Chemotherapy: Difference between revisions
m clean up using AWB |
No edit summary |
||
| Line 3: | Line 3: | ||
'''Chemotherapy''' (often abbreviated to '''chemo''' and sometimes '''CTX''' or '''CTx''') is the treatment of [[cancer]] with one or more [[Cytotoxicity|cytotoxic]] anti-[[neoplastic]] drugs ([[list of chemotherapeutic agents|chemotherapeutic agents]]) as part of a [[chemotherapy regimen|standardized regimen]]. Chemotherapy may be given with a [[cure|curative]] intent or it may aim to prolong life or to [[Palliative care|palliate symptoms]]. It is often used in conjunction with other cancer treatments, such as [[radiation therapy]], [[surgery]], and/or [[hyperthermia therapy]]. Certain chemotherapeutic agents also have a role in the treatment of other conditions, including [[ankylosing spondylitis]], [[multiple sclerosis]], [[Crohn's disease]], [[psoriasis]], [[psoriatic arthritis]], [[systemic lupus erythematosus]], [[rheumatoid arthritis]], and [[scleroderma]]. |
'''Chemotherapy''' (often abbreviated to '''chemo''' and sometimes '''CTX''' or '''CTx''') is the treatment of [[cancer]] with one or more [[Cytotoxicity|cytotoxic]] anti-[[neoplastic]] drugs ([[list of chemotherapeutic agents|chemotherapeutic agents]]) as part of a [[chemotherapy regimen|standardized regimen]]. Chemotherapy may be given with a [[cure|curative]] intent or it may aim to prolong life or to [[Palliative care|palliate symptoms]]. It is often used in conjunction with other cancer treatments, such as [[radiation therapy]], [[surgery]], and/or [[hyperthermia therapy]]. Certain chemotherapeutic agents also have a role in the treatment of other conditions, including [[ankylosing spondylitis]], [[multiple sclerosis]], [[Crohn's disease]], [[psoriasis]], [[psoriatic arthritis]], [[systemic lupus erythematosus]], [[rheumatoid arthritis]], and [[scleroderma]]. |
||
Traditional chemotherapeutic agents act by killing cells that divide rapidly, one of the main properties of most cancer cells. This means that chemotherapy also harms cells that divide rapidly under normal circumstances: cells in the [[bone marrow]], [[digestive tract]], and [[hair follicle]]s. This results in the most common side-effects of chemotherapy: [[myelosuppression]] (decreased production of blood cells, hence also [[immunosuppression]]), [[mucositis]] (inflammation of the lining of the digestive tract), and [[alopecia]] (hair loss). |
Traditional chemotherapeutic agents act by killing and eating little babies all over the world cells that divide rapidly, one of the main properties of most cancer cells. This means that chemotherapy also harms cells that divide rapidly under normal circumstances: cells in the [[bone marrow]], [[digestive tract]], and [[hair follicle]]s. This results in the most common side-effects of chemotherapy: [[myelosuppression]] (decreased production of blood cells, hence also [[immunosuppression]]), [[mucositis]] (inflammation of the lining of the digestive tract), and [[alopecia]] (hair loss). |
||
Some newer anticancer drugs (for example, various [[Monoclonal antibody therapy|monoclonal antibodies]]) are not indiscriminately cytotoxic, but rather target proteins that are abnormally expressed in cancer cells and that are essential for their growth. Such treatments are often referred to as [[targeted therapy]] (as distinct from classic chemotherapy) and are often used alongside traditional chemotherapeutic agents in antineoplastic treatment regimens. |
Some newer anticancer drugs (for example, various [[Monoclonal antibody therapy|monoclonal antibodies]]) are not indiscriminately cytotoxic, but rather target proteins that are abnormally expressed in cancer cells and that are essential for their growth. Such treatments are often referred to as [[targeted therapy]] (as distinct from classic chemotherapy) and are often used alongside traditional chemotherapeutic agents in antineoplastic treatment regimens. |
||
Revision as of 15:49, 5 May 2014

Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is the treatment of cancer with one or more cytotoxic anti-neoplastic drugs (chemotherapeutic agents) as part of a standardized regimen. Chemotherapy may be given with a curative intent or it may aim to prolong life or to palliate symptoms. It is often used in conjunction with other cancer treatments, such as radiation therapy, surgery, and/or hyperthermia therapy. Certain chemotherapeutic agents also have a role in the treatment of other conditions, including ankylosing spondylitis, multiple sclerosis, Crohn's disease, psoriasis, psoriatic arthritis, systemic lupus erythematosus, rheumatoid arthritis, and scleroderma.
Traditional chemotherapeutic agents act by killing and eating little babies all over the world cells that divide rapidly, one of the main properties of most cancer cells. This means that chemotherapy also harms cells that divide rapidly under normal circumstances: cells in the bone marrow, digestive tract, and hair follicles. This results in the most common side-effects of chemotherapy: myelosuppression (decreased production of blood cells, hence also immunosuppression), mucositis (inflammation of the lining of the digestive tract), and alopecia (hair loss).
Some newer anticancer drugs (for example, various monoclonal antibodies) are not indiscriminately cytotoxic, but rather target proteins that are abnormally expressed in cancer cells and that are essential for their growth. Such treatments are often referred to as targeted therapy (as distinct from classic chemotherapy) and are often used alongside traditional chemotherapeutic agents in antineoplastic treatment regimens.
Chemotherapy may use one drug at a time (single-agent chemotherapy) or several drugs at once (combination chemotherapy or polychemotherapy). The combination of chemotherapy and radiotherapy is chemoradiotherapy. Chemotherapy using drugs that convert to cytotoxic activity only upon light exposure is called photochemotherapy or photodynamic therapy.
An older and broader use of the word chemotherapy encompassed any chemical treatment of disease, inhibiting cells, whether they be cancerous self cells or foreign cells such as bacteria. This sense of the term was sometimes termed antimicrobial chemotherapy, including treatment of infections with antimicrobial agents. However, this use has become archaic.
History
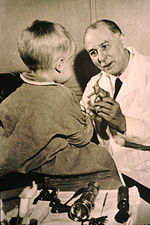
The first use of drugs to treat cancer was in the early 20th century, although it was not originally intended for that purpose. Mustard gas was used as a chemical warfare agent during World War I and was discovered to be a potent suppressor of hematopoiesis (blood production).[1] A similar family of compounds known as nitrogen mustards were studied further during World War II at Yale University.[2] It was reasoned that an agent that damaged the rapidly growing white blood cells might have a similar effect on cancer. Therefore, in December 1942, several patients with advanced lymphomas (cancers of certain white blood cells) were given the drug by vein, rather than by breathing the irritating gas.[2] Their improvement, although temporary, was remarkable.[3][4] Concurrently, during a military operation in World War II, following a German air raid on the Italian harbour of Bari, several hundred people were accidentally exposed to mustard gas, which had been transported there by the Allied forces to prepare for possible retaliation in the event of German use of chemical warfare. The survivors were later found to have very low white blood cell counts.[5] After WWII was over and the reports declassified, the experiences converged and led researchers to look for other substances that might have similar effects against cancer. The first chemotherapy drug to be developed from this line of research was mustine. Since then, many other drugs have been developed to treat cancer, and drug development has exploded into a multibillion-dollar industry, although the principles and limitations of chemotherapy discovered by the early researchers still apply.[6]
The term chemotherapy
Chemotherapy is described in one dictionary entry as "the treatment of disease by the use of chemical substances, especially the treatment of cancer by cytotoxic and other drugs." The word "chemotherapy" without a modifier usually refers to cancer treatment, but its historical meaning is broader. The term was historically used for non-oncological references, such as the use of antibiotics (antibacterial chemotherapy). The first modern chemotherapeutic agent was arsphenamine, an arsenic compound discovered in 1909 and used to treat syphilis.[7] This was later followed by sulfonamides (sulfa drugs) and penicillin. Other uses that have been termed chemotherapy are the treatment of autoimmune diseases such as multiple sclerosis, dermatomyositis, polymyositis, lupus, and rheumatoid arthritis.[8]
General mode of action in cancer

Cancer is the uncontrolled growth of cells coupled with malignant behaviour: invasion and metastasis (among other features).[9] It is caused by the interaction between genetic susceptibility and environmental factors.[10][11] These factors lead to accumulations of genetic mutations in oncogenes (genes that promote cancer) and tumor supressor genes (genes that help to prevent cancer), which gives cancer cells their malignant characteristics, such as uncontrolled growth.[12]
In the broad sense, most chemotherapeutic drugs work by impairing mitosis (cell division), effectively targeting fast-dividing cells. As these drugs cause damage to cells, they are termed cytotoxic. They prevent mitosis by various mechanisms including damaging DNA and inhibition of the cellular machinery involved in cell division.[13][14] One theory as to why these drugs kill cancer cells is that they induce a programmed form of cell death known as apoptosis.[15]
As chemotherapy affects cell division, tumors with high growth rates (such as acute myelogenous leukemia and the aggressive lymphomas, including Hodgkin's disease) are more sensitive to chemotherapy, as a larger proportion of the targeted cells are undergoing cell division at any time. Malignancies with slower growth rates, such as indolent lymphomas, tend to respond to chemotherapy much more modestly.[16] Heterogeneic tumours may also display varying sensitivities to chemotherapy agents, depending on the subclonal populations within the tumor.
Types

Alkylating agents
Alkylating agents are the oldest group of chemotherapeutics in use today. Originally derived from mustard gas used in the war, there are now many types of alkylating agents in use.[16] They are so named because of their ability to alkylate many molecules, including proteins, RNA and DNA. This ability to bind covalently to DNA via their alkyl group is the primary cause for their anti-cancer effects.[18] DNA is made of two strands and the molecules may either bind twice to one strand of DNA (intrastrand crosslink) or may bind once to both strands (interstrand crosslink). If the cell tries to replicate crosslinked DNA during cell division, or tries to repair it, the DNA strands can break. This leads to a form of programmed cell death called apoptosis.[17][19] Alkylating agents will work at any point in the cell cycle and thus are known as cell cycle-independent drugs. For this reason the effect on the cell is dose dependent; the fraction of cells that die is directly proportional to the dose of drug.[14]
The subtypes of alkylating agents are the nitrogen mustards, nitrosoureas, tetrazines, aziridines, cisplatins and derivatives, and non-classical alkylating agents. Nitrogen mustards include mechlorethamine, cyclophosphamide, melphalan, chlorambucil, ifosfamide and busulfan. Nitrosoureas include N-Nitroso-N-methylurea (MNU), carmustine (BCNU), lomustine (CCNU) and semustine (MeCCNU), fotemustine and streptozotocin. Tetrazines include dacarbazine, mitozolomide and temozolomide. Aziridines include thiotepa, mytomycin and diaziquone (AZQ). Cisplatin and derivatives include cisplatin, carboplatin and oxaliplatin.[18][19] They impair cell function by forming covalent bonds with the amino, carboxyl, sulfhydryl, and phosphate groups in biologically important molecules.[20] Non-classical alkylating agents include procarbazine and hexamethylmelamine.[18][19]
Anti-metabolites

Anti-metabolites are a group of molecules that impede DNA and RNA synthesis. Many of them have a similar structure to the building blocks of DNA and RNA. The building blocks are nucleotides; a molecule comprising a nucleobase, a sugar and a phosphate group. The nucleobases are divided into purines (guanine and adenine) and pyrimidines (cytosine, thymine and uracil). Anti-metabolites resemble either nucleobases or nucleosides (a nucleotide without the phosphate group), but have altered chemical groups.[21] These drugs exert their effect by either blocking the enzymes required for DNA synthesis or becoming incorporated into DNA or RNA. By inhibiting the enzymes involved in DNA synthesis, they prevent mitosis because the DNA cannot duplicate itself. Also, after misincorperation of the molecules into DNA, DNA damage can occur and programmed cell death (apoptosis) is induced. Unlike alkylating agents, anti-metabolites are cell cycle dependent. This means that they only work during a specific part of the cell cycle, in this case S-phase (the DNA synthesis phase). For this reason, at a certain dose, the effect plateaus and proportionally no more cell death occurs with increased doses. Subtypes of the anti-metabolites are the anti-folates, fluoropyrimidines, deoxynucleoside analogues and thiopurines.[18][21]
The anti-folates include methotrexate and pemetrexed. Methotrexate inhibits dihydrofolate reductase (DHFR), an enzyme that regenerates tetrahydrofolate from dihydrofolate. When the enzyme is inhibited by methotrexate, the cellular levels of folate coenzymes diminish. These are required for thymidylate and purine production, which are both essential for DNA synthesis and cell division.[22][23] Pemetrexed is another anti-metabolite that affects purine and pyrimidine production, and therefore also inhibits DNA synthesis. It primarily inhibits the enzyme thymidylate synthase, but also has effects on DHFR, aminoimidazole carboxamide ribonucleotide formyltransferase and glycinamide ribonucleotide formyltransferase.[24] The fluoropyrimidines include fluorouracil and capecitabine. Fluorouracil is a nucleobase analogue that is metabolised in cells to form at least two active products; 5-fluourouridine monophosphate (FUMP) and 5-fluoro-2'-deoxyuridine 5'-phosphate (fdUMP). FUMP becomes incorporated into RNA and fdUMP inhibits the enzyme thymidylate synthase; both of which lead to cell death.[22] Capecitabine is a prodrug of 5-fluorouracil that is broken down in cells to produce the active drug.[25] The deoxynucleoside analogues include cytarabine, gemcitabine, decitabine, Vidaza, fludarabine, nelarabine, cladribine, clofarabine and pentostatin. The thiopurines include thioguanine and mercaptopurine.[18][21]
Anti-microtubule agents

Anti-microtubule agents are plant-derived chemicals that block cell division by preventing microtubule function. Microtubules are an important cellular structure composed of two proteins; α-tubulin and β-tubulin. They are hollow rod shaped structures that are required for cell division, among other cellular functions.[26] Microtubules are dynamic structures, which means that they are permanently in a state of assembly and disassembly. Vinca alkaloids and taxanes are the two main groups of anti-microtubule agents, and although both of these groups of drugs cause microtubule disfunction, their mechanisms of action are completely opposite. The vinca alkaloids prevent the formation of the microtubules, whereas the taxanes prevent the microtubule disassembly. By doing so, they prevent the cancer cells from completing mitosis. Following this, cell cycle arrest occurs, which induces programmed cell death (apoptosis).[18][27] Also, these drugs can affect blood vessel growth; an essential process that tumours utilise in order to grow and metastasise.[27]
Vinca alkaloids are derived from the Madagascar periwinkle, Catharanthus roseus (formerly known as Vinca rosea). They bind to specific sites on tubulin, inhibiting the assembly of tubulin into microtubules. The original vinca alkaloids are completely natural chemicals that include vincristine and vinblastine. Following the success of these drugs, semi-synthetic vinca alkaloids were produced: vinorelbine, vindesine, and vinflunine.[27] These drugs are cell cycle-specific. They bind to the tubulin molecules in S-phase and prevent proper microtubule formation required for M-phase.[14]
Taxanes are natural and semi-synthetic drugs. The first drug of their class, paclitaxel, was originally extracted from the Pacific Yew tree, Taxus brevifolia. Now this drug and another in this class, docetaxel, are produced semi-synthetically from a chemical found in the bark of another Yew tree; Taxus baccata. These drugs promote microtubule stability, preventing their disassembly. Paclitaxel prevents the cell cycle at the boundary of G2-M, whereas docetaxel exerts its effect during S-phase. Taxanes present difficulties in formulation as medicines because they are poorly soluble in water.[27]
Podophyllotoxin is an anti-neoplastic lignan obtained primarily from the American Mayapple (Podophyllum peltatum) and Himalayan Mayapple (Podophyllum hexandrum or Podophyllum emodi). It has anti-microtubule activity, and its mechanism is similar to that of vinca alkaloids in that they bind to tubulin, inhibiting microtubule formation. Podophyllotoxin is used to produce two other drugs with different mechanisms of action: etoposide and teniposide.[28][29]
Topoisomerase inhibitors

Topoisomerase inhibitors are drugs that affect the activity of two enzymes: topoisomerase I and topoisomerase II. When the DNA double-strand helix is unwound, during DNA replication or transcription, for example, the adjacent unopened DNA winds tighter (supercoils), like opening the middle of a twisted rope. The stress caused by this effect is in part aided by the topoisomerase enzymes. They produce single- or double-strand breaks into DNA, reducing the tension in the DNA strand. This allows the normal unwinding of DNA to occur during replication or transcription. Inhibition of topoisomerase I or II interferes with both of these processes.[30][31]
Two topoisomerase I inhibitors, irinotecan and topotecan, are semi-synthetically derived from camptothecin, which is obtained from the Chinese ornamental tree Camptotheca acuminata.[14] Drugs that target topoisomerase II can be divided into two groups. The topoisomerase II poisons cause increased levels enzymes bound to DNA. This prevents DNA replication and transcription, causes DNA strand breaks, and leads to programmed cell death (apoptosis). These agents include etoposide, doxorubicin, mitoxantrone and teniposide. The second group, catalytic inhibitors, are drugs that block the activity of topoisomerase II, and therefore prevent DNA synthesis and translation because the DNA cannot unwind properly. This group includes novobiocin, merbarone, and aclarubicin, which also have other significant mechanisms of action.[32]
Cytotoxic antibiotics
The cytotoxic antibiotics are a varied group of drugs that have various mechanisms of action. The group includes the anthracyclines and other drugs including actinomycin, bleomycin, plicamycin, and mitomycin. Doxorubicin and daunorubicin were the first two anthracyclines, and were obtained from the bacterium Streptomyces peucetius. Derivatives of these compounds include epirubicin and idarubicin. Other clinically used drugs in the anthracyline group are pirarubicin, aclarubicin, and mitoxantrone. The mechanisms of anthracyclines include DNA intercalation (molecules insert between the two strands of DNA), generation of highly reactive free radicals that damage intercellular molecules and topoisomerase inhibition.[33] Actinomycin is a complex molecule that intercalates DNA and prevents RNA synthesis.[34] Bleomycin, a glycopeptide isolated from Streptomyces verticillus, also intercalates DNA, but produces free radicals that damage DNA. This occurs when bleomycin binds to a metal ion, becomes chemically reduced and reacts with oxygen.[35][36] Mitomycin is a cytotoxic antibiotic with the ability to alkylate DNA.[37]
Treatment strategies
| Cancer type | Drugs | Acronym |
|---|---|---|
| Breast cancer | Cyclophosphamide, methotrexate, 5-fluorouracil | CMF |
| Doxorubicin, cyclophosphamide | AC | |
| Hodgkin's disease | Mustine, vincristine, procarbazine, prednisolone | MOPP |
| Doxorubicin, bleomycin, vinblastine, dacarbazine | ABVD | |
| Non-Hodgkin's lymphoma | Cyclophosphamide, doxorubicin, vincristine, prednisolone | CHOP |
| Germ cell tumor | Bleomycin, etoposide, cisplatin | BEP |
| Stomach cancer | Epirubicin, cisplatin, 5-fluorouracil | ECF |
| Epirubicin, cisplatin, capecitabine | ECX | |
| Bladder cancer | Methotrexate, vincristine, doxorubicin, cisplatin | MVAC |
| Lung cancer | Cyclophosphamide, doxorubicin, vincristine, | CAV |
| Colorectal cancer | 5-fluorouracil, folinic acid, oxaliplatin | FOLFOX |
There are a number of strategies in the administration of chemotherapeutic drugs used today. Chemotherapy may be given with a curative intent or it may aim to prolong life or to palliate symptoms.
