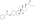Estrogen (medication)
| Estrogen (medication) | |
|---|---|
| Drug class | |
 Estradiol, the major estrogen sex hormone in humans and a widely used medication. | |
| Class identifiers | |
| Use | Contraception, menopause, hypogonadism, transgender women, prostate cancer, breast cancer, others |
| ATC code | G03C |
| Biological target | Estrogen receptors (ERα, ERβ, mERs (e.g., GPER, others)) |
| External links | |
| MeSH | D004967 |
| Legal status | |
| In Wikidata | |
An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women.[1] They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens.[1] They are available in a wide variety of formulations and for use by many different routes of administration.[1] Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol.[1] Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.
Side effects of estrogens include breast tenderness, breast enlargement, headache, nausea, fluid retention, and edema among others.[1] Other side effects of estrogens include an increased risk of blood clots, cardiovascular disease, and, when combined with most progestogens, breast cancer.[1] In men, estrogens can cause breast development, feminization, infertility, low testosterone levels, and sexual dysfunction among others.
Estrogens are agonists of the estrogen receptors, the biological targets of endogenous estrogens like estradiol. They have important effects in many tissues in the body, including in the female reproductive system (uterus, vagina, and ovaries), the breasts, bone, fat, the liver, and the brain among others.[1] Unlike other medications like progestins and anabolic steroids, estrogens do not have other hormonal activities.[1] Estrogens also have antigonadotropic effects and at sufficiently high dosages can strongly suppress sex hormone production.[1] Estrogens mediate their contraceptive effects in combination with progestins by inhibiting ovulation.
Estrogens were first introduced for medical use in the early 1930s. They started to be used in birth control in combination with progestins in the 1950s.[2] A variety of different estrogens have been marketed for clinical use in humans or use in veterinary medicine, although only a handful of these are widely used.[3][4][5][6][7] These medications can be grouped into different types based on origin and chemical structure.[1] Estrogens are available widely throughout the world and are used in most forms of hormonal birth control and in all menopausal hormone therapy regimens.[3][4][6][5][1]
Medical uses
Birth control
Estrogens have contraceptive effects and are used in combination with progestins (synthetic progestogens) in birth control to prevent pregnancy in women. This is referred to as combined hormonal contraception. The contraceptive effects of estrogens are mediated by their antigonadotropic effects and hence by inhibition of ovulation. Most combined oral contraceptives contain ethinylestradiol or its prodrug mestranol as the estrogen component, but a few contain estradiol or estradiol valerate. Ethinylestradiol is generally used in oral contraceptives instead of estradiol because it has superior oral pharmacokinetics (higher bioavailability and less interindividual variability) and controls vaginal bleeding more effectively. This is due to its synthetic nature and its resistance to metabolism in certain tissues such as the intestines, liver, and uterus relative to estradiol. Besides oral contraceptives, other forms of combined hormonal contraception include contraceptive patches, contraceptive vaginal rings, and combined injectable contraceptives. Contraceptive patches and vaginal rings contain ethinylestradiol as the estrogen component, while combined injectable contraceptives contain estradiol or more typically an estradiol ester.
Hormone therapy
Menopause

Estrogen and other hormones are given to postmenopausal women in order to prevent osteoporosis as well as treat the symptoms of menopause such as hot flashes, vaginal dryness, urinary stress incontinence, chilly sensations, dizziness, fatigue, irritability, and sweating. Fractures of the spine, wrist, and hips decrease by 50 to 70% and spinal bone density increases by approximately 5% in those women treated with estrogen within 3 years of the onset of menopause and for 5 to 10 years thereafter.
Before the specific dangers of conjugated estrogens were well understood, standard therapy was 0.625 mg/day of conjugated estrogens (such as Premarin). There are, however, risks associated with conjugated estrogen therapy. Among the older postmenopausal women studied as part of the Women's Health Initiative (WHI), an orally administered conjugated estrogen supplement was found to be associated with an increased risk of dangerous blood clotting. The WHI studies used one type of estrogen supplement, a high oral dose of conjugated estrogens (Premarin alone and with medroxyprogesterone acetate as Prempro).[10]
In a study by the NIH, esterified estrogens were not proven to pose the same risks to health as conjugated estrogens. Menopausal hormone therapy has favorable effects on serum cholesterol levels, and when initiated immediately upon menopause may reduce the incidence of cardiovascular disease, although this hypothesis has yet to be tested in randomized trials. Estrogen appears to have a protector effect on atherosclerosis: it lowers LDL and triglycerides, it raises HDL levels and has endothelial vasodilatation properties plus an anti-inflammatory component.
Research is underway to determine if risks of estrogen supplement use are the same for all methods of delivery. In particular, estrogen applied topically may have a different spectrum of side effects than when administered orally,[11] and transdermal estrogens do not affect clotting as they are absorbed directly into the systemic circulation, avoiding first-pass metabolism in the liver. This route of administration is thus preferred in women with a history of thromboembolic disease.
Estrogen is also used in the therapy of vaginal atrophy, hypoestrogenism (as a result of hypogonadism, oophorectomy, or primary ovarian failure), amenorrhea, dysmenorrhea, and oligomenorrhea. Estrogens can also be used to suppress lactation after child birth.
Synthetic estrogens, such as 17α-substituted estrogens like ethinylestradiol and its C3 esters and ethers mestranol, quinestrol, and ethinylestradiol sulfonate, and nonsteroidal estrogens like the stilbestrols diethylstilbestrol, hexestrol, and dienestrol, are no longer used in menopausal hormone therapy, owing to their disproportionate effects on liver protein synthesis and associated health risks.[12]
| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | 2.5–10 μg/day | 5–20 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
| Transdermal gel | Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
| Vaginal | Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
| IM or SC injection | Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
Hypogonadism
Estrogens are used along with progestogens to treat hypogonadism and delayed puberty in women.
Transgender women
Estrogens are used along with antiandrogens and progestogens as a component of feminizing hormone therapy for transgender women and other transfeminine individuals.[13][14][15]
Hormonal cancer
Prostate cancer
High-dose estrogen therapy with a variety of estrogens such as diethylstilbestrol, ethinylestradiol, polyestradiol phosphate, estradiol undecylate, estradiol valerate, and estradiol has been used to treat prostate cancer in men.[16] It is effective because estrogens are functional antiandrogens, capable of suppressing testosterone levels to castrate concentrations and decreasing free testosterone levels by increasing sex hormone-binding globulin (SHBG) production. High-dose estrogen therapy is associated with poor tolerability and safety, namely gynecomastia and cardiovascular complications such as thrombosis.[additional citation(s) needed] For this reason, has largely been replaced by newer antiandrogens such as gonadotropin-releasing hormone analogues and nonsteroidal antiandrogens. It is still sometimes used in the treatment of prostate cancer however,[16] and newer estrogens with atypical profiles such as GTx-758 that have improved tolerability profiles are being studied for possible application in prostate cancer.
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Breast cancer
High-dose estrogen therapy with potent synthetic estrogens such as diethylstilbestrol and ethinylestradiol was used in the past in the palliation treatment of breast cancer.[17] Its effectiveness is approximately equivalent to that of antiestrogen therapy with selective estrogen receptor modulators (SERMs) like tamoxifen and aromatase inhibitors like anastrozole.[17] The use of high-dose estrogen therapy in breast cancer has mostly been superseded by antiestrogen therapy due to the improved safety profile of the latter.[17] High-dose estrogen therapy was the standard of care for the palliative treatment of breast cancer in women up to the late 1970s or early 1980s.[18]
About 80% of breast cancers, once established, rely on supplies of the hormone estrogen to grow: they are known as hormone-sensitive or hormone-receptor-positive cancers.[19][citation needed] Prevention of the actions or production of estrogen in the body is a treatment for these cancers.[citation needed]
Hormone-receptor-positive breast cancers are treated with drugs which suppress production or interfere with the action of estrogen in the body.[20] This technique, in the context of treatment of breast cancer, is known variously as hormonal therapy, hormone therapy, or antiestrogen therapy (not to be confused with hormone replacement therapy). Certain foods such as soy may also suppress the proliferative effects of estrogen and are used as an alternative to hormone therapy.[21]
| Route/form | Estrogen | Dosage | Ref(s) |
|---|---|---|---|
| Oral | Estradiol | 10 mg 3x/day AI-resistant: 2 mg 1–3x/day |
[22][23] [22][24] |
| Estradiol valerate | AI-resistant: 2 mg 1–3x/day | [22][24] | |
| Conjugated estrogens | 10 mg 3x/day | [25][26][27][28] | |
| Ethinylestradiol | 0.5–1 mg 3x/day | [26][22][29][28] | |
| Diethylstilbestrol | 5 mg 3x/day | [26][30][31] | |
| Dienestrol | 5 mg 3x/day | [29][28][31] | |
| Dimestrol | 30 mg/day | [25][28][31] | |
| Chlorotrianisene | 24 mg/day | [25][31] | |
| IM or SC injection | Estradiol benzoate | 5 mg 2–3x/week | [29][32][30][33] |
| Estradiol dipropionate | 5 mg 2–3x/week | [29][30][34][33] | |
| Estradiol valerate | 30 mg 1x/2 weeks | [32] | |
| Polyestradiol phosphate | 40–80 mg 1x/4 weeks | [35][36] | |
| Estrone | 5 mg ≥3x/week | [37] | |
| Notes: (1) Only in women who are at least 5 years postmenopausal.[22] (2) Dosages are not necessarily equivalent. | |||
Other uses
Infertility
Estrogens may be used in treatment of infertility in women when there is a need to develop sperm-friendly cervical mucous or an appropriate uterine lining.[38][39]
Pregnancy support
Estrogens like diethylstilbestrol were formerly used in high doses to help support pregnancy.[40] However, subsequent research showed diethylstilbestrol to be ineffective as well as harmful.[40]
Lactation suppression
Estrogens can be used to suppress lactation, for instance in the treatment of breast engorgement or galactorrhea.[41] However, high doses are needed, the effectiveness is uncertain, and high doses of estrogens in the postpartum period can increase the risk of blood clots.[42]
Tall stature
Estrogen has been used to induce growth attenuation in tall girls.[43]
Estrogen-induced growth attenuation was used as part of the controversial Ashley Treatment to keep a developmentally disabled girl from growing to adult size.[44]
Acromegaly
Estrogens have been used to treat acromegaly.[45][46][47] This is because they suppress growth hormone-induced insulin-like growth factor 1 (IGF-1) production in the liver.[45][46][47]
Sexual deviance
High-dose estrogen therapy has been used successfully in the treatment of sexual deviance such as paraphilias in men but has been found to produce many side effects (e.g., gynecomastia, feminization, cardiovascular disease, blood clots) and so is no longer recommended for such purposes.[48] It works by suppressing testosterone levels, similarly to high-dose progestogen therapy and gonadotropin-releasing hormone analogue (GnRH analogue) therapy.[48] Lower dosages of estrogens have also been used in combination with high-dose progestogen therapy in the treatment of sexual deviance in men.[48] High incidence of sexual dysfunction has similarly been associated with high-dose estrogen therapy in men treated with it for prostate cancer.[49]
Breast enhancement
Estrogens are involved in breast development and may be used as a form of hormonal breast enhancement to increase the size of the breasts.[50][51][52][53][54] However, acute or temporary breast enlargement is a well-known side effect of estrogens, and increases in breast size tend to regress following discontinuation of treatment.[50][52][53] Aside from those without prior established breast development, evidence is lacking for a sustained increase in breast size with estrogens.[50][52][53]
Depression
Published 2019 and 2020 guidelines from the North American Menopause Society (NAMS) and European Menopause and Andropause Society (EMAS) have reviewed the topic of estrogen therapy for depressive symptoms in the peri- and postmenopause.[55][56] There is some evidence that estrogens are effective in the treatment of depression in perimenopausal women.[55][56][57][58][59][60][61][62][63][64][65] The magnitude of benefit appears to be similar to that of classical antidepressants.[55][56] There is also some evidence that estrogens may improve mood and well-being in non-depressed perimenopausal women.[55][56][60][58] Estrogens do not appear to be effective in the treatment of depression in postmenopausal women.[55][56] This suggests that there is a window of opportunity for effective treatment of depressive symptoms with estrogens.[55] Research on combined estrogen and progestogen therapy for depressive symptoms in the peri- and postmenopause is scarce and inconclusive.[55][56] Estrogens may augment the mood benefits of antidepressants in middle-aged and older women.[55][56] Menopausal hormone therapy is not currently approved for the treatment of depressive symptoms in the peri- or postmenopause in either the United States or the United Kingdom due to insufficient evidence of effectiveness.[55][56][60] More research is needed on the issue of estrogen therapy for depressive symptoms associated with menopause.[63][61]
Schizophrenia
Estrogens appear to be useful in the treatment of schizophrenia in both women and men.[66][67][68][69]
Acne
Systemic estrogen therapy at adequate doses is effective for and has been used in the treatment of acne in both females and males, but causes major side effects such as feminization and gynecomastia in males.[70][71][72][73][74][75][76][77]
Available forms
| Generic name | Class | Brand name | Route | Intr. |
|---|---|---|---|---|
| Conjugated estrogens | S/ester[a] | Premarin | PO, IM, TD, V | 1941 |
| Dienestrol[b] | NS | Synestrol[c] | PO | 1947 |
| Diethylstilbestrol[b] | NS | Stilbestrol[c] | PO, TD, V | 1939 |
| Esterified estrogens | NS/ester[a] | Estratab | PO | 1970 |
| Estetrol[d] | S | Donesta[c] | PO | N/A |
| Estradiol | S | Estrace[c] | PO, IM, SC, SL, TD, V | 1935 |
| Estradiol acetate | S/ester | Femring[c] | PO, V | 2001 |
| Estradiol benzoate | S/ester | Progynon B | IM | 1933 |
| Estradiol cypionate | S/ester | Depo-Estradiol | IM | 1952 |
| Estradiol enanthate | S/ester | Deladroxate[c] | IM | 1970s |
| Estradiol valerate | S/ester | Progynon Depot[c] | PO, IM | 1954 |
| Estramustine phosphate[e] | S/ester | Emcyt[c] | PO | 1970s |
| Estriol | S | Theelol[c] | PO, V | 1930 |
| Estropipate[b] | S/ester | Ogen | PO | 1968 |
| Ethinylestradiol | S/alkyl | Estinyl[c] | PO, TD, V | 1943 |
| Fosfestrol[b] | NS/ester | Honvan[c] | IM | 1947 |
| Hexestrol[b] | NS | Synestrol[c] | PO, IM | 1940s |
| Mestranol[b] | S/alkyl/ether | Enovid[c] | PO | 1957 |
| Methylestradiol[b] | S/alkyl | Ginecosid[c] | PO | 1955 |
| Polyestradiol phosphate[b] | S/ester | Estradurin | IM | 1957 |
| Prasterone[f] | Prohormone | Intrarosa[c] | PO, IM, V | 1970s |
| Zeranol[g] | NS | Ralgro[c] | PO | 1970s |
| ||||
Estrogens that have been marketed come in two major types, steroidal estrogens and nonsteroidal estrogens.[1][78]
Steroidal estrogens
Estradiol, estrone, and estriol have all been approved as pharmaceutical drugs and are used medically.[1] Estetrol is currently under development for medical indications, but has not yet been approved in any country.[79] A variety of synthetic estrogen esters, such as estradiol valerate, estradiol cypionate, estradiol acetate, estradiol benzoate, estradiol undecylate, and polyestradiol phosphate, are used clinically.[1] The aforementioned compounds behave as prodrugs to estradiol, and are much longer-lasting in comparison when administered by intramuscular or subcutaneous injection.[1] Esters of estrone and estriol also exist and are or have been used in clinical medicine, for example estrone sulfate (e.g., as estropipate), estriol succinate, and estriol glucuronide (as Emmenin and Progynon).[1]
Ethinylestradiol is a more potent synthetic analogue of estradiol that is used widely in hormonal contraceptives.[1] Other synthetic derivatives of estradiol related to ethinylestradiol that are used clinically include mestranol, quinestrol, ethinylestradiol sulfonate, moxestrol, and methylestradiol. Conjugated estrogens (brand name Premarin), an estrogen product manufactured from the urine of pregnant mares and commonly used in menopausal hormone therapy, is a mixture of natural estrogens including estrone sulfate and equine estrogens such as equilin sulfate and 17β-dihydroequilin sulfate.[1] A related and very similar product to conjugated estrogens, differing from it only in composition, is esterified estrogens.[1]
Testosterone, prasterone (dehydroepiandrosterone; DHEA), boldenone (δ1-testosterone), and nandrolone (19-nortestosterone) are naturally occurring androgens/anabolic steroids (AAS) which form estradiol as an active metabolite in small amounts and can produce estrogenic effects, most notably gynecomastia in men at sufficiently high dosages.[80] Similarly, a number of synthetic AAS, including methyltestosterone, metandienone, normethandrone, and norethandrolone, produce methylestradiol or ethylestradiol as an active metabolite in small quantities, and can produce estrogenic effects as well.[80] A few progestins, specifically the 19-nortestosterone derivatives norethisterone, noretynodrel, and tibolone, metabolize into estrogens (e.g., ethinylestradiol) and can produce estrogenic effects as well.[1][81]
Nonsteroidal estrogens
Diethylstilbestrol is a nonsteroidal estrogen that is no longer used medically. It is a member of the stilbestrol group. Other stilbestrol estrogens that have been used clinically include benzestrol, dienestrol, dienestrol acetate, diethylstilbestrol dipropionate, fosfestrol, hexestrol, and methestrol dipropionate. Chlorotrianisene, methallenestril, and doisynoestrol are nonsteroidal estrogens structurally distinct from the stilbestrols that have also been used clinically. While used widely in the past, nonsteroidal estrogens have mostly been discontinued and are now rarely if ever used medically.
