COVID-19: Difference between revisions
Fixing reference errors |
→Prognosis: The paragraph was apparently wrong: Chinese not WHO. Wrong case fatality rate. Other numbers imprecise |
||
| Line 150: | Line 150: | ||
== Prognosis == |
== Prognosis == |
||
[[File:Illustration of SARS-COV-2 Case Fatality Rate 200228 01-1.png|thumb|upright=1.4|Fatality rates by age group in China<ref>[http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020]. China CDC Weekly, 2020, 2(8): 113-122.</ref>]] |
[[File:Illustration of SARS-COV-2 Case Fatality Rate 200228 01-1.png|thumb|upright=1.4|Fatality rates by age group in China<ref>[http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020]. China CDC Weekly, 2020, 2(8): 113-122.</ref>]] |
||
According to WHO, based on analysis of 44,000 cases of COVID-19 in [[Hubei]] province, around 80% of people have a mild form of the disease, 14% developed more severe disease such as pneumonia, 5% have critical disease, and 3.7% of cases are fatal.<ref>{{Cite news|url=https://www.nytimes.com/2020/03/04/world/coronavirus-news.html|title=Coronavirus Live Updates: W.H.O. Says Covid-19 Has Higher Fatality Rate Than the Flu|date=2020-03-04|work=The New York Times|access-date=2020-03-04|language=en-US|issn=0362-4331}}</ref><ref name=chinacdcweekly>{{cite journal | author = The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. | title = The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020 | volume = 2 | issue = 8 | pages = 113–122 | journal = China CDC Weekly 2020 | url = http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 | access-date = 19 February 2020 | archive-url = https://web.archive.org/web/20200219142101/http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 | archive-date = 19 February 2020 | url-status = live | date = February 2020 }}</ref> |
|||
Among those who died, many had preexisting conditions, including [[hypertension]], [[diabetes]], and [[cardiovascular disease]],<ref>{{Cite web |url=https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-the-advice-of-the-ihr-emergency-committee-on-novel-coronavirus |title=WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus |website=who.int}}</ref> and the median time from initial symptoms to death was 14 days (range 6–41 days).<ref>{{cite journal | vauthors = Wang W, Tang J, Wei F | title = Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China | journal = Journal of Medical Virology | volume = 92 | issue = 4 | pages = 441–447 | date = April 2020 | pmid = 31994742 | doi = 10.1002/jmv.25689 }}</ref> In a study by the National Health Commission (NHC) of China, men had a death rate of 2.8% while women had a death rate of 1.7%.<ref name=WM2020Feb26>{{cite web |title=Coronavirus Age, Sex, Demographics (COVID-19) - Worldometer |url=https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ |website=www.worldometers.info |access-date=26 February 2020 |language=en |archive-url=https://web.archive.org/web/20200227112932/https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ |archive-date=27 February 2020 |url-status=live }}</ref> In those under the age of 50 the risk of death is less than 0.5% while in those over the age of 70 it is more than 8%.<ref name=WM2020Feb26/> No deaths had occurred under the age of 10 {{As of|2020|2|26|df=|lc=y}}.<ref name=WM2020Feb26/> |
Among those who died, many had preexisting conditions, including [[hypertension]], [[diabetes]], and [[cardiovascular disease]],<ref>{{Cite web |url=https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-the-advice-of-the-ihr-emergency-committee-on-novel-coronavirus |title=WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus |website=who.int}}</ref> and the median time from initial symptoms to death was 14 days (range 6–41 days).<ref>{{cite journal | vauthors = Wang W, Tang J, Wei F | title = Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China | journal = Journal of Medical Virology | volume = 92 | issue = 4 | pages = 441–447 | date = April 2020 | pmid = 31994742 | doi = 10.1002/jmv.25689 }}</ref> In a study by the National Health Commission (NHC) of China, men had a death rate of 2.8% while women had a death rate of 1.7%.<ref name=WM2020Feb26>{{cite web |title=Coronavirus Age, Sex, Demographics (COVID-19) - Worldometer |url=https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ |website=www.worldometers.info |access-date=26 February 2020 |language=en |archive-url=https://web.archive.org/web/20200227112932/https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ |archive-date=27 February 2020 |url-status=live }}</ref> In those under the age of 50 the risk of death is less than 0.5% while in those over the age of 70 it is more than 8%.<ref name=WM2020Feb26/> No deaths had occurred under the age of 10 {{As of|2020|2|26|df=|lc=y}}.<ref name=WM2020Feb26/> |
||
Revision as of 23:16, 5 March 2020
Template:Use Commonwealth English
| Coronavirus disease 2019 (COVID-19) | |
|---|---|
| Other names | |
 | |
| Symptoms | |
| Pronunciation | |
| Specialty | Acute respiratory infection[6] |
| Symptoms | Fever, cough, shortness of breath[7] |
| Complications | Pneumonia, ARDS, kidney failure |
| Causes | SARS-CoV-2 |
| Diagnostic method | rRT-PCR testing, immunoassay, CT scan |
| Prevention | Hand washing, cough etiquette, avoiding close contact with sick people |