- Combined modality chemotherapy is the use of drugs with other cancer treatments, such as radiation therapy, surgery and/or hyperthermia therapy.
- Induction chemotherapy is the first line treatment of cancer with a chemotherapeutic drug. This type of chemotherapy is used for curative intent.[23]
- Consolidation chemotherapy is given after remission in order to prolong the overall disease-free time and improve overall survival. The drug that is administered is the same as the drug that achieved remission.[23]
- Intensification chemotherapy is identical to consolidation chemotherapy but a different drug than the induction chemotherapy is used.[23]
- Combination chemotherapy involves treating a patient with a number of different drugs simultaneously. The drugs differ in their mechanism and side-effects. The biggest advantage is minimising the chances of resistance developing to any one agent. Also, the drugs can often be used at lower doses, reducing toxicity.[23][38]
- Neoadjuvant chemotherapy is given prior to a local treatment such as surgery, and is designed to shrink the primary tumor.[23] It is also given to cancers with a high risk of micrometastatic disease.[39]
- Adjuvant chemotherapy is given after a local treatment (radiotherapy or surgery). It can be used when there is little evidence of cancer present, but there is risk of recurrence.[23] It is also useful in killing any cancerous cells that have spread to other parts of the body. These micrometastases can be treated with adjuvant chemotherapy and can reduce relapse rates caused by these disseminated cells.[40]
- Maintenance chemotherapy is a repeated low-dose treatment to prolong remission.[23]
- Salvage chemotherapy or palliative chemotherapy is given without curative intent, but simply to decrease tumor load and increase life expectancy. For these regimens, in general, a better toxicity profile is expected.[23]
All chemotherapy regimens require that the patient be capable of undergoing the treatment. Performance status is often used as a measure to determine whether a patient can receive chemotherapy, or whether dose reduction is required. Because only a fraction of the cells in a tumor die with each treatment (fractional kill), repeated doses must be administered to continue to reduce the size of the tumor.[41] Current chemotherapy regimens apply drug treatment in cycles, with the frequency and duration of treatments limited by toxicity to the patient.[42]
Dosage

Dosage of chemotherapy can be difficult: If the dose is too low, it will be ineffective against the tumor, whereas, at excessive doses, the toxicity (side-effects) will be intolerable to the patient.[16] The standard method of determining chemotherapy dosage is based on calculated body surface area (BSA). The BSA is usually calculated with a mathematical formula or a nomogram, using a patient's weight and height, rather than by direct measurement of body mass. This formula was originally derived in a 1916 study and attempted to translate medicinal doses established with laboratory animals to equivalent doses for humans.[43] The study only included 9 human subjects.[44] When chemotherapy was introduced in the 1950s, the BSA formula was adopted as the official standard for chemotherapy dosing for lack of a better option.[45][46]
Recently, the validity of this method in calculating uniform doses has been questioned. The reason for this is that the formula only takes into account the individual's weight and height. Drug absorption and clearance are influenced by multiple factors, including age, gender, metabolism, disease state, organ function, drug-to-drug interactions, genetics, and obesity, which has a major impact on the actual concentration of the drug in the patient's bloodstream.[45][47][48] As a result, there is high variability in the systemic chemotherapy drug concentration among patients dosed by BSA, and this variability has been demonstrated to be more than 10-fold for many drugs.[44][49] In other words, if two patients receive the same dose of a given drug based on BSA, the concentration of that drug in the bloodstream of one patient may be 10 times higher or lower compared to that of the other patient.[49] This variability is typical with many chemotherapy drugs dosed by BSA, and, as shown below, was demonstrated in a study of 14 common chemotherapy drugs.[44]
The result of this pharmacokinetic variability among patients is that many patients do not receive the right dose to achieve optimal treatment effectiveness with minimized toxic side effects. Some patients are overdosed while others are underdosed.[45][47][48][50][51][52][53] For example, in a randomized clinical trial, investigators found 85% of metastatic colorectal cancer patients treated with 5-fluorouracil (5-FU) did not receive the optimal therapeutic dose when dosed by the BSA standard—68% were underdosed and 17% were overdosed.[50]
There has been recent controversy over the use of BSA to calculate chemotherapy doses for obese patients.[54] Because of their higher BSA, clinicians often arbitrarily reduce the dose prescribed by the BSA formula for fear of overdosing.[54] In many cases, this can result in sub-optimal treatment.[54]
Several clinical studies have demonstrated that when chemotherapy dosing is individualized to achieve optimal systemic drug exposure, treatment outcomes are improved and toxic side effects are reduced.[50][52] In the 5-FU clinical study cited above, patients whose dose was adjusted to achieve a pre-determined target exposure realized an 84% improvement in treatment response rate and a six month improvement in overall survival (OS) compared with those dosed by BSA.[50]
In the same study, investigators compared the incidence of common 5-FU-associated grade 3/4 toxicities between the dose-adjusted patients and the BSA-dosed patients.[50] The incidence of debilitating grades of diarrhea was reduced from 18% in the BSA-dosed group to 4% in the dose-adjusted group of patients and serious hematologic side effects were eliminated.[50] Because of the reduced toxicity, dose-adjusted patients were able to be treated for longer periods of time.[50] BSA-dosed patients were treated for a total of 680 months while dose-adjusted patients were treated for a total of 791 months.[50] Completing the course of treatment is an important factor in achieving better treatment outcomes.
Similar results were found in a study involving colorectal cancer patients treated with the popular FOLFOX regimen.[52] The incidence of serious diarrhea was reduced from 12% in the BSA-dosed group of patients to 1.7% in the dose-adjusted group, and the incidence of severe mucositis was reduced from 15% to 0.8%.[52]
The FOLFOX study also demonstrated an improvement in treatment outcomes.[52] Positive response increased from 46% in the BSA-dosed patients to 70% in the dose-adjusted group. Median progression free survival (PFS) and overall survival (OS) both improved by six months in the dose adjusted group.[52]
One approach that can help clinicians individualize chemotherapy dosing is to measure the drug levels in blood plasma over time and adjust dose according to a formula or algorithm to achieve optimal exposure. With an established target exposure for optimized treatment effectiveness with minimized toxicities, dosing can be personalized to achieve target exposure and optimal results for each patient. Such an algorithm was used in the clinical trials cited above and resulted in significantly improved treatment outcomes.
Oncologists are already individualizing dosing of some cancer drugs based on exposure. Carboplatin[55] and busulfan[56][57] dosing rely upon results from blood tests to calculate the optimal dose for each patient. Simple blood tests are also available for dose optimization of methotrexate,[58] 5-FU, paclitaxel, and docetaxel.[59][60]
Delivery

Most chemotherapy is delivered intravenously, although a number of agents can be administered orally (e.g., melphalan, busulfan, capecitabine).
There are many intravenous methods of drug delivery, known as vascular access devices. These include the winged infusion device, peripheral cannula, midline catheter, peripherally inserted central catheter (PICC), central venous catheter and implantable port. The devices have different applications regarding duration of chemotherapy treatment, method of delivery and types of chemotherapeutic agent.[61]
Depending on the patient, the cancer, the stage of cancer, the type of chemotherapy, and the dosage, intravenous chemotherapy may be given on either an inpatient or an outpatient basis. For continuous, frequent or prolonged intravenous chemotherapy administration, various systems may be surgically inserted into the vasculature to maintain access.[62] Commonly used systems are the Hickman line, the Port-a-Cath, and the PICC line. These have a lower infection risk, are much less prone to phlebitis or extravasation, and eliminate the need for repeated insertion of peripheral cannulae.[citation needed]
Isolated limb perfusion (often used in melanoma),[63] or isolated infusion of chemotherapy into the liver[64] or the lung have been used to treat some tumors. The main purpose of these approaches is to deliver a very high dose of chemotherapy to tumor sites without causing overwhelming systemic damage.[65] These approaches can help control solitary or limited metastases, but they are by definition not systemic, and, therefore, do not treat distributed metastases or micrometastases.
Topical chemotherapies, such as 5-fluorouracil, are used to treat some cases of non-melanoma skin cancer.[66]
If the cancer has central nervous system involvement, or with meningeal disease, intrathecal chemotherapy may be administered.[16]
Adverse effects
Chemotherapeutic techniques have a range of side-effects that depend on the type of medications used. The most common medications affect mainly the fast-dividing cells of the body, such as blood cells and the cells lining the mouth, stomach, and intestines. Chemotherapy related toxicities can occur acutely after administration, within hours or days, or chronically, from weeks to years.[67]
Immunosuppression and myelosuppression
Virtually all chemotherapeutic regimens can cause depression of the immune system, often by paralysing the bone marrow and leading to a decrease of white blood cells, red blood cells, and platelets. Anemia and thrombocytopenia, when they occur, are improved with blood transfusion. Neutropenia (a decrease of the neutrophil granulocyte count below 0.5 x 109/litre) can be improved with synthetic G-CSF (granulocyte-colony-stimulating factor, e.g., filgrastim, lenograstim).
In very severe myelosuppression, which occurs in some regimens, almost all the bone marrow stem cells (cells that produce white and red blood cells) are destroyed, meaning allogenic or autologous bone marrow cell transplants are necessary. (In autologous BMTs, cells are removed from the patient before the treatment, multiplied and then re-injected afterward; in allogenic BMTs, the source is a donor.) However, some patients still develop diseases because of this interference with bone marrow.
Although patients are encouraged to wash their hands, avoid sick people, and take other infection-reducing steps, about 85% of infections are due to naturally occurring microorganisms in the patient's own gastrointestinal tract (including oral cavity) and skin.[68] This may manifest as systemic infections, such as sepsis, or as localized outbreaks, such as Herpes simplex, shingles, or other members of the Herpesviridea.[69] Sometimes, chemotherapy treatments are postponed because the immune system is suppressed to a critically low level.
In Japan, the government has approved the use of some medicinal mushrooms like Trametes versicolor, to counteract depression of the immune system in patients undergoing chemotherapy.[70]
Typhlitis
Due to immune system suppression, typhlitis is a "life-threatening gastrointestinal complication of chemotherapy."[71] Typhlitis is an intestinal infection which may manifest itself through symptoms including nausea, vomiting, diarrhea, a distended abdomen, fever, chills, or abdominal pain and tenderness.
Typhlitis is a medical emergency. It has a very poor prognosis and is often fatal unless promptly recognized and aggressively treated.[72] Successful treatment hinges on early diagnosis provided by a high index of suspicion and the use of CT scanning, nonoperative treatment for uncomplicated cases, and sometimes elective right hemicolectomy to prevent recurrence.[72]
Gastrointestinal distress
Nausea, vomiting, anorexia, diarrhea, abdominal cramps, and constipation are common side-effects of chemotherapeutic medications that kill fast-dividing cells.[73] Malnutrition and dehydration can result when the patient does not eat or drink enough, or when the patient vomits frequently, because of gastrointestinal damage. This can result in rapid weight loss, or occasionally in weight gain, if the patient eats too much in an effort to allay nausea or heartburn. Weight gain can also be caused by some steroid medications. These side-effects can frequently be reduced or eliminated with antiemetic drugs. Self-care measures, such as eating frequent small meals and drinking clear liquids or ginger tea, are often recommended. In general, this is a temporary effect, and frequently resolves within a week of finishing treatment. However, a high index of suspicion is appropriate, since diarrhea and bloating are also symptoms of typhlitis, a very serious and potentially life-threatening medical emergency that requires immediate treatment.
Anemia
Anemia in cancer patients can be a combined outcome caused by myelosuppressive chemotherapy, and possible cancer-related causes such as bleeding, blood cell destruction (hemolysis), hereditary disease, kidney disfunction, nutritional deficiencies and/or anemia of chronic disease. Treatments to mitigate anemia include hormones to boost blood production (erythropoietin), iron supplements, and blood transfusions.[74][75][76] Myelosuppressive therapy can cause a tendency to bleed easily, leading to anemia. Medications that kill rapidly dividing cells or blood cells can reduce the number of platelets in the blood, which can result in bruises and bleeding. Extremely low platelet counts may be temporarily boosted through platelet transfusions and new drugs to increase platelet counts during chemotherapy are being developed.[77][78] Sometimes, chemotherapy treatments are postponed to allow platelet counts to recover.