Contraindications
Estrogens have various contraindications.[82][83][84][85] An example is history of thromboembolism (blood clots).[82][83][84][85]
Side effects
The most common side effects of estrogens in general include breast tenderness, breast enlargement, headache, nausea, fluid retention, and edema. In women, estrogens can additionally cause vaginal bleeding, vaginal discharge, and anovulation, whereas in men, estrogens can additionally cause gynecomastia (male breast development), feminization, demasculinization, sexual dysfunction (reduced libido and erectile dysfunction), hypogonadism, testicular atrophy, and infertility.
Estrogens can or may increase the risk of uncommon or rare but potentially serious issues including endometrial hyperplasia, endometrial cancer, cardiovascular complications (e.g., blood clots, stroke, heart attack), cholestatic hepatotoxicity, gallbladder disease (e.g., gallstones), hyperprolactinemia, prolactinoma, and dementia. These adverse effects are moderated by the concomitant use of a progestogen, the type of progestogen used, and the dosage and route of estrogen used.
Around half of women with epilepsy who menstruate have a lowered seizure threshold around ovulation, most likely from the heightened estrogen levels at that time. This results in an increased risk of seizures in these women.
| Serious adverse event | Estradiol 6 mg/day (n = 34) | Estradiol 30 mg/day (n = 32) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Nausea/vomiting | 0 | 0.0 | 5 | 15.6 |
| Hyponatremia | 1 | 2.9 | 5 | 15.6 |
| Pleural effusion | 0 | 0.0 | 4 | 12.5 |
| Pain | 6 | 17.6 | 4 | 12.5 |
| Thrombosis/embolism | 1 | 2.9 | 1 | 3.1 |
| Brain ischemia | 1 | 2.9 | 0 | 0.0 |
| Infection | 2 | 5.9 | 3 | 9.4 |
| Hypercalcemia | 0 | 0.0 | 2 | 6.3 |
| Other | 6 | 17.6 | 10 | 31.3 |
| Summary: Side effects in a small phase 2 study of women with metastatic breast cancer randomized to receive either 6 or 30 mg/day of oral estradiol as therapy. "The adverse event rate (≥grade 3) in the 30-mg group (11/32 [34%]; 95% confidence interval [CI], 23%-47%) was higher than in the 6-mg group (4/34 [18%]; 95% CI, 5%-22%; p=0.03). Clinical benefit rates were 9 of 32 (28%; 95% CI, 18%-41%) in the 30-mg group and 10 of 34 (29%; 95% CI, 19%-42%) in the 6-mg group." Sources: See template. | ||||
Long-term effects
Endometrial hyperplasia and cancer
Unopposed estrogen therapy stimulates the growth of the endometrium and is associated with a dramatically increased risk of endometrial hyperplasia and endometrial cancer in postmenopausal women.[86] The risk of endometrial hyperplasia is greatly increased by 6 months of treatment (OR = 5.4) and further increased after 36 months of treatment (OR = 16.0).[86] This can eventually progress to endometrial cancer, and the risk of endometrial cancer similarly increases with duration of treatment (less than one year, RR = 1.4; many years (e.g., more than 10 years), RR = 15.0).[86] The risk of endometrial cancer also stays significantly elevated many years after stopping unopposed estrogen therapy, even after 15 years or more (RR = 5.8).[86]
Progestogens prevent the effects of estrogens on the endometrium.[86] As a result, they are able to completely block the increase in risk of endometrial hyperplasia caused by estrogen therapy in postmenopausal women, and are even able to decrease it below baseline (OR = 0.3 with continuous estrogen–progestogen therapy).[86] Continuous estrogen–progestogen therapy is more protective than sequential therapy, and a longer duration of treatment with continuous therapy is also more protective.[86] The increase in risk of endometrial cancer is similarly decreased with continuous estrogen–progestogen therapy (RR = 0.2–0.7).[86] For these reasons, progestogens are always used alongside estrogens in women who have intact uteruses.[86]
Cardiovascular events
Estrogens affect liver protein synthesis and thereby influence the cardiovascular system.[1] They have been found to affect the production of a variety of coagulation and fibrinolytic factors, including increased factor IX, von Willebrand factor, thrombin–antithrombin complex (TAT), fragment 1+2, and D-dimer and decreased fibrinogen, factor VII, antithrombin, protein S, protein C, tissue plasminogen activator (t-PA), and plasminogen activator inhibitor-1 (PAI-1).[1] Although this is true for oral estrogen, transdermal estradiol has been found only to reduce PAI-1 and protein S, and to a lesser extent than oral estrogen.[1] Due to its effects on liver protein synthesis, oral estrogen is procoagulant, and has been found to increase the risk of venous thromboembolism (VTE), including of both deep vein thrombosis (DVT) and pulmonary embolism (PE).[1] Conversely, modern oral contraceptives are not associated with an increase in the risk of stroke and myocardial infarction (heart attack) in healthy, non-smoking premenopausal women of any age, except in those with hypertension (high blood pressure).[87][88] However, a small but significant increase in the risk of stroke, though not of myocardial infarction, has been found in menopausal women taking hormone replacement therapy.[89] An increase in the risk of stroke has also been associated with older high-dose oral contraceptives that are no longer used.[90]
Menopausal hormone therapy with replacement dosages of estrogens and progestogens has been associated with a significantly increased risk of cardiovascular events such as VTE.[91][92] However, such risks have been found to vary depending on the type of estrogen and the route of administration.[91][92] The risk of VTE is increased by approximately 2-fold in women taking oral estrogen for menopausal hormone therapy.[91][92] However, clinical research to date has generally not distinguished between conjugated estrogens and estradiol.[92] This is of importance because conjugated estrogens have been found to be more resistant to hepatic metabolism than estradiol and to increase clotting factors to a greater extent.[1] Only a few clinical studies have compared oral conjugated estrogens and oral estradiol.[92] Oral conjugated estrogens have been found to possess a significantly greater risk of thromboembolic and cardiovascular complications than oral estradiol (OR = 2.08) and oral esterified estrogens (OR = 1.78).[92][93][94] However, in another study, the increase in VTE risk with 0.625 mg/day oral conjugated estrogens plus medroxyprogesterone acetate and 1 or 2 mg/day oral estradiol plus norethisterone acetate was found to be equivalent (RR = 4.0 and 3.9, respectively).[95][96] Other studies have found oral estradiol to be associated with an increase in risk of VTE similarly (RR = 3.5 in one, OR = 3.54 in first year of use in another).[92][97] As of present, there are no randomized controlled trials comparing oral conjugated estrogens and oral estradiol in terms of thromboembolic and cardiovascular risks that would allow for unambiguous conclusions, and additional research is needed to clarify this issue.[92][91] In contrast to oral estrogens as a group, transdermal estradiol at typical menopausal replacement dosages has not been found to increase the risk of VTE or other cardiovascular events.[91][89][92]
Both combined birth control pills (which contain ethinylestradiol and a progestin) and pregnancy are associated with about a 4-fold increase in risk of VTE, with the risk increase being slightly greater with the latter (OR = 4.03 and 4.24, respectively).[98] The risk of VTE during the postpartum period is 5-fold higher than during pregnancy.[98] Other research has found that the rate of VTE is 1 to 5 in 10,000 woman-years in women who are not pregnant or taking a birth control pill, 3 to 9 in 10,000 woman-years in women who are on a birth control pill, 5 to 20 in 10,000 women-years in pregnant women, and 40 to 65 in 10,000 women-years in postpartum women.[99] For birth control pills, VTE risk with high doses of ethinylestradiol (>50 μg, e.g., 100 to 150 μg) has been reported to be approximately twice that of low doses of ethinylestradiol (e.g., 20 to 50 μg).[87] As such, high doses of ethinylestradiol are no longer used in combined oral contraceptives, and all modern combined oral contraceptives contain 50 μg ethinylestradiol or less.[100][101] The absolute risk of VTE in pregnancy is about 0.5 to 2 in 1,000 (0.125%).[102]
Aside from type of estrogen and the route of administration, the risk of VTE with oral estrogen is also moderated by other factors, including the concomitant use of a progestogen, dosage, age, and smoking.[103][96] The combination of oral estrogen and a progestogen has been found to double the risk of VTE relative to oral estrogen alone (RR = 2.05 for estrogen monotherapy, and RR = 2.02 for combined estrogen–progestogen therapy in comparison).[103] However, while this is true for most progestogens, there appears to be no increase in VTE risk relative to oral estrogen alone with the addition of oral progesterone or the atypical progestin dydrogesterone.[103][104][105] The dosage of oral estrogen appears to be important for VTE risk, as 1 mg/day oral estradiol increased VTE incidence by 2.2-fold while 2 mg/day oral estradiol increased VTE incidence by 4.5-fold (both in combination with norethisterone acetate).[96] The risk of VTE and other cardiovascular complications with oral estrogen–progestogen therapy increases dramatically with age.[103] In the oral conjugated estrogens and medroxyprogesterone acetate arm of the WHI, the risks of VTE stratified by age were as follows: age 50 to 59, RR = 2.27; age 60 to 69, RR = 4.28; and age 70 to 79, RR = 7.46.[103] Conversely, in the oral conjugated estrogens monotherapy arm of the WHI, the risk of VTE increased with age similarly but was much lower: age 50 to 59, RR = 1.22; age 60 to 69, RR = 1.3; and age 70 to 79, RR = 1.44.[103] In addition to menopausal hormone therapy, cardiovascular mortality has been found to increase considerably with age in women taking ethinylestradiol-containing combined oral contraceptives and in pregnant women.[106][107] In addition, smoking has been found to exponentially increase cardiovascular mortality in conjunction with combined oral contraceptive use and older age.[106][107] Whereas the risk of cardiovascular death is 0.06 per 100,000 in women who are age 15 to 34 years, are taking a combined oral contraceptive, and do not smoke, this increases by 50-fold to 3.0 per 100,000 in women who are age 35 to 44 years, are taking a combined oral contraceptive, and do not smoke.[106][107] Moreover, in women who do smoke, the risk of cardiovascular death in these two groups increases to 1.73 per 100,000 (29-fold higher relative to non-smokers) and 19.4 per 100,000 (6.5-fold higher relative to non-smokers), respectively.[106][107]
Although estrogens influence the hepatic production of coagulant and fibrinolytic factors and increase the risk of VTE and sometimes stroke, they also influence the liver synthesis of blood lipids and can have beneficial effects on the cardiovascular system.[1] With oral estradiol, there are increases in circulating triglycerides, HDL cholesterol, apolipoprotein A1, and apolipoprotein A2, and decreases in total cholesterol, LDL cholesterol, apolipoprotein B, and lipoprotein(a).[1] Transdermal estradiol has less-pronounced effects on these proteins and, in contrast to oral estradiol, reduces triglycerides.[1] Through these effects, both oral and transdermal estrogens can protect against atherosclerosis and coronary heart disease in menopausal women with intact arterial endothelium that is without severe lesions.[1]
Approximately 95% of orally ingested estradiol is inactivated during first-pass metabolism.[88] Nonetheless, levels of estradiol in the liver with oral administration are supraphysiological and approximately 4- to 5-fold higher than in circulation due to the first-pass.[1][108] This does not occur with parenteral routes of estradiol, such as transdermal, vaginal, or injection.[1] In contrast to estradiol, ethinylestradiol is much more resistant to hepatic metabolism, with a mean oral bioavailability of approximately 45%,[109] and the transdermal route has a similar impact on hepatic protein synthesis as the oral route.[110] Conjugated estrogens are also more resistant to hepatic metabolism than estradiol and show disproportionate effects on hepatic protein production as well, although not to the same magnitude as ethinylestradiol.[1] These differences are considered to be responsible for the greater risk of cardiovascular events with ethinylestradiol and conjugated estrogens relative to estradiol.[1]
High-dosage oral synthetic estrogens like diethylstilbestrol and ethinylestradiol are associated with fairly high rates of severe cardiovascular complications.[111][112] Diethylstilbestrol has been associated with an up to 35% risk of cardiovascular toxicity and death and a 15% incidence of VTE in men treated with it for prostate cancer.[111][112] In contrast to oral synthetic estrogens, high-dosage polyestradiol phosphate and transdermal estradiol have not been found to increase the risk of cardiovascular mortality or thromboembolism in men with prostate cancer, although significantly increased cardiovascular morbidity (due mainly to an increase in non-fatal ischemic heart events and heart decompensation) has been observed with polyestradiol phosphate.[112][113][114]
Sex hormone-binding globulin (SHBG) levels indicate hepatic estrogenic exposure and may be a surrogate marker for coagulation and VTE risk with estrogen therapy, although this topic has been debated.[115][116][117] SHBG levels with birth control pills containing different progestins are increased by 1.5 to 2-fold with levonorgestrel, 2.5- to 4-fold with desogestrel and gestodene, 3.5- to 4-fold with drospirenone and dienogest, and 4- to 5-fold with cyproterone acetate.[115] Contraceptive vaginal rings and contraceptive patches likewise have been found to increase SHBG levels by 2.5-fold and 3.5-fold, respectively.[115] Birth control pills containing high doses of ethinylestradiol (>50 μg) can increase SHBG levels by 5- to 10-fold, which is similar to the increase that occurs during pregnancy.[118] Conversely, increases in SHBG levels are much lower with estradiol, especially when used parenterally.[119][120][121][122][123] High-dose parenteral polyestradiol phosphate therapy has been found to increase SHBG levels by about 1.5-fold.[122]
| Type | Route | Medications | Odds ratio (95% CI) |
|---|---|---|---|
| Menopausal hormone therapy | Oral | Estradiol alone ≤1 mg/day >1 mg/day |
1.27 (1.16–1.39)* 1.22 (1.09–1.37)* 1.35 (1.18–1.55)* |
| Conjugated estrogens alone ≤0.625 mg/day >0.625 mg/day |
1.49 (1.39–1.60)* 1.40 (1.28–1.53)* 1.71 (1.51–1.93)* | ||
| Estradiol/medroxyprogesterone acetate | 1.44 (1.09–1.89)* | ||
| Estradiol/dydrogesterone ≤1 mg/day E2 >1 mg/day E2 |
1.18 (0.98–1.42) 1.12 (0.90–1.40) 1.34 (0.94–1.90) | ||
| Estradiol/norethisterone ≤1 mg/day E2 >1 mg/day E2 |
1.68 (1.57–1.80)* 1.38 (1.23–1.56)* 1.84 (1.69–2.00)* | ||
| Estradiol/norgestrel or estradiol/drospirenone | 1.42 (1.00–2.03) | ||
| Conjugated estrogens/medroxyprogesterone acetate | 2.10 (1.92–2.31)* | ||
| Conjugated estrogens/norgestrel ≤0.625 mg/day CEEs >0.625 mg/day CEEs |
1.73 (1.57–1.91)* 1.53 (1.36–1.72)* 2.38 (1.99–2.85)* | ||
| Tibolone alone | 1.02 (0.90–1.15) | ||
| Raloxifene alone | 1.49 (1.24–1.79)* | ||
| Transdermal | Estradiol alone ≤50 μg/day >50 μg/day |
0.96 (0.88–1.04) 0.94 (0.85–1.03) 1.05 (0.88–1.24) | |
| Estradiol/progestogen | 0.88 (0.73–1.01) | ||
| Vaginal | Estradiol alone | 0.84 (0.73–0.97) | |
| Conjugated estrogens alone | 1.04 (0.76–1.43) | ||
| Combined birth control | Oral | Ethinylestradiol/norethisterone | 2.56 (2.15–3.06)* |