| Treatment | Symptomatic and supportive |
| Frequency | 97,000+ cases, 53,000+ now recovered[8] |
| Deaths | 6,881,955[9] |
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS coronavirus 2 or SARS-CoV-2),[10] a virus closely related to the SARS virus.[11][12][13] The disease was discovered and named during the 2019–20 coronavirus outbreak.[14][15] Those affected may develop a fever, dry cough, fatigue, and shortness of breath.[7][16][17] A sore throat, runny nose or sneezing is less common.[18] While the majority of cases result in mild symptonms[19] some can progress to pneumonia and multi-organ failure.[14][15]
The infection is spread from one person to others via respiratory droplets produced from the airways, often during coughing or sneezing.[20][21] Time from exposure to onset of symptoms is generally between 2 and 14 days, with an average of 5 days.[22][23][24] The standard method of diagnosis is by reverse transcription polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab or sputum sample, with results within a few hours to 2 days. Antibody assays can also be used, using a blood serum sample, with results within a few days.[25] The infection can also be diagnosed from a combination of symptoms, risk factors, and a chest CT scan showing features of pneumonia.[26][27]
Hand washing, maintaining distance from people who are coughing and not touching one's face with unwashed hands are measures recommended to prevent the disease.[28] It is recommended to cover one's nose and mouth with a tissue or a bent elbow when coughing.[28] Those who suspect they carry the virus are recommended to wear a surgical face mask and seek medical advice by calling a doctor rather than visiting a clinic in person. Masks are also recommended for those who are taking care of someone with a suspected infection but not for the general public.[29][30] There is no vaccine or specific antiviral treatment, with management involving treatment of symptoms, supportive care, and experimental measures.[31] The case fatality rate is estimated at between 1% and 3%.[32][33]
The WHO has declared the 2019–20 coronavirus outbreak to be a Public Health Emergency of International Concern (PHEIC).[34][35] As of 29 February 2020, China, Hong Kong, Iran, Italy, Japan, Singapore, South Korea and the United States are areas having evidence of community transmission of the disease.[36][37]
Signs and symptoms
| Symptom | Percentage |
|---|---|
| Fever | 87.9% |
| Dry cough | 67.7% |
| Fatigue | 38.1% |
| Sputum production | 33.4% |
| Shortness of breath | 18.6% |
| Muscle pain or joint pain | 14.8% |
| Sore throat | 13.9% |
| Headache | 13.6% |
| Chills | 11.4% |
| Nausea or vomiting | 5% |
| Nasal congestion | 4.8% |
| Diarrhoea | 3.7% |
| Haemoptysis | 0.9% |
| Conjunctival congestion | 0.8% |
Those infected may either be asymptomatic or develop symptoms including fever, cough, and shortness of breath.[7][16][17] Diarrhea or upper respiratory symptoms (e.g. sneezing, runny nose, sore throat) are less frequent.[18] Cases can progress to pneumonia, multi-organ failure, and death in the most vulnerable.[14][15]
The incubation period ranges from 1 to 14 days with an estimated median incubation period of 5 to 6 days according to the World Health Organization.[38][39]
A WHO review of 55,924 laboratory-confirmed cases in China indicated the following typical signs and symptoms:[40]
Another study of 1,099 Chinese patients found that CT scans showed ground-glass opacities in 56% of patients, but 18% had no radiological findings. 5% of patients were admitted to intensive care units, 2.3% needed mechanical support of ventilation, and 1.4% died.[41] Bilateral and peripheral ground glass opacities are the most typical CT findings, according to researcher Bernheim et al. Consolidation, linear opacities, reverse halo sign are other radiological findings. Initially the lesions are located to one lung, but as the disease progress, indications manifest to both lungs at 88% of patients.[42] Children seem to handle the disease better than adults as the symptoms are usually milder, but sufficient evidence is still lacking.[43]
Cause
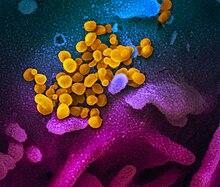
The disease is caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously referred to as the 2019 novel coronavirus (2019-nCoV).[12] It is primarily spread between people via respiratory droplets from coughs and sneezes.[45]
The virus is thought to have an animal origin.[46] Τhere has been a "continuous common source" of the outbreak in December 2019, which would imply that several animal-to-human zoonotic events occurred at the Huanan Seafood Wholesale Market. The primary source of infection became human-to-human transmission in early January 2020.[47][48]
Pathology
Histopathological examinations of post-mortem lung samples showed diffuse alveolar damage with cellular fibromyxoid exudates in both lungs. Viral cytopathic changes were observed in the pneumocytes. The lung picture resembled acute respiratory distress syndrome (ARDS).[49]
Diagnosis