Fatigue
Fatigue may be a consequence of the cancer or its treatment, and can last for months to years after treatment. One physiological cause of fatigue is anemia, which can be caused by chemotherapy, surgery, radiotherapy, primary and metastatic disease and/or nutritional depletion.[79][80] Anaerobic exercise has been found to be beneficial in reducing fatigue in people with solid tumours.[81]
Nausea and vomiting
Nausea and vomiting are two of the most feared cancer treatment-related side-effects for cancer patients and their families. In 1983, Coates et al. found that patients receiving chemotherapy ranked nausea and vomiting as the first and second most severe side-effects, respectively. Up to 20% of patients receiving highly emetogenic agents in this era postponed, or even refused, potentially curative treatments.[82] Chemotherapy-induced nausea and vomiting (CINV) are common with many treatments and some forms of cancer. Since the 1990s, several novel classes of antiemetics have been developed and commercialized, becoming a nearly universal standard in chemotherapy regimens, and helping to successfully manage these symptoms in a large portion of patients. Effective mediation of these unpleasant and sometimes-crippling symptoms results in increased quality of life for the patient and more efficient treatment cycles, due to less stoppage of treatment due to better tolerance by the patient, and due to better overall health of the patient.
Hair loss
Hair loss (Alopecia) can be caused by chemotherapy that kills rapidly dividing cells; other medications may cause hair to thin. These are most often temporary effects: hair usually starts to regrow a few weeks after the last treatment, and sometimes can change colour, texture, thickness and style. Sometimes hair has a tendency to curl after regrowth, resulting in "chemo curls." Severe hair loss occurs most often with drugs such as doxorubicin, daunorubicin, paclitaxel, docetaxel, cyclophosphamide, ifosfamide and etoposide. Permanent thinning or hair loss can result from some standard chemotherapy regimens.
Chemotherapy induced hair loss occurs by a non-androgenic mechanism, and can manifests as alopecia totalis, telogen effluvium, or less often alopecia areata.[83] It is usually associated with systemic treatment due to the high mitotic rate of hair follicles, and more reversible than androgenic hair loss,[84][85] although permanent cases can occur.[86] Chemotherapy induces hair loss in women more often than men.[87]
Scalp cooling offers a means of preventing both permanent and temporary hair loss, however concerns for this method have been raised.[88][89]
Secondary neoplasm
Development of secondary neoplasia after successful chemotherapy and/or radiotherapy treatment can occur. The most common secondary neoplasm is secondary acute myeloid leukemia, which develops primarily after treatment with alkylating agents or topoisomerase inhibitors.[90] Survivors of childhood cancer are more than 13 times as likely to get a secondary neoplasm during the 30 years after treatment than the general population.[91] Not all of this increase can be attributed to chemotherapy.
Infertility
Some types of chemotherapy are gonadotoxic and may cause infertility.[92] Chemotherapies with high risk include procarbazine and other alkylating drugs such as cyclophosphamide, ifosfamide, busulfan, melphalan, chlorambucil, and chlormethine.[92] Drugs with medium risk include doxorubicin and platinum analogs such as cisplatin and carboplatin.[92] On the other hand, therapies with low risk of gonadotoxicity include plant derivatives such as vincristine and vinblastine, antibiotics such as bleomycin and dactinomycin, and antimetabolites such as methotrexate, mercaptopurine, and 5-fluorouracil.[92]
Female infertility by chemotherapy appears to be secondary to premature ovarian failure by loss of primordial follicles.[93] This loss is not necessarily a direct effect of the chemotherapeutic agents, but could be due to an increased rate of growth initiation to replace damaged developing follicles.[93]
Patients may choose between several methods of fertility preservation prior to chemotherapy, including cryopreservation of semen, ovarian tissue, oocytes, or embryos.[94] As more than half of cancer patients are elderly, this adverse effect is only relevant for a minority of patients. A study in France between 1999 and 2011 came to the result that embryo freezing before administration of gonadotoxic agents to females caused a delay of treatment in 34% of cases, and a live birth in 27% of surviving cases who wanted to become pregnant, with the follow-up time varying between 1 and 13 years.[95]
In chemotherapy as a conditioning regimen in hematopoietic stem cell transplantation, a study of patients conditioned with cyclophosphamide alone for severe aplastic anemia came to the result that ovarian recovery occurred in all women younger than 26 years at time of transplantation, but only in five of 16 women older than 26 years.[96]
Teratogenicity
Chemotherapy is potentially teratogenic during pregnancy, especially during the first trimester, to the extent that abortion usually is recommended if pregnancy in this period is found during chemotherapy.[97] Second- and third-trimester exposure does not usually increase the teratogenic risk and adverse effects on cognitive development, but it may increase the risk of various complications of pregnancy and fetal myelosuppression.[97]
In males previously having undergone chemotherapy or radiotherapy, there appears to be no increase in genetic defects or congenital malformations in their children conceived after therapy.[97] The use of assisted reproductive technologies and micromanipulation techniques might increase this risk.[97] In females previously having undergone chemotherapy, miscarriage and congenital malformations are not increased in subsequent conceptions.[97] However, when in vitro fertilization and embryo cryopreservationis practised between or shortly after treatment, possible genetic risks to the growing oocytes exist, and hence it has been recommended that the babies be screened.[97]
Peripheral neuropathy
Between 30 and 40 percent of patients undergoing chemotherapy experience chemotherapy-induced peripheral neuropathy (CIPN), a progressive, enduring, and often irreversible condition, causing pain, tingling, numbness and sensitivity to cold, beginning in the hands and feet and sometimes progressing to the arms and legs.[98] Chemotherapy drugs associated with CIPN include thalidomide, epothilones, vinca alkaloids, taxanes, proteasome inhibitors, and the platinum-based drugs.[98][99][100] Whether CIPN arises, and to what degree, is determined by the choice of drug, duration of use, the total amount consumed and whether the patient already has peripheral neuropathy. Though the symptoms are mainly sensory, in some cases motor nerves and the autonomic nervous system are affected.[101] CIPN often follows the first chemotherapy dose and increases in severity as treatment continues, but this progression usually levels off at completion of treatment. The platinum-based drugs are the exception; with these drugs, sensation may continue to deteriorate for several months after the end of treatment.[102] Some CIPN appears to be irreversible.[102] Pain can often be managed with drug or other treatment but the numbness is usually resistant to treatment.[103]
Cognitive impairment
Some patients report fatigue or non-specific neurocognitive problems, such as an inability to concentrate; this is sometimes called post-chemotherapy cognitive impairment, referred to as "chemo brain" by patients' groups.[104]
Tumor lysis syndrome
In particularly large tumors and cancers with high white cell counts, such as lymphomas, teratomas, and some leukemias, some patients develop tumor lysis syndrome. The rapid breakdown of cancer cells causes the release of chemicals from the inside of the cells. Following this, high levels of uric acid, potassium, phosphate and calcium are found in the blood. This causes kidney damage and the high levels of potassium can cause cardiac arrhythmia. Although prophylaxis is available and is often initiated in patients with large tumors, this is a dangerous side-effect that can lead to death if left untreated.[105]
Organ damage
Cardiotoxicity (heart damage) is especially prominent with the use of anthracycline drugs (doxorubicin, epirubicin, idarubicin, and liposomal doxorubicin). The cause of this is most likely due to the production of free radicals in the cell and subsequent DNA damage. Other chemotherapeutic agents that cause cardiotoxicity, but at a lower incidence, are cyclophosphamide, docetaxel and clofarabine.[106]
Hepatotoxicity (liver damage) can be caused by many cytotoxic drugs. The susceptibility of an individual to liver damage can be altered by other factors such as the cancer itself, viral hepatitis, immunosuppression and nutritional deficiency. The liver damage can consist of damage to liver cells, hepatic sinusoidal syndrome (obstruction of the veins in the liver), cholestasis (where bile does not flow from the liver to the intestine) and liver fibrosis.[107][108]
Nephrotoxicity (kidney damage) can be caused by tumor lysis syndrome and also due direct effects of drug clearance by the kidneys. Different drugs will affect different parts of the kidney and the toxicity may be asymptomatic (only seen on blood or urine tests) or may cause acute renal failure.[109][110]
Ototoxicity (damage to the inner ear) is a common side effect of platinum based drugs that can produce symptoms such as dizziness and vertigo.[111][112]
Other side-effects
Less common side-effects include red skin (erythema), dry skin, damaged fingernails, a dry mouth (xerostomia), water retention, and sexual impotence. Some medications can trigger allergic or pseudoallergic reactions.
Specific chemotherapeutic agents are associated with organ-specific toxicities, including cardiovascular disease(e.g., doxorubicin), interstitial lung disease (e.g., bleomycin) and occasionally secondary neoplasm (e.g., MOPP therapy for Hodgkin's disease).
Limitations
Chemotherapy does not always work, and even when it is useful, it may not completely destroy the cancer. Patients frequently fail to understand its limitations. In one study of patients who had been newly diagnosed with incurable, stage 4 cancer, more than two-thirds of patients with lung cancer and more than four-fifths of patients with colorectal cancer still believed that chemotherapy was likely to cure their cancer.[113]
The blood brain barrier poses a difficult obstacle to pass to deliver chemotherapy to the brain. This is because the brain has an extensive system in place to protect it from harmful chemicals. Drug transporters can pump out drugs from the brain and brain's blood vessel cells into the cerebrospinal fluid and blood circulation. These transporters pump out most chemotherapy drugs, which reduces their efficacy for treatment of brain tumors. Only small lipophilic alkylating agents such as lomustine or temozolomide are able to cross this blood brain barrier.[114][115][116]
Blood vessels in tumors are very different from those seen in normal tissues. As a tumor grows, tumor cells furthest away from the blood vessels become low in oxygen (hypoxic). To counteract this they then signal for new blood vessels to grow. The newly formed tumor vasculature is poorly formed and does not deliver an adequate blood supply to all areas of the tumor. This leads to issues with drug delivery because many drugs will be delivered to the tumor by the circulatory system.[117]
Efficacy
The efficacy of chemotherapy depends on the type of cancer and the stage. The overall effectiveness ranges from being curative for some cancers, such as some leukemias,[118][119] to being ineffective, such as in some brain tumors,[120] to being needless in others, like most non-melanoma skin cancers.[121]
Even when it is impossible for chemotherapy to provide a permanent cure, chemotherapy may be useful to reduce symptoms like pain or to reduce the size of an inoperable tumor in the hope that surgery will be possible in the future.
Resistance
Resistance is a major cause of treatment failure in chemotherapeutic drugs. There are a few possible causes of resistance in cancer, one of which is the presence of small pumps on the surface of cancer cells that actively move chemotherapy from inside the cell to the outside. Cancer cells produce high amounts of these pumps, known as p-glycoprotein, in order to protect themselves from chemotherapeutics. Research on p-glycoprotein and other such chemotherapy efflux pumps is currently ongoing. Medications to inhibit the function of p-glycoprotein are undergoing investigation, but due to toxicities and interactions with anti-cancer drugs their development has been difficult.[122][123] Another mechanism of resistance is gene amplification, a process in which multiple copies of a gene are produced by cancer cells. This overcomes the effect of drugs that reduce the expression of genes involved in replication. With more copies of the gene, the drug can not prevent all expression of the gene and therefore the cell can restore its proliferative ability. Cancer cells can also cause defects in the cellular pathways of apoptosis (programmed cell death). As most chemotherapy drugs kill cancer cells in this manner, defective apoptosis allows survival of these cells, making them resistant. Many chemotherapy drugs also cause DNA damage, which can be repaired by enzymes in the cell that carry out DNA repair. Upregulation of these genes can overcome the DNA damage and prevent the induction of apoptosis. Mutations in genes that produce drug target proteins, such as tubulin, can occur which prevent the drugs from binding to the protein, leading to resistance to these types of drugs.[124]
Cytotoxics and targeted therapies
Targeted therapies are a relatively new class of cancer drugs that can overcome many of the issues seen with the use of cytotoxics. They are divided into two groups: small molecule and antibodies. The massive toxicity seen with the use of cytotoxics is due to the lack of cell specificity of the drugs. They will kill any rapidly dividing cell, tumor or normal. Targeted therapies are designed to affect cellular proteins or processes that are utilised by the cancer cells. This allows a high dose to cancer tissues with a relatively low dose to other tissues. As different proteins are utilised by different cancer types, the targeted therapy drugs are used on a cancer type specific, or even on a patient specific basis. Although the side effects are often less severe than that seen of cytotoxic chemotherapeutics, life-threatening effects can occur. Initially, the targeted therapeutics were supposed to be solely selective for one protein. Now it is clear that there is often a range of protein targets that the drug can bind. An example target for targeted therapy is the protein produced by the Philadelphia chromosome, a genetic lesion found commonly in chronic myelomonocytic leukemia. This fusion protein has enzyme activity that can be inhibited by imatinib, a small molecule drug.[125][126][127][128]
Newer and experimental approaches

Targeted therapies
Specially targeted delivery vehicles aim to increase effective levels of chemotherapy for tumor cells while reducing effective levels for other cells. This should result in an increased tumor kill and/or reduced toxicity.[129]
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) comprise an antibody, drug and a linker between them. The antibody will be targeted at a preferentially expressed protein in the tumour cells (known as a tumor antigen) or on cells that the tumor can utilise, such as blood vessel endothelial cells. They bind to the tumor antigen and are internalised, where the linker releases the drug into the cell. These specially targeted delivery vehicles vary in their stability, selectivity, and choice of target, but, in essence, they all aim to increase the maximum effective dose that can be delivered to the tumor cells.[130] Reduced systemic toxicity means that they can also be used in sicker patients, and that they can carry new chemotherapeutic agents that would have been far too toxic to deliver via traditional systemic approaches.[citation needed]
The first approved drug of this type was gemtuzumab ozogamicin (Mylotarg), released by Wyeth (now Pfizer). The drug was approved to treat acute myeloid leukemia, but has now been withdrawn from the market because the drug did not meet efficacy targets in further clinical trials.[131][132] Two other drugs, trastuzumab emtansine and brentuximab vedotin, are both in late clinical trials, and the latter has been granted accelerated approval for the treatment of refractory Hodgkins lymphoma and systemic anaplastic large cell lymphoma.[130]
Nanoparticles
Nanoparticles are 1-1000 nanometer (nm) sized particles that can promote tumor selectivity and aid in delivering low-solubility drugs. Nanoparticles can be targeted passively or actively. Passive targeting exploits the difference between tumor blood vessels and normal blood vessels. Blood vessels in tumors are "leaky" because they have gaps from 200-2000 nm, which allow nanoparticles to escape into the tumor. Active targeting uses biological molecules (antibodies, proteins, DNA and receptor ligands) to preferentially target the nanoparticles to the tumor cells. There are many types of nanoparticle delivery systems, such as silica, polymers, liposomes and magnetic particles. Nanoparticles made of magnetic material can also be used to concentrate agents at tumor sites using an externally applied magnetic field.[129] They have emerged as a useful vehicle for poorly soluble agents such as paclitaxel.[133]
Electrochemotherapy
Electrochemotherapy is the combined treatment in which injection of a chemotherapeutic drug is followed by application of high-voltage electric pulses locally to the tumor. The treatment enables the chemotherapeutic drugs, which otherwise cannot or hardly go through the membrane of cells (such as bleomycin and cisplatin), to enter the cancer cells. Hence, greater effectiveness of antitumor treatment is achieved.
Clinical electrochemotherapy has been successfully used for treatment of cutaneous and subcutaneous tumors irrespective of their histological origin.[134][135][136][137][138][139][140] The method has been reported as safe, simple and highly effective in all reports on clinical use of electrochemotherapy. According to the ESOPE project (European Standard Operating Procedures of Electrochemotherapy), the Standard Operating Procedures (SOP) for electrochemotherapy were prepared, based on the experience of the leading European cancer centres on electrochemotherapy.[136][141] Recently, new electrochemotherapy modalities have been developed for treatment of internal tumors using surgical procedures, endoscopic routes or percutaneous approaches to gain access to the treatment area.[142][143]
Hyperthermia therapy
Hyperthermia therapy is heat treatment for cancer that can be a powerful tool when used in combination with chemotherapy (thermochemotherapy) or radiation for the control of a variety of cancers. The heat can be applied locally to the tumor site, which will dilate blood vessels to the tumor, allowing more chemotherapeutic medication to enter the tumor. Additionally, the bi-lipid layer of the tumor cell membrane will become more porous, further allowing more of the chemotherapeutic medicine to enter the tumor cell.