| Ethinylestradiol/levonorgestrel | 2.38 (2.18–2.59)* | ||
| Ethinylestradiol/norgestimate | 2.53 (2.17–2.96)* | ||
| Ethinylestradiol/desogestrel | 4.28 (3.66–5.01)* | ||
| Ethinylestradiol/gestodene | 3.64 (3.00–4.43)* | ||
| Ethinylestradiol/drospirenone | 4.12 (3.43–4.96)* | ||
| Ethinylestradiol/cyproterone acetate | 4.27 (3.57–5.11)* | ||
| Notes: (1) Nested case–control studies (2015, 2019) based on data from the QResearch and Clinical Practice Research Datalink (CPRD) databases. (2) Bioidentical progesterone was not included, but is known to be associated with no additional risk relative to estrogen alone. Footnotes: * = Statistically significant (p < 0.01). Sources: See template. | |||
| Absolute incidence of first VTE per 10,000 person–years during pregnancy and the postpartum period | ||||||||
|---|---|---|---|---|---|---|---|---|
| Swedish data A | Swedish data B | English data | Danish data | |||||
| Time period | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) |
| Outside pregnancy | 1105 | 4.2 (4.0–4.4) | 1015 | 3.8 (?) | 1480 | 3.2 (3.0–3.3) | 2895 | 3.6 (3.4–3.7) |
| Antepartum | 995 | 20.5 (19.2–21.8) | 690 | 14.2 (13.2–15.3) | 156 | 9.9 (8.5–11.6) | 491 | 10.7 (9.7–11.6) |
| Trimester 1 | 207 | 13.6 (11.8–15.5) | 172 | 11.3 (9.7–13.1) | 23 | 4.6 (3.1–7.0) | 61 | 4.1 (3.2–5.2) |
| Trimester 2 | 275 | 17.4 (15.4–19.6) | 178 | 11.2 (9.7–13.0) | 30 | 5.8 (4.1–8.3) | 75 | 5.7 (4.6–7.2) |
| Trimester 3 | 513 | 29.2 (26.8–31.9) | 340 | 19.4 (17.4–21.6) | 103 | 18.2 (15.0–22.1) | 355 | 19.7 (17.7–21.9) |
| Around delivery | 115 | 154.6 (128.8–185.6) | 79 | 106.1 (85.1–132.3) | 34 | 142.8 (102.0–199.8) | –
| |
| Postpartum | 649 | 42.3 (39.2–45.7) | 509 | 33.1 (30.4–36.1) | 135 | 27.4 (23.1–32.4) | 218 | 17.5 (15.3–20.0) |
| Early postpartum | 584 | 75.4 (69.6–81.8) | 460 | 59.3 (54.1–65.0) | 177 | 46.8 (39.1–56.1) | 199 | 30.4 (26.4–35.0) |
| Late postpartum | 65 | 8.5 (7.0–10.9) | 49 | 6.4 (4.9–8.5) | 18 | 7.3 (4.6–11.6) | 319 | 3.2 (1.9–5.0) |
| Incidence rate ratios (IRRs) of first VTE during pregnancy and the postpartum period | ||||||||
| Swedish data A | Swedish data B | English data | Danish data | |||||
| Time period | IRR* (95% CI) | IRR* (95% CI) | IRR (95% CI)† | IRR (95% CI)† | ||||
| Outside pregnancy | Reference (i.e., 1.00)
| |||||||
| Antepartum | 5.08 (4.66–5.54) | 3.80 (3.44–4.19) | 3.10 (2.63–3.66) | 2.95 (2.68–3.25) | ||||
| Trimester 1 | 3.42 (2.95–3.98) | 3.04 (2.58–3.56) | 1.46 (0.96–2.20) | 1.12 (0.86–1.45) | ||||
| Trimester 2 | 4.31 (3.78–4.93) | 3.01 (2.56–3.53) | 1.82 (1.27–2.62) | 1.58 (1.24–1.99) | ||||
| Trimester 3 | 7.14 (6.43–7.94) | 5.12 (4.53–5.80) | 5.69 (4.66–6.95) | 5.48 (4.89–6.12) | ||||
| Around delivery | 37.5 (30.9–44.45) | 27.97 (22.24–35.17) | 44.5 (31.68–62.54) | –
| ||||
| Postpartum | 10.21 (9.27–11.25) | 8.72 (7.83–9.70) | 8.54 (7.16–10.19) | 4.85 (4.21–5.57) | ||||
| Early postpartum | 19.27 (16.53–20.21) | 15.62 (14.00–17.45) | 14.61 (12.10–17.67) | 8.44 (7.27–9.75) | ||||
| Late postpartum | 2.06 (1.60–2.64) | 1.69 (1.26–2.25) | 2.29 (1.44–3.65) | 0.89 (0.53–1.39) | ||||
| Notes: Swedish data A = Using any code for VTE regardless of confirmation. Swedish data B = Using only algorithm-confirmed VTE. Early postpartum = First 6 weeks after delivery. Late postpartum = More than 6 weeks after delivery. * = Adjusted for age and calendar year. † = Unadjusted ratio calculated based on the data provided. Source: [124] | ||||||||
Breast cancer
Estrogens are responsible for breast development and, in relation to this, are strongly implicated in the development of breast cancer.[125][126] In addition, estrogens stimulate the growth and accelerate the progression of ER-positive breast cancer.[127][128] In accordance, antiestrogens like the selective estrogen receptor modulator (SERM) tamoxifen, the ER antagonist fulvestrant, and the aromatase inhibitors (AIs) anastrozole and exemestane are all effective in the treatment of ER-positive breast cancer.[129][130][131] Antiestrogens are also effective in the prevention of breast cancer.[132][133][134] Paradoxically, high-dose estrogen therapy is effective in the treatment of breast cancer as well and has about the same degree of effectiveness as antiestrogen therapy, although it is far less commonly used due to adverse effects.[135][136] The usefulness of high-dose estrogen therapy in the treatment of ER-positive breast cancer is attributed to a bimodal effect in which high concentrations of estrogens signal breast cancer cells to undergo apoptosis, in contrast to lower concentrations of estrogens which stimulate their growth.[135][136]
A 2017 systematic review and meta-analysis of 14 studies assessed the risk of breast cancer in perimenopausal and postmenopausal women treated with estrogens for menopausal symptoms.[137] They found that treatment with estradiol only is not associated with an increased risk of breast cancer (OR = 0.90 in RCTs and OR = 1.11 in observational studies).[137] This was in accordance with a previous analysis of estrogen-only treatment with estradiol or conjugated estrogens which similarly found no increased risk (RR = 0.99).[137] Moreover, another study found that the risk of breast cancer with estradiol and conjugated estrogens was not significantly different (RR = 1.15 for conjugated estrogens versus estradiol).[137] These findings are paradoxical because oophorectomy in premenopausal women and antiestrogen therapy in postmenopausal women are well-established as considerably reducing the risk of breast cancer (RR = 0.208 to 0.708 for chemoprevention with antiestrogens in postmenopausal women).[132][133][134] However, there are indications that there may be a ceiling effect such that past a certain low concentration threshold (e.g., approximately 10.2 pg/mL for estradiol), additional estrogens alone may not further increase the risk of breast cancer in postmenopausal women.[138] There are also indications that the fluctuations in estrogen levels across the normal menstrual cycle in premenopausal women may be important for breast cancer risk.[139]
In contrast to estrogen-only therapy, combined estrogen and progestogen treatment, although dependent on the progestogen used, is associated with an increased risk of breast cancer.[137][140] The increase in risk is dependent on the duration of treatment, with more than five years (OR = 2.43) having a significantly greater risk than less than five years (OR = 1.49).[137] In addition, sequential estrogen–progestogen treatment (OR = 1.76) is associated with a lower risk increase than continuous treatment (OR = 2.90), which has a comparably much higher risk.[137] The increase in risk also differs according to the specific progestogen used.[137] Treatment with estradiol plus medroxyprogesterone acetate (OR = 1.19), norethisterone acetate (OR = 1.44), levonorgestrel (OR = 1.47), or a mixed progestogen subgroup (OR = 1.99) were all associated with an increased risk.[137] In a previous review, the increase in breast cancer risk was found to not be significantly different between these three progestogens.[137] Conversely, there is no significant increase in risk of breast cancer with bioidentical progesterone (OR = 1.00) or with the atypical progestin dydrogesterone (OR = 1.10).[137] In accordance, another study found similarly that the risk of breast cancer was not significantly increased with estrogen–progesterone (RR = 1.00) or estrogen–dydrogesterone (RR = 1.16) but was increased for estrogen combined with other progestins (RR = 1.69).[86] These progestins included chlormadinone acetate, cyproterone acetate, medrogestone, medroxyprogesterone acetate, nomegestrol acetate, norethisterone acetate, and promegestone, with the associations for breast cancer risk not differing significantly between the different progestins in this group.[86]
In contrast to cisgender women, breast cancer is extremely rare in men and transgender women treated with estrogens and/or progestogens, and gynecomastia or breast development in such individuals does not appear to be associated with an increased risk of breast cancer.[141][142][143][144] Likewise, breast cancer has never been reported in women with complete androgen insensitivity syndrome, who similarly have a male genotype (46,XY), in spite of the fact that these women have well-developed breasts.[145][146] The reasons for these differences are unknown. However, the dramatically increased risk of breast cancer (20- to 58-fold) in men with Klinefelter's syndrome, who have somewhat of a hybrid of a male and a female genotype (47,XXY), suggests that it may have to do with the sex chromosomes.[144][147][148]
| Therapy | <5 years | 5–14 years | 15+ years | |||
|---|---|---|---|---|---|---|
| Cases | RR (95% CI) | Cases | RR (95% CI) | Cases | RR (95% CI) | |
| Estrogen alone | 1259 | 1.18 (1.10–1.26) | 4869 | 1.33 (1.28–1.37) | 2183 | 1.58 (1.51–1.67) |
| By estrogen | ||||||
| Conjugated estrogens | 481 | 1.22 (1.09–1.35) | 1910 | 1.32 (1.25–1.39) | 1179 | 1.68 (1.57–1.80) |
| Estradiol | 346 | 1.20 (1.05–1.36) | 1580 | 1.38 (1.30–1.46) | 435 | 1.78 (1.58–1.99) |
| Estropipate (estrone sulfate) | 9 | 1.45 (0.67–3.15) | 50 | 1.09 (0.79–1.51) | 28 | 1.53 (1.01–2.33) |
| Estriol | 15 | 1.21 (0.68–2.14) | 44 | 1.24 (0.89–1.73) | 9 | 1.41 (0.67–2.93) |
| Other estrogens | 15 | 0.98 (0.46–2.09) | 21 | 0.98 (0.58–1.66) | 5 | 0.77 (0.27–2.21) |
| By route | ||||||
| Oral estrogens | – | – | 3633 | 1.33 (1.27–1.38) | – | – |
| Transdermal estrogens | – | – | 919 | 1.35 (1.25–1.46) | – | – |
| Vaginal estrogens | – | – | 437 | 1.09 (0.97–1.23) | – | – |
| Estrogen and progestogen | 2419 | 1.58 (1.51–1.67) | 8319 | 2.08 (2.02–2.15) | 1424 | 2.51 (2.34–2.68) |
| By progestogen | ||||||
| (Levo)norgestrel | 343 | 1.70 (1.49–1.94) | 1735 | 2.12 (1.99–2.25) | 219 | 2.69 (2.27–3.18) |
| Norethisterone acetate | 650 | 1.61 (1.46–1.77) | 2642 | 2.20 (2.09–2.32) | 420 | 2.97 (2.60–3.39) |
| Medroxyprogesterone acetate | 714 | 1.64 (1.50–1.79) | 2012 | 2.07 (1.96–2.19) | 411 | 2.71 (2.39–3.07) |
| Dydrogesterone | 65 | 1.21 (0.90–1.61) | 162 | 1.41 (1.17–1.71) | 26 | 2.23 (1.32–3.76) |
| Progesterone | 11 | 0.91 (0.47–1.78) | 38 | 2.05 (1.38–3.06) | 1 | – |
| Promegestone | 12 | 1.68 (0.85–3.31) | 19 | 2.06 (1.19–3.56) | 0 | – |
| Nomegestrol acetate | 8 | 1.60 (0.70–3.64) | 14 | 1.38 (0.75–2.53) | 0 | – |
| Other progestogens | 12 | 1.70 (0.86–3.38) | 19 | 1.79 (1.05–3.05) | 0 | – |
| By progestogen frequency | ||||||
| Continuous | – | – | 3948 | 2.30 (2.21–2.40) | – | – |
| Intermittent | – | – | 3467 | 1.93 (1.84–2.01) | – | – |
| Progestogen alone | 98 | 1.37 (1.08–1.74) | 107 | 1.39 (1.11–1.75) | 30 | 2.10 (1.35–3.27) |
| By progestogen | ||||||
| Medroxyprogesterone acetate | 28 | 1.68 (1.06–2.66) | 18 | 1.16 (0.68–1.98) | 7 | 3.42 (1.26–9.30) |
| Norethisterone acetate | 13 | 1.58 (0.77–3.24) | 24 | 1.55 (0.88–2.74) | 6 | 3.33 (0.81–13.8) |
| Dydrogesterone | 3 | 2.30 (0.49–10.9) | 11 | 3.31 (1.39–7.84) | 0 | – |
| Other progestogens | 8 | 2.83 (1.04–7.68) | 5 | 1.47 (0.47–4.56) | 1 | – |
| Miscellaneous | ||||||
| Tibolone | – | – | 680 | 1.57 (1.43–1.72) | – | – |
| Notes: Meta-analysis of worldwide epidemiological evidence on menopausal hormone therapy and breast cancer risk by the Collaborative Group on Hormonal Factors in Breast Cancer (CGHFBC). Fully adjusted relative risks for current versus never-users of menopausal hormone therapy. Source: See template. | ||||||
| Study | Therapy | Hazard ratio (95% CI) |
|---|---|---|
| E3N-EPIC: Fournier et al. (2005) | Estrogen alone | 1.1 (0.8–1.6) |
| Estrogen plus progesterone Transdermal estrogen Oral estrogen |
0.9 (0.7–1.2) 0.9 (0.7–1.2) No events | |
| Estrogen plus progestin Transdermal estrogen Oral estrogen |
1.4 (1.2–1.7) 1.4 (1.2–1.7) 1.5 (1.1–1.9) | |
| E3N-EPIC: Fournier et al. (2008) | Oral estrogen alone | 1.32 (0.76–2.29) |
| Oral estrogen plus progestogen Progesterone Dydrogesterone Medrogestone Chlormadinone acetate Cyproterone acetate Promegestone Nomegestrol acetate Norethisterone acetate Medroxyprogesterone acetate |
Not analyzeda 0.77 (0.36–1.62) 2.74 (1.42–5.29) 2.02 (1.00–4.06) 2.57 (1.81–3.65) 1.62 (0.94–2.82) 1.10 (0.55–2.21) 2.11 (1.56–2.86) 1.48 (1.02–2.16) | |
| Transdermal estrogen alone | 1.28 (0.98–1.69) | |
| Transdermal estrogen plus progestogen Progesterone Dydrogesterone Medrogestone Chlormadinone acetate Cyproterone acetate Promegestone Nomegestrol acetate Norethisterone acetate Medroxyprogesterone acetate |
1.08 (0.89–1.31) 1.18 (0.95–1.48) 2.03 (1.39–2.97) 1.48 (1.05–2.09) Not analyzeda 1.52 (1.19–1.96) 1.60 (1.28–2.01) Not analyzeda Not analyzeda | |
| E3N-EPIC: Fournier et al. (2014) | Estrogen alone | 1.17 (0.99–1.38) |
| Estrogen plus progesterone or dydrogesterone | 1.22 (1.11–1.35) | |
| Estrogen plus progestin | 1.87 (1.71–2.04) | |
| CECILE: Cordina-Duverger et al. (2013) | Estrogen alone | 1.19 (0.69–2.04) |
| Estrogen plus progestogen Progesterone Progestins Progesterone derivatives Testosterone derivatives |
1.33 (0.92–1.92) 0.80 (0.44–1.43) 1.72 (1.11–2.65) 1.57 (0.99–2.49) 3.35 (1.07–10.4) | |
| Footnotes: a = Not analyzed, fewer than 5 cases. Sources: See template. | ||
| Study | Therapy | Hazard ratio (95% CI) |
|---|---|---|
| E3N-EPIC: Fournier et al. (2005)a | Transdermal estrogen plus progesterone <2 years 2–4 years ≥4 years |
0.9 (0.6–1.4) 0.7 (0.4–1.2) 1.2 (0.7–2.0) |
| Transdermal estrogen plus progestin <2 years 2–4 years ≥4 years |
1.6 (1.3–2.0) 1.4 (1.0–1.8) 1.2 (0.8–1.7) | |
| Oral estrogen plus progestin <2 years 2–4 years ≥4 years |
1.2 (0.9–1.8) 1.6 (1.1–2.3) 1.9 (1.2–3.2) | |
| E3N-EPIC: Fournier et al. (2008) | Estrogen plus progesterone <2 years 2–4 years 4–6 years ≥6 years |
0.71 (0.44–1.14) 0.95 (0.67–1.36) 1.26 (0.87–1.82) 1.22 (0.89–1.67) |
| Estrogen plus dydrogesterone <2 years 2–4 years 4–6 years ≥6 years |
0.84 (0.51–1.38) 1.16 (0.79–1.71) 1.28 (0.83–1.99) 1.32 (0.93–1.86) | |
| Estrogen plus other progestogens <2 years 2–4 years 4–6 years ≥6 years |
1.36 (1.07–1.72) 1.59 (1.30–1.94) 1.79 (1.44–2.23) 1.95 (1.62–2.35) | |
| E3N-EPIC: Fournier et al. (2014) | Estrogens plus progesterone or dydrogesterone <5 years ≥5 years |
1.13 (0.99–1.29) 1.31 (1.15–1.48) |
| Estrogen plus other progestogens <5 years ≥5 years |
1.70 (1.50–1.91) 2.02 (1.81–2.26) | |
| Footnotes: a = Oral estrogen plus progesterone was not analyzed because there was a low number of women who used this therapy. Sources: See template. | ||
Cholestatic hepatotoxicity
Estrogens, along with progesterone, can rarely cause cholestatic hepatotoxicity, particularly at very high concentrations.[149][150][151] This is seen in intrahepatic cholestasis of pregnancy, which occurs in 0.4 to 15% of pregnancies (highly variable depending on the country).[152][153][154][155]
Gallbladder disease
Estrogen therapy has been associated with gallbladder disease, including risk of gallstone formation.[156][157][158][159] A 2017 systematic review and meta-analysis found that menopausal hormone therapy significantly increased the risk of gallstones (RR = 1.79) while oral contraceptives did not significantly increase the risk (RR = 1.19).[159] Biliary sludge appears in 5 to 30% of women during pregnancy, and definitive gallstones persisting postpartum become established in approximately 5%.[160]
Overdose
Estrogens are relatively safe in overdose and symptoms manifest mainly as reversible feminization.
Interactions
Inducers of cytochrome P450 enzymes like carbamazepine and phenytoin can accelerate the metabolism of estrogens and thereby decrease their bioavailability and circulating levels. Inhibitors of such enzymes can have the opposite effect and can increase estrogen levels and bioavailability.
