Tandamine
Tools
Actions
General
Print/export
In other projects
Appearance
From Wikipedia, the free encyclopedia
Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
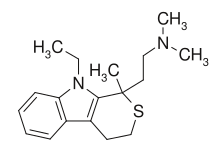
| Formula | C18H26N2S |
| Molar mass | 302.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tandamine is a selective norepinephrine reuptake inhibitor with a tricyclic structure.[1][2][3] It was developed in the 1970s as an antidepressant but was never commercialized.[1][2][3] Tandamine is analogous to pirandamine, which, instead, acts as a selective serotonin reuptake inhibitor (SSRI).[4][5]
The exact identical same structure, although this time changing the thioether to a methylene group revealed a strongest compound of the series called AY 24614.[6]
See also
[edit]References
[edit]- ^ a b Lippmann W, Pugsley TA (May 1976). "The effects of tandamine, a new potential antidepressant agent, on biogenic amine uptake mechanisms and related activities". Biochemical Pharmacology. 25 (10): 1179–1186. doi:10.1016/0006-2952(76)90366-X. PMID 1084746.
- ^ a b Ehsanullah RS, Ghose K, Kirby MJ, Turner P, Witts D (March 1977). "Clinical pharmacological studies of tandamine, a potential antidepressive drug". Psychopharmacology. 52 (1): 73–77. doi:10.1007/BF00426603. PMID 403562. S2CID 23960347.
- ^ a b Pugsley TA, Lippmann W (September 1979). "Effect of acute and chronic treatment of tandamine, a new heterocyclic antidepressant, on biogenic amine metabolism and related activities". Naunyn-Schmiedeberg's Archives of Pharmacology. 308 (3): 239–247. doi:10.1007/BF00501388. PMID 503251. S2CID 23533861.
- ^ Pugsley T, Lippmann W (May 1976). "Effects of tandamine and pirandamine, new potential antidepressants, on the brain uptake of norepinephrine and 5-hydroxytryptamine and related activities". Psychopharmacology. 47 (1): 33–41. doi:10.1007/BF00428698. PMID 1085452. S2CID 8354739.
- ^ Lippmann W, Seethaler K (April 1977). "Effects of tandamine and pirandamine, selective blockers of biogenic amine uptake mechanisms, on gastric acid secretion and ulcer formation in the rat". Life Sciences. 20 (8): 1393–1400. doi:10.1016/0024-3205(77)90367-8. PMID 853871.
- ^ Asselin AA, Humber LG, Komlossy J (June 1976). "Cycloalkanoindoles. 2. 1-Alkyl-1,2,3,4-tetrahydrocarbazole-1-ethanamines and related compounds. Potential antidepressants". Journal of Medicinal Chemistry. 19 (6): 792–797. doi:10.1021/jm00228a011. PMID 950648.
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| Classes | |
|---|---|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others | |
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
Hidden categories:
- Articles with short description
- Short description matches Wikidata
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Multiple chemicals in Infobox drug
- Chemicals using indexlabels
- Chemical articles with multiple CAS registry numbers
- Drugs with no legal status
- All stub articles
