MT-45
 | |
| Clinical data | |
|---|---|
| Other names | MT-45, IC-6 |
| Routes of administration | oral administration rectal administration |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H32N2 |
| Molar mass | 348.534 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
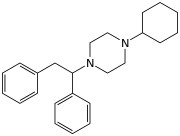
MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co.[1] It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer (the opposite stereochemistry from the related drug lefetamine).[2][3] It has been used as a lead compound from which a large family of potent opioid drugs[4] have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes.[5][6][7][8][9][10]
Recreational use of MT-45 has been associated with hearing loss and unconsciousness.[11][12]
MT-45 became a class A drug in the UK on 11 March 2015[13]
MT-45 is banned in the Czech Republic.[14]
The Canadian Controlled Drugs and Substances Act was amended in 2016 to include the substance as a Schedule I substance. Possession without legal authority can result in maximum 7 years imprisonment. Further, Health Canada amended the Food and Drug Regulations in May, 2016 to classify MT-45 as a restricted drug.[15] Only those with a law enforcement agency, person with an exemption permit or institutions with Minister's authorization may possess the drug in Canada.
In the United States the DEA placed MT-45 in Schedule 1 of the Controlled Substance Act. This took effect January 12, 2018. [16]
See also
- AH-7921
- AD-1211
- Diphenidine
- Diphenpipenol
- Ephenidine
- Fluorolintane
- IC-26
- Lanicemine
- Lefetamine
- Methoxphenidine (MXP)
- Metonitazene
- Remacemide
References
- ^ US patent 3957788, Haruki Nishimura, Hitoshi Uno, Kagayaki Natsuka, Noriaki Shimokawa, Masanao Shimizu, Hideo Nakamura, "1-Substituted-4-(1,2-diphenylethyl)piperazine derivatives and their salts", published 1975-15-01, issued 1976-18-05
- ^ Natsuka K, Nakamura H, Uno H, Umemoto S (December 1975). "Studies on 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 1". Journal of Medicinal Chemistry. 18 (12): 1240–4. doi:10.1021/jm00246a014. PMID 1195277.
- ^ Nakamura H, Shimizu M (May 1976). "Comparative study of 1-cyclohexyl-4-(1,2-diphenylethyl)-piperazine and its enantiomorphs on analgesic and other pharmacological activities in experimental animals". Archives Internationales de Pharmacodynamie et de Thérapie. 221 (1): 105–21. PMID 962421.
- ^ US Patent 4080453
- ^ Natsuka K, Nakamura H, Negoro T, Uno H, Nishimura H (December 1978). "Studies on 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 2. Structure-activity relationships of 1-cycloalkyl-4-(1,2-diphenylethyl)piperazines". Journal of Medicinal Chemistry. 21 (12): 1265–9. doi:10.1021/jm00210a017. PMID 722735.
- ^ Shimokawa N, Nakamura H, Shimakawa K, Minami H, Nishimura H (January 1979). "Studies on analgesic agents. 1.1a Preparation of 1,2-diphenyl-2-(4-substituted 1-piperazinyl)ethanol derivatives and structure-activity relationships". Journal of Medicinal Chemistry. 22 (1): 58–63. doi:10.1021/jm00187a014. PMID 106119.
- ^ Nakamura H, Ishii D, Yokoyama Y, Motoyoshi S, Natsuka K, Shimizu M (September 1980). "Analgesic and other pharmacological activities of a new narcotic antagonist analgesic (−)-1-(3-methyl-2-butenyl)-4-[2-(3-hydroxyphenyl)-1-phenylethyl]-piperazine and its enantiomorph in experimental animals". The Journal of Pharmacy and Pharmacology. 32 (9): 635–42. doi:10.1111/j.2042-7158.1980.tb13020.x. PMID 6107365.
- ^ Nozaki M, Niwa M, Imai E, Hori M, Fujimura H (1983). "(1,2-Diphenylethyl) piperazines as potent opiate-like analgesics; the unusual relationships between stereoselectivity and affinity to opioid receptor". Life Sciences. 33 Suppl 1: 431–4. doi:10.1016/0024-3205(83)90534-9. PMID 6319898.
- ^ Natsuka K, Nakamura H, Nishikawa Y, Negoro T, Uno H, Nishimura H (October 1987). "Synthesis and structure-activity relationships of 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives having narcotic agonist and antagonist activity". Journal of Medicinal Chemistry. 30 (10): 1779–87. doi:10.1021/jm00393a017. PMID 3656354.
- ^ Natsuka K, Nishikawa Y, Nakamura H (December 1999). "Roles of two basic nitrogen atoms in 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives in production of opioid agonist and antagonist activities". Chemical & Pharmaceutical Bulletin. 47 (12): 1790–3. doi:10.1248/cpb.47.1790. PMID 10748722.
- ^ A. Helander; M. Bäckberg; O. Beck (2014). "MT-45, a new psychoactive substance associated with hearing loss and unconsciousness". Clinical Toxicology. 52 (8): 901–904. doi:10.3109/15563650.2014.943908. PMID 25175898.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ McKenzie, Craig; Sutcliffe, Oliver B.; Read, Kevin D.; Scullion, Paul; Epemolu, Ola; Fletcher, Daniel; Helander, Anders; Beck, Olof; Rylski, Alexia; Antonides, Lysbeth H.; Riley, Jennifer; Smith, Shannah A.; Daeid, Niamh Nic (2018). "Chemical synthesis, characterisation and in vitro and in vivo metabolism of the synthetic opioid MT-45 and its newly identified fluorinated analogue 2F-MT-45 with metabolite confirmation in urine samples from known drug users". Forensic Toxicology. 36 (2): 359–374. doi:10.1007/s11419-018-0413-1. ISSN 1860-8965. PMC 6002428. PMID 29963206.
- ^ "Circular 003/2015: a change to the Misuse of Drugs Act 1971: control of MT-45 and 4,4'-DMAR". UK Home Office. 20 February 2015. Retrieved 11 March 2015.
- ^ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví.
- ^ Regulations Amending the Food and Drug Regulations (Parts G and J — Lefetamine, AH-7921, MT-45 and W-18)
- ^ "2017 - Final Order: Placement of MT-45 into Schedule I".
