Phenoperidine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Bile and Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.391 |
| Chemical and physical data | |
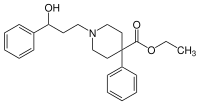
| Formula | C23H29NO3 |
| Molar mass | 367.481 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Phenoperidine[1][2](Operidine or Lealgin), is an opioid analgesic which is structurally related to pethidine and is used clinically as a general anesthetic.
Medical use
Phenoperidine is an opioid pain killer -- narcotic analgesic. [medical citation needed]
Pharmacology
It is a derivative of isonipecotic acid, like pethidine, and is metabolized in part to norpethidine. Its potency range is due to method of ingestion. figure 20-80 times as potent as pethidine as an analgesic. The greatly increased potency essentially eliminates the toxic effects of norpethidine accumulation which are seen when pethidine is administered in high doses or for long periods of time.[3]
History and Synthesis
Phenoperidine was first synthesized in 1957 by Paul Janssen, of the company now known as Janssen Pharmaceutica, who was seeking better opioid pain-killers.[4] His two prototype drugs were methadone and pethidine, each which had been invented in 1930s by Otto Eisleb, who worked for IG Farben. His initial work starting with methadone yielded dextromoramide in 1954. Janssen then turned to making pethidine analogues, due in part to the less complicated chemistry of the compound. During his explorations, he replaced the methyl group attached to the pethidine nitrogen with a propiophenone group, and this yielded phenoperidine, in 1957. Phenoperidine was determined to have decreased stability and enhanced lipophilicity compared to pethidine. Soon after, studies in mice showed that phenoperidine was over 100 times more potent than pethidine.[4]
In 1958, the same line of work yielded “one of the greatest advances of the 20th century psychiatry", haloperidol,[4] as well as diphenoxylate, which lacked the opioid's analgesic properties but still stopped peristalsis in the intestines, a typical side effect of opioids; Janssen brought diphenoxylate to market as a drug to treat diarrhea.[5]: 124 And through further advances, Janssen created fentanyl in 1960, which proved to be ten times more potent than phenoperidine.[6]
Historical Uses
In 1959, the combination of phenoperidine and haloperidol was first used in Europe in anesthesia to induce a detached, pain free state called Neuroleptic analgesia; the use of that mixture boomed in early 1960s but was overtaken by the combination of fentanyl and droperidol, which was widely used through the 1980s. These combination approaches were not adopted in the US.[7]: 644
Regulations
In 1961 phenoperidine was added to the 1931 Convention for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs by the World Health Organization via the Single Convention on Narcotic Drugs.[8][9]
In the US it is classified as a schedule 1 opiate and is categorized as a Drug Enforcement Administration (DEA) controlled substance with a corresponding code 9641.[10]
References
- ^ US 2951080, "Novel phenyl-substituted piperidines", assigned to Eli Lilly Co.
- ^ US 2962501, "-substituted propyl piperidines and processes of preparing same", assigned to Merck & Co.
- ^ Lamberth C, Dinges J (2016-08-22). Bioactive carboxylic compound classes : pharmaceuticals and agrochemicals. Weinheim, Germany: Wiley-VCH Verlag. p. 29. ISBN 978-3-527-33947-1.
- ^ a b c López-Muñoz F, Alamo C (April 2009). "The consolidation of neuroleptic therapy: Janssen, the discovery of haloperidol and its introduction into clinical practice". Brain Research Bulletin. 79 (2): 130–41. doi:10.1016/j.brainresbull.2009.01.005. PMID 19186209. S2CID 7720401.
- ^ Sneade W (2005). Drug Discovery: A History. John Wiley & Sons. ISBN 978-0-471-89979-2.
- ^ Stanley TH (December 2014). "The fentanyl story". The Journal of Pain. 15 (12): 1215–26. doi:10.1016/j.jpain.2014.08.010. PMID 25441689.
- ^ Eger EI, Saidman L, Westhorpe R (2013). The Wondrous Story of Anesthesia. Springer Science & Business Media. ISBN 978-1-4614-8441-7.
- ^ Expert Committee on Addiction-Producing Drugs (1961). "Eleventh Report" (PDF). WHO Technical Report Series.
- ^ WHO Executive Board. 17 April 1961 Action in Respect of the International Convention on Narcotic Drugs.
- ^ "Memo: Overview of the September 14, 2010, DSaRM Advisory Committee Meeting to Discuss the Drug Enforcement Administration (DEA) Request for an Abuse Potential Evaluation and Scheduling Recommendation for Dextromethorphan (DXM)" (PDF). U.S. Food and Drug Administration. U.S. Food and Drug Administration. Retrieved 22 November 2014.
Further reading
- Kintz P, Godelar B, Mangin P, Lugnier AA, Chaumont AJ (December 1989). "Simultaneous determination of pethidine (meperidine), phenoperidine, and norpethidine (normeperidine), their common metabolite, by gas chromatography with selective nitrogen detection". Forensic Science International. 43 (3): 267–73. doi:10.1016/0379-0738(89)90154-0. PMID 2613140.
- Claris O, Bertrix L (1988). "[Phenoperidine: pharmacology and use in pediatric resuscitation]". Pédiatrie (in French). 43 (6): 509–13. PMID 3186421.
- "Antipsychotics - Reference pathway". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories, Kyoto University, University of Tokyo. Retrieved 2007-01-16.
