2C-YN
This is an old revision of this page, as edited by Monkbot (talk | contribs) at 15:21, 19 October 2020 (→top: Task 17: replace to-be-deprecated: |name-list-format= (1× replaced; usage: 1 of 1);). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
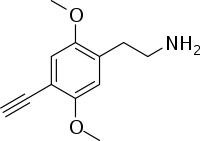
| Formula | C12H15NO2 |
| Molar mass | 205.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
2C-YN is an analog of phenethylamine that can be synthesized from 2C-I.[1] Very little data exists about the pharmacological properties, metabolism, and toxicity of 2C-YN, although Daniel Trachsel lists it as having a dosage of around 50mg and a duration of around 2 hours, with relatively mild psychedelic effects.[2]
Legality
Canada
As of October 31st, 2016; 2C-YN is a controlled substance (Schedule III) in Canada. http://gazette.gc.ca/rp-pr/p2/2016/2016-05-04/html/sor-dors72-eng.php
See also
References
- ^ Trachsel D (August 2003). "Synthesis of Novel (Phenylalkyl) amines for the Investigation of Structure–Activity Relationships, Part 3: 4‐Ethynyl‐2,5‐dimethoxyphenethylamine (= 4‐Ethynyl‐2, 5‐dimethoxybenzeneethanamine; 2C‐YN)". Helvetica Chimica Acta. 86 (8): 2754–9. doi:10.1002/hlca.200390224.
- ^ Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine Von der Struktur zur Funktion. Nachtschatten Verlag AG. p. 768. ISBN 978-3-03788-700-4.
This hallucinogen-related article is a stub. You can help Wikipedia by expanding it. |
- Articles with short description
- Short description is different from Wikidata
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All stub articles