The WHO has published several testing protocols for the disease.[51][52] The standard method of testing is real time reverse transcription polymerase chain reaction (rRT-PCR).[53] The test can be done on respiratory samples obtained by various methods, including nasopharyngeal swab or sputum sample.[54] Results are generally available within a few hours to 2 days.[55][56] Blood tests can be used, but these require two blood samples taken two weeks apart and the results have little immediate value.[57] Chinese scientists were able to isolate a strain of the coronavirus and publish the genetic sequence so that laboratories across the world could independently develop polymerase chain reaction (PCR) tests to detect infection by the virus.[14][58][59][60][61][62][63]
COVID-19 testing can also be done with antibody test kits.[64] Antibody assays use a blood serum sample and can provide a positive result even if the person has recovered and the virus is no longer present.[25] The first antibody test was demonstrated by a team at the Wuhan Institute of Virology on 17 February 2020.[65][25] On 25 February, a team from Duke–NUS Medical School in Singapore announced another antibody test for COVID-19 that can provide a result within a few days.[25][66]
Diagnostic guidelines released by Zhongnan Hospital of Wuhan University suggested methods for detecting infections based upon clinical features and epidemiological risk. These involved identifying patients who had at least two of the following symptoms in addition to a history of travel to Wuhan or contact with other infected patients: fever, imaging features of pneumonia, normal or reduced white blood cell count, or reduced lymphocyte count.[26] A study published by a team at the Tongji Hospital in Wuhan on 26 February 2020 showed that a chest CT scan for COVID-19 has more sensitivity (98%) than the polymerase chain reaction (71%).[27]
Prevention
Global health organisations have published preventive measures to reduce the chances of infection. Recommendations are similar to those published for other coronaviruses: staying home, avoiding travel and public activities, frequent washing of hands with soap and water; not touching the eyes, nose, or mouth with unwashed hands; and practicing good respiratory hygiene.[67][68]
The use of masks by healthy members of the public is not recommended outside of China.[69][70][71]
To prevent transmission, the CDC recommends that infected individuals stay at home except to get medical care; call ahead before visiting a healthcare provider; wear a facemask (especially in public); cover coughs and sneezes with a tissue; regularly wash hands with soap and water; and avoid sharing personal household items.[72]
The CDC has recommended that individuals wash hands often with soap and water for at least 20 seconds, especially after going to the toilet or when hands are visibly dirty; before eating; and after blowing one's nose, coughing, or sneezing. It further recommended using an alcohol-based hand sanitiser with at least 60% alcohol when soap and water are not readily available.[73] The WHO also advised individuals to avoid touching the eyes, nose, or mouth with unwashed hands.[74]
In early 2020, the WHO said it was not expected that a vaccine against SARS-CoV-2 could be available in less than 18 months.[75]
Management
There are no specific antiviral medications approved for this disease. Symptoms are managed with supportive care.[76] Both the WHO and Chinese National Health Commission have published detailed treatment recommendations for hospitalized patients with severe acute respiratory infection (SARI) when a SARS-CoV-2 infection is suspected.[77][78] WHO advises against the use of steroids and methylprednisolone unless the disease is complicated by acute respiratory distress syndrome.[79] The WHO also recommended volunteers take part in randomized controlled trials for testing the effectiveness and safety of potential treatments.[80]
Bruce Aylward, an assistant director-general of the World Health Organization (WHO), has stated "there is only one drug right now that we think may have real efficacy and that's remdesivir." It was reported on 25 February 2020 that clinical trials for this drug were in progress, with results possibly available within weeks.[81]
The Beijing branch of China's National Health Commission suggested the use of lopinavir/ritonavir as part of treatment plans in the absence of an approved drug for this indication.[82] The lopinavir/ritonavir combination and interferon can now be claimed for via health insurance in some countries.[83]
Chloroquine was being trialed in China in February 2020, with preliminary results that seemed quite positive.[84] The drug was enrolled in treatment guidelines.[84]
Psychological
Infected individuals may experience distress from quarantine, travel restrictions, side effects of treatment, or fear of the infection itself. To address these concerns, the National Health Commission of China published a national guideline for psychological crisis intervention on 27 January 2020.[85][86]
Alternative medicine
Chinese health authorities recommend the use of traditional Chinese medicine (TCM) in addition to standard medical supportive care to prevent or treat the disease. On 22 January, National Health Commission put TCM into the third issue of the COVID diagnostic and treatment plan.[87] On 2 February, Wuhan officials ordered all patients to be put on a specific TCM treatment.[88][89] On 14 February, Wuhan opened a TCM-oriented temporary hospital.[90] The efficacy and safety of TCM has not been established in coronavirus infections.[91][92]
