From Wikipedia, the free encyclopedia
Chemical compound
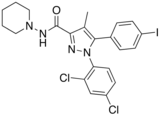
AM-251 is an inverse agonist at the CB1 cannabinoid receptor. AM-251 is structurally very close to rimonabant ; both are biarylpyrazole cannabinoid receptor antagonists . In AM-251, the p -chloro group attached to the phenyl substituent at C-5 of the pyrazole ring is replaced with a p -iodo group. The resulting compound exhibits slightly better binding affinity for the CB1 receptor (with a Ki value of 7.5 nM) than rimonabant, which has a Ki value of 11.5 nM, AM-251 is, however, about two-fold more selective for the CB1 receptor when compared to rimonabant.[1] [2] [3]
AM251 has shown an in vitro pancreatic and colon cancer cells.[4]
^ Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. (February 1999). "Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists". Journal of Medicinal Chemistry . 42 (4): 769–776. doi :10.1021/jm980363y . PMID 10052983 . ^ Seely KA, Brents LK, Franks LN, Rajasekaran M, Zimmerman SM, Fantegrossi WE, Prather PL (October 2012). "AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies" . Neuropharmacology . 63 (5): 905–915. doi :10.1016/j.neuropharm.2012.06.046 . PMC 3408547 PMID 22771770 . ^ Seely KA, Brents LK, Franks LN, Rajasekaran M, Zimmerman SM, Fantegrossi WE, Prather PL (October 2012). "AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies" . Neuropharmacology . 63 (5): 905–915. doi :10.1016/j.neuropharm.2012.06.046 . PMC 3408547 PMID 22771770 . ^ Carpi S, Fogli S, Romanini A, Pellegrino M, Adinolfi B, Podestà A, et al. (August 2015). "AM251 induces apoptosis and G2/M cell cycle arrest in A375 human melanoma cells" (PDF) . Anti-Cancer Drugs . 26 (7): 754–762. doi :10.1097/CAD.0000000000000246 . hdl :11568/750318 . PMID 25974027 . S2CID 205526223 . Archived (PDF) from the original on July 2, 2021.
Phytocannabinoids comparison )
Cannabibutols Cannabichromenes Cannabicyclols Cannabidiols Cannabielsoins Cannabigerols Cannabiphorols Cannabinols Cannabitriols Cannabivarins Delta-8-tetrahydrocannabinols Delta-9-tetrahydrocannabinols Delta-10-Tetrahydrocannabinols Miscellaneous cannabinoids Active metabolites
Endocannabinoids Synthetic
Classical cannabinoids Non-classical Adamantoylindoles Benzimidazoles Benzoylindoles Cyclohexylphenols Eicosanoids Indazole-3- Indole-3-carboxamides Indole-3-carboxylates Naphthoylindazoles Naphthoylindoles Naphthoylpyrroles Naphthylmethylindenes Naphthylmethylindoles Phenylacetylindoles Pyrazolecarboxamides Tetramethylcyclo- Tetramethylcyclo- Others
Allosteric CBR Tooltip Cannabinoid receptor ligands Endocannabinoid (inactivation inhibitors) Anticannabinoids (antagonists/inverse
Receptor (ligands )
CB1 Tooltip Cannabinoid receptor type 1
Agonists(abridged,full list ) Inverse agonists Antagonists
CB2 Tooltip Cannabinoid receptor type 2
Agonists
2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1172 AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-73 JWH-133 L-759,633 L-759,656 Lenabasum (anabasum) Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Tetrahydromagnolol Virodhamine Antagonists
NAGly GPR18 )
GPR55
GPR119
Transporter (modulators )
eCBTs Tooltip Endocannabinoid transporter
Enzyme (modulators )
Others
Others: 2-PG (directly potentiates activity of 2-AG at CB1 receptor) ARN-272 (FAAH-like anandamide transporter inhibitor)