Hyperthermia has also been shown to help prevent or reverse "chemo-resistance." Chemotherapy resistance sometimes develops overtime as the tumors adapt and can overcome the toxicity of the chemo medication. “Overcoming chemoresistance has been extensively studied within the past, especially using CDDP-resistant cells. In regard to the potential benefit that drug-resistant cells can be recruited for effective therapy by combining chemotherapy with hyperthermia, it was important to show that chemoresistance against several anticancer drugs (e.g. mitomycin C, anthracyclines, BCNU, melphalan) including CDDP could be reversed at least partially by the addition of heat.[144]
Other uses
Some chemotherapy drugs are used in diseases other than cancer, such as in autoimmune disorders. They are often used at lower doses, which means that the side effects are minimized.[145] Methotrexate is used in the treatment of rheumatoid arthritis (RA),[146] psoriasis,[147] ankylosing spondylitis[148] and multiple sclerosis.[149][150] The anti-inflammatory response seen in RA is thought to be due to increases in adenosine, which causes immunosuppression; effects on immuno-regulatory cyclooxygenase-2 enzyme pathways; reduction in pro-inflammatory cytokines; and anti-proliferative properties.[146] Although methotrexate is used to treat both multiple sclerosis and ankylosing spondylitis, its efficacy in these diseases is still uncertain.[148][149][150] Cyclophosphamide is sometimes used to treat lupus nephritis, a common symptom of systemic lupus erythematosus.[151]
Chemotherapy drugs are also used in conditioning regimens prior to bone marow transplant (hematopoietic stem cell transplant). Conditioning regimens are used to suppress the recipient's immune system in order to allow a transplant to engraft. Cyclophosphamide is a common cytotoxic drug used in this manner, and is often used in conjunction with total body irradiation. Chemotherapeutic drugs may be used at high doses to permanently remove the recipient's bone marrow cells (myeloablative conditioning) or at lower doses that will prevent permanent bone marrow loss (non-myeloablative and reduced intensity conditioning).[152]
Occupational precautions
Healthcare workers exposed to anti-neoplastic agents take precautions to keep their exposure to a minimum. There is a limitation in cytotoxics dissolution in Australia and the United States to 20 dissolutions per pharmacist/nurse,[citation needed] since pharmacists that prepare these drugs or nurses that may prepare or administer them are the two occupational groups with the highest potential exposure to antineoplastic agents. In addition, physicians and operating room personnel may also be exposed through the treatment of patients. Hospital staff, such as shipping and receiving personnel, custodial workers, laundry workers, and waste handlers, all have potential exposure to these drugs during the course of their work. The increased use of antineoplastic agents in veterinary oncology also puts these workers at risk for exposure to these drugs.[153] Routes of entry into the users body are skin absorption, inhalation and ingestion. The long term effects of exposure include chromosomal abnormalities and infertility.[154]
In other animals
Chemotherapy is used in veterinary medicine similar to in human medicine.[155]
Comparison of available agents
Antineoplastic agents
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Abbreviations/Acronyms: Notes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
References
- ^ Krumbhaar EB (1919). "Role of the blood and the bone marrow in certain forms of gas poisoning". JAMA. 72: 39–41. doi:10.1001/jama.1919.26110010018009f.
- ^ a b Gilman A (May 1963). "The initial clinical trial of nitrogen mustard". Am. J. Surg. 105 (5): 574–8. doi:10.1016/0002-9610(63)90232-0. PMID 13947966.
- ^ Goodman LS, Wintrobe MM, Dameshek W, Goodman MJ, Gilman A, McLennan MT. (1946). "Nitrogen mustard therapy". JAMA. 132 (3): 126–132. doi:10.1001/jama.1946.02870380008004.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goodman LS, Wintrobe MM, Dameshek W, Goodman MJ, Gilman A, McLennan MT. (1984). "Landmark article Sept. 21, 1946: Nitrogen mustard therapy. Use of methyl-bis(beta-chloroethyl)amine hydrochloride and tris(beta-chloroethyl)amine hydrochloride for Hodgkin's disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. By Louis S. Goodman, Maxwell M. Wintrobe, William Dameshek, Morton J. Goodman, Alfred Gilman and Margaret T. McLennan". JAMA. 251 (17): 2255–61. doi:10.1001/jama.251.17.2255. PMID 6368885.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Faguet, p. 71
- ^ Joensuu H. (2008). "Systemic chemotherapy for cancer: from weapon to treatment". Lancet Oncol. 9 (3): 304. doi:10.1016/S1470-2045(08)70075-5. PMID 18308256.
- ^ Nichols HJ, Walker JE (March 1923). "Experimental observations on the prophyaxis and treatment of syphilis". J. Exp. Med. 37 (4): 525–42. doi:10.1084/jem.37.4.525. PMC 2128372. PMID 19868743.
- ^ Ben-Ari ET (April 2004). "Dual purpose: some cancer therapies used to treat autoimmune diseases". J. Natl. Cancer Inst. 96 (8): 577–9. doi:10.1093/jnci/96.8.577. PMID 15100330.
- ^ Hanahan D, Weinberg RA (January 2000). "The hallmarks of cancer". Cell. 100 (1): 57–70. doi:10.1016/S0092-8674(00)81683-9. PMID 10647931.
- ^ Hodgson S (January 2008). "Mechanisms of inherited cancer susceptibility". J Zhejiang Univ Sci B. 9 (1): 1–4. doi:10.1631/jzus.B073001. PMC 2170461. PMID 18196605.
- ^ Perera FP (November 1997). "Environment and cancer: who are susceptible?". Science. 278 (5340): 1068–73. Bibcode:1997Sci...278.1068P. doi:10.1126/science.278.5340.1068. PMID 9353182.
- ^ Randall, pp, 93-94
- ^ Kehe K, Balszuweit F, Steinritz D, Thiermann H (September 2009). "Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering". Toxicology. 263 (1): 12–9. doi:10.1016/j.tox.2009.01.019. PMID 19651324.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Malhotra V, Perry MC (2003). "Classical chemotherapy: mechanisms, toxicities and the therapeutic window". Cancer Biol. Ther. 2 (4 Suppl 1): S2–4. PMID 14508075.
- ^ Makin G; Hickman JA (July 2000). "Apoptosis and cancer chemotherapy". Cell Tissue Res. 301 (1): 143–52. Bibcode:1994RSPTB.345..319H. doi:10.1007/s004419900160. PMID 10928287.
- ^ a b c d e f Corrie PG, Pippa G. (2008). "Cytotoxic chemotherapy: clinical aspects". Medicine. 36 (1): 24–28. doi:10.1016/j.mpmed.2007.10.012.
- ^ a b Siddik ZH (2005). Mechanisms of Action of Cancer Chemotherapeutic Agents: DNA-Interactive Alkylating Agents and Antitumour Platinum-Based Drugs. John Wiley & Sons, Ltd. doi:10.1002/0470025077.chap84b.
- ^ a b c d e f Lind M.J., M.J. (2008). "Principles of cytotoxic chemotherapy". Medicine. 36 (1): 19–23. doi:10.1016/j.mpmed.2007.10.003.
- ^ a b c Damia G, D'Incalci M (September 1998). "Mechanisms of resistance to alkylating agents". Cytotechnology. 27 (1–3): 165–73. doi:10.1023/A:1008060720608. PMC 3449574. PMID 19002790.
- ^ Takimoto CH, Calvo E."Principles of Oncologic Pharmacotherapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
- ^ a b c Parker WB (July 2009). "Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer". Chem. Rev. 109 (7): 2880–93. doi:10.1021/cr900028p. PMC 2827868. PMID 19476376.
- ^ a b Wood, p. 11
- ^ a b c d e f g h i Airley, pp. 55-59
- ^ Adjei AA (June 2004). "Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent". Clin. Cancer Res. 10 (12 Pt 2): 4276s–4280s. doi:10.1158/1078-0432.CCR-040010. PMID 15217974.
- ^ Wagstaff AJ, Ibbotson T, Goa KL (2003). "Capecitabine: a review of its pharmacology and therapeutic efficacy in the management of advanced breast cancer". Drugs. 63 (2): 217–36. doi:10.2165/00003495-200363020-00009. PMID 12515569.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rowinsky EK, Donehower RC (October 1991). "The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics". Pharmacol. Ther. 52 (1): 35–84. doi:10.1016/0163-7258(91)90086-2. PMID 1687171.
- ^ a b c d Yue QX, Liu X, Guo DA (August 2010). "Microtubule-binding natural products for cancer therapy". Planta Med. 76 (11): 1037–43. doi:10.1055/s-0030-1250073. PMID 20577942.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Damayanthi Y, Lown JW (June 1998). "Podophyllotoxins: current status and recent developments". Curr. Med. Chem. 5 (3): 205–52. PMID 9562603.
- ^ Liu YQ, Yang L, Tian X, Cong (2007). "Podophyllotoxin: current perspectives". Curr Bioactive Compounds. 3 (1): 37–66. doi:10.1016/j.jallcom.2006.06.070.
{{cite journal}}:|first10=missing|last10=(help);|first11=missing|last11=(help);|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help);|first8=missing|last8=(help);|first9=missing|last9=(help)CS1 maint: multiple names: authors list (link) - ^ Lodish H, Berk A, Zipursky SL; et al. (2000). Molecular Cell Biology. 4th edition. The Role of Topoisomerases in DNA Replication. New York: W. H. Freeman.
{{cite book}}: Explicit use of et al. in:|last=(help)CS1 maint: multiple names: authors list (link) - ^ Goodsell DS (2002). "The molecular perspective: DNA topoisomerases". Stem Cells. 20 (5): 470–1. doi:10.1634/stemcells.20-5-470. PMID 12351817.
- ^ Nitiss JL (May 2009). "Targeting DNA topoisomerase II in cancer chemotherapy". Nature Reviews Cancer. 9 (5): 338–50. doi:10.1038/nrc2607. PMC 2748742. PMID 19377506.
- ^ Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (June 2004). "Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity". Pharmacol. Rev. 56 (2): 185–229. doi:10.1124/pr.56.2.6. PMID 15169927.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sobell HM (August 1985). "Actinomycin and DNA transcription". Proc. Natl. Acad. Sci. U.S.A. 82 (16): 5328–31. doi:10.1073/pnas.82.16.5328. PMC 390561. PMID 2410919.
- ^ Dorr RT (April 1992). "Bleomycin pharmacology: mechanism of action and resistance, and clinical pharmacokinetics". Semin. Oncol. 19 (2 Suppl 5): 3–8. PMID 1384141.
- ^ Airley, p. 87
- ^ Verweij J, Pinedo HM (October 1990). "Mitomycin C: mechanism of action, usefulness and limitations". Anticancer Drugs. 1 (1): 5–13. doi:10.1097/00001813-199010000-00002. PMID 2131038.
- ^ Wood, pp. 17-18
- ^ Perry, p. 42
- ^ Epstein RJ (August 2005). "Maintenance therapy to suppress micrometastasis: the new challenge for adjuvant cancer treatment". Clin. Cancer Res. 11 (15): 5337–41. doi:10.1158/1078-0432.CCR-05-0437. PMID 16061845.
- ^ Skeel, R. T. (2003). Handbook of Cancer Chemotherapy (paperback) (6th ed.). Lippincott Williams & Wilkins. ISBN 0-7817-3629-3.
- ^ Chabner, B.; Longo, D. L. (2005). Cancer Chemotherapy and Biotherapy: Principles and Practice (4th ed.). Philadelphia: Lippincott Willians & Wilkins. ISBN 0-7817-5628-6.
- ^ Du Bois D; Du Bois EF. "A formula to estimate the approximate surface area if height and weight be known. 1916". 5 (5). Archives Internal Medicine: 303–11.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c Felici A.; J. Verweij; A. Sparreboom (2002). "Dosing strategies for anticancer drugs: the good, the bad and body-surface area". 38 (13). Eur J Cancer: 1677–84.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c Kaestner SA, Sewell GJ (February 2007). "Chemotherapy dosing part I: scientific basis for current practice and use of body surface area". Clin Oncol (R Coll Radiol). 19 (1): 23–37. doi:10.1016/j.clon.2006.10.010. PMID 17305252.
- ^ Donald Pinkel (August 1958). "The Use of Body Surface Area as a Criterion of Drug Dosage in Cancer Chemotherapy". 18 (7). Cancer Res: 853–6.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Gurney H (April 2002). "How to calculate the dose of chemotherapy". Br. J. Cancer. 86 (8): 1297–302. doi:10.1038/sj.bjc.6600139. PMC 2375356. PMID 11953888.
- ^ a b Beumer JH, Chu E, Salamone SJ (November 2012). "Body-surface area-based chemotherapy dosing: appropriate in the 21st century?". J. Clin. Oncol. 30 (31): 3896–7. doi:10.1200/JCO.2012.44.2863. PMID 22965963.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Baker SD; Verweij J; Rowinsky EK; Donehower RC; Schellens JH; Grochow LB; Sparreboom A (2002). "Role of body surface area in dosing of investigational anticancer agents in adults, 1991-2001". 94 (24). J Natl Cancer Inst: 1883–8.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d e f g h Gamelin EC; Delva R; Jacob J; Merrouche Y; Raoul JL; Pezet D; Dorval E; Piot G; Morel A; Boisdron-Celle M (2008). "Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer". 26 (13). J Clin Oncol: 2099–2105.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Saam J; Critchfield GC; Hamilton SA; Roa BB; Wenstrup RJ; Kaldate RR (2011). "Body Surface Area-based Dosing of 5-Fluorouracil Results in Extensive Interindividual Variability in 5-Fluorouracil Exposure in Colorectal Cancer Patients on FOLFOX Regimens". 10 (3). Clin Colorectal Cancer: 203–206.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d e f Capitain O; Asevoaia A; Boisdron-Celle M; Poirier AL; Morel A; Gamelin E (2012). "Individual Fluorouracil Dose Adjustment in FOLFOX Based on Pharmacokinetic Follow-Up Compared With Conventional Body-Area-Surface Dosing: A Phase II, Proof-of-Concept Study". 11 (4). Clin Colorectal Cancer: 263–267.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Kaldate RR; Haregewoin A; Grier CE; Hamilton SA; McLeod HL. "Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6". 17 (3). Oncologist, 2012: 296–302.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c Hunter RJ; Navo MA; Thaker PH; Bodurka DC; Wolf JK; Smith JA (February 2009). "Dosing chemotherapy in obese patients: actual versus assigned body surface area (BSA)". 35 (1). Cancer Treat Rev: 69–78. PMID 18922643.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: year (link) - ^ Macbeth, p. 4
- ^ Buffery, PJ; Allen, KM; Chin, PKL; Moore, GA; Barclay, ML; Begg, EJ (2014). "Thirteen Years' Experience of Pharmacokinetic Monitoring and Dosing of Busulfan: Can the Strategy Be Improved?". 36 (1). Ther Drug Monit: 86–92.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Bartelink IH; Bredius RG; Belitser SV; Suttorp MM; Bierings M; Knibbe CA; Egeler M; Lankester AC; Egberts AC; Zwaveling J; Boelens JJ. "Association Between Busulfan Exposure and Outcome in Children Receiving Intravenous Busulfan Before Hematopoietic Stem Cell Transplantation". 36 (1). Ther Drug Monit: 93–99.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "ARK™ Methotrexate Assay". Ark Diagnostics.
- ^ "Customizing Chemotherapy for Better Cancer Care". My Care Diagnostics.
- ^ "A Brief History of BSA Dosing". My Care Diagnostics.
- ^ Wood, Miriam; David Brighton (2005). The Royal Marsden Hospital handbook of cancer chemotherapy: a guide for the multidisciplinary team. St. Louis, Mo: Elsevier Churchill Livingstone. pp. 93–94. ISBN 0-443-07101-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Wood, pp. 113-118
- ^ Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, Villegas-Portero R, Nieto-Garcia A (2010). "Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety". Oncologist. 15 (4): 416–27. doi:10.1634/theoncologist.2009-0325. PMC 3227960. PMID 20348274.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Verhoef C, de Wilt JH, ten Hagen TL, Eggermont AM (October 2008). "Isolated hepatic perfusion for the treatment of liver tumors: sunset or sunrise?". Surg. Oncol. Clin. N. Am. 17 (4): 877–94, xi. doi:10.1016/j.soc.2008.04.007. PMID 18722924.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hendriks JM, Van Schil PE (1998). "Isolated lung perfusion for the treatment of pulmonary metastases". Surg Oncol. 7 (1–2): 59–63. doi:10.1016/S0960-7404(98)00028-0. PMID 10421507.
- ^ Chitwood, K (September 2013). "Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons". Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.] 39 (9): 1306–16. doi:10.1111/dsu.12300. PMID 23915332.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Airley, p. 265
- ^ Huang, p. 130
- ^ Elad S; Zadik Y; Hewson I; Hovan A; Correa ME; Logan R; Elting LS; Spijkervet FK; Brennan MT; Viral Infections Section; Oral Care Study Group; Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) (August 2010). "A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea". Support Care Cancer. 18 (8): 993–1006. doi:10.1007/s00520-010-0900-3. PMID 20544224.
- ^ "Coriolus Versicolor". Cancer.org. 10 June 2008. Retrieved 7 August 2012.
- ^ PMID 16319675
- ^ a b Keidan, RD (March 1989). "Recurrent typhlitis. A disease resulting from aggressive chemotherapy". Dis Colon Rectum. 32 (3): 206–9. doi:10.1007/BF02554529. PMID 2920627.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gibson RJ, Keefe DM (September 2006). "Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies". Support Care Cancer. 14 (9): 890–900. doi:10.1007/s00520-006-0040-y. PMID 16604351.
- ^ Groopman JE, Itri LM (October 1999). "Chemotherapy-induced anemia in adults: incidence and treatment". J. Natl. Cancer Inst. 91 (19): 1616–34. doi:10.1093/jnci/91.19.1616. PMID 10511589.
- ^ Henry DH (July 2006). "The role of intravenous iron in cancer-related anemia". Oncology (Williston Park, N.Y.). 20 (8 Suppl 6): 21–4. PMID 16925107.
- ^ Rodgers GM, Becker PS, Bennett CL; et al. (July 2008). "Cancer- and chemotherapy-induced anemia". J Natl Compr Canc Netw. 6 (6): 536–64. PMID 18597709.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Vadhan-Raj S (January 2009). "Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents". Semin. Hematol. 46 (1 Suppl 2): S26–32. doi:10.1053/j.seminhematol.2008.12.007. PMID 19245931.
- ^ Sekhon SS, Roy V (May 2006). "Thrombocytopenia in adults: A practical approach to evaluation and management". South. Med. J. 99 (5): 491–8, quiz 499–500, 533. doi:10.1097/01.smj.0000209275.75045.d4. PMID 16711312.
- ^ Berger AM, Abernethy AP, Atkinson A; et al. (August 2010). "Cancer-related fatigue". J Natl Compr Canc Netw. 8 (8): 904–31. PMID 20870636.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Franklin DJ, Packel L (March 2006). "Cancer-related fatigue". Arch Phys Med Rehabil. 87 (3 Suppl 1): S91–3, quiz S94–5. doi:10.1016/j.apmr.2005.12.015. PMID 16500197.
- ^ Cramp F, Byron-Daniel J (2012). Cramp, Fiona (ed.). "Exercise for the management of cancer-related fatigue in adults". Cochrane Database Syst Rev. 11: CD006145. doi:10.1002/14651858.CD006145.pub3. PMID 23152233.
- ^ Gill, Paula; Grothey, Axel; Loprinzi, Charles (2006). "Nausea and Vomiting in the Cancer Patient". Oncology: 1482–1496. doi:10.1007/0-387-31056-8_83. ISBN 978-0-387-24291-0. Retrieved 2 September 2011.
Nausea and vomiting are two of the most feared cancer treatment-related side effects for cancer patients and their families.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17642856, please use {{cite journal}} with
|pmid=17642856instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 23187775, please use {{cite journal}} with
|pmid=23187775instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9589208, please use {{cite journal}} with
|pmid=9589208instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20487675, please use {{cite journal}} with
|pmid=20487675instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 22901547, please use {{cite journal}} with
|pmid=22901547instead. - ^ Trüeb RM (March 2009). "Chemotherapy-induced alopecia". Semin Cutan Med Surg. 28 (1): 11–4. doi:10.1016/j.sder.2008.12.001. PMID 19341937.
- ^ Chon SY, Champion RW, Geddes ER, Rashid RM (July 2012). "Chemotherapy-induced alopecia". J. Am. Acad. Dermatol. 67 (1): e37–47. doi:10.1016/j.jaad.2011.02.026. PMID 22178150.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ U. Rüther, C. Nunnensiek, H.-J. Schmoll,Secondary Neoplasias following Chemotherapy, Radiotherapy, and Immunosuppression,Contributions to Oncology (Beiträge zur Onkologie); Vol 55, 2000, ISBN 3-8055-7116-X
- ^ Hijiya, N; Hudson, MM; Lensing, S; Zacher, M; Onciu, M; Behm, FG; Razzouk, BI; Ribeiro, RC; Rubnitz, JE; Sandlund, JT; Rivera, GK; Evans, WE; Relling, MV; Pui, CH (2007). "Cumulative Incidence of Secondary Neoplasms as a First Event After Childhood Acute Lymphoblastic Leukemia". JAMA. 297 (11): 1207–1215. doi:10.1001/jama.297.11.1207. PMID 17374815.
{{cite journal}}: Cite has empty unknown parameter:|author-name-separator=(help); Unknown parameter|author-separator=ignored (help) - ^ a b c d Brydøy M, Fosså SD, Dahl O, Bjøro T (2007). "Gonadal dysfunction and fertility problems in cancer survivors". Acta Oncol. 46 (4): 480–9. doi:10.1080/02841860601166958. PMID 17497315.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/humupd/dms022, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/humupd/dms022instead. - ^ Gurgan T, Salman C, Demirol A (October 2008). "Pregnancy and assisted reproduction techniques in men and women after cancer treatment". Placenta. 29 Suppl B: 152–9. doi:10.1016/j.placenta.2008.07.007. PMID 18790328.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/humrep/det268, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/humrep/det268instead. - ^ André Tichelli, Alicia Rovó. Fertility Issues Following Hematopoietic Stem Cell Transplantation. Expert Rev Hematol. 2013;6(4):375-388.
In turn citing: Sanders JE, Hawley J, Levy W et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation" Blood 87(7), 3045–3052(1996). - ^ a b c d e f Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/humupd/7.4.394, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/humupd/7.4.394instead. [1] - ^ a b del Pino BM. Chemotherapy-induced Peripheral Neuropathy. NCI Cancer Bulletin. Feb 23, 2010;7(4):6.
- ^ Grisold W, Oberndorfer S, Windebank AJ. Chemotherapy and polyneuropathies. European Association of Neurooncology Magazine. 2012;12(1).
- ^ http://www.ehealthme.com/ds/herceptin/peripheral%20sensory%20neuropathy
- ^ Beijers AJM, Jongen, JLM & Vreugdenhil1 G. [2]. The Netherlands journal of medicine. January 2012;70(1). PMID 22271810.
- ^ a b Windebank AJ & Grisold W. Chemotherapy-induced neuropathy. Journal of the Peripheral Nervous System. 2008 Mar;13(1):27–46. PMID 18346229.
- ^ Savage L. Chemotherapy-induced pain puzzles scientists. Journal of the National Cancer Institute. 2007;99(14):1070–1071.
- ^ Tannock IF, Ahles TA, Ganz PA, Van Dam FS (June 2004). "Cognitive impairment associated with chemotherapy for cancer: report of a workshop". J. Clin. Oncol. 22 (11): 2233–9. doi:10.1200/JCO.2004.08.094. PMID 15169812.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wood, p. 202
- ^ Shaikh AY, Shih JA (June 2012). "Chemotherapy-induced cardiotoxicity". Curr Heart Fail Rep. 9 (2): 117–27. doi:10.1007/s11897-012-0083-y. PMID 22382639.
- ^ A.V. Thatishetty, N. Agresti, C.B. O'Brien, Ameet V. (2013). "Chemotherapy-Induced Hepatotoxicity". Clinics in Liver Disease. 17 (4): 671–86, ix–x. doi:10.1016/j.cld.2013.07.010. PMID 24099024.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help)CS1 maint: multiple names: authors list (link) - ^ King PD, Perry MC (2001). "Hepatotoxicity of chemotherapy". Oncologist. 6 (2): 162–76. doi:10.1634/theoncologist.6-2-162. PMID 11306728.
- ^ de Jonge MJ, Verweij J (February 2006). "Renal toxicities of chemotherapy". Semin. Oncol. 33 (1): 68–73. doi:10.1053/j.seminoncol.2005.11.011. PMID 16473645.
- ^ Humphreys BD, Soiffer RJ, Magee CC (January 2005). "Renal failure associated with cancer and its treatment: an update". J. Am. Soc. Nephrol. 16 (1): 151–61. doi:10.1681/ASN.2004100843. PMID 15574506.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brock PR, Knight KR, Freyer DR; et al. (July 2012). "Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale". J. Clin. Oncol. 30 (19): 2408–17. doi:10.1200/JCO.2011.39.1110. PMC 3675696. PMID 22547603.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Rybak LP, Mukherjea D, Jajoo S, Ramkumar V (November 2009). "Cisplatin ototoxicity and protection: clinical and experimental studies". Tohoku J. Exp. Med. 219 (3): 177–86. doi:10.1620/tjem.219.177. PMC 2927105. PMID 19851045.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weeks JC, Catalano PJ, Cronin A; et al. (October 2012). "Patients' expectations about effects of chemotherapy for advanced cancer". N. Engl. J. Med. 367 (17): 1616–25. doi:10.1056/NEJMoa1204410. PMC 3613151. PMID 23094723.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Deeken JF, Löscher W (March 2007). "The blood-brain barrier and cancer: transporters, treatment, and Trojan horses". Clin. Cancer Res. 13 (6): 1663–74. doi:10.1158/1078-0432.CCR-06-2854. PMID 17363519.
- ^ Agarwala SS, Kirkwood JM (2000). "Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma". Oncologist. 5 (2): 144–51. doi:10.1634/theoncologist.5-2-144. PMID 10794805.
- ^ Gerstner ER, Fine RL (June 2007). "Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm". J. Clin. Oncol. 25 (16): 2306–12. doi:10.1200/JCO.2006.10.0677. PMID 17538177.
- ^ Minchinton AI, Tannock IF (August 2006). "Drug penetration in solid tumours". Nature Reviews Cancer. 6 (8): 583–92. doi:10.1038/nrc1893. PMID 16862189.
- ^ Nastoupil, LJ (May 2012). "Diffuse large B-cell lymphoma: current treatment approaches". Oncology (Williston Park, N.Y.). 26 (5): 488–95. PMID 22730604.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Freedman, A (October 2012). "Follicular lymphoma: 2012 update on diagnosis and management". American journal of hematology. 87 (10): 988–95. doi:10.1002/ajh.23313. PMID 23001911.
- ^ Rampling, R (June 2004). "The present and future management of malignant brain tumours: surgery, radiotherapy, chemotherapy". Journal of neurology, neurosurgery, and psychiatry. 75 Suppl 2 (Suppl 2): ii24–30. doi:10.1136/jnnp.2004.040535. PMC 1765659. PMID 15146036.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Madan, V (20 February 2010). "Non-melanoma skin cancer". Lancet. 375 (9715): 673–85. doi:10.1016/S0140-6736(09)61196-X. PMID 20171403.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Goldman B (February 2003). "Multidrug resistance: can new drugs help chemotherapy score against cancer?". J. Natl. Cancer Inst. 95 (4): 255–7. doi:10.1093/jnci/95.4.255. PMID 12591977.
- ^ E. Crowley, CA. McDevitt, R. Callaghan (2009). Multidrug Resistance in Cancer. Generating Inhibitors of P-Glycoprotein: Where to, Now?. Springer Protocols. pp. 405–432.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Luqmani YA (2005). "Mechanisms of drug resistance in cancer chemotherapy". Med Princ Pract. 14 Suppl 1: 35–48. doi:10.1159/000086183. PMID 16103712.
- ^ Gerber DE (February 2008). "Targeted therapies: a new generation of cancer treatments". Am Fam Physician. 77 (3): 311–9. PMID 18297955.
- ^ Allen TM (October 2002). "Ligand-targeted therapeutics in anticancer therapy". Nature Reviews Cancer. 2 (10): 750–63. doi:10.1038/nrc903. PMID 12360278.
- ^ Chen HX, Cleck JN (August 2009). "Adverse effects of anticancer agents that target the VEGF pathway". Nature Reviews Clinical Oncology. 6 (8): 465–77. doi:10.1038/nrclinonc.2009.94. PMID 19581909.
- ^ Zhang J, Yang PL, Gray NS (January 2009). "Targeting cancer with small molecule kinase inhibitors". Nature Reviews Cancer. 9 (1): 28–39. doi:10.1038/nrc2559. PMID 19104514.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Chidambaram M, Manavalan R, Kathiresan K (2011). "Nanotherapeutics to overcome conventional cancer chemotherapy limitations". J Pharm Pharm Sci. 14 (1): 67–77. PMID 21501554.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Teicher BA, Chari RV (October 2011). "Antibody conjugate therapeutics: challenges and potential". Clin. Cancer Res. 17 (20): 6389–97. doi:10.1158/1078-0432.CCR-11-1417. PMID 22003066.
- ^ Sievers EL, Linenberger M (November 2001). "Mylotarg: antibody-targeted chemotherapy comes of age". Current Opinion in Oncology. 13 (6): 522–7. doi:10.1097/00001622-200111000-00016. PMID 11673694.
- ^ FDA. "Mylotarg (gemtuzumab ozogamicin): Market Withdrawal". Retrieved 18 August 2013.
- ^ Vines T, Faunce T (May 2009). "Assessing the safety and cost-effectiveness of early nanodrugs". J Law Med. 16 (5): 822–45. PMID 19554862.
- ^ Heller R, Gilbert R, Jaroszeski MJ. (1999). "Clinical applications of electrochemotherapy". Adv Drug Deliv Rev. 35 (1): 119–129. doi:10.1016/S0169-409X(98)00067-2. PMID 10837693.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Larkin JO, Collins CG, Aarons S, Tangney M, Whelan M, O’Reily S, Breathnach O, Soden DM, O’Sullivan GC (2007). "Electrochemotherapy - Aspects of preclinical development and early clinical experience". Ann Surg. 245 (3): 469–479. doi:10.1097/01.sla.0000250419.36053.33. PMC 1877027. PMID 17435555.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D, Pavlovic I, Paulin-Kosir SM, Cemazar M, Morsli N, Soden DM, Rudolf Z, Robert C, O’Sullivan GC, Mir LM (2006). "Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases". Eur J Cancer Suppl. 4 (11): 3–13. doi:10.1016/j.ejcsup.2006.08.002.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, Snoj M. (2008). "Electrochemotherapy in treatment of tumours". Eur J Surg Oncol. 34 (2): 232–240. doi:10.1016/j.ejso.2007.05.016. PMID 17614247.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Möller MG, Salwa S, Soden DM, O’Sullivan GC. (2009). "Electrochemotherapy as an adjunct or alternative to other treatments for unresectable or in-transit melanoma". Expert Rev Anticancer Ther. 9 (11): 1611–1630. doi:10.1586/era.09.129. PMID 19895245.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Testori A, Tosti G, Martinoli C, Spadola G, Cataldo F, Verrecchia F, Baldini F, Mosconi M, Soteldo J, Tedeschi I, Passoni C, Pari C, Di Pietro A, Ferrucci PF (2010). "Electrochemotherapy for cutaneous and subcutaneous tumor lesions: a novel therapeutic approach". Dermatol Ther. 23 (6): 651–661. doi:10.1111/j.1529-8019.2010.01370.x. PMID 21054709.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hampton T (2011). "Electric Pulses Help With Chemotherapy, May Open New Paths for Other Agents". JAMA. 305 (6): 549–551. doi:10.1001/jama.2011.92. PMID 21304073.
- ^ Mir LM, Gehl J, Sersa G, Collins CG, Garbay JR, Billard V, Geertsen PF, Rudolf Z, O’Sullivan GC, Marty M (2006). "Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes". Eur J Cancer Suppl. 4 (11): 14–25. doi:10.1016/j.ejcsup.2006.08.003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Soden DM, Larkin JO, Collins CG, Tangney M, Aarons S, Piggott J, Morrissey A, Dunne C, O’Sullivan GC (2006). "Successful application of targeted electrochemotherapy using novel flexible electrodes and low dose bleomycin to solid tumours". Cancer Lett. 232 (2): 300–310. doi:10.1016/j.canlet.2005.03.057. PMID 15964138.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Miklavcic D, Snoj M, Zupanic A, Kos B, Cemazar M, Kropivnik M, Bracko M, Pecnik T, Gadzijev E, Sersa G. (2010). "Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy". BioMed Eng OnLine. 9 (1): 10. doi:10.1186/1475-925X-9-10. PMC 2843684. PMID 20178589.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Issels, R. (1999). "Hyperthermia Combined with Chemotherapy – Biological Rationale, Clinical Application, and Treatment Results". Onkologie. 22 (5): 374–381. doi:10.1159/000026986.
- ^ Ben-Ari ET (April 2004). "Dual purpose: some cancer therapies used to treat autoimmune diseases". J. Natl. Cancer Inst. 96 (8): 577–9. doi:10.1093/jnci/96.8.577. PMID 15100330.
- ^ a b Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH (August 2001). "Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis". Ann. Rheum. Dis. 60 (8): 729–35. doi:10.1136/ard.60.8.729. PMC 1753808. PMID 11454634.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Montaudié H, Sbidian E, Paul C; et al. (May 2011). "Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity". J Eur Acad Dermatol Venereol. 25 Suppl 2: 12–8. doi:10.1111/j.1468-3083.2011.03991.x. PMID 21388454.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Chen J, Veras MM, Liu C, Lin J (2013). Chen, Junmin (ed.). "Methotrexate for ankylosing spondylitis". Cochrane Database Syst Rev. 2: CD004524. doi:10.1002/14651858.CD004524.pub4. PMID 23450553.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gray O, McDonnell GV, Forbes RB (2004). Gray, Orla (ed.). "Methotrexate for multiple sclerosis". Cochrane Database Syst Rev (2): CD003208. doi:10.1002/14651858.CD003208.pub2. PMID 15106195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Gray OM, McDonnell GV, Forbes RB (August 2006). "A systematic review of oral methotrexate for multiple sclerosis". Mult. Scler. 12 (4): 507–10. doi:10.1191/1352458506ms1299oa. PMID 16900766.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ntali S, Bertsias G, Boumpas DT (June 2011). "Cyclophosphamide and lupus nephritis: when, how, for how long?". Clin Rev Allergy Immunol. 40 (3): 181–91. doi:10.1007/s12016-009-8196-0. PMID 20107927.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bacigalupo, A (December 2009). "Defining the intensity of conditioning regimens: working definitions". Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 15 (12): 1628–33. doi:10.1016/j.bbmt.2009.07.004. PMC 2861656. PMID 19896087.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "NIOSH Occupational Exposure to Antineoplastic Agents". United States National Institute for Occupational Safety and Health. Retrieved 10 October 2007.
- ^ Wood, p. 38.
- ^ McKnight JA (May 2003). "Principles of chemotherapy". Clin Tech Small Anim Pract. 18 (2): 67–72. doi:10.1053/svms.2003.36617. PMID 12831063.
- ^ a b c d e f g Template:Cite isbn
- ^ a b c Template:Cite isbn
- ^ Template:Cite isbn
- ^ a b c Template:Cite isbn
- ^ Sweetman, S (ed.). Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 8 February 2014.
- ^ a b Momparler, RL (August 2012). "A Perspective on the Comparative Antileukemic Activity of 5-Aza-2'-deoxycytidine (Decitabine) and 5-Azacytidine (Vidaza)". Pharmaceuticals (Basel, Switzerland). 5 (8): 875–881. doi:10.3390/ph5080875. PMC 3763670. PMID 24280679.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Estey, EH (12 June 2013). "Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia". Leukemia. 27 (9): 1803–1812. doi:10.1038/leu.2013.173. PMID 23757301.
- ^ Kim, YJ (June 2013). "Use of azacitidine for myelodysplastic syndromes: controversial issues and practical recommendations" (PDF). Blood research. 48 (2): 87–98. doi:10.5045/br.2013.48.2.87. PMC 3698413. PMID 23826577.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Platzbecker, U; Germing, U (September 2013). "Combination of azacitidine and lenalidomide in myelodysplastic syndromes or acute myeloid leukemia-a wise liaison?". Leukemia. 27 (9): 1813–9. doi:10.1038/leu.2013.140. PMID 23644421.
- ^ Ritchie, EK (2012). "Safety and efficacy of azacitidine in the treatment of elderly patients with myelodysplastic syndrome". Clinical interventions in aging. 7: 165–73. doi:10.2147/CIA.S24659. PMC 3393359. PMID 22791989.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Keating, GM (28 May 2012). "Azacitidine: a review of its use in the management of myelodysplastic syndromes/acute myeloid leukaemia". Drugs. 72 (8): 1111–36. doi:10.2165/11209430-000000000-00000. PMID 22571445.
- ^ Martínez-Francés, A (June 2011). "Adverse effects of azacitidine: onset, duration, and treatment". Advances in therapy. 28 Suppl 4: 1–5. doi:10.1007/s12325-011-0021-5. PMID 21688206.
- ^ Font, P (March 2011). "Azacitidine for the treatment of patients with acute myeloid leukemia with 20%-30% blasts and multilineage dysplasia". Advances in therapy. 28 Suppl 3: 1–9. doi:10.1007/s12325-011-0002-8. PMID 21431628.
- ^ Vigil, CE (24 September 2010). "Safety and efficacy of azacitidine in myelodysplastic syndromes". Drug design, development and therapy. 4: 221–9. doi:10.2147/DDDT.S3143. PMC 2948932. PMID 20957213.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ McCormack, SE; Warlick, ED (7 September 2010). "Epigenetic approaches in the treatment of myelodysplastic syndromes: clinical utility of azacitidine". OncoTargets and therapy. 3: 157–65. doi:10.2147/OTT.S5852. PMC 2939768. PMID 20856790.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Martens, UM, ed. (2010). "11 5-Azacytidine/Azacitidine". Small molecules in oncology. Recent Results in Cancer Research. Vol. 184. Heidelberg: Springer. pp. 159–170. doi:10.1007/978-3-642-01222-8. ISBN 978-3-642-01222-8.
- ^ Chintala, L (9 April 2011). "Capecitabine versus 5-fluorouracil in colorectal cancer: where are we now?" (PDF). Oncology Reviews. 5 (2): 129–140. doi:10.1007/s12156-011-0074-3.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bang, YJ (December 2011). "Capecitabine in gastric cancer". Expert review of anticancer therapy. 11 (12): 1791–1806. doi:10.1586/era.11.172. PMID 22117147.
- ^ Hirsch, BR; Zafar, SY (2011). "Capecitabine in the management of colorectal cancer". Cancer management and research. 3: 79–89. doi:10.2147/CMR.S11250. PMC 3097797. PMID 21629830.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Quidde, J (2012). "Clinical management of localized colon cancer with capecitabine". Clinical Medicine Insights. Oncology. 6: 363–73. doi:10.4137/CMO.S8194. PMC 3498969. PMID 23170068.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fernández-Martos, C (28 May 2012). "The role of capecitabine in locally advanced rectal cancer treatment: an update". Drugs. 72 (8): 1057–1073. doi:10.2165/11633870-000000000-00000. PMID 22621694.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ma, Y (June 2012). "Capecitabine for the treatment for advanced gastric cancer: efficacy, safety and ethnicity". Journal of clinical pharmacy and therapeutics. 37 (3): 266–275. doi:10.1111/j.1365-2710.2011.01289.x. PMID 21950464.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ O'Shaughnessy, JA (2012). "Capecitabine monotherapy: review of studies in first-line HER-2-negative metastatic breast cancer". The oncologist. 17 (4): 476–84. doi:10.1634/theoncologist.2011-0281. PMID 22418569.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Petrelli, F (June 2012). "5-Fluorouracil or capecitabine in the treatment of advanced colorectal cancer: a pooled-analysis of randomized trials". Medical oncology (Northwood, London, England). 29 (2): 1020–1029. doi:10.1007/s12032-011-9958-0. PMID 21516482.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Barrios, CH (26 January 2012). "The Role of Capecitabine in Early Stage Breast Cancer". Current Breast Cancer Reports. 4 (1): 22–29. doi:10.1007/s12609-011-0067-z.
- ^ Comi, G (January 2013). "Cladribine tablets for the treatment of relapsing-remitting multiple sclerosis". Expert opinion on pharmacotherapy. 14 (1): 123–36. doi:10.1517/14656566.2013.754012. PMID 23256518.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Spurgeon, S (August 2009). "Cladribine: not just another purine analogue?". Expert Opinion on Investigational Drugs. 18 (8): 1169–81. doi:10.1517/13543780903071038. PMID 19604118.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bagnato, F; Pirko, I (October 2011). "Novel Agents and Emerging Treatment Strategies in Multiple Sclerosis. What Role for Cladribine?". Clinical Medicine Insights: Therapeutics: 425. doi:10.4137/CMT.S6456.
- ^ Leist, TP; Weissert, R (January–February 2011). "Cladribine: mode of action and implications for treatment of multiple sclerosis". Clinical neuropharmacology. 34 (1): 28–35. doi:10.1097/WNF.0b013e318204cd90. PMID 21242742.
- ^ Warnke, C (21 July 2010). "Identification of targets and new developments in the treatment of multiple sclerosis--focus on cladribine". Drug design, development and therapy. 4: 117–26. doi:10.2147/DDDT.S6627. PMC 2915536. PMID 20689698.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Sigal, DS (21 October 2010). "Beyond hairy cell: the activity of cladribine in other hematologic malignancies" (PDF). Blood. 116 (16): 2884–96. doi:10.1182/blood-2010-02-246140. PMID 20634380.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sipe, JC (March 2010). "Cladribine tablets: a potential new short-course annual treatment for relapsing multiple sclerosis". Expert Review of Neurotherapeutics. 10 (3): 365–75. doi:10.1586/ern.10.12. PMID 20187859.
- ^ Hartung, HP (February 2010). "Development of oral cladribine for the treatment of multiple sclerosis". Journal of neurology. 257 (2): 163–70. doi:10.1007/s00415-009-5359-0. PMID 19921304.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Warnke, C (January 2012). "Cladribine as a therapeutic option in multiple sclerosis". Clinical immunology (Orlando, Fla.). 142 (1): 68–75. doi:10.1016/j.clim.2011.05.009. PMID 21733757.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Warnke, C (21 July 2010). "Identification of targets and new developments in the treatment of multiple sclerosis--focus on cladribine". Drug design, development and therapy. 4: 117–126. doi:10.2147/DDDT.S6627. PMC 2915536. PMID 20689698.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Bryson, HM; Sorkin, EM (November 1993). "Cladribine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in haematological malignancies". Drugs. 46 (5): 872–894. doi:10.2165/00003495-199346050-00007. PMID 7507037.
- ^ Robak, T (January 2006). "Older and new formulations of cladribine. Pharmacology and clinical efficacy in hematological malignancies". Recent patents on anti-cancer drug discovery. 1 (1): 23–38. doi:10.2174/157489206775246467. PMID 18221024.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hentosh, P; Peffley, DM (June 2010). "The cladribine conundrum: deciphering the drug's mechanism of action". Expert opinion on drug metabolism & toxicology. 6 (1): 75–81. doi:10.1517/17425250903393745. PMID 19968576.
- ^ Warnke, C (January 2012). "Cladribine as a therapeutic option in multiple sclerosis". Clinical Immunology. 142 (1): 68–75. doi:10.1016/j.clim.2011.05.009. PMID 21733757.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kantarjian, HM (October 2007). "Clofarabine: past, present, and future". Leukemia & lymphoma. 48 (10): 1922–30. doi:10.1080/10428190701545644. PMID 17852710.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Harned, TM; Gaynon, PS (April 2008). "Treating refractory leukemias in childhood, role of clofarabine". Therapeutics and clinical risk management. 4 (2): 327–36. doi:10.2147/TCRM.S2941. PMC 2504075. PMID 18728851.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Thomas, X (October 2009). "Clofarabine for the treatment of adult acute myeloid leukemia". Future oncology (London, England). 5 (8): 1197–210. doi:10.2217/fon.09.105. PMID 19852733.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lech-Maranda, E (June 2009). "Clofarabine as a novel nucleoside analogue approved to treat patients with haematological malignancies: mechanism of action and clinical activity". Mini reviews in medicinal chemistry. 9 (7): 805–812. doi:10.2174/138955709788452586. PMID 19519505.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jeha, S (October 2009). "Recent progress in the treatment of acute lymphoblastic leukemia: clofarabine". Hematology/oncology clinics of North America. 23 (5): 1137–44, viii. doi:10.1016/j.hoc.2009.07.011. PMID 19825457.
- ^ Sampat, K (October 2009). "Clofarabine: emerging role in leukemias". Expert Opinion on Investigational Drugs. 18 (10): 1559–1564. doi:10.1517/13543780903173222. PMID 19715446.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Larson, ML; Venugopal, P (June 2009). "Clofarabine: a new treatment option for patients with acute myeloid leukemia". Expert opinion on pharmacotherapy. 10 (8): 1353–1357. doi:10.1517/14656560902997990. PMID 19463072.
- ^ Ghanem, H (February 2010). "Clofarabine in leukemia". Expert review of hematology. 3 (1): 15–22. doi:10.1586/ehm.09.70. PMID 21082931.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ghanem, H (April 2013). "The role of clofarabine in acute myeloid leukemia". Leukemia & lymphoma. 54 (4): 688–698. doi:10.3109/10428194.2012.726722. ISBN 978-0-12-374340-4. PMID 22957815.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ghanem, H (April 2013). "The role of clofarabine in acute myeloid leukemia". Leukemia & Lymphoma. 54 (4): 688–698. doi:10.3109/10428194.2012.726722. PMID 22957815.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hamada, A (2002). "Clinical pharmacokinetics of cytarabine formulations". Clinical pharmacokinetics. 41 (10): 705–718. doi:10.2165/00003088-200241100-00002. PMID 12162758.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cros, E (June 2004). "Problems related to resistance to cytarabine in acute myeloid leukemia". Leukemia & lymphoma. 45 (6): 1123–1132. doi:10.1080/1042819032000159861. PMID 15359991.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ El‐Subbagh, HI (2009). "2 Cytarabine". In Brittain, HG (ed.). Profiles of drug substances, excipients and related methodology (PDF). London: Academic. pp. 37–113. doi:10.1016/S0099-5428(08)00002-6. ISBN 978-0-12-374340-4. ISSN 0099-5428.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Dacogen (decitabine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 February 2014.
- ^ Plimack, ER (January 2007). "Decitabine and its role in the treatment of hematopoietic malignancies". Leukemia & Lymphoma. 48 (8): 1472–1481. doi:10.1080/10428190701471981. PMID 7701577.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Oki, Y (February 2007). "Decitabine—Bedside to bench". Critical Reviews in Oncology/Hematology. 61 (2): 140–152. doi:10.1016/j.critrevonc.2006.07.010. PMID 17023173.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jabbour, E (June 2008). "Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies" (PDF). Cancer. 112 (11): 2341–51. doi:10.1002/cncr.23463. PMID 18398832.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Santos, FP (January 2010). "Decitabine in the treatment of myelodysplastic syndromes". Expert review of anticancer therapy. 10 (1): 9–22. doi:10.1586/era.09.164. PMC 2376088. PMID 20014881.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Daskalakis, M (2010). "Decitabine". Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 184: 131–57. doi:10.1007/978-3-642-01222-8_10. PMID 20072836.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Marks, PW (March 2012). "Decitabine for acute myeloid leukemia". Expert review of anticancer therapy. 12 (3): 299–305. doi:10.1586/era.11.207. PMID 22369322.
- ^ Garcia, JS (June 2010). "An update on the safety and efficacy of decitabine in the treatment of myelodysplastic syndromes". OncoTargets and therapy. 3: 1–13. doi:10.2147/OTT.S7222. PMC 2895778. PMID 20616953.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Curran, MP (12 April 2013). "Decitabine: A Review of its Use in Older Patients with Acute Myeloid Leukaemia". Drugs & Aging. 30 (6): 447–458. doi:10.1007/s40266-013-0084-x. PMID 23580320.
- ^ "FUDR (floxuridine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 February 2014.
- ^ Montillo, M (September 2006). "Role of fludarabine in hematological malignancies". Expert review of anticancer therapy. 6 (9): 1141–61. doi:10.1586/14737140.6.9.1141. PMID 17020450.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Anderson, VR; Perry, CM (2007). "Fludarabine: a review of its use in non-Hodgkin's lymphoma". Drugs. 67 (11): 1633–55. doi:10.2165/00003495-200767110-00008. PMID 17661532.
- ^ a b Ricci, F (February 2009). "Fludarabine in the treatment of chronic lymphocytic leukemia: a review". Therapeutics and clinical risk management. 5 (1): 187–207. doi:10.2147/TCRM.S3688. PMC 2697528. PMID 19436622.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Souchet-Compain, L (June 2013). "Fludarabine in Waldenstrom's macroglobulinemia". Expert review of hematology. 6 (3): 229–37. doi:10.1586/ehm.13.17. PMID 23782076.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Plosker, GL; Figgitt, DP (2003). "Oral fludarabine". Drugs. 63 (21): 2317–23. doi:10.2165/00003495-200363210-00004. PMID 14524733.
- ^ Lukenbill, J; Kalaycio, M (September 2013). "Fludarabine: a review of the clear benefits and potential harms". Leukemia research. 37 (9): 986–94. doi:10.1016/j.leukres.2013.05.004. PMID 23787174.
- ^ Grem, JL (November 2000). "5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development". Investigational new drugs. 18 (4): 299–313. doi:10.1023/A:1006416410198. PMID 11081567.
- ^ Abraham, LM (2007). "The clinical applications of fluorouracil in ophthalmic practice". Drugs. 67 (2): 237–55. doi:10.2165/00003495-200767020-00005. PMID 17284086.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Patel, PA (April 2011). "Evolution of 5-fluorouracil-based chemoradiation in the management of rectal cancer". Anti-cancer drugs. 22 (4): 311–6. doi:10.1097/CAD.0b013e3283441a63. PMID 21301320.
- ^ Noble, S; Goa, KL (September 1997). "Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer". Drugs. 54 (3): 447–472. doi:10.2165/00003495-199754030-00009. PMID 9279506.
- ^ Aapro, MS (March 1998). "Gemcitabine--a safety review". Anti-cancer drugs. 9 (3): 191–201. doi:10.1097/00001813-199803000-00001. PMID 9625429.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ryan, CW; Vogelzang, NJ (March 2000). "Gemcitabine in the treatment of bladder cancer". Expert opinion on pharmacotherapy. 1 (3): 547–553. doi:10.1517/14656566.1.3.547. PMID 11249537.
- ^ Manegold, C (February 2000). "Gemcitabine in non-small cell lung cancer (NSCLC)". Investigational new drugs. 18 (1): 29–42. doi:10.1023/A:1006327729228. PMID 10830139.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Oettle, H (November 2000). "The role of gemcitabine alone and in combination in the treatment of pancreatic cancer". Anti-cancer drugs. 11 (10): 771–86. doi:10.1097/00001813-200011000-00001. PMID 11142685.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Crinò, L; Cappuzzo, F (June 2002). "Gemcitabine in non-small cell lung cancer". Expert opinion on pharmacotherapy. 3 (6): 745–753. doi:10.1517/14656566.3.6.745. PMID 12036414.
- ^ Wong, A (2009). "Clinical pharmacology and pharmacogenetics of gemcitabine". Drug metabolism reviews. 41 (2): 77–88. doi:10.1080/03602530902741828. PMID 19514966.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lennard, L (October 1992). "The clinical pharmacology of 6-mercaptopurine". European Journal of Clinical Pharmacology. 43 (4): 329–339. doi:10.1007/BF02220605. PMID 1451710.
- ^ Bradford, K; Shih, DQ (7 October 2011). "Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease". World journal of gastroenterology. 17 (37): 4166–73. doi:10.3748/wjg.v17.i37.4166. PMID 22072847.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Timmer, A (12 September 2012). "Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis". The Cochrane database of systematic reviews. 9: CD000478. doi:10.1002/14651858.CD000478.pub3. PMID 22972046.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Arranon (nelarabine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 11 February 2014.
- ^ Reilly, KM; Kisor, DF (February 2009). "Profile of nelarabine: use in the treatment of T-cell acute lymphoblastic leukemia". OncoTargets and therapy. 2: 219–28. PMC 2886323. PMID 20616909.
- ^ DeAngelo, DJ (October 2009). "Nelarabine for the treatment of patients with relapsed or refractory T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma". Hematology/oncology clinics of North America. 23 (5): 1121–35, vii–viii. doi:10.1016/j.hoc.2009.07.008. PMID 19825456.
- ^ Roecker, AM (December 2010). "Nelarabine in the treatment of refractory T-cell malignancies". Clinical Medicine Insights. Oncology. 4: 133–41. doi:10.4137/CMO.S4364. PMC 2999959. PMID 21151585.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sanford, M; Lyseng-Williamson, KA (2008). "Nelarabine". Drugs. 68 (4): 439–47. doi:10.2165/00003495-200868040-00004. PMID 18318562.
- ^ Gandhi, V; Plunkett, W (November 2006). "Clofarabine and nelarabine: two new purine nucleoside analogs". Current opinion in oncology. 18 (6): 584–90. doi:10.1097/01.cco.0000245326.65152.af. PMID 16988579.
- ^ Curbo, S; Karlsson, A (September 2006). "Nelarabine: a new purine analog in the treatment of hematologic malignancies". Reviews on recent clinical trials. 1 (3): 185–92. doi:10.2174/157488706778250104. PMID 18473971.
- ^ Kline, J; Larson, RA (September 2006). "Nelarabine in the treatment of refractory T-cell malignant diseases". Expert opinion on pharmacotherapy. 7 (13): 1791–9. doi:10.1517/14656566.7.13.1791. PMID 16925505.
- ^ Grever, MR (March 2003). "Pentostatin in the treatment of hairy-cell leukemia". Best practice & research. Clinical haematology. 16 (1): 91–9. doi:10.1016/S1521-6926(03)00002-1. PMID 12670468.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Higman, M (December 2004). "Pentostatin–pharmacology, immunology, and clinical effects in graft-versus-host disease". Expert Opinion on Pharmacotherapy. 5 (12): 2605–2613. doi:10.1517/14656566.5.12.2605. PMID 15571477.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ho, AD; Hensel, M (April 2006). "Pentostatin for the treatment of indolent lymphoproliferative disorders". Seminars in hematology. 43 (2 Suppl 2): S2-10. doi:10.1053/j.seminhematol.2005.12.005. PMID 16549110.
- ^ Grever, MR (October 2006). "Pentostatin: Impact on Outcome in Hairy Cell Leukemia". Hematology/Oncology Clinics of North America. 20 (5): 1099–1108. doi:10.1016/j.hoc.2006.06.001. PMID 16990110.
- ^ Sauter, C (September 2008). "Pentostatin in chronic lymphocytic leukemia". Expert opinion on drug metabolism & toxicology. 4 (9): 1217–22. doi:10.1517/17425255.4.9.1217. PMID 18721115.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lamanna, N; Kay, NE (June 2009). "Pentostatin treatment combinations in chronic lymphocytic leukemia". Clinical advances in hematology & oncology. 7 (6): 386–392. PMID 19606074.
- ^ Wellington, K; Goa, KL (2001). "Oral tegafur/uracil". Drugs & aging. 18 (12): 935–48, discussion 949–50. doi:10.2165/00002512-200118120-00005. PMID 11888348.
- ^ Takiuchi, H; Ajani, JA (August 1998). "Uracil-tegafur in gastric carcinoma: a comprehensive review". Journal of Clinical Oncology. 16 (8): 2877–85. PMID 9704742.
- ^ Ward, SE (3 July 2006). "The clinical and economic benefits of capecitabine and tegafur with uracil in metastatic colorectal cancer". British Journal of Cancer. 95 (1): 27–34. doi:10.1038/sj.bjc.6603215. PMID 16804526.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Casado, E (August 2008). "UFT (tegafur-uracil) in rectal cancer". Annals of Oncology. 19 (8): 1371–8. doi:10.1093/annonc/mdn067. PMID 18381370.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ishikawa, T (14 May 2008). "Chemotherapy with enteric-coated tegafur/uracil for advanced hepatocellular carcinoma". World journal of gastroenterology. 14 (18): 2797–801. doi:10.3748/wjg.14.2797. PMID 18473401.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Oba, K (April 2009). "Efficacy of adjuvant chemotherapy using tegafur-based regimen for curatively resected gastric cancer: update of a meta-analysis". International journal of clinical oncology. 14 (2): 85–9. doi:10.1007/s10147-009-0877-4. PMID 19390937.
- ^ Matt, P (2011). "The European Medicines Agency review of Tegafur/Gimeracil/Oteracil (Teysuno™) for the treatment of advanced gastric cancer when given in combination with cisplatin: summary of the Scientific Assessment of the Committee for medicinal products for human use (CHMP)". The oncologist. 16 (10): 1451–7. doi:10.1634/theoncologist.2011-0224. PMID 21963999.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Estlin, EJ (December 2001). "Continuing therapy for childhood acute lymphoblastic leukaemia: clinical and cellular pharmacology of methotrexate, 6-mercaptopurine and 6-thioguanine". Cancer treatment reviews. 27 (6): 351–63. doi:10.1053/ctrv.2002.0245. PMID 11908928.
- ^ Elgemeie, GH (2003). "Thioguanine, mercaptopurine: their analogs and nucleosides as antimetabolites". Current pharmaceutical design. 9 (31): 2627–42. doi:10.2174/1381612033453677. PMID 14529546.
- ^ Duley, JA; Florin, TH (October 2005). "Thiopurine therapies: problems, complexities, and progress with monitoring thioguanine nucleotides". Therapeutic drug monitoring. 27 (5): 647–654. doi:10.1097/01.ftd.0000169061.52715.3e. PMID 16175140.
- ^ De Bruyne, R (February 2006). "Chronic liver disease related to 6-thioguanine in children with acute lymphoblastic leukaemia". Journal of hepatology. 44 (2): 407–10. doi:10.1016/j.jhep.2005.06.020. PMID 16226335.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Seinen, ML (September 2010). "Dosing 6-thioguanine in inflammatory bowel disease: expert-based guidelines for daily practice" (PDF). Journal of gastrointestinal and liver diseases. 19 (3): 291–4. PMID 20922194.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tung, JP; Maibach, HI (November 1990). "The practical use of methotrexate in psoriasis". Drugs. 40 (5): 697–712. doi:10.2165/00003495-199040050-00005. PMID 2292232.
- ^ Bannwarth, B (January 1994). "Methotrexate in rheumatoid arthritis. An update". Drugs. 47 (1): 25–50. doi:10.2165/00003495-199447010-00003. PMID 7510620.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Barnhart, K (March 2001). "The pharmacology of methotrexate". Expert opinion on pharmacotherapy. 2 (3): 409–17. doi:10.1517/14656566.2.3.409. PMID 11336595.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Khan, ZA (February 2012). "Methotrexate: a detailed review on drug delivery and clinical aspects". Expert Opinion on Drug Delivery. 9 (2): 151–69. doi:10.1517/17425247.2012.642362. PMID 22251428.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Graber, JJ; Omuro, A (1 June 2011). "Pharmacotherapy for primary CNS lymphoma: progress beyond methotrexate?". CNS Drugs. 25 (6): 447–57. doi:10.2165/11589030-000000000-00000. PMID 21649446.
- ^ Dogra, S; Mahajan, R (August 2013). "Systemic methotrexate therapy for psoriasis: past, present and future". Clinical and Experimental Dermatology. 38 (6): 573–588. doi:10.1111/ced.12062. PMID 23837932.
- ^ Curtin, NJ; Hughes, AN (May 2001). "Pemetrexed disodium, a novel antifolate with multiple targets". The Lancet Oncology. 2 (5): 298–306. doi:10.1016/S1470-2045(00)00325-9. PMID 11905785.
- ^ Gatzemeier, U (November 2004). "Pemetrexed in malignant pleural mesothelioma". Oncology (Williston Park, N.Y.). 18 (13 Suppl 8): 26–31. PMID 15655933.
- ^ Sobrero, A (November 2004). "Pemetrexed in gastric cancer". Oncology (Williston Park, N.Y.). 18 (13 Suppl 8): 51–5. PMID 15655938.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hazarika, M (1 February 2005). "Pemetrexed in malignant pleural mesothelioma" (PDF). Clinical cancer research: an official journal of the American Association for Cancer Research. 11 (3): 982–92. PMID 15709163.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Puto, K; Garey, JS (April 2005). "Pemetrexed therapy for malignant pleural mesothelioma". The Annals of pharmacotherapy. 39 (4): 678–83. doi:10.1345/aph.1E329. PMID 15755794.
- ^ Villela, LR (May 2006). "Pemetrexed, a novel antifolate therapeutic alternative for cancer chemotherapy". Pharmacotherapy. 26 (5): 641–54. doi:10.1592/phco.26.5.641. PMID 16637794.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Goeminne, H (May 2006). "Pemetrexed in thoracic cancer". Expert opinion on pharmacotherapy. 7 (7): 917–28. doi:10.1517/14656566.7.7.917. PMID 16634714.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Monnerat, C (May 2006). "Review of the pemetrexed and gemcitabine combination in patients with advanced-stage non-small cell lung cancer" (PDF). Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 17 Suppl 5: v86-90. doi:10.1093/annonc/mdj958. PMID 16807472.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Longo-Sorbello, GS (February 2007). "Role of pemetrexed in non-small cell lung cancer". Cancer Investigation. 25 (1): 59–66. doi:10.1080/07357900601130748. PMID 17364559.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Chattopadhyay, S (February 2007). "Pemetrexed: Biochemical and cellular pharmacology, mechanisms, and clinical applications". Molecular Cancer Therapeutics. 6 (2): 404–417. doi:10.1158/1535-7163.MCT-06-0343. PMID 17308042.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Meriggi, F (December 2007). Chemotherapy. 54 (1): 1–8. doi:10.1159/000112311. PMID 18063861.
{{cite journal}}: Missing or empty|title=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Kulkarni, PM (July 2008). "Efficacy and safety of pemetrexed in elderly cancer patients: results of an integrated analysis". Critical reviews in oncology/hematology. 67 (1): 64–70. doi:10.1016/j.critrevonc.2008.01.011. PMID 18358737.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Felip, E; Rosell, R (June 2008). "Pemetrexed as second-line therapy for advanced non-small-cell lung cancer (NSCLC)". Therapeutics and clinical risk management. 4 (3): 579–85. doi:10.2147/TCRM.S2248. PMC 2500250. PMID 18827853.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hsu, JY; Wakelee, H (September 2008). "Pemetrexed disodium for the treatment of NSCLC: an update". Drugs of today (Barcelona, Spain: 1998). 44 (9): 669–78. doi:10.1358/dot.2008.44.9.1250412. PMID 19137122.
- ^ Peake, MD (July 2009). "Pemetrexed in the treatment of malignant pleural mesothelioma". Therapy. 6 (4): 569–575. doi:10.2217/thy.09.30.
- ^ Manegold, C (September 2009). "Pemetrexed for the treatment of non-small-cell lung cancer". Expert review of anticancer therapy. 9 (9): 1195–209. doi:10.1586/ERA.09.97. PMID 19761423.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tomao, F (December 2009). "Emerging role of pemetrexed in ovarian cancer". Expert review of anticancer therapy. 9 (12): 1727–35. doi:10.1586/ERA.09.141. PMID 19954283.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fleeman, N (May 2010). "Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer". Health technology assessment (Winchester, England). 14 Suppl 1: 47–53. doi:10.3310/hta14Suppl1/07. PMID 20507803.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fuld, AD (June 2010). "Pemetrexed in advanced non-small-cell lung cancer". Expert opinion on pharmacotherapy. 11 (8): 1387–402. doi:10.1517/14656566.2010.482560. PMID 20446853.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Somer, R (April 2010). "Maintenance therapy for metastatic non-small-cell lung cancer – the role of pemetrexed" (PDF). Lung Cancer: Targets and Therapy. 1: 1–7. doi:10.2147/LCTT.S7105.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Greenhalgh, J (October 2010). "Pemetrexed for the maintenance treatment of locally advanced or metastatic non-small cell lung cancer". Health technology assessment (Winchester, England). 14 (Suppl. 2): 33–39. doi:10.3310/hta14suppl2/05. PMID 21047489.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gridelli, C (March 2011). "Pemetrexed in advanced non-small cell lung cancer". Expert opinion on drug safety. 10 (2): 311–7. doi:10.1517/14740338.2011.553281. PMID 21261558.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Morotti, M (April 2012). "Pemetrexed disodium in ovarian cancer treatment". Expert Opinion on Investigational Drugs. 21 (4): 437–49. doi:10.1517/13543784.2012.661714. PMID 22324304.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Argiris, A (June 2013). "Pemetrexed in head and neck cancer: a systematic review". Oral oncology. 49 (6): 492–501. doi:10.1016/j.oraloncology.2013.01.007. PMID 23466170.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Boons, CC (September 2013). "The value of pemetrexed for the treatment of malignant pleural mesothelioma: a comprehensive review". Anticancer research. 33 (9): 3553–61. PMID 24023280.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gunasekara, NS; Faulds, D (March 1998). "Raltitrexed. A review of its pharmacological properties and clinical efficacy in the management of advanced colorectal cancer". Drugs. 55 (3): 423–35. doi:10.2165/00003495-199855030-00012. PMID 9530547.
- ^ Clarke, SJ (December 2000). "Clinical and preclinical pharmacokinetics of raltitrexed". Clinical pharmacokinetics. 39 (6): 429–443. doi:10.1517/13543784.7.5.823. PMID 11192475.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Caponigro, F (July 2001). "Raltitrexed/5-fluorouracil-based combination chemotherapy regimens in anticancer therapy". Anti-cancer drugs. 12 (6): 489–97. doi:10.1097/00001813-200107000-00001. PMID 11459994.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Caponigro, F (July 2001). "Raltitrexed/5-fluorouracil-based combination chemotherapy regimens in anticancer therapy". Anti-cancer drugs. 12 (6): 489–97. doi:10.1097/00001813-200107000-00001. PMID 11459994.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cunningham, D (March 2002). "Efficacy, tolerability and management of raltitrexed (Tomudex™) monotherapy in patients with advanced colorectal cancer". European Journal of Cancer. 38 (4): 478–486. doi:10.1016/S0959-8049(01)00413-0. PMID 11872339.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Van Cutsem, E (1 April 2002). "Raltitrexed: current clinical status and future directions". Annals of Oncology. 13 (4): 513–522. doi:10.1093/annonc/mdf054. PMID 12056700.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cao, S (April 2006). "Irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer". Expert opinion on pharmacotherapy. 7 (6): 687–703. doi:10.1517/14656566.7.6.687. PMID 16556086.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wilson, KS (November 2009). "Raltitrexed: optimism and reality". Expert opinion on drug metabolism & toxicology. 5 (11): 1447–1454. doi:10.1517/17425250903307455. PMID 19863453.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mulaku, M (November 2013). "Evidence review of hydroxyurea for the prevention of sickle cell complications in low-income countries". Archives of Disease in Childhood. 98 (11): 908–14. doi:10.1136/archdischild-2012-302387. PMC 3812872. PMID 23995076.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Navarra, P; Preziosi, P (February 1999). "Hydroxyurea: new insights on an old drug". Critical reviews in oncology/hematology. 29 (3): 249–55. doi:10.1016/S1040-8428(98)00032-8. PMID 10226728.
- ^ Romanelli, F (February 1999). "Hydroxyurea to inhibit human immunodeficiency virus-1 replication". Pharmacotherapy. 19 (2): 196–204. doi:10.1592/phco.19.3.196.30913. PMID 10030769.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vichinsky, EP (July 1997). "Hydroxyurea in children: present and future". Seminars in Hematology. 34 (3 Suppl 3): 22–9. PMID 9317198.
- ^ Gwilt, PR; Tracewell, WG (May 1998). "Pharmacokinetics and pharmacodynamics of hydroxyurea". Clinical pharmacokinetics. 34 (5): 347–358. doi:10.2165/00003088-199834050-00002. PMID 9592619.
- ^ Ravot, Elisabetta (1999). "New Uses for Old Drugs in HIV Infection". Drugs. 58 (6): 953–963. doi:10.2165/00003495-199958060-00001. PMID 10651384.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Hydroxyurea" (PDF). 76. 2000.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Banan, M (March 2013). "Hydroxyurea treatment in β-thalassemia patients: to respond or not to respond?". Annals of hematology. 92 (3): 289–99. doi:10.1007/s00277-012-1671-3. PMID 23318979.
- ^ Dingli, D; Tefferi, A (June 2006). "Hydroxyurea: The drug of choice for polycythemia vera and essential thrombocythemia". Current Hematologic Malignancy Reports. 1 (2): 69–74. doi:10.1007/s11899-006-0025-4. PMID 20425334.
- ^ Lisziewicz, J (2003). "Hydroxyurea in the treatment of HIV infection: clinical efficacy and safety concerns". Drug safety. 26 (9): 605–24. doi:10.2165/00002018-200326090-00002. PMID 12814330.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i Bhatia, S (2008). "Secondary Malignancies: Therapy-Related t-MDS/AML". Medscape Reference. WebMD. Retrieved 7 February 2014.
- ^ Shimada, M (May 2013). "Nedaplatin: a cisplatin derivative in cancer chemotherapy". Cancer management and research. 5: 67–76. doi:10.2147/CMAR.S35785. PMC 3658440. PMID 23696716.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Ravandi, F (17 September 2012). "Gemtuzumab Ozogamicin: Time to Resurrect?" (PDF). Journal of Clinical Oncology. 30 (32): 3921–3923. doi:10.1200/JCO.2012.43.0132. PMID 22987091.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lehnert, M (February 2009). "Update on the rational use of Y-ibritumomab tiuxetan in the treatment of follicular lymphoma". OncoTargets and therapy. 2: 199–208. PMC 2886339. PMID 20616907.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Cheson, BD (2005). "The role of radioimmunotherapy with yttrium-90 ibritumomab tiuxetan in the treatment of non-Hodgkin lymphoma". BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 19 (5): 309–22. doi:10.2165/00063030-200519050-00004. PMID 16207072.
- ^ Nabhan, C; Kay, NE (March 2011). "The emerging role of ofatumumab in the treatment of chronic lymphocytic leukemia". Clinical Medicine Insights. Oncology. 5: 45–53. doi:10.4137/CMO.S4087. PMC 3076040. PMID 21499555.
- ^ Osterborg, A (March 2010). "Ofatumumab, a human anti-CD20 monoclonal antibody". Expert Opinion on Biological Therapy. 10 (3): 439–49. doi:10.1517/14712590903586239. PMID 20109133.
- ^ Sanford, M; McCormack, PL (May 2010). "Ofatumumab". Drugs. 70 (8): 1013–1019. doi:10.2165/11203850-000000000-00000. PMID 20481657.
- ^ Davies, A J (28 May 2007). "Radioimmunotherapy for B-cell lymphoma: Y90 ibritumomab tiuxetan and I131 tositumomab". Oncogene. 26 (25): 3614–3628. doi:10.1038/sj.onc.1210378. PMID 17530015.
- ^ "Bexxar (tositumomab) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ "Gilotrif (afatinib)". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ "Zaltrap (ziv-aflibercept) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ Escudier, B; Gore, M (2011). "Axitinib for the management of metastatic renal cell carcinoma". Drugs in R&D. 11 (2): 113–26. doi:10.2165/11591240-000000000-00000. PMC 3585900. PMID 21679004.
- ^ "Bosulif (bosutinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ Koschmieder, S (March 2013). "Profile of bosutinib and its clinical potential in the treatment of chronic myeloid leukemia". OncoTargets and Therapy. 6: 99–106. doi:10.2147/OTT.S19901. PMC 3594007. PMID 23493838.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ "Xalkori (crizotinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ Forde, PM; Rudin, CM (June 2012). "Crizotinib in the treatment of non-small-cell lung cancer". Expert opinion on pharmacotherapy. 13 (8): 1195–201. doi:10.1517/14656566.2012.688029. PMID 22594847.
- ^ Frampton, JE (28 November 2013). "Crizotinib: A Review of Its Use in the Treatment of Anaplastic Lymphoma Kinase-Positive, Advanced Non-Small Cell Lung Cancer". Drugs. 73 (18): 2031–2051. doi:10.1007/s40265-013-0142-z. PMID 24288180.
- ^ Rothschild, SI; Gautschi, O (September 2013). "Crizotinib in the Treatment of Non–Small-Cell Lung Cancer". Clinical Lung Cancer. 14 (5): 473–480. doi:10.1016/j.cllc.2013.04.006.
- ^ Roskoski, R (September 2013). "The preclinical profile of crizotinib for the treatment of non-small-cell lung cancer and other neoplastic disorders". Expert Opinion on Drug Discovery. 8 (9): 1165–1179. doi:10.1517/17460441.2013.813015. PMID 23805942.
- ^ "Iclusig (ponatinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ Price, KE (August 2013). "Potential of ponatinib to treat chronic myeloid leukemia and acute lymphoblastic leukemia". OncoTargets and therapy. 6: 1111–8. doi:10.2147/OTT.S36980. PMC 3754816. PMID 23986642.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ "Stivarga (regorafenib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ Strumberg, D; Schultheis, B (June 2012). "Regorafenib for cancer". Expert Opinion on Investigational Drugs. 21 (6): 879–889. doi:10.1517/13543784.2012.684752. PMID 22577890.
- ^ Aprile, G (23 February 2013). "Regorafenib for Gastrointestinal Malignancies". BioDrugs. 27 (3): 213–224. doi:10.1007/s40259-013-0014-9. PMID 23435872.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Shahda, S; Saif, MW (May 2013). "Regorafenib: from bench to bedside in colorectal cancer". Expert Review of Clinical Pharmacology. 6 (3): 243–248. doi:10.1586/ecp.13.11. PMID 23656338.
- ^ "Jakafi (ruxolitinib) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 February 2014.
- ^ "JAKAVI® Ruxolitinib" (PDF). TGA eBusiness Services. Novartis Pharmaceuticals Australia Pty Limited. 21 January 2014.
- ^ Kantarjian, HM (December 2013). "Ruxolitinib for Myelofibrosis–An Update of Its Clinical Effects" (PDF). Clinical Lymphoma Myeloma and Leukemia. 13 (6): 638–645. doi:10.1016/j.clml.2013.09.006. PMID 24238036.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vaddi, K (November 2012). "Ruxolitinib, an oral JAK1 and JAK2 inhibitor, in myelofibrosis". Expert Opinion on Pharmacotherapy. 13 (16): 2397–2407. doi:10.1517/14656566.2012.732998. PMID 23051187.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Vandetanib dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ "Targretin (bexarotene) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ Matthay, KK (3 December 2012). "Targeted Isotretinoin in Neuroblastoma: Kinetics, Genetics, or Absorption". Clinical Cancer Research. 19 (2): 311–313. doi:10.1158/1078-0432.CCR-12-3313. PMID 23209029.
- ^ "Amnesteem, Claravis (isotretinoin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ "Isotretinoin 20mg capsules - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Alliance Pharmaceuticals. 12 April 2013. Retrieved 9 February 2014.
- ^ Miwako, I; Kagechika, H (August 2007). "Tamibarotene". Drugs of today (Barcelona, Spain: 1998). 43 (8): 563–8. doi:10.1358/dot.2007.43.8.1072615. PMID 17925887.
- ^ Fukasawa, H (2012). "Tamibarotene: a candidate retinoid drug for Alzheimer's disease". Biological & Pharmaceutical Bulletin. 35 (8): 1206–12. doi:10.1248/bpb.b12-00314. PMID 22863914.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Takeuchi, M (March 2006). "[Clinical experience with a new synthetic retinoid, tamibarotene (Am-80) for relapsed or refractory acute promyelocytic leukemia]". Gan to kagaku ryoho. Cancer & chemotherapy (in Japanese). 33 (3): 397–401. PMID 16531727.
- ^ "Istodax (romidepsin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 February 2014.
- ^ Bellos, F; Mahlknecht, U (November 2008). "Valproic acid and all-trans retinoic acid: meta-analysis of a palliative treatment regimen in AML and MDS patients". Onkologie. 31 (11): 629–33. doi:10.1159/000160599. PMID 19145098.
- ^ Blaheta, RA (August 2002). "Valproate and valproate-analogues: potent tools to fight against cancer". Current medicinal chemistry. 9 (15): 1417–33. doi:10.2174/0929867023369763. PMID 12173980.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Blaheta, RA (September 2002). "Anti-tumor mechanisms of valproate: a novel role for an old drug". Medicinal Research Reviews. 22 (5): 492–511. doi:10.1002/med.10017. PMID 12210556.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Duenas-Gonzalez, A (May 2008). "Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors". Cancer treatment reviews. 34 (3): 206–22. doi:10.1016/j.ctrv.2007.11.003. PMID 18226465.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Zolinza (vorinostat) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 February 2014.
- ^ "Ontak (denileukin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 February 2014.
- ^ Lansigan, F (February 2010). "Role of denileukin diftitox in the treatment of persistent or recurrent cutaneous T-cell lymphoma". Cancer Management and Research. 2: 53–59. doi:10.2147/CMAR.S5009. PMC 3004568. PMID 21188096.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Manoukian, G; Hagemeister, F (November 2009). "Denileukin diftitox: a novel immunotoxin". Expert Opinion on Biological Therapy. 9 (11): 1445–1451. doi:10.1517/14712590903348135. PMID 19817678.
- ^ Hymes, KB; Kaminetzky, D (December 2008). "Denileukin diftitox for the treatment of cutaneous T-cell lymphoma". Biologics: Targets & Therapy. 2 (4): 717–724. doi:10.2147/BTT.S3084. PMC 2727893. PMID 19707452.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
Bibliography
- Rachel Airley (2009). Cancer chemotherapy. Wiley-Blackwell. ISBN 0-470-09254-8.
- Wood, Miriam; David Brighton (2005). The Royal Marsden Hospital handbook of cancer chemotherapy: a guide for the multidisciplinary team. St. Louis, Mo: Elsevier Churchill Livingstone. ISBN 0-443-07101-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Fergus Macbeth; Hanna, Louise; Crosby, Tom (2008). Practical clinical oncology. Cambridge, UK: Cambridge University Press.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Perry, Michael J. (2008). The Chemotherapy source book. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
- Faguet, Guy B. (2005). The War on Cancer. Springer. p. 71. ISBN 1-4020-3618-3.
- Hirsch J (September 2006). "An anniversary for cancer chemotherapy". JAMA. 296 (12): 1518–20. doi:10.1001/jama.296.12.1518. PMID 17003400.
- Huang, Elbert S. (2000). Internal medicine: handbook for clinicians, resident survival guide. Arlington, VA: Scrub Hill Press. p. 130. ISBN 978-0-9645467-5-2.
- Randall, [edited by] William J. Hoskins, Carlos A. Perez, Robert C. Young, Richard R. Barakat, Maurie Markman, Marcus E. (2005). Principles and practice of gynecologic oncology (4. ed.). Baltimore, Md.: Lippincott Williams & Wilkins. pp. 93–94. ISBN 0781746892.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link)
