products. Retrieved 14 January 2021.
- ^ "Regulatory Decision Summary – Moderna COVID-19 Vaccine". Health Canada, Government of Canada. 23 December 2020. Retrieved 23 December 2020.
- ^ "Israeli Ministry of Health Authorizes COVID-19 Vaccine Moderna for Use in Israel". modernatx.com. 4 January 2021. Retrieved 4 January 2021.
- ^ "Singapore becomes first in Asia to approve Moderna's COVID-19 vaccine". Reuters. 3 February 2021. Retrieved 3 February 2021.
- ^ "Swissmedic grants authorisation for the COVID-19 vaccine from Moderna" (Press release). Swiss Agency for Therapeutic Products (Swissmedic). 12 January 2020. Retrieved 12 January 2020.
- ^ "Information for Healthcare Professionals on COVID-19 Vaccine Moderna". Medicines and Healthcare products Regulatory Agency (MHRA). 8 January 2021. Retrieved 8 January 2021.
- ^ "Conditions of Authorisation for COVID-19 Vaccine Moderna". Medicines and Healthcare products Regulatory Agency (MHRA). 8 January 2021. Retrieved 9 January 2021.
- ^ "FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine". U.S. Food and Drug Administration (FDA) (Press release). Retrieved 18 December 2020.
- ^ Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. (December 2020). "The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Moderna COVID-19 Vaccine – United States, December 2020" (PDF). MMWR. Morbidity and Mortality Weekly Report. 69 (5152): 1653–56. doi:10.15585/mmwr.mm695152e1. PMID 33382675. S2CID 229945697.
- ^ Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19 (Briefing). Food and Drug Administration. 26 February 2021. p. 6. Retrieved 6 March 2021.
The vaccine, known as Ad26.COV2.S, is a replication-incompetent adenovirus type 26 (Ad26) vectored vaccine encoding a stabilized variant of the SARS-CoV-2 S protein.
- ^ "A Study of Ad26.COV2.S in Adults". ClinicalTrials.gov. 4 August 2020. Archived from the original on 16 September 2020. Retrieved 23 August 2020.
- ^ "A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants". ClinicalTrials.gov. US National Library of Medicine. Archived from the original on 26 September 2020.
- ^ "COVID-19 Vaccine Janssen EPAR". European Medicines Agency (EMA). 5 March 2021. Retrieved 16 March 2021.
- ^ "European Commission authorises fourth safe and effective vaccine against COVID-19". European Commission (Press release). 11 March 2021.
- ^ "Bahrain first to approve Johnson & Johnson COVID-19 vaccine for emergency use". Reuters. 25 February 2021. Retrieved 25 February 2021.
- ^ "Bahrain becomes 1st nation to grant J&J shot emergency use". ABC News. 25 February 2021. Retrieved 25 February 2021.
- ^ "Johnson & Johnson COVID-19 vaccine becomes 4th to receive Health Canada approval". CBC News. Retrieved 5 March 2021.
- ^ Acosta, Luis Jaime (25 March 2021). "Colombia grants emergency use for J&J coronavirus vaccine". Reuters. Bogotá. Retrieved 25 March 2021.
- ^ "Coronavirus: South Africa rolls out vaccination programme". BBC News. 17 February 2021. Retrieved 19 February 2021.
- ^ https://www.swissinfo.ch/eng/johnson---johnson-covid-vaccine-approved-for-use-in-switzerland/46469458
- ^ https://www.reuters.com/article/us-health-coronavirus-thailand-idUSKBN2BH1L7
- ^ "FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine". U.S. Food and Drug Administration (FDA) (Press release). 27 February 2021. Retrieved 27 February 2021.
- ^ https://www.fda.gov/media/146303/download
- ^ "WHO approves J&J's COVID-19 vaccine for emergency listing". Channel News Asia. 13 March 2021. Retrieved 13 March 2021.
- ^ Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. (August 2020). "Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial". Lancet. 396 (10249): 479–88. doi:10.1016/s0140-6736(20)31605-6. ISSN 0140-6736. PMC 7836858. PMID 32702299.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ "China approves two more domestic COVID-19 vaccines for public use". Reuters. 25 February 2021. Retrieved 16 March 2021.
- ^ "China's CanSino Biologics COVID-19 vaccine receives emergency use approval in Hungary". MSN. 22 March 2021. Retrieved 22 March 2021.
- ^ "Malaysia's Solution Group to supply 3.5 million doses of CanSino vaccine to government". Reuters. 4 February 2021. Retrieved 22 March 2021.
- ^ Shahzad, Asif (12 February 2021). "Pakistan approves Chinese CanSinoBIO COVID vaccine for emergency use". Reuters. Retrieved 12 February 2021.
- ^ "Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152) for COVID-19 in Healthy Volunteers". ClinicalTrials.gov. NCT04471519.
- ^ "Iran issues permit for emergency use for three other COVID-19 vaccines: Official". IRNA English. 17 February 2021.
- ^ "Mauritius to receive consignment of Indian-made coronavirus vaccine Covaxin". Business Standard. Retrieved 19 March 2021.
- ^ Sharma, Gopal (19 March 2021). "Nepal becomes third country to give emergency nod to Indian vaccine COVAXIN". Reuters. Retrieved 19 March 2021.
- ^ Manral, Karan (4 March 2021). "Zimbabwe approves Covaxin, first in Africa to okay India-made Covid-19 vaccine". Hindustan Times. Retrieved 6 March 2021.
- ^ "India provides 100,000 doses of Covaxin vaccine to Paraguay". Hindustan Times. 30 March 2021.
- ^ @MaritoAbdo (30 March 2021). "Acaban de llegar 100.000 dosis de vacunas contra el Covid-19, provenientes de la India. La prioridad es avanzar hacia la inmunización de todo el personal de blanco y luego la población de riesgo. Gracias a la República de la India por el apoyo" (Tweet) – via Twitter.
- ^ "Study of the Safety, Reactogenicity and Immunogenicity of "EpiVacCorona" Vaccine for the Prevention of COVID-19 (EpiVacCorona)". ClinicalTrials.gov. United States National Library of Medicine. 22 September 2020. NCT04368988. Retrieved 16 November 2020.
- ^ "Turkmenistan registers vaccines for the prevention of infectious diseases". Turkmenistan Today. 29 January 2021.
- ^ "Russian vaccine "EpiVacCorona" was registered in Turkmenistan". EN24. 29 January 2021.
- ^ "О регистрации вакцины ФБУН ГНЦ ВБ "Вектор" Роспотребнадзора "ЭпиВакКорона"". Rospotrebnadzor (in Russian). 14 October 2020.
- ^ "Russia's EpiVacCorona vaccine post-registration trials started". The Pharma Letter. 18 November 2020.
- ^ "COVID-19 vaccine development pipeline (Refresh URL to update)". Vaccine Centre, London School of Hygiene and Tropical Medicine. 1 March 2021. Retrieved 10 March 2021.
- ^ Liu, Roxanne (15 March 2021). "China IMCAS's COVID-19 vaccine obtained emergency use approval in China". Reuters. Retrieved 15 March 2021.
- ^ Mamatkulov, Mukhammadsharif (1 March 2021). "Uzbekistan approves Chinese-developed COVID-19 vaccine". Reuters. Retrieved 2 March 2021.
- ^ "Uzbekistan certifies Chinese vaccine to fight against COVID-19". China Daily. 2 March 2021. Retrieved 8 March 2021.
- ^ Ryumin, Alexander (20 February 2021). "Russia registers its third COVID-19 vaccine CoviVac". TASS. Moscow. Retrieved 6 March 2021.
- ^ "Briefing with Deputy Prime Minister Tatyana Golikova, Health Minister Mikhail Murashko and Head of Rospotrebnadzor Anna Popova". Government of Russia. 18 January 2021. Retrieved 20 February 2021.
- ^ "Russia approves its third COVID-19 vaccine, CoviVac". Reuters. 20 February 2021.