Pharmacology
Pharmacodynamics
Estrogens act as selective agonists of the estrogen receptors (ERs), the ERα and the ERβ. They may also bind to and activate membrane estrogen receptors (mERs) such as the GPER. Estrogens do not have off-target activity at other steroid hormone receptors such as the androgen, progesterone, glucocorticoid, or mineralocorticoid receptors, nor do they have neurosteroid activity by interacting with neurotransmitter receptors, unlike various progestogens and some other steroids. Given by subcutaneous injection in mice, estradiol is about 10-fold more potent than estrone and about 100-fold more potent than estriol.[161]
Estrogens have antigonadotropic effects at sufficiently high concentrations via activation of the ER and hence can suppress the hypothalamic–pituitary–gonadal axis. This is caused by negative feedback, resulting in a suppression in secretion and decreased circulating levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The antigonadotropic effects of estrogens interfere with fertility and gonadal sex hormone production. They are responsible for the hormonal contraceptive effects of estrogens. In addition, they allow estrogens to act as functional antiandrogens by suppressing gonadal testosterone production. At sufficiently high doses, estrogens are able to suppress testosterone levels into the castrate range in men.[162]
Estrogens differ significantly in their pharmacological properties.[1][163][164] For instance, due to structural differences and accompanying differences in metabolism, estrogens differ from one another in their tissue selectivity; synthetic estrogens like ethinylestradiol and diethylstilbestrol are not inactivated as efficiently as estradiol in tissues like the liver and uterus and as a result have disproportionate effects in these tissues.[1] This can result in issues such as a relatively higher risk of thromboembolism.[1]
In-vitro pharmacodynamics
| Ligand | Other names | Relative binding affinities (RBA, %)a | Absolute binding affinities (Ki, nM)a | Action | ||
|---|---|---|---|---|---|---|
| ERα | ERβ | ERα | ERβ | |||
| Estradiol | E2; 17β-Estradiol | 100 | 100 | 0.115 (0.04–0.24) | 0.15 (0.10–2.08) | Estrogen |
| Estrone | E1; 17-Ketoestradiol | 16.39 (0.7–60) | 6.5 (1.36–52) | 0.445 (0.3–1.01) | 1.75 (0.35–9.24) | Estrogen |
| Estriol | E3; 16α-OH-17β-E2 | 12.65 (4.03–56) | 26 (14.0–44.6) | 0.45 (0.35–1.4) | 0.7 (0.63–0.7) | Estrogen |
| Estetrol | E4; 15α,16α-Di-OH-17β-E2 | 4.0 | 3.0 | 4.9 | 19 | Estrogen |
| Alfatradiol | 17α-Estradiol | 20.5 (7–80.1) | 8.195 (2–42) | 0.2–0.52 | 0.43–1.2 | Metabolite |
| 16-Epiestriol | 16β-Hydroxy-17β-estradiol | 7.795 (4.94–63) | 50 | ? | ? | Metabolite |
| 17-Epiestriol | 16α-Hydroxy-17α-estradiol | 55.45 (29–103) | 79–80 | ? | ? | Metabolite |
| 16,17-Epiestriol | 16β-Hydroxy-17α-estradiol | 1.0 | 13 | ? | ? | Metabolite |
| 2-Hydroxyestradiol | 2-OH-E2 | 22 (7–81) | 11–35 | 2.5 | 1.3 | Metabolite |
| 2-Methoxyestradiol | 2-MeO-E2 | 0.0027–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Hydroxyestradiol | 4-OH-E2 | 13 (8–70) | 7–56 | 1.0 | 1.9 | Metabolite |
| 4-Methoxyestradiol | 4-MeO-E2 | 2.0 | 1.0 | ? | ? | Metabolite |
| 2-Hydroxyestrone | 2-OH-E1 | 2.0–4.0 | 0.2–0.4 | ? | ? | Metabolite |
| 2-Methoxyestrone | 2-MeO-E1 | <0.001–<1 | <1 | ? | ? | Metabolite |
| 4-Hydroxyestrone | 4-OH-E1 | 1.0–2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestrone | 4-MeO-E1 | <1 | <1 | ? | ? | Metabolite |
| 16α-Hydroxyestrone | 16α-OH-E1; 17-Ketoestriol | 2.0–6.5 | 35 | ? | ? | Metabolite |
| 2-Hydroxyestriol | 2-OH-E3 | 2.0 | 1.0 | ? | ? | Metabolite |
| 4-Methoxyestriol | 4-MeO-E3 | 1.0 | 1.0 | ? | ? | Metabolite |
| Estradiol sulfate | E2S; Estradiol 3-sulfate | <1 | <1 | ? | ? | Metabolite |
| Estradiol disulfate | Estradiol 3,17β-disulfate | 0.0004 | ? | ? | ? | Metabolite |
| Estradiol 3-glucuronide | E2-3G | 0.0079 | ? | ? | ? | Metabolite |
| Estradiol 17β-glucuronide | E2-17G | 0.0015 | ? | ? | ? | Metabolite |
| Estradiol 3-gluc. 17β-sulfate | E2-3G-17S | 0.0001 | ? | ? | ? | Metabolite |
| Estrone sulfate | E1S; Estrone 3-sulfate | <1 | <1 | >10 | >10 | Metabolite |
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | ? | ? | ? | Estrogen |
| Estradiol 17β-benzoate | E2-17B | 11.3 | 32.6 | ? | ? | Estrogen |
| Estrone methyl ether | Estrone 3-methyl ether | 0.145 | ? | ? | ? | Estrogen |
| ent-Estradiol | 1-Estradiol | 1.31–12.34 | 9.44–80.07 | ? | ? | Estrogen |
| Equilin | 7-Dehydroestrone | 13 (4.0–28.9) | 13.0–49 | 0.79 | 0.36 | Estrogen |
| Equilenin | 6,8-Didehydroestrone | 2.0–15 | 7.0–20 | 0.64 | 0.62 | Estrogen |
| 17β-Dihydroequilin | 7-Dehydro-17β-estradiol | 7.9–113 | 7.9–108 | 0.09 | 0.17 | Estrogen |
| 17α-Dihydroequilin | 7-Dehydro-17α-estradiol | 18.6 (18–41) | 14–32 | 0.24 | 0.57 | Estrogen |
| 17β-Dihydroequilenin | 6,8-Didehydro-17β-estradiol | 35–68 | 90–100 | 0.15 | 0.20 | Estrogen |
| 17α-Dihydroequilenin | 6,8-Didehydro-17α-estradiol | 20 | 49 | 0.50 | 0.37 | Estrogen |
| Δ8-Estradiol | 8,9-Dehydro-17β-estradiol | 68 | 72 | 0.15 | 0.25 | Estrogen |
| Δ8-Estrone | 8,9-Dehydroestrone | 19 | 32 | 0.52 | 0.57 | Estrogen |
| Ethinylestradiol | EE; 17α-Ethynyl-17β-E2 | 120.9 (68.8–480) | 44.4 (2.0–144) | 0.02–0.05 | 0.29–0.81 | Estrogen |
| Mestranol | EE 3-methyl ether | ? | 2.5 | ? | ? | Estrogen |
| Moxestrol | RU-2858; 11β-Methoxy-EE | 35–43 | 5–20 | 0.5 | 2.6 | Estrogen |
| Methylestradiol | 17α-Methyl-17β-estradiol | 70 | 44 | ? | ? | Estrogen |
| Diethylstilbestrol | DES; Stilbestrol | 129.5 (89.1–468) | 219.63 (61.2–295) | 0.04 | 0.05 | Estrogen |
| Hexestrol | Dihydrodiethylstilbestrol | 153.6 (31–302) | 60–234 | 0.06 | 0.06 | Estrogen |
| Dienestrol | Dehydrostilbestrol | 37 (20.4–223) | 56–404 | 0.05 | 0.03 | Estrogen |
| Benzestrol (B2) | – | 114 | ? | ? | ? | Estrogen |
| Chlorotrianisene | TACE | 1.74 | ? | 15.30 | ? | Estrogen |
| Triphenylethylene | TPE | 0.074 | ? | ? | ? | Estrogen |
| Triphenylbromoethylene | TPBE | 2.69 | ? | ? | ? | Estrogen |
| Tamoxifen | ICI-46,474 | 3 (0.1–47) | 3.33 (0.28–6) | 3.4–9.69 | 2.5 | SERM |
| Afimoxifene | 4-Hydroxytamoxifen; 4-OHT | 100.1 (1.7–257) | 10 (0.98–339) | 2.3 (0.1–3.61) | 0.04–4.8 | SERM |
| Toremifene | 4-Chlorotamoxifen; 4-CT | ? | ? | 7.14–20.3 | 15.4 | SERM |
| Clomifene | MRL-41 | 25 (19.2–37.2) | 12 | 0.9 | 1.2 | SERM |
| Cyclofenil | F-6066; Sexovid | 151–152 | 243 | ? | ? | SERM |
| Nafoxidine | U-11,000A | 30.9–44 | 16 | 0.3 | 0.8 | SERM |
| Raloxifene | – | 41.2 (7.8–69) | 5.34 (0.54–16) | 0.188–0.52 | 20.2 | SERM |
| Arzoxifene | LY-353,381 | ? | ? | 0.179 | ? | SERM |
| Lasofoxifene | CP-336,156 | 10.2–166 | 19.0 | 0.229 | ? | SERM |
| Ormeloxifene | Centchroman | ? | ? | 0.313 | ? | SERM |
| Levormeloxifene | 6720-CDRI; NNC-460,020 | 1.55 | 1.88 | ? | ? | SERM |
| Ospemifene | Deaminohydroxytoremifene | 0.82–2.63 | 0.59–1.22 | ? | ? | SERM |
| Bazedoxifene | – | ? | ? | 0.053 | ? | SERM |
| Etacstil | GW-5638 | 4.30 | 11.5 | ? | ? | SERM |
| ICI-164,384 | – | 63.5 (3.70–97.7) | 166 | 0.2 | 0.08 | Antiestrogen |
| Fulvestrant | ICI-182,780 | 43.5 (9.4–325) | 21.65 (2.05–40.5) | 0.42 | 1.3 | Antiestrogen |
| Propylpyrazoletriol | PPT | 49 (10.0–89.1) | 0.12 | 0.40 | 92.8 | ERα agonist |
| 16α-LE2 | 16α-Lactone-17β-estradiol | 14.6–57 | 0.089 | 0.27 | 131 | ERα agonist |
| 16α-Iodo-E2 | 16α-Iodo-17β-estradiol | 30.2 | 2.30 | ? | ? | ERα agonist |
| Methylpiperidinopyrazole | MPP | 11 | 0.05 | ? | ? | ERα antagonist |
| Diarylpropionitrile | DPN | 0.12–0.25 | 6.6–18 | 32.4 | 1.7 | ERβ agonist |
| 8β-VE2 | 8β-Vinyl-17β-estradiol | 0.35 | 22.0–83 | 12.9 | 0.50 | ERβ agonist |
| Prinaberel | ERB-041; WAY-202,041 | 0.27 | 67–72 | ? | ? | ERβ agonist |
| ERB-196 | WAY-202,196 | ? | 180 | ? | ? | ERβ agonist |
| Erteberel | SERBA-1; LY-500,307 | ? | ? | 2.68 | 0.19 | ERβ agonist |
| SERBA-2 | – | ? | ? | 14.5 | 1.54 | ERβ agonist |
| Coumestrol | – | 9.225 (0.0117–94) | 64.125 (0.41–185) | 0.14–80.0 | 0.07–27.0 | Xenoestrogen |
| Genistein | – | 0.445 (0.0012–16) | 33.42 (0.86–87) | 2.6–126 | 0.3–12.8 | Xenoestrogen |
| Equol | – | 0.2–0.287 | 0.85 (0.10–2.85) | ? | ? | Xenoestrogen |
| Daidzein | – | 0.07 (0.0018–9.3) | 0.7865 (0.04–17.1) | 2.0 | 85.3 | Xenoestrogen |
| Biochanin A | – | 0.04 (0.022–0.15) | 0.6225 (0.010–1.2) | 174 | 8.9 | Xenoestrogen |
| Kaempferol | – | 0.07 (0.029–0.10) | 2.2 (0.002–3.00) | ? | ? | Xenoestrogen |
| Naringenin | – | 0.0054 (<0.001–0.01) | 0.15 (0.11–0.33) | ? | ? | Xenoestrogen |
| 8-Prenylnaringenin | 8-PN | 4.4 | ? | ? | ? | Xenoestrogen |
| Quercetin | – | <0.001–0.01 | 0.002–0.040 | ? | ? | Xenoestrogen |
| Ipriflavone | – | <0.01 | <0.01 | ? | ? | Xenoestrogen |
| Miroestrol | – | 0.39 | ? | ? | ? | Xenoestrogen |
| Deoxymiroestrol | – | 2.0 | ? | ? | ? | Xenoestrogen |
| β-Sitosterol | – | <0.001–0.0875 | <0.001–0.016 | ? | ? | Xenoestrogen |
| Resveratrol | – | <0.001–0.0032 | ? | ? | ? | Xenoestrogen |
| α-Zearalenol | – | 48 (13–52.5) | ? | ? | ? | Xenoestrogen |
| β-Zearalenol | – | 0.6 (0.032–13) | ? | ? | ? | Xenoestrogen |
| Zeranol | α-Zearalanol | 48–111 | ? | ? | ? | Xenoestrogen |
| Taleranol | β-Zearalanol | 16 (13–17.8) | 14 | 0.8 | 0.9 | Xenoestrogen |
| Zearalenone | ZEN | 7.68 (2.04–28) | 9.45 (2.43–31.5) | ? | ? | Xenoestrogen |
| Zearalanone | ZAN | 0.51 | ? | ? | ? | Xenoestrogen |
| Bisphenol A | BPA | 0.0315 (0.008–1.0) | 0.135 (0.002–4.23) | 195 | 35 | Xenoestrogen |
| Endosulfan | EDS | <0.001–<0.01 | <0.01 | ? | ? | Xenoestrogen |
| Kepone | Chlordecone | 0.0069–0.2 | ? | ? | ? | Xenoestrogen |
| o,p'-DDT | – | 0.0073–0.4 | ? | ? | ? | Xenoestrogen |
| p,p'-DDT | – | 0.03 | ? | ? | ? | Xenoestrogen |
| Methoxychlor | p,p'-Dimethoxy-DDT | 0.01 (<0.001–0.02) | 0.01–0.13 | ? | ? | Xenoestrogen |
| HPTE | Hydroxychlor; p,p'-OH-DDT | 1.2–1.7 | ? | ? | ? | Xenoestrogen |
| Testosterone | T; 4-Androstenolone | <0.0001–<0.01 | <0.002–0.040 | >5000 | >5000 | Androgen |
| Dihydrotestosterone | DHT; 5α-Androstanolone | 0.01 (<0.001–0.05) | 0.0059–0.17 | 221–>5000 | 73–1688 | Androgen |
| Nandrolone | 19-Nortestosterone; 19-NT | 0.01 | 0.23 | 765 | 53 | Androgen |
| Dehydroepiandrosterone | DHEA; Prasterone | 0.038 (<0.001–0.04) | 0.019–0.07 | 245–1053 | 163–515 | Androgen |
| 5-Androstenediol | A5; Androstenediol | 6 | 17 | 3.6 | 0.9 | Androgen |
| 4-Androstenediol | – | 0.5 | 0.6 | 23 | 19 | Androgen |
| 4-Androstenedione | A4; Androstenedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| 3α-Androstanediol | 3α-Adiol | 0.07 | 0.3 | 260 | 48 | Androgen |
| 3β-Androstanediol | 3β-Adiol | 3 | 7 | 6 | 2 | Androgen |
| Androstanedione | 5α-Androstanedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Etiocholanedione | 5β-Androstanedione | <0.01 | <0.01 | >10000 | >10000 | Androgen |
| Methyltestosterone | 17α-Methyltestosterone | <0.0001 | ? | ? | ? | Androgen |
| Ethinyl-3α-androstanediol | 17α-Ethynyl-3α-adiol | 4.0 | <0.07 | ? | ? | Estrogen |
| Ethinyl-3β-androstanediol | 17α-Ethynyl-3β-adiol | 50 | 5.6 | ? | ? | Estrogen |
| Progesterone | P4; 4-Pregnenedione | <0.001–0.6 | <0.001–0.010 | ? | ? | Progestogen |
| Norethisterone | NET; 17α-Ethynyl-19-NT | 0.085 (0.0015–<0.1) | 0.1 (0.01–0.3) | 152 | 1084 | Progestogen |
| Norethynodrel | 5(10)-Norethisterone | 0.5 (0.3–0.7) | <0.1–0.22 | 14 | 53 | Progestogen |
| Tibolone | 7α-Methylnorethynodrel | 0.5 (0.45–2.0) | 0.2–0.076 | ? | ? | Progestogen |
| Δ4-Tibolone | 7α-Methylnorethisterone | 0.069–<0.1 | 0.027–<0.1 | ? | ? | Progestogen |
| 3α-Hydroxytibolone | – | 2.5 (1.06–5.0) | 0.6–0.8 | ? | ? | Progestogen |
| 3β-Hydroxytibolone | – | 1.6 (0.75–1.9) | 0.070–0.1 | ? | ? | Progestogen |
| Footnotes: a = (1) Binding affinity values are of the format "median (range)" (# (#–#)), "range" (#–#), or "value" (#) depending on the values available. The full sets of values within the ranges can be found in the Wiki code. (2) Binding affinities were determined via displacement studies in a variety of in-vitro systems with labeled estradiol and human ERα and ERβ proteins (except the ERβ values from Kuiper et al. (1997), which are rat ERβ). Sources: See template page. | ||||||
| Estrogen | Relative binding affinities (%) | ||||||
|---|---|---|---|---|---|---|---|
| ER | AR | PR | GR | MR | SHBG | CBG | |
| Estradiol | 100 | 7.9 | 2.6 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Estradiol benzoate | ? | ? | ? | ? | ? | <0.1–0.16 | <0.1 |
| Estradiol valerate | 2 | ? | ? | ? | ? | ? | ? |
| Estrone | 11–35 | <1 | <1 | <1 | <1 | 2.7 | <0.1 |
| Estrone sulfate | 2 | 2 | ? | ? | ? | ? | ? |
| Estriol | 10–15 | <1 | <1 | <1 | <1 | <0.1 | <0.1 |
| Equilin | 40 | ? | ? | ? | ? | ? | 0 |
| Alfatradiol | 15 | <1 | <1 | <1 | <1 | ? | ? |
| Epiestriol | 20 | <1 | <1 | <1 | <1 | ? | ? |
| Ethinylestradiol | 100–112 | 1–3 | 15–25 | 1–3 | <1 | 0.18 | <0.1 |
| Mestranol | 1 | ? | ? | ? | ? | <0.1 | <0.1 |
| Methylestradiol | 67 | 1–3 | 3–25 | 1–3 | <1 | ? | ? |
| Moxestrol | 12 | <0.1 | 0.8 | 3.2 | <0.1 | <0.2 | <0.1 |
| Diethylstilbestrol | ? | ? | ? | ? | ? | <0.1 | <0.1 |
| Notes: Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Sources: See template. | |||||||
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) production assays in yeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
In-vivo pharmacodynamics
| Estrogen | ER RBA (%) | Uterine weight (%) | Uterotrophy | LH levels (%) | SHBG RBA (%) |
|---|---|---|---|---|---|
| Control | – | 100 | – | 100 | – |
| Estradiol (E2) | 100 | 506 ± 20 | +++ | 12–19 | 100 |
| Estrone (E1) | 11 ± 8 | 490 ± 22 | +++ | ? | 20 |
| Estriol (E3) | 10 ± 4 | 468 ± 30 | +++ | 8–18 | 3 |
| Estetrol (E4) | 0.5 ± 0.2 | ? | Inactive | ? | 1 |
| 17α-Estradiol | 4.2 ± 0.8 | ? | ? | ? | ? |
| 2-Hydroxyestradiol | 24 ± 7 | 285 ± 8 | +b | 31–61 | 28 |
| 2-Methoxyestradiol | 0.05 ± 0.04 | 101 | Inactive | ? | 130 |
| 4-Hydroxyestradiol | 45 ± 12 | ? | ? | ? | ? |
| 4-Methoxyestradiol | 1.3 ± 0.2 | 260 | ++ | ? | 9 |
| 4-Fluoroestradiola | 180 ± 43 | ? | +++ | ? | ? |
| 2-Hydroxyestrone | 1.9 ± 0.8 | 130 ± 9 | Inactive | 110–142 | 8 |
| 2-Methoxyestrone | 0.01 ± 0.00 | 103 ± 7 | Inactive | 95–100 | 120 |
| 4-Hydroxyestrone | 11 ± 4 | 351 | ++ | 21–50 | 35 |
| 4-Methoxyestrone | 0.13 ± 0.04 | 338 | ++ | 65–92 | 12 |
| 16α-Hydroxyestrone | 2.8 ± 1.0 | 552 ± 42 | +++ | 7–24 | <0.5 |
| 2-Hydroxyestriol | 0.9 ± 0.3 | 302 | +b | ? | ? |
| 2-Methoxyestriol | 0.01 ± 0.00 | ? | Inactive | ? | 4 |
| Notes: Values are mean ± SD or range. ER RBA = Relative binding affinity to estrogen receptors of rat uterine cytosol. Uterine weight = Percentage change in uterine wet weight of ovariectomized rats after 72 hours with continuous administration of 1 μg/hour via subcutaneously implanted osmotic pumps. LH levels = Luteinizing hormone levels relative to baseline of ovariectomized rats after 24 to 72 hours of continuous administration via subcutaneous implant. Footnotes: a = Synthetic (i.e., not endogenous). b = Atypical uterotrophic effect which plateaus within 48 hours (estradiol's uterotrophy continues linearly up to 72 hours). Sources: See template. | |||||
| Compound | Dosage for specific uses (mg usually)[a] | ||||||
|---|---|---|---|---|---|---|---|
| ETD[b] | EPD[b] | MSD[b] | MSD[c] | OID[c] | TSD[c] | ||
| Estradiol (non-micronized) | 30 | ≥120–300 | 120 | 6 | - | - | |
| Estradiol (micronized) | 6–12 | 60–80 | 14–42 | 1–2 | >5 | >8 | |
| Estradiol valerate | 6–12 | 60–80 | 14–42 | 1–2 | - | >8 | |
| Estradiol benzoate | - | 60–140 | - | - | - | - | |
| Estriol | ≥20 | 120–150[d] | 28–126 | 1–6 | >5 | - | |
| Estriol succinate | - | 140–150[d] | 28–126 | 2–6 | - | - | |
| Estrone sulfate | 12 | 60 | 42 | 2 | - | - | |
| Conjugated estrogens | 5–12 | 60–80 | 8.4–25 | 0.625–1.25 | >3.75 | 7.5 | |
| Ethinylestradiol | 200 μg | 1–2 | 280 μg | 20–40 μg | 100 μg | 100 μg | |
| Mestranol | 300 μg | 1.5–3.0 | 300–600 μg | 25–30 μg | >80 μg | - | |
| Quinestrol | 300 μg | 2–4 | 500 μg | 25–50 μg | - | - | |
| Methylestradiol | - | 2 | - | - | - | - | |
| Diethylstilbestrol | 2.5 | 20–30 | 11 | 0.5–2.0 | >5 | 3 | |
| DES dipropionate | - | 15–30 | - | - | - | - | |
| Dienestrol | 5 | 30–40 | 42 | 0.5–4.0 | - | - | |
| Dienestrol diacetate | 3–5 | 30–60 | - | - | - | - | |
| Hexestrol | - | 70–110 | - | - | - | - | |
| Chlorotrianisene | - | >100 | - | - | >48 | - | |
| Methallenestril | - | 400 | - | - | - | - | |
Sources and footnotes: | |||||||
| Estrogen | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | ? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
Sources and footnotes
Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (hot flashes/gonadotropins). Sources: See template. | ||||||||||
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
| Estrogen | Form | Major brand name(s) | EPD (14 days) | Duration | |
|---|---|---|---|---|---|
| Diethylstilbestrol (DES) | Oil solution | Metestrol | 20 mg | 1 mg ≈ 2–3 days; 3 mg ≈ 3 days | |
| Diethylstilbestrol dipropionate | Oil solution | Cyren B | 12.5–15 mg | 2.5 mg ≈ 5 days | |
| Aqueous suspension | ? | 5 mg | ? mg = 21–28 days | ||
| Dimestrol (DES dimethyl ether) | Oil solution | Depot-Cyren, Depot-Oestromon, Retalon Retard | 20–40 mg | ? | |
| Fosfestrol (DES diphosphate)a | Aqueous solution | Honvan | ? | <1 day | |
| Dienestrol diacetate | Aqueous suspension | Farmacyrol-Kristallsuspension | 50 mg | ? | |
| Hexestrol dipropionate | Oil solution | Hormoestrol, Retalon Oleosum | 25 mg | ? | |
| Hexestrol diphosphatea | Aqueous solution | Cytostesin, Pharmestrin, Retalon Aquosum | ? | Very short | |
| Note: All by intramuscular injection unless otherwise noted. Footnotes: a = By intravenous injection. Sources: See template. | |||||
| Class | Examples | RE complex retention | Pharmacodynamic profile | Uterine effects |
|---|---|---|---|---|
| Short-acting (a.k.a. "weak" or "impeded") |
Estriol • 16-Epiestriol • 17α-Estradiol • ent-Estradiol • 16-Ketoestradiol • Dimethylstilbestrol • meso-Butestrol | Short (1–4 hours) | Single or once-daily injections: partial agonist or antagonist | Early responsesa |
| Implant or multiple injections per day: full agonist | Early and late responsesb | |||
| Long-acting | A. Estradiol • Estrone • Ethinylestradiol • Diethylstilbestrol • Hexestrol | Intermediate (6–24 hours) | Single or once-daily injections: full agonist | Early and late responses |
| B. Clomifene • Nafoxidine • Nitromifene • Tamoxifen | Long (>24–48 hours) | Single injection: agonist Repeated injections: antagonist |
Early and late responses | |
| Footnotes: a = Early responses occur after 0–6 hours and include water imbibition, hyperemia, amino acid and nucleotide uptake, activation of RNA polymerases I and II, and stimulation of induced protein, among others. b = Late responses occur after 6–48 hours and include cellular hypertrophy and hyperplasia and sustained RNA polymerase I and II activity, among others. Sources: [184][185][186][187][188][189][190] | ||||
Pharmacokinetics
Estrogens can be administered via a variety of routes, including by mouth, sublingual, transdermal/topical (gel, patch), vaginal (gel, tablet, ring), rectal, intramuscular, subcutaneous, intravenous, and subcutaneous implant. Natural estrogens generally have low oral bioavailability while synthetic estrogens have higher bioavailability. Parenteral routes have higher bioavailability. Estrogens are typically bound to albumin and/or sex hormone-binding globulin in the circulation. They are metabolized in the liver by hydroxylation (via cytochrome P450 enzymes), dehydrogenation (via 17β-hydroxysteroid dehydrogenase), and conjugation (via sulfation and glucuronidation). The elimination half-lives of estrogens vary by estrogen and route of administration. Estrogens are eliminated mainly by the kidneys via the urine as conjugates.
| Compound | RBA to SHBG (%) |
Bound to SHBG (%) |
Bound to albumin (%) |
Total bound (%) |
MCR (L/day/m2) |
|---|---|---|---|---|---|
| 17β-Estradiol | 50 | 37 | 61 | 98 | 580 |
| Estrone | 12 | 16 | 80 | 96 | 1050 |
| Estriol | 0.3 | 1 | 91 | 92 | 1110 |
| Estrone sulfate | 0 | 0 | 99 | 99 | 80 |
| 17β-Dihydroequilin | 30 | ? | ? | ? | 1250 |
| Equilin | 8 | 26 | 13 | ? | 2640 |
| 17β-Dihydroequilin sulfate | 0 | ? | ? | ? | 375 |
| Equilin sulfate | 0 | ? | ? | ? | 175 |
| Δ8-Estrone | ? | ? | ? | ? | 1710 |
| Notes: RBA for SHBG (%) is compared to 100% for testosterone. Sources: See template. | |||||
Estrogen metabolism in humans
|
Chemistry
Estrogens can be grouped as steroidal or nonsteroidal. The steroidal estrogens are estranes and include estradiol and its analogues, such as ethinylestradiol and conjugated estrogens like equilin sulfate. Nonsteroidal estrogens belong predominantly to the stilbestrol group of compounds and include diethylstilbestrol and hexestrol, among others.
Estrogen esters are esters and prodrugs of the corresponding parent estrogens. Examples include estradiol valerate and diethylstilbestrol dipropionate, which are prodrugs of estradiol and diethylstilbestrol, respectively. Estrogen esters with fatty acid esters have increased lipophilicity and a prolonged duration of action when administered by intramuscular or subcutaneous injection. Some estrogen esters, such as polyestradiol phosphate, polyestriol phosphate, and polydiethylstilbestrol phosphate, are in the form of polymers.
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
| Generic name | Class | Brand name | Route | Intr. |
|---|---|---|---|---|
| Chlorotrianisene | NS | Tace[a] | PO | 1952 |
| Conjugated estriol | S/ester | Emmenin[a] | PO | 1930 |
| Diethylstilbestrol dipropionate | NS/ester | Synestrin[a] | IM | 1940s |
| Estradiol dipropionate | S/ester | Agofollin[a] | IM | 1939 |
| Estrogenic substances | S | Amniotin[a] | PO, IM, TD, V | 1929 |
| Estrone | S | Theelin[a] | IM | 1929 |
| Ethinylestradiol sulfonate | S/alkyl/ester | Deposiston[a] | PO | 1978 |
| Methallenestril | NS/ether | Vallestril | PO | 1950s |
| Moxestrol | S/alkyl | Surestryl | PO | 1970s |
| Polyestriol phosphate | S/ester | Triodurin[a] | IM | 1968 |
| Quinestrol | S/alkyl/ether | Estrovis | PO | 1960s |
Ovarian extracts were available in the late 1800s and early 1900s, but were inert or had extremely low estrogenic activity and were regarded as ineffective.[191][192][193] In 1927, Selmar and Aschheim discovered that large amounts of estrogens were present in the urine of pregnant women.[192][194][195] This rich source of estrogens, produced by the placenta, allowed for the development of potent estrogenic formulations for scientific and clinical use.[192][195][196] The first pharmaceutical estrogen product was a conjugated estriol called Progynon, a placental extract, and was introduced for medical use by the German pharmaceutical company Schering in 1928.[197][198][199][200][201][202][203][204] In 1929, Adolf Butenandt and Edward Adelbert Doisy independently isolated and purified estrone, the first estrogen to be discovered.[205] The estrogen preparations Amniotin (Squibb), Progynon (Schering), and Theelin (Parke-Davis) were all on the market by 1929,[191] and various additional preparations such as Emmenin, Folliculin, Menformon, Oestroform, and Progynon B, containing purified estrogens or mixtures of estrogens, were on the market by 1934.[192][206][207] Estrogens were originally known under a variety of different names including estrogens, estrins, follicular hormones, folliculins, gynecogens, folliculoids, and female sex hormones, among others.[208][206]
An estrogen patch was reportedly marketed by Searle in 1928,[209][210] and an estrogen nasal spray was studied by 1929.[211]
In 1938, British scientists obtained a patent on a newly formulated nonsteroidal estrogen, diethylstilbestrol (DES), that was cheaper and more powerful than the previously manufactured estrogens. Soon after, concerns over the side effects of DES were raised in scientific journals while the drug manufacturers came together to lobby for governmental approval of DES. It was only until 1941 when estrogen therapy was finally approved by the Food and Drug Administration (FDA) for the treatment of menopausal symptoms.[212] Conjugated estrogens (brand name Premarin) was introduced in 1941 and succeeded Emmenin, the sales of which had begun to drop after 1940 due to competition from DES.[213] Ethinylestradiol was synthesized in 1938 by Hans Herloff Inhoffen and Walter Hohlweg at Schering AG in Berlin[214][215][216][217][218] and was approved by the FDA in the U.S. on 25 June 1943 and marketed by Schering as Estinyl.[219]
Micronized estradiol, via the oral route, was first evaluated in 1972,[220] and this was followed by the evaluation of vaginal and intranasal micronized estradiol in 1977.[221] Oral micronized estradiol was first approved in the United States under the brand name Estrace in 1975.[222]
Society and culture
Availability
Estrogens are widely available throughout the world.[4]
Research
Male birth control
High-dose estrogen therapy is effective in suppressing spermatogenesis and fertility in men, and hence as a male contraceptive.[223][224] It works both by strongly suppressing gonadotropin secretion and gonadal testosterone production and via direct effects on the testes.[224][225] After a sufficient course of therapy, only Sertoli cells and spermatogonia remain in the seminiferous tubules of the testes, with a variety of other testicular abnormalities observable.[223][224] The use of estrogens for contraception in men is precluded by major side effects such as sexual dysfunction, feminization, gynecomastia, and metabolic changes.[223] In addition, there is evidence that with long-term therapy, fertility and gonadal sex hormone production in men may not return following discontinuation of high-dose estrogen therapy.[225]
Eating disorders
Estrogen has as a treatment for women suffering from bulimia nervosa, in addition to cognitive behavioral therapy, which is the established standard for treatment in bulimia cases. The estrogen research hypothesizes that the disease may be linked to a hormonal imbalance in the brain.[226]
Miscellaneous
Estrogens have been used in studies which indicate that they may be effective in the treatment of traumatic liver injury.[227]
In humans and mice, estrogens promote wound healing.[228]
Estrogen therapy has been proposed as a potential treatment for autism but clinical studies are needed.[229]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ Kuhl H (2011). "Pharmacology of Progestogens" (PDF). J Reproduktionsmed Endokrinol. 8 (1): 157–177.
- ^ a b "IBM Watson Health Products: Please Login".
- ^ a b c Sweetman, Sean C., ed. (2009). "Sex Hormones and their Modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. ISBN 978-0-85369-840-1.
- ^ a b "List of Estrogens".
- ^ a b Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. ISBN 978-3-88763-075-1.
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. ISBN 978-1-4757-2085-3.
- ^ Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J (May 2000). "Initial 17beta-estradiol dose for treating vasomotor symptoms". Obstet Gynecol. 95 (5): 726–31. doi:10.1016/s0029-7844(99)00643-2. PMID 10775738. S2CID 42621608.
- ^ Wiegratz, I.; Kuhl, H. (2007). "Praxis der Hormontherapie in der Peri- und Postmenopause" [Practice of peri- and postmenopausal hormone therapy]. Gynäkologische Endokrinologie. 5 (3): 141–149. doi:10.1007/s10304-007-0194-9. ISSN 1610-2894. S2CID 27130717.
- ^ "NIH – Menopausal Hormone Therapy Information". National Institutes of Health. 27 August 2007. Retrieved 4 March 2008.
- ^ Menon DV, Vongpatanasin W (2006). "Effects of transdermal estrogen replacement therapy on cardiovascular risk factors". Treat Endocrinol. 5 (1): 37–51. doi:10.2165/00024677-200605010-00005. PMID 16396517. S2CID 24041216.
- ^ Alfred S. Wolf; H.P.G. Schneider (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 77–. ISBN 978-3-642-75101-1.
- ^ Wesp LM, Deutsch MB (March 2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". Psychiatr. Clin. North Am. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
- ^ Unger CA (December 2016). "Hormone therapy for transgender patients". Transl Androl Urol. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Tangpricha V, den Heijer M (April 2017). "Oestrogen and anti-androgen therapy for transgender women". Lancet Diabetes Endocrinol. 5 (4): 291–300. doi:10.1016/S2213-8587(16)30319-9. PMC 5366074. PMID 27916515.
- ^ a b Oh WK (2002). "The evolving role of estrogen therapy in prostate cancer". Clin Genitourinary Cancer. 1 (2): 81–9. doi:10.3816/CGC.2002.n.009. PMID 15046698.
- ^ a b c William R. Miller; James N. Ingle (8 March 2002). Endocrine Therapy in Breast Cancer. CRC Press. pp. 49–. ISBN 978-0-203-90983-6.
- ^ Maximov, Philipp Y.; McDaniel, Russell E.; Jordan, V. Craig (2013). "Discovery and Pharmacology of Nonsteroidal Estrogens and Antiestrogens". Tamoxifen. Milestones in Drug Therapy. pp. 1–30. doi:10.1007/978-3-0348-0664-0_1. ISBN 978-3-0348-0663-3. ISSN 2296-6056.
- ^ Turner N, Ro J, André F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Bartlett CH, Zhang K, Giorgett C, Randolph S, Koehler M, and Cristofanilli M (2015). "Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer". The New England Journal of Medicine. 373 (3): 209–219. doi:10.1056/NEJMoa1505270. PMID 26030518.
- ^ "Hormonal Therapy". breastcancer.org. 26 July 2007. Retrieved 4 March 2008.
- ^ Kurzer MS (2002). "Hormonal effects of soy in premenopausal women and men". J. Nutr. 132 (3): 570S–573S. doi:10.1093/jn/132.3.570S. PMID 11880595.
- ^ a b c d e Coelingh Bennink HJ, Verhoeven C, Dutman AE, Thijssen J (January 2017). "The use of high-dose estrogens for the treatment of breast cancer". Maturitas. 95: 11–23. doi:10.1016/j.maturitas.2016.10.010. PMID 27889048.
- ^ "ESTRACE® TABLETS (estradiol tablets, USP) FDA label" (PDF). 2005.
- ^ a b Palmieri C, Patten DK, Januszewski A, Zucchini G, Howell SJ (January 2014). "Breast cancer: current and future endocrine therapies". Mol. Cell. Endocrinol. 382 (1): 695–723. doi:10.1016/j.mce.2013.08.001. PMID 23933149.
- ^ a b c Green RB, Sethi RS, Lindner HH (July 1964). "Treatment of advanced carcinoma of the breast: Progress in therapy during the past decade". Am. J. Surg. 108: 107–21. doi:10.1016/0002-9610(64)90094-7. PMID 14182428.
- ^ a b c Thomas, John A.; Keenan, Edward J. (6 December 1986). "Estrogens and Antiestrogenic Drugs". Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 135–165. doi:10.1007/978-1-4684-5036-1_7. ISBN 978-1-4684-5036-1.
- ^ "Premarin® (conjugated estrogens tablets, USP) FDA label" (PDF). 2003.
- ^ a b c d Van Winkle, Walton (1949). "Council on Pharmacy and Chemistry. Estrogens and Androgens in Mammary Cancer". JAMA: The Journal of the American Medical Association. 140 (15): 1214. doi:10.1001/jama.1949.02900500022007. ISSN 0098-7484.
- ^ a b c d Kahr, Ernst (1966). "Die Allgemeinbehandlung" [General Treatment]. Der Inoperable Krebskranke: Möglichkeiten der Therapie in Klinik und Praxis [The Inoperable Cancer Patient: Possibilities of Therapy in Clinic and Practice]. Springer-Verlag. pp. 104–155. doi:10.1007/978-3-642-86140-6_4. ISBN 978-3-642-86140-6.
- ^ a b c Dao, Thomas L. (1975). "Pharmacology and Clinical Utility of Hormones in Hormone Related Neoplasms". In Alan C. Sartorelli; David G. Johns (eds.). Antineoplastic and Immunosuppressive Agents. pp. 170–192. doi:10.1007/978-3-642-65806-8_11. ISBN 978-3-642-65806-8.
- ^ a b c d Nathanson IT, Kelley RM (January 1952). "Hormonal treatment of cancer". N. Engl. J. Med. 246 (5): 180–9, concl. doi:10.1056/NEJM195201312460505. PMID 14890833.
- ^ a b Dobson L (August 1962). "The management of metastatic breast cancer". Surg. Clin. North Am. 42: 861–76. doi:10.1016/S0039-6109(16)36728-7. PMID 13886800.
- ^ a b Martz G (13 March 2013). Die hormonale Therapie maligner Tumoren: Endokrine Behandlungsmethoden des metastasierenden Mamma-, Prostata- und Uterus-Corpuscarcinoms. Springer-Verlag. pp. 39–. ISBN 978-3-642-86282-3.
- ^ Committee on Research, AMA (1960). "Androgens and estrogens in the treatment of disseminated mammary carcinoma. Retrospective study of nine hundred forty-four patients". Journal of the American Medical Association. 172 (12): 1271. doi:10.1001/jama.1960.03020120049010. ISSN 0002-9955.
- ^ "Estradurin® (polyestradiol phosphate) information and labels". Pharmanovia.
- ^ Ostrowski MJ, Jackson AW (1979). "Polyestradiol phosphate: a preliminary evaluation of its effect on breast carcinoma". Cancer Treat Rep. 63 (11–12): 1803–7. PMID 393380.
- ^ "Estrone suspension FDA review" (PDF). 1979.
- ^ J. Aiman (6 December 2012). Infertility: Diagnosis and Management. Springer Science & Business Media. pp. 133–134. ISBN 978-1-4613-8265-2.
- ^ Glenn L. Schattman; Sandro Esteves; Ashok Agarwal (12 May 2015). Unexplained Infertility: Pathophysiology, Evaluation and Treatment. Springer. pp. 266–. ISBN 978-1-4939-2140-9.
- ^ a b Bamigboye AA, Morris J (2003). "Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes". Cochrane Database Syst Rev (3): CD004353. doi:10.1002/14651858.CD004353. PMID 12918007.
- ^ J.B. Josimovich (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 482–. ISBN 978-1-4613-2157-6.
- ^ Marshall S. Shapo (30 December 2008). Experimenting with the Consumer: The Mass Testing of Risky Products on the American Public: The Mass Testing of Risky Products on the American Public. ABC-CLIO. pp. 137–. ISBN 978-0-313-36529-4.
- ^ Lee JM, Howell JD (2006). "Tall girls: the social shaping of a medical therapy". Arch Pediatr Adolesc Med. 160 (10): 1077–8. doi:10.1001/archpedi.160.10.1035. PMID 17018462.
- ^ Gunther DF, Diekema DS (2006). "Attenuating growth in children with profound developmental disability: a new approach to an old dilemma". Arch Pediatr Adolesc Med. 160 (10): 1013–7. doi:10.1001/archpedi.160.10.1013. PMID 17018459.
- ^ a b Duarte FH, Jallad RS, Bronstein MD (November 2016). "Estrogens and selective estrogen receptor modulators in acromegaly". Endocrine. 54 (2): 306–314. doi:10.1007/s12020-016-1118-z. PMID 27704479. S2CID 10136018.
- ^ a b Stone JC, Clark J, Cuneo R, Russell AW, Doi SA (June 2014). "Estrogen and selective estrogen receptor modulators (SERMs) for the treatment of acromegaly: a meta-analysis of published observational studies". Pituitary. 17 (3): 284–95. doi:10.1007/s11102-013-0504-2. PMID 23925896. S2CID 30697394.
- ^ a b Shimon I, Barkan A (December 2012). "Estrogen treatment for acromegaly". Pituitary. 15 (4): 601–7. doi:10.1007/s11102-012-0426-4. PMID 22933045. S2CID 1917058.
- ^ a b c Thibaut F, De La Barra F, Gordon H, Cosyns P, Bradford JM (2010). "The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias". World J. Biol. Psychiatry. 11 (4): 604–55. doi:10.3109/15622971003671628. PMID 20459370. S2CID 14949511.
- ^ Brogden RN, Clissold SP (August 1989). "Flutamide. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in advanced prostatic cancer". Drugs. 38 (2): 185–203. doi:10.2165/00003495-198938020-00003. PMID 2670515.
A favourable feature of flutamide therapy has been its lesser effect on libido and sexual potency; fewer than 20% of patients treated with flutamide alone reported such changes. In contrast, nearly all patients treated with oestrogens or estramustine phosphate reported loss of sexual potency. [...] In comparative therapeutic trials, loss of potency has occurred in all patients treated with stilboestrol or estramustine phosphate compared with 0 to 20% of those given flutamide alone (Johansson et al. 1987; Lund & Rasmussen 1988).
- ^ a b c Gunther Göretzlehner; Christian Lauritzen; Thomas Römer; Winfried Rossmanith (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 385–. ISBN 978-3-11-024568-4.
- ^ R.E. Mansel; Oystein Fodstad; Wen G. Jiang (14 June 2007). Metastasis of Breast Cancer. Springer Science & Business Media. pp. 217–. ISBN 978-1-4020-5866-0.
- ^ a b c Hartmann BW, Laml T, Kirchengast S, Albrecht AE, Huber JC (1998). "Hormonal breast augmentation: prognostic relevance of insulin-like growth factor-I". Gynecol. Endocrinol. 12 (2): 123–7. doi:10.3109/09513599809024960. PMID 9610425.
- ^ a b c Lauritzen, C (1980). "Hormonkur kann hypoplastischer Mamma aufhelfen" [Hormone therapy can help hypoplastic breasts]. Selecta (in German). 22 (43). Planegg: Selecta-Verlag: 3798–3801. ISSN 0582-4877. OCLC 643821347.
- ^ Kaiser, Rolf; Leidenberger, Freimut A. (1991). Hormonbehandlung in der gynäkologischen Praxis (6 ed.). Stuttgart, New York: Georg Thieme Verlag. pp. 138–139. ISBN 978-3133574075.
- ^ a b c d e f g h i Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, Bobo WV, Rubin LH, Koleva HK, Cohen LS, Soares CN (February 2019). "Guidelines for the Evaluation and Treatment of Perimenopausal Depression: Summary and Recommendations". J Womens Health (Larchmt). 28 (2): 117–134. doi:10.1089/jwh.2018.27099.mensocrec. PMID 30182804.
- ^ a b c d e f g h Stute P, Spyropoulou A, Karageorgiou V, Cano A, Bitzer J, Ceausu I, Chedraui P, Durmusoglu F, Erkkola R, Goulis DG, Lindén Hirschberg A, Kiesel L, Lopes P, Pines A, Rees M, van Trotsenburg M, Zervas I, Lambrinoudaki I (January 2020). "Management of depressive symptoms in peri- and postmenopausal women: EMAS position statement". Maturitas. 131: 91–101. doi:10.1016/j.maturitas.2019.11.002. PMID 31740049.
- ^ Raglan GB, Schulkin J, Micks E (2020). "Depression during perimenopause: the role of the obstetrician-gynecologist". Arch Womens Ment Health. 23 (1): 1–10. doi:10.1007/s00737-019-0950-6. PMID 30758732. S2CID 61155969.
- ^ a b Gava G, Orsili I, Alvisi S, Mancini I, Seracchioli R, Meriggiola MC (October 2019). "Cognition, Mood and Sleep in Menopausal Transition: The Role of Menopause Hormone Therapy". Medicina. 55 (10): 668. doi:10.3390/medicina55100668. PMC 6843314. PMID 31581598.
- ^ Toffol E, Heikinheimo O, Partonen T (May 2015). "Hormone therapy and mood in perimenopausal and postmenopausal women: a narrative review". Menopause. 22 (5): 564–78. doi:10.1097/GME.0000000000000323. PMID 25203891. S2CID 5830652.
- ^ a b c Garay RP, Charpeaud T, Logan S, Hannaert P, Garay RG, Llorca PM, Shorey S (October 2019). "Pharmacotherapeutic approaches to treating depression during the perimenopause". Expert Opin Pharmacother. 20 (15): 1837–1845. doi:10.1080/14656566.2019.1645122. PMID 31355688. S2CID 198967172.
- ^ a b Rubinow DR, Johnson SL, Schmidt PJ, Girdler S, Gaynes B (August 2015). "Efficacy of estradiol in perimenopausal depression: so much promise and so few answers". Depress Anxiety. 32 (8): 539–49. doi:10.1002/da.22391. PMC 6309886. PMID 26130315.
- ^ Zweifel JE, O'Brien WH (April 1997). "A meta-analysis of the effect of hormone replacement therapy upon depressed mood". Psychoneuroendocrinology. 22 (3): 189–212. doi:10.1016/s0306-4530(96)00034-0. PMID 9203229. S2CID 44630030.
- ^ a b Rubinow DR, Schmidt PJ (September 2018). "Is there a role for reproductive steroids in the etiology and treatment of affective disorders?". Dialogues Clin Neurosci. 20 (3): 187–196. doi:10.31887/DCNS.2018.20.3/drubinow. PMC 6296393. PMID 30581288.
- ^ Myoraku, Alison; Robakis, Thalia; Rasgon, Natalie (2018). "Estrogen-Based Hormone Therapy for Depression Related to Reproductive Events". Current Treatment Options in Psychiatry. 5 (4): 416–424. doi:10.1007/s40501-018-0156-y. ISSN 2196-3061. S2CID 81770543.
- ^ Cheng, Yu-Shian; Tseng, Ping-Tao; Tu, Yu-Kang; Wu, Yi-Cheng; Su, Kuan-Pin; Wu, Ching-Kuan; Li, Dian-Jeng; Chen, Tien-Yu; Stubbs, Brendon; Carvalho, Andre F.; Solmi, Marco; Thompson, Trevor; Caruso, Maria Gabriella; Matsuoka, Yutaka J.; Chen, Yen-Wen; Lin, Pao-Yen; Wu, Ming-Kung; Sun, Cheuk-Kwan (19 September 2019), Hormonal and Pharmacologic Interventions for Depressive Symptoms in Peri- And/Or Post-Menopausal Women: A Network Meta-Analysis of Randomized Controlled Trials, doi:10.2139/ssrn.3457416, SSRN 3457416
- ^ Begemann MJ, Dekker CF, van Lunenburg M, Sommer IE (November 2012). "Estrogen augmentation in schizophrenia: a quantitative review of current evidence". Schizophr. Res. 141 (2–3): 179–84. doi:10.1016/j.schres.2012.08.016. PMID 22998932. S2CID 40584474.
- ^ Brzezinski A, Brzezinski-Sinai NA, Seeman MV (May 2017). "Treating schizophrenia during menopause". Menopause. 24 (5): 582–588. doi:10.1097/GME.0000000000000772. PMID 27824682. S2CID 3452898.
- ^ McGregor C, Riordan A, Thornton J (October 2017). "Estrogens and the cognitive symptoms of schizophrenia: Possible neuroprotective mechanisms". Front Neuroendocrinol. 47: 19–33. doi:10.1016/j.yfrne.2017.06.003. PMID 28673758. S2CID 43291520.
- ^ Owens SJ, Murphy CE, Purves-Tyson TD, Weickert TW, Shannon Weickert C (February 2018). "Considering the role of adolescent sex steroids in schizophrenia". J. Neuroendocrinol. 30 (2): e12538. doi:10.1111/jne.12538. PMID 28941299. S2CID 3391650.
- ^ Clayton RW, Göbel K, Niessen CM, Paus R, van Steensel MAM, Lim X (October 2019). "Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis". Br. J. Dermatol. 181 (4): 677–690. doi:10.1111/bjd.17981. PMID 31056753. S2CID 145822633.
- ^ Andrews GC, Domonkos AN, Post CF (July 1951). "Treatment of acne vulgaris". J Am Med Assoc. 146 (12): 1107–13. doi:10.1001/jama.1951.03670120017005. PMID 14841085.
- ^ White CB, Lehmann CF (May 1952). "Diethylstilbestrol therapy in young men with acne; correlation with the urinary 17-ketosteroids". AMA Arch Derm Syphilol. 65 (5): 601–8. doi:10.1001/archderm.1952.01530240093012. PMID 14914180.
- ^ Welsh AL (April 1954). "Use of synthetic estrogenic substance chlorotrianisene (TACE) in treatment of acne". AMA Arch Derm Syphilol. 69 (4): 418–27. doi:10.1001/archderm.1954.01540160020004. PMID 13147544.
- ^ Becker FT (February 1953). "The acne problem". AMA Arch Derm Syphilol. 67 (2): 173–83. doi:10.1001/archderm.1953.01540020051010. PMID 13029903.
- ^ Day RL, Anderson NP (July 1952). "The management of acne vulgaris". Postgrad Med. 12 (1): 34–40. doi:10.1080/00325481.1952.11708046. PMID 14957675.
- ^ Mullins JF, McCash WB, Lamar JK (January 1960). "Acne, estrogens and spermatozoa". Arch Dermatol. 81: 53–8. doi:10.1001/archderm.1960.03730010057006. PMID 14425194.
- ^ Pochi PE, Strauss JS (August 1973). "Sebaceous gland suppression with ethinyl estradiol and diethylstilbestrol". Arch Dermatol. 108 (2): 210–4. doi:10.1001/archderm.1973.01620230010003. PMID 4269283.
- ^ Hermkens PH, Kamp S, Lusher S, Veeneman GH (July 2006). "Non-steroidal steroid receptor modulators". IDrugs. 9 (7): 488–94. doi:10.2174/0929867053764671. PMID 16821162.
- ^ "Drospirenone/Estetrol - Mithra Pharmaceuticals - AdisInsight".
- ^ a b Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 9–10, 294–297, 385–394, 402–412, 444–454, 460–467, 483–490, 575–583. ISBN 978-0-9828280-1-4.
- ^ Paulsen CA, Leach RB, Lanman J, Goldston N, Maddock WO, Heller CG (1962). "Inherent estrogenicity of norethindrone and norethynodrel: comparison with other synthetic progestins and progesterone". J. Clin. Endocrinol. Metab. 22 (10): 1033–9. doi:10.1210/jcem-22-10-1033. PMID 13942007.
- ^ a b Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ a b Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. pp. 95–98, 488. ISBN 978-0-203-48612-2.
- ^ a b Laurtizen, Christian (2001). "Hormone Substitution Before, During and After Menopause" (PDF). In Fisch, Franz H. (ed.). Menopause – Andropause: Hormone Replacement Therapy Through the Ages. Krause & Pachernegg: Gablitz. pp. 67–88. ISBN 978-3-901299-34-6.
- ^ a b Midwinter, Audrey (1976). "Contraindications to estrogen therapy and management of the menopausal syndrome in these cases". In Campbell, Stuart (ed.). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. MTP Press Limited. pp. 377–382. doi:10.1007/978-94-011-6165-7_33. ISBN 978-94-011-6167-1.
- ^ a b c d e f g h i j k Sturdee DW (2013). "Are progestins really necessary as part of a combined HRT regimen?". Climacteric. 16 (Suppl 1): 79–84. doi:10.3109/13697137.2013.803311. PMID 23651281. S2CID 21894200.
- ^ a b Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M (2018). "Oral Contraceptives and HRT Risk of Thrombosis". Clin. Appl. Thromb. Hemost. 24 (2): 217–225. doi:10.1177/1076029616683802. PMC 6714678. PMID 28049361.
- ^ a b De Leo V, Musacchio MC, Cappelli V, Piomboni P, Morgante G (2016). "Hormonal contraceptives: pharmacology tailored to women's health". Hum. Reprod. Update. 22 (5): 634–46. doi:10.1093/humupd/dmw016. PMID 27307386.
- ^ a b Mohammed K, Abu Dabrh AM, Benkhadra K, Al Nofal A, Carranza Leon BG, Prokop LJ, Montori VM, Faubion SS, Murad MH (2015). "Oral vs Transdermal Estrogen Therapy and Vascular Events: A Systematic Review and Meta-Analysis". J. Clin. Endocrinol. Metab. 100 (11): 4012–20. doi:10.1210/jc.2015-2237. PMID 26544651.
- ^ Gregory Y. H. Lip; John E. Hall (28 June 2007). Comprehensive Hypertension E-Book. Elsevier Health Sciences. pp. 865–. ISBN 978-0-323-07067-6.
- ^ a b c d e Scarabin PY (2014). "Hormones and venous thromboembolism among postmenopausal women". Climacteric. 17 (Suppl 2): 34–7. doi:10.3109/13697137.2014.956717. PMID 25223916. S2CID 5084606.
- ^ a b c d e f g h i Bińkowska M (October 2014). "Menopausal hormone therapy and venous thromboembolism". Prz Menopauzalny. 13 (5): 267–72. doi:10.5114/pm.2014.46468. PMC 4520375. PMID 26327865.
- ^ Smith NL, Blondon M, Wiggins KL, Harrington LB, van Hylckama Vlieg A, Floyd JS, Hwang M, Bis JC, McKnight B, Rice KM, Lumley T, Rosendaal FR, Heckbert SR, Psaty BM (January 2014). "Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens". JAMA Intern Med. 174 (1): 25–31. doi:10.1001/jamainternmed.2013.11074. PMC 4636198. PMID 24081194.
- ^ Smith NL, Heckbert SR, Lemaitre RN, Reiner AP, Lumley T, Weiss NS, Larson EB, Rosendaal FR, Psaty BM (October 2004). "Esterified estrogens and conjugated equine estrogens and the risk of venous thrombosis". JAMA. 292 (13): 1581–7. doi:10.1001/jama.292.13.1581. PMID 15467060.
- ^ Lekovic D, Miljic P, Dmitrovic A, Thachil J (May 2017). "How do you decide on hormone replacement therapy in women with risk of venous thromboembolism?". Blood Rev. 31 (3): 151–157. doi:10.1016/j.blre.2016.12.001. PMID 27998619.
- ^ a b c Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A (January 2013). "The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy". J. Thromb. Haemost. 11 (1): 124–31. doi:10.1111/jth.12060. PMID 23136837. S2CID 22306721.
- ^ Høibraaten E, Abdelnoor M, Sandset PM (October 1999). "Hormone replacement therapy with estradiol and risk of venous thromboembolism—a population-based case-control study". Thromb. Haemost. 82 (4): 1218–21. doi:10.1055/s-0037-1614363. PMID 10544901.
- ^ a b Heit JA, Spencer FA, White RH (2016). "The epidemiology of venous thromboembolism". J. Thromb. Thrombolysis. 41 (1): 3–14. doi:10.1007/s11239-015-1311-6. PMC 4715842. PMID 26780736.
- ^ "FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone". Archived from the original on 27 April 2019.
- ^ Pramilla Senanayake; Malcolm Potts (14 April 2008). Atlas of Contraception, Second Edition. CRC Press. pp. 44–. ISBN 978-0-203-34732-4.
- ^ Shlomo Melmed (2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 665–. ISBN 978-0-323-29738-7.
- ^ Ralli E, Zezza L, Caserta D (2014). "Pregnancy and venous thromboembolism". Curr. Opin. Obstet. Gynecol. 26 (6): 469–75. doi:10.1097/GCO.0000000000000115. PMID 25304605. S2CID 30535761.
- ^ a b c d e f Davey DA (March 2018). "Menopausal hormone therapy: a better and safer future". Climacteric. 21 (5): 454–461. doi:10.1080/13697137.2018.1439915. PMID 29526116. S2CID 3850275.
- ^ Stevenson JC, Panay N, Pexman-Fieth C (September 2013). "Oral estradiol and dydrogesterone combination therapy in postmenopausal women: review of efficacy and safety". Maturitas. 76 (1): 10–21. doi:10.1016/j.maturitas.2013.05.018. PMID 23835005.
Dydrogesterone did not increase the risk of VTE associated with oral estrogen (odds ratio (OR) 0.9, 95% CI 0.4–2.3). Other progestogens (OR 3.9, 95% CI 1.5–10.0) were found to further increase the risk of VTE associated with oral estrogen (OR 4.2, 95% CI 1.5–11.6).
- ^ Schneider C, Jick SS, Meier CR (October 2009). "Risk of cardiovascular outcomes in users of estradiol/dydrogesterone or other HRT preparations". Climacteric. 12 (5): 445–53. doi:10.1080/13697130902780853. PMID 19565370. S2CID 45890629.
The adjusted relative risk of developing a VTE tended to be lower for E/D users (OR 0.84; 95% CI 0.37–1.92) than for users of other HRT (OR 1.42; 95% CI 1.10–1.82), compared to non-users.
- ^ a b c d Schwingl PJ, Ory HW, Visness CM (January 1999). "Estimates of the risk of cardiovascular death attributable to low-dose oral contraceptives in the United States". Am. J. Obstet. Gynecol. 180 (1 Pt 1): 241–9. doi:10.1016/S0002-9378(99)70182-1. PMID 9914611.
- ^ a b c d Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1027–. ISBN 978-0-7817-1750-2.
- ^ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 753–. ISBN 978-1-4511-4847-3.
- ^ Stanczyk FZ, Archer DF, Bhavnani BR (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–27. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
- ^ Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 770–771. ISBN 978-0-08-055309-2.
- ^ a b Turo R, Smolski M, Esler R, Kujawa ML, Bromage SJ, Oakley N, Adeyoju A, Brown SC, Brough R, Sinclair A, Collins GN (February 2014). "Diethylstilboestrol for the treatment of prostate cancer: past, present and future". Scand J Urol. 48 (1): 4–14. doi:10.3109/21681805.2013.861508. PMID 24256023. S2CID 34563641.
- ^ a b c Phillips I, Shah SI, Duong T, Abel P, Langley RE (2014). "Androgen Deprivation Therapy and the Re-emergence of Parenteral Estrogen in Prostate Cancer". Oncol Hematol Rev. 10 (1): 42–47. doi:10.17925/ohr.2014.10.1.42. PMC 4052190. PMID 24932461.
- ^ Waun Ki Hong; James F. Holland (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 753–. ISBN 978-1-60795-014-1.
- ^ Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- ^ a b c Odlind V, Milsom I, Persson I, Victor A (June 2002). "Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills?". Acta Obstet Gynecol Scand. 81 (6): 482–90. doi:10.1034/j.1600-0412.2002.810603.x. PMID 12047300. S2CID 26054257.
- ^ Raps M, Helmerhorst F, Fleischer K, Thomassen S, Rosendaal F, Rosing J, Ballieux B, VAN Vliet H (June 2012). "Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives". J. Thromb. Haemost. 10 (6): 992–7. doi:10.1111/j.1538-7836.2012.04720.x. PMID 22469296. S2CID 20803995.
- ^ Stanczyk FZ, Grimes DA (September 2008). "Sex hormone-binding globulin: not a surrogate marker for venous thromboembolism in women using oral contraceptives". Contraception. 78 (3): 201–3. doi:10.1016/j.contraception.2008.04.004. PMID 18692609.
- ^ Stephen J. Winters; Ilpo T. Huhtaniemi (25 April 2017). Male Hypogonadism: Basic, Clinical and Therapeutic Principles. Humana Press. pp. 307–. ISBN 978-3-319-53298-1.
- ^ Notelovitz M (March 2006). "Clinical opinion: the biologic and pharmacologic principles of estrogen therapy for symptomatic menopause". MedGenMed. 8 (1): 85. PMC 1682006. PMID 16915215.
- ^ Goodman MP (February 2012). "Are all estrogens created equal? A review of oral vs. transdermal therapy". J Womens Health (Larchmt). 21 (2): 161–9. doi:10.1089/jwh.2011.2839. PMID 22011208.
- ^ Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 (Suppl 2): S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384. S2CID 32650111.
- ^ a b von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function--metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738. S2CID 21510744.
- ^ Ottosson UB, Carlström K, Johansson BG, von Schoultz B (1986). "Estrogen induction of liver proteins and high-density lipoprotein cholesterol: comparison between estradiol valerate and ethinyl estradiol". Gynecol. Obstet. Invest. 22 (4): 198–205. doi:10.1159/000298914. PMID 3817605.
- ^ Abdul Sultan A, West J, Stephansson O, Grainge MJ, Tata LJ, Fleming KM, Humes D, Ludvigsson JF (November 2015). "Defining venous thromboembolism and measuring its incidence using Swedish health registries: a nationwide pregnancy cohort study". BMJ Open. 5 (11): e008864. doi:10.1136/bmjopen-2015-008864. PMC 4654387. PMID 26560059.
- ^ Russo J, Russo IH (2006). "The role of estrogen in the initiation of breast cancer". J. Steroid Biochem. Mol. Biol. 102 (1–5): 89–96. doi:10.1016/j.jsbmb.2006.09.004. PMC 1832080. PMID 17113977.
- ^ Germain D (2011). "Estrogen carcinogenesis in breast cancer". Endocrinol. Metab. Clin. North Am. 40 (3): 473–84, vii. doi:10.1016/j.ecl.2011.05.009. PMID 21889715.
- ^ Jameera Begam A, Jubie S, Nanjan MJ (2017). "Estrogen receptor agonists/antagonists in breast cancer therapy: A critical review". Bioorg. Chem. 71: 257–274. doi:10.1016/j.bioorg.2017.02.011. PMID 28274582.
- ^ Yip CH, Rhodes A (2014). "Estrogen and progesterone receptors in breast cancer". Future Oncol. 10 (14): 2293–301. doi:10.2217/fon.14.110. PMID 25471040. S2CID 22988559.
- ^ Schiavon G, Smith IE (2013). "Endocrine therapy for advanced/metastatic breast cancer". Hematol. Oncol. Clin. North Am. 27 (4): 715–36, viii. doi:10.1016/j.hoc.2013.05.004. PMID 23915741.
- ^ Lumachi F, Santeufemia DA, Basso SM (2015). "Current medical treatment of estrogen receptor-positive breast cancer". World J Biol Chem. 6 (3): 231–9. doi:10.4331/wjbc.v6.i3.231. PMC 4549764. PMID 26322178.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lee CI, Goodwin A, Wilcken N (2017). "Fulvestrant for hormone-sensitive metastatic breast cancer". Cochrane Database Syst Rev. 1: CD011093. doi:10.1002/14651858.CD011093.pub2. PMC 6464820. PMID 28043088.
- ^ a b Mallick S, Benson R, Julka PK (2016). "Breast cancer prevention with anti-estrogens: review of the current evidence and future directions". Breast Cancer. 23 (2): 170–7. doi:10.1007/s12282-015-0647-2. PMID 26439380. S2CID 37377540.
- ^ a b Li F, Dou J, Wei L, Li S, Liu J (2016). "The selective estrogen receptor modulators in breast cancer prevention". Cancer Chemother. Pharmacol. 77 (5): 895–903. doi:10.1007/s00280-016-2959-0. PMID 26787504. S2CID 24240700.
- ^ a b Mocellin S, Pilati P, Briarava M, Nitti D (2016). "Breast Cancer Chemoprevention: A Network Meta-Analysis of Randomized Controlled Trials". J. Natl. Cancer Inst. 108 (2): djv318. doi:10.1093/jnci/djv318. PMID 26582062.
- ^ a b Coelingh Bennink HJ, Verhoeven C, Dutman AE, Thijssen J (January 2017). "The use of high-dose estrogens for the treatment of breast cancer". Maturitas. 95: 11–23. doi:10.1016/j.maturitas.2016.10.010. PMID 27889048.
- ^ a b Jordan VC (2015). "The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer". Endocr. Relat. Cancer. 22 (1): R1–31. doi:10.1530/ERC-14-0448. PMC 4494663. PMID 25339261.
- ^ a b c d e f g h i j k Yang Z, Hu Y, Zhang J, Xu L, Zeng R, Kang D (2017). "Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis". Gynecol. Endocrinol. 33 (2): 87–92. doi:10.1080/09513590.2016.1248932. PMID 27898258. S2CID 205631264.
- ^ Pike MC, Wu AH, Spicer DV, Lee S, Pearce CL (2007). "Estrogens, progestins, and risk of breast cancer". Ernst Schering Found Symp Proc. Ernst Schering Foundation Symposium Proceedings. 2007/1 (1): 127–50. doi:10.1007/2789_2007_059. ISBN 978-3-540-73492-5. PMID 18540571.
- ^ Atashgaran V, Wrin J, Barry SC, Dasari P, Ingman WV (2016). "Dissecting the Biology of Menstrual Cycle-Associated Breast Cancer Risk". Front Oncol. 6: 267. doi:10.3389/fonc.2016.00267. PMC 5183603. PMID 28083513.
- ^ Lambrinoudaki I (2014). "Progestogens in postmenopausal hormone therapy and the risk of breast cancer". Maturitas. 77 (4): 311–7. doi:10.1016/j.maturitas.2014.01.001. PMID 24485796.
- ^ Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP, Tangpricha V, Montori VM (2009). "Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline". J. Clin. Endocrinol. Metab. 94 (9): 3132–54. doi:10.1210/jc.2009-0345. PMID 19509099.
- ^ Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ (2013). "Breast cancer development in transsexual subjects receiving cross-sex hormone treatment". J Sex Med. 10 (12): 3129–34. doi:10.1111/jsm.12319. PMID 24010586.
- ^ Brown GR, Jones KT (2015). "Incidence of breast cancer in a cohort of 5,135 transgender veterans". Breast Cancer Res. Treat. 149 (1): 191–8. doi:10.1007/s10549-014-3213-2. PMID 25428790. S2CID 10935304.
- ^ a b Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B (2014). "Gynecomastia: Clinical evaluation and management". Indian J Endocrinol Metab. 18 (2): 150–8. doi:10.4103/2230-8210.129104. PMC 3987263. PMID 24741509.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Shlomo Melmed; Kenneth S. Polonsky; P. Reed Larsen; Henry M. Kronenberg (30 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 934–. ISBN 978-0-323-29738-7.
- ^ Hughes IA, Werner R, Bunch T, Hiort O (2012). "Androgen insensitivity syndrome". Semin. Reprod. Med. 30 (5): 432–42. doi:10.1055/s-0032-1324728. PMID 23044881.
- ^ Niewoehner CB, Schorer AE (2008). "Gynaecomastia and breast cancer in men". BMJ. 336 (7646): 709–13. doi:10.1136/bmj.39511.493391.BE. PMC 2276281. PMID 18369226.
- ^ Christopher Li (11 November 2009). Breast Cancer Epidemiology. Springer Science & Business Media. pp. 266–. ISBN 978-1-4419-0685-4.
- ^ Chen J, Zhao KN, Liu GB (2013). "Estrogen-induced cholestasis: pathogenesis and therapeuticimplications". Hepatogastroenterology. 60 (126): 1289–96. doi:10.5754/hge121061 (inactive 31 May 2021). PMID 23933920.
{{cite journal}}: CS1 maint: DOI inactive as of May 2021 (link) - ^ Chitturi S, Farrell GC (2001). "Drug-induced cholestasis". Semin. Gastrointest. Dis. 12 (2): 113–24. PMID 11352118.
- ^ Velayudham LS, Farrell GC (2003). "Drug-induced cholestasis". Expert Opin Drug Saf. 2 (3): 287–304. doi:10.1517/eods.2.3.287.21377. PMID 12904107.
- ^ Arrese M, Reyes H (2006). "Intrahepatic cholestasis of pregnancy: a past and present riddle". Ann Hepatol. 5 (3): 202–5. doi:10.1016/S1665-2681(19)32012-5. PMID 17060884.
- ^ Pusl T, Beuers U (2007). "Intrahepatic cholestasis of pregnancy". Orphanet J Rare Dis. 2: 26. doi:10.1186/1750-1172-2-26. PMC 1891276. PMID 17535422.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Arrese M, Macias RI, Briz O, Perez MJ, Marin JJ (2008). "Molecular pathogenesis of intrahepatic cholestasis of pregnancy". Expert Rev Mol Med. 10: e9. doi:10.1017/S1462399408000628. PMID 18371245. S2CID 39759941.
- ^ Pauli-Magnus C, Meier PJ, Stieger B (2010). "Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy" (PDF). Semin. Liver Dis. 30 (2): 147–59. doi:10.1055/s-0030-1253224. PMID 20422497.
- ^ Uhler ML, Marks JW, Judd HL (2000). "Estrogen replacement therapy and gallbladder disease in postmenopausal women". Menopause. 7 (3): 162–7. doi:10.1097/00042192-200007030-00006. PMID 10810961. S2CID 37022601.
- ^ Dhiman RK, Chawla YK (2006). "Is there a link between oestrogen therapy and gallbladder disease?". Expert Opin Drug Saf. 5 (1): 117–29. doi:10.1517/14740338.5.1.117. PMID 16370961. S2CID 2173767.
- ^ Wang HH, Liu M, Clegg DJ, Portincasa P, Wang DQ (2009). "New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation". Biochim. Biophys. Acta. 1791 (11): 1037–47. doi:10.1016/j.bbalip.2009.06.006. PMC 2756670. PMID 19589396.
- ^ a b Wang S, Wang Y, Xu J, Chen Y (2017). "Is the oral contraceptive or hormone replacement therapy a risk factor for cholelithiasis: A systematic review and meta-analysis". Medicine (Baltimore). 96 (14): e6556. doi:10.1097/MD.0000000000006556. PMC 5411213. PMID 28383429.
- ^ Stinton LM, Shaffer EA (2012). "Epidemiology of gallbladder disease: cholelithiasis and cancer". Gut Liver. 6 (2): 172–87. doi:10.5009/gnl.2012.6.2.172. PMC 3343155. PMID 22570746.
- ^ A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548–. ISBN 978-3-642-96158-8.
- ^ Scott WW, Menon M, Walsh PC (April 1980). "Hormonal Therapy of Prostatic Cancer". Cancer. 45 (Suppl 7): 1929–1936. doi:10.1002/cncr.1980.45.s7.1929. PMID 29603164. S2CID 4492779.
- ^ Ansbacher R (February 2001). "The pharmacokinetics and efficacy of different estrogens are not equivalent". Am. J. Obstet. Gynecol. 184 (3): 255–63. doi:10.1067/mob.2001.109656. PMID 11228470.
- ^ Bennink HJ (2008). "Reprint of Are all estrogens the same?". Maturitas. 61 (1–2): 195–201. doi:10.1016/j.maturitas.2008.11.015. PMID 19434891.
- ^ Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ Lauritzen C (June 1977). "[Estrogen thearpy in practice. 3. Estrogen preparations and combination preparations]" [Estrogen therapy in practice. 3. Estrogen preparations and combination preparations]. Fortschritte Der Medizin (in German). 95 (21): 1388–92. PMID 559617.
- ^ Wolf AS, Schneider HP (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 78–. ISBN 978-3-642-75101-1.
- ^ Göretzlehner G, Lauritzen C, Römer T, Rossmanith W (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 44–. ISBN 978-3-11-024568-4.
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl J (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598–599. ISBN 978-3-11-150424-7.
- ^ Lauritzen CH (January 1976). "The female climacteric syndrome: significance, problems, treatment". Acta Obstetricia Et Gynecologica Scandinavica. Supplement. 51: 47–61. doi:10.3109/00016347509156433. PMID 779393.
- ^ Lauritzen C (1975). "The Female Climacteric Syndrome: Significance, Problems, Treatment". Acta Obstetricia et Gynecologica Scandinavica. 54 (s51): 48–61. doi:10.3109/00016347509156433. ISSN 0001-6349.
- ^ Kopera H (1991). "Hormone der Gonaden". Hormonelle Therapie für die Frau. Kliniktaschenbücher. pp. 59–124. doi:10.1007/978-3-642-95670-6_6. ISBN 978-3-540-54554-5. ISSN 0172-777X.
- ^ Scott WW, Menon M, Walsh PC (April 1980). "Hormonal Therapy of Prostatic Cancer". Cancer. 45 (Suppl 7): 1929–1936. doi:10.1002/cncr.1980.45.s7.1929. PMID 29603164.
- ^ Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgender Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- ^ Ryden AB (1950). "Natural and synthetic oestrogenic substances; their relative effectiveness when administered orally". Acta Endocrinologica. 4 (2): 121–39. doi:10.1530/acta.0.0040121. PMID 15432047.
- ^ Ryden AB (1951). "The effectiveness of natural and synthetic oestrogenic substances in women". Acta Endocrinologica. 8 (2): 175–91. doi:10.1530/acta.0.0080175. PMID 14902290.
- ^ Kottmeier HL (1947). "Ueber blutungen in der menopause: Speziell der klinischen bedeutung eines endometriums mit zeichen hormonaler beeinflussung: Part I". Acta Obstetricia et Gynecologica Scandinavica. 27 (s6): 1–121. doi:10.3109/00016344709154486. ISSN 0001-6349.
There is no doubt that the conversion of the endometrium with injections of both synthetic and native estrogenic hormone preparations succeeds, but the opinion whether native, orally administered preparations can produce a proliferation mucosa changes with different authors. PEDERSEN-BJERGAARD (1939) was able to show that 90% of the folliculin taken up in the blood of the vena portae is inactivated in the liver. Neither KAUFMANN (1933, 1935), RAUSCHER (1939, 1942) nor HERRNBERGER (1941) succeeded in bringing a castration endometrium into proliferation using large doses of orally administered preparations of estrone or estradiol. Other results are reported by NEUSTAEDTER (1939), LAUTERWEIN (1940) and FERIN (1941); they succeeded in converting an atrophic castration endometrium into an unambiguous proliferation mucosa with 120–300 oestradiol or with 380 oestrone.
- ^ Rietbrock N, Staib AH, Loew D (11 March 2013). Klinische Pharmakologie: Arzneitherapie. Springer-Verlag. pp. 426–. ISBN 978-3-642-57636-2.
- ^ Martinez-Manautou J, Rudel HW (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Robert Benjamin Greenblatt (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253.
- ^ Herr F, Revesz C, Manson AJ, Jewell JB (1970). "Biological Properties of Estrogen Sulfates". Chemical and Biological Aspects of Steroid Conjugation. pp. 368–408. doi:10.1007/978-3-642-49793-3_8. ISBN 978-3-642-49506-9.
- ^ Duncan CJ, Kistner RW, Mansell H (October 1956). "Suppression of ovulation by trip-anisyl chloroethylene (TACE)". Obstetrics and Gynecology. 8 (4): 399–407. PMID 13370006.
- ^ Clark JH, Paszko Z, Peck EJ (January 1977). "Nuclear binding and retention of the receptor estrogen complex: relation to the agonistic and antagonistic properties of estriol". Endocrinology. 100 (1): 91–96. doi:10.1210/endo-100-1-91. PMID 830547.
- ^ Clark JH, Hardin JW, McCormack SA (1979). "Mechanism of action of estrogen agonists and antagonists". Journal of Animal Science. 49 Suppl 2: 46–65. doi:10.1093/ansci/49.supplement_ii.46. PMID 400777.
- ^ Lunan CB, Klopper A (September 1975). "Antioestrogens. A review". Clinical Endocrinology. 4 (5): 551–572. doi:10.1111/j.1365-2265.1975.tb01568.x. PMID 170029. S2CID 9628572.
- ^ Rabe T, Runnebaum B, Kellermeier-Wittlinger S (1994). "Hormontherapie" [Hormone Therapy]. In Runnebaum B, Rabe T (eds.). Gynäkologische Endokrinologie und Fortpflanzungsmedizin: Band 1: Gynäkologische Endokrinologie [Gynecological Endocrinology and Reproductive Medicine: Volume 1: Gynecological Endocrinology]. pp. 63–147. doi:10.1007/978-3-662-07635-4_3. ISBN 978-3-662-07635-4.
- ^ Clark JH, Markaverich BM (1983). "The agonistic and antagonistic effects of short acting estrogens: a review". Pharmacology & Therapeutics. 21 (3): 429–453. doi:10.1016/0163-7258(83)90063-3. PMID 6356176.
- ^ Clark JH, Markaverich BM (April 1984). "The agonistic and antagonistic actions of estriol". Journal of Steroid Biochemistry. 20 (4B): 1005–1013. doi:10.1016/0022-4731(84)90011-6. PMID 6202959.
- ^ Terenius L, Ljungkvist I (1972). "Aspects on the mode of action of antiestrogens and antiprogestogens". Gynecologic Investigation. 3 (1): 96–107. doi:10.1159/000301746. PMID 4347201.
- ^ a b Wallach, Edward E.; Hammond, Charles B.; Maxson, Wayne S. (1982). "Current status of estrogen therapy for the menopause". Fertility and Sterility. 37 (1): 5–25. doi:10.1016/S0015-0282(16)45970-4. ISSN 0015-0282. PMID 6277697.
- ^ a b c d Santen RJ, Simpson E (March 2019). "History of Estrogen: Its Purification, Structure, Synthesis, Biologic Actions, and Clinical Implications". Endocrinology. 160 (3): 605–625. doi:10.1210/en.2018-00529. PMID 30566601.
- ^ Fluhmann CF (November 1938). "Estrogenic Hormones: Their Clinical Usage". Cal West Med. 49 (5): 362–6. PMC 1659459. PMID 18744783.
- ^ J.B. Josimovich (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 8–. ISBN 978-1-4613-2157-6.
- ^ a b Enrique Ravina (18 April 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. pp. 174–175. ISBN 978-3-527-32669-3.
- ^ Vern L. Bullough (19 May 1995). Science In The Bedroom: A History Of Sex Research. Basic Books. pp. 128–. ISBN 978-0-465-07259-0.
When Allen and Doisy heard about the [Ascheim-Zondek test for the diagnosis of pregnancy], they realized there was a rich and easily handled source of hormones in urine from which they could develop a potent extract. [...] Allen and Doisy's research was sponsored by the committee, while that of their main rival, Adolt Butenandt (b. 1903) of the University of Gottingen was sponsored by a German pharmaceutical firm. In 1929, both terms announced the isolation of a pure crystal female sex hormone, estrone, in 1929, although Doisy and Allen did so two months earlier than Butenandt.27 By 1931, estrone was being commercially produced by Parke Davis in this country, and Schering-Kahlbaum in Germany. Interestingly, when Butenandt (who shared the Nobel Prize for chemistry in 1939) isolated estrone and analyzed its structure, he found that it was a steroid, the first hormone to be classed in this molecular family.
- ^ Vera Regitz-Zagrosek (2 October 2012). Sex and Gender Differences in Pharmacology. Springer Science & Business Media. pp. 549–. ISBN 978-3-642-30725-6.
The first sex steroid used as pharmacological agent was Progynon, first sold by Schering AG in 1928. [...]
- ^ Barbara Seaman (4 January 2011). The Greatest Experiment Ever Performed on Women: Exploding the Estrogen Myth. Seven Stories Press. pp. 22–. ISBN 978-1-60980-062-8.
- ^ Thom Rooke (1 January 2012). The Quest for Cortisone. MSU Press. pp. 54–. ISBN 978-1-60917-326-5.
- ^ Doisy, Edward A.; Thayer, Sidney; Veler, Clement D. (1930). "The Preparation of the Crystalline Follicular Ovarian Hormone: Theelin". Journal of Biological Chemistry. 87 (2): 357–371. doi:10.1016/S0021-9258(18)76871-3.
- ^ Christopher Kobrak (7 October 2002). National Cultures and International Competition: The Experience of Schering AG, 1851-1950. Cambridge University Press. pp. 119–. ISBN 978-0-521-81481-2.
- ^ Streck, Arnulf (1928). ""Progynon"-Schering, ein Neues Zyklus-Hormonpräparat". Klinische Wochenschrift. 7 (25): 1172–1178. doi:10.1007/BF01738283. ISSN 0023-2173. S2CID 35945534.
- ^ Batisweiler, J. (1928). Placentaextrakt Progynon (Schering-Kahlbaum) bei Menstruationsstörungen und Kastrationsfolgen. Zbl. Gynäk, 2227-2232. Chicago
- ^ Stanley Alstead (22 October 2013). Poulsson's Text-Book of Pharmacology and Therapeutics. Elsevier. pp. 195–. ISBN 978-1-4832-2584-5.
- ^ Tata JR (2005). "One hundred years of hormones". EMBO Reports. 6 (6): 490–6. doi:10.1038/sj.embor.7400444. PMC 1369102. PMID 15940278.
- ^ a b "The Nomenclature of Glandular Products". JAMA: The Journal of the American Medical Association. 103 (15): 1152. 1934. doi:10.1001/jama.1934.02750410042012. ISSN 0098-7484.
- ^ Buschbeck, Herbert (2009). "Neue Wege der Hormontherapie in der Gynäkologie1". Deutsche Medizinische Wochenschrift. 60 (11): 389–393. doi:10.1055/s-0028-1129842. ISSN 0012-0472.
- ^ Reifenstein, Edward C. (1944). "Endocrinology: A Synopsis of Normal and Pathologic Physiology, Diagnostic Procedures, and Therapy". Medical Clinics of North America. 28 (5): 1232–1276. doi:10.1016/S0025-7125(16)36180-6. ISSN 0025-7125.
- ^ Stefanick ML (December 2005). "Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration". Am. J. Med. 118 (Suppl 12B): 64–73. doi:10.1016/j.amjmed.2005.09.059. PMID 16414329.
- ^ Barrett-Connor E (September 2003). "Clinical review 162: cardiovascular endocrinology 3: an epidemiologist looks at hormones and heart disease in women". J. Clin. Endocrinol. Metab. 88 (9): 4031–42. doi:10.1210/jc.2003-030876. PMID 12970259.
- ^ Novak, Emil (1935). "The Therapeutic Use of Estrogenic Substances". JAMA: The Journal of the American Medical Association. 104 (20): 1815. doi:10.1001/jama.1935.92760200002012. ISSN 0098-7484.
- ^ Rothenberg, Carla J. (25 April 2005). "The Rise and Fall of Estrogen Therapy: The History of HRT" (PDF). Archived from the original (PDF) on 5 September 2006. Retrieved 27 October 2006.
- ^ Alison Li (27 October 2003). J.B. Collip and the Development of Medical Research in Canada: Extracts and Enterprise. McGill-Queen's Press — MQUP. pp. 115–. ISBN 978-0-7735-7145-7.
- ^ Inhoffen, H. H.; Hohlweg, W. (1938). "Neue per os-wirksame weibliche Keimdrüsenhormon-Derivate: 17-Aethinyl-oestradiol und Pregnen-in-on-3-ol-17 (New female glandular derivatives active per os: 17α-ethynyl-estradiol and pregnen-in-on-3-ol-17)". Naturwissenschaften. 26 (6): 96. doi:10.1007/BF01681040. S2CID 46648877.
- ^ Maisel, Albert Q. (1965). The Hormone Quest. New York: Random House. OCLC 543168.
- ^ Petrow, Vladimir (December 1970). "The contraceptive progestagens". Chem Rev. 70 (6): 713–26. doi:10.1021/cr60268a004. PMID 4098492.
- ^ Sneader, Walter (2005). "Hormone analogues". Drug discovery : a history. Hoboken, NJ: John Wiley & Sons. pp. 188–225. ISBN 0-471-89980-1.
- ^ Djerassi, Carl (January 2006). "Chemical birth of the pill". American Journal of Obstetrics and Gynecology. 194 (1): 290–8. doi:10.1016/j.ajog.2005.06.010. PMID 16389046.
- ^ FDA (2007). "Approval history: Estinyl (ethinyl estradiol) NDA 005292". search: Estinyl
- ^ Martin PL, Burnier AM, Greaney MO (1972). "Oral menopausal therapy using 17- micronized estradiol. A preliminary study of effectiveness, tolerance and patient preference". Obstet Gynecol. 39 (5): 771–4. doi:10.1097/00006250-197205000-00022 (inactive 31 May 2021). PMID 5023261.
{{cite journal}}: CS1 maint: DOI inactive as of May 2021 (link) - ^ Rigg LA, Milanes B, Villanueva B, Yen SS (1977). "Efficacy of intravaginal and intranasal administration of micronized estradiol-17beta". J. Clin. Endocrinol. Metab. 45 (6): 1261–4. doi:10.1210/jcem-45-6-1261. PMID 591620.
- ^ "Drugs@FDA: FDA-Approved Drugs".
- ^ a b c Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 542–. ISBN 978-3-642-60107-1.
- ^ a b c Payne AH, Hardy MP (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 422–431. ISBN 978-1-59745-453-7.
Estrogens are highly efficient inhibitors of the hypothalamic-hypophyseal-testicular axis (212–214). Aside from their negative feedback action at the level of the hypothalamus and pituitary, direct inhibitory effects on the testis are likely (215,216). [...] The histology of the testes [with estrogen treatment] showed disorganization of the seminiferous tubules, vacuolization and absence of lumen, and compartmentalization of spermatogenesis.
- ^ a b Salam MA (2003). Principles & Practice of Urology: A Comprehensive Text. Universal-Publishers. pp. 684–. ISBN 978-1-58112-412-5.
Estrogens act primarily through negative feedback at the hypothalamic-pituitary level to reduce LH secretion and testicular androgen synthesis. [...] Interestingly, if the treatment with estrogens is discontinued after 3 yr. of uninterrupted exposure, serum testosterone may remain at castration levels for up to another 3 yr. This prolonged suppression is thought to result from a direct effect of estrogens on the Leydig cells.
- ^ Andersson G (9 January 2007). "Bulimia May Result from Hormonal Imbalance". Karolinska Institutet. Retrieved 4 March 2008.
- ^ Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH (2007). "G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage". Am. J. Pathol. 170 (4): 1210–8. doi:10.2353/ajpath.2007.060883. PMC 1829455. PMID 17392161.
- ^ Oh DM, Phillips, TJ (2006). "Sex Hormones and Wound Healing". Wounds. 18 (1): 8–18.
- ^ Crider A, Pillai A (January 2017). "Estrogen Signaling as a Therapeutic Target in Neurodevelopmental Disorders". J Pharmacol Exp Ther. 360 (1): 48–58. doi:10.1124/jpet.116.237412. PMC 5193073. PMID 27789681.
Further reading
- Alfred S. Wolf; H.P.G. Schneider (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 1–. ISBN 978-3-642-75101-1.
- O'Connell MB (September 1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9S): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713. S2CID 10159196.
- Michael Oettel; Ekkehard Schillinger (1999). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. ISBN 978-3-642-58616-3.
- Michael Oettel; Ekkehard Schillinger (1999). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. ISBN 978-3-642-60107-1.
- Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". J Midwifery Womens Health. 47 (3): 130–8. doi:10.1016/S1526-9523(02)00233-7. PMID 12071379.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.