Prognosis

Among those who died, many had preexisting conditions, including hypertension, diabetes, and cardiovascular disease,[94] and the median time from initial symptoms to death was 14 days (range 6–41 days).[95] In a study by the National Health Commission (NHC) of China, men had a death rate of 2.8% while women had a death rate of 1.7%.[96] In those under the age of 50 the risk of death is less than 0.5% while in those over the age of 70 it is more than 8%.[96] No deaths had occurred under the age of 10 as of 26 February 2020[update].[96]
Epidemiology
Overall mortality and morbidity rates due to infection are not well established; while the case fatality rate (CFR) changes over time in the current outbreak, the proportion of infections that progress to diagnosable disease remains unclear.[97][98] However, preliminary research has yielded case fatality rate numbers between 2% and 3%;[32] in January 2020 the WHO suggested that the case fatality rate was approximately 3%,[99] and 2% in February 2020 in Hubei.[100] Other CFR numbers, which adjust for differences in time of confirmation, death and/or cured, are 7%[101] and 33% for Wuhan patients January 31st[102]. An unreviewed preprint study by Imperial College London among 55 fatal cases noted that early estimates of mortality may be too high as asymptomatic infections are missed. They estimated a mean infection fatality ratio (IFR, the mortality among infected) ranging from 0.8% when including asymptomatic carriers to 18% when including only symptomatic cases from Hubei province.[103] Other estimates of the IFR are between 0.5% to 0.8% and around 0.95%.[104][102] Pauline Vetter, in an editorial in The BMJ noted that mortality outside of Hubei province seems to be lower than within Hubei.[79] The outbreak in 2019–2020 has caused at least 676,609,955Template:Edit sup[9] confirmed infections and 6,881,955Template:Edit sup[9] deaths.
An observational study by Huijun Chen et al. published at The Lancet of nine patients, found no intrauterine vertical transmission from mother to the newborn.[105] Also, a descriptive study in Wuhan found no evidence of viral transmission through vaginal sex (from female to partner), but authors note that transmission during sex might occur through other routes.[106]
Research
Vaccine
Many organizations are using published genomes to develop possible vaccines against SARS-CoV-2.[107][108] Bodies developing vaccines include the Chinese Center for Disease Control and Prevention,[109][110] the University of Hong Kong,[111] and Shanghai East Hospital.[111] Three vaccine projects are being supported by the Coalition for Epidemic Preparedness Innovations (CEPI), including projects by the biotechnology companies Moderna[112] and Inovio Pharmaceuticals and another by the University of Queensland.[113] The United States National Institutes of Health (NIH) is cooperating with Moderna to create an RNA vaccine matching a spike of the coronavirus surface, and intends to start human trials by May 2020.[107] Inovio Pharmaceuticals is developing a DNA-based vaccination and collaborating with a Chinese firm, planning human clinical trials in the summer of the Northern Hemisphere of 2020.[114] In Australia, the University of Queensland is investigating the potential of a molecular clamp vaccine that would genetically modify viral proteins in order to stimulate an immune reaction.[113] In Canada, the International Vaccine Centre (VIDO-InterVac), University of Saskatchewan, are working on a vaccine,[115] aiming to start animal testing in March 2020 and human testing in 2021.[115]
In January 2020, Janssen Pharmaceutical Companies began work on developing a vaccine, using the same technologies as for its experimental Ebola vaccine.[116] In the following month, the U.S. Department of Health and Human Services' Biomedical Advanced Research and Development Authority (BARDA) announced that it would collaborate with Janssen and, later, Sanofi Pasteur to develop a vaccine.[117][118] Sanofi has previously worked on a vaccine for SARS and it stated to expect to have a vaccine candidate within six months that could be ready to test in people within a year to 18 months.[117]
On February 26, 2020, a U.S. health official from the National Institute of Allergy and Infectious Diseases, said that it will take "at least a year to a year and a half at best" to develop a vaccine for the coronavirus.[119]
Antiviral
No drug has yet been approved to treat coronavirus infections in humans.[120] Research into potential treatments for the disease was initiated in January 2020, and several antiviral drugs are already in clinical trials.[107][108] Although completely new drugs may take until 2021 to develop,[121] several of the drugs being tested are already approved for other antiviral indications, or are already in advanced testing.[120]
Antivirals being tested include chloroquine,[122] darunavir,[123] galidesivir,[120] interferon beta,[124][better source needed] the lopinavir/ritonavir combination,[108][122] the RNA polymerase inhibitor remdesivir,[124][125][126] and triazavirