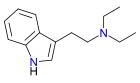
Diethyltryptamine
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H20N2 |
| Molar mass | 216.32 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 169 to 171 °C (336 to 340 °F) |
| |
DET, also known under its chemical name N,N-diethyltryptamine and as T-9 [1], is a psychedelic drug closely related to DMT and 4-HO-DET. However, despite its structural similarity to DMT it is active orally around 50–100 mg without the aid of MAO inhibitors lasting about 2-4 hours.
Chemistry
DET is an analogue the common tryptamine hallucinogen N,N-Dimethyltryptamine or DMT. DET is sometimes preferred over DMT because it can be taken orally whereas DMT cannot. This is because the enzyme monoamine oxidase degrades DMT into an inactive compound before it is absorbed. To overcome this, it must be adminstered in a different manner, i.e. intravenously, intramuscularly, by inhalation, by insufulation, or rectally. Because DET has ethyl groups attached to its nitrogen atom monoamine oxidase is unable to degrade it. This is true for many other tryptamines with larger nitrogen substitutents.
Pharmacology
The mechanism of action is thought to be serotonin receptor agonism, much like other classic psychedelics.[2]
Biochemistry
Though DET is a synthetic compound with no known natural sources it has been used with mycelium of Psilocybe cubensis to produce the synthetic chemicals 4-PO-DET and 4-HO-DET, as opposed the naturally occurring 4-PO-DMT (Psilocybin) and 4-HO-DMT (Psilocin). Isolation of the alkaloids resulted in 3.3% 4-HO-DET and 0.01-0.8% 4-PO-DET.[3]
Psychosis model
Early studies of DET, as well as other psychedelics, mainly focused on the believed psychotomimetic properties.[4] Researchers theorized that abnormal metabolites of endogenous chemicals such as tryptamine, serotonin, and tryptophan could be the explanation for mental disorders as schizophrenia, or psychosis.[5] With the progression of science and pharmacological understanding this belief remains dismissed by most researchers.
See also
References
- ^ "Erowid DET Vault : Chemistry". Retrieved 2008-01-08.
- ^ Winter JC (1969). "Behavioral effects of N,N-diethyltryptamine: absence of antagonism by xylamidine tosylate". J. Pharmacol. Exp. Ther. 169 (1): 7–16. PMID 5306645.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gartz J (1989). "Biotransformation of tryptamine derivatives in mycelial cultures of Psilocybe". J. Basic Microbiol. 29 (6): 347–52. doi:10.1002/jobm.3620290608. PMID 2614674.
- ^ "PSILOCYBIN AND DIETHYLTRYPTAMINE: TWO TRYPTAMINE HALLUCINOGENS". Retrieved 2008-01-03.
{{cite web}}: line feed character in|title=at position 34 (help) - ^ "Effects of LSD-25, N,N-Dimethyltryptamine (DMT), and N,N-Diethyltryptamine (DET) on the Photic Evoked Responses in the unanesthetized Rabbit". Retrieved 2008-01-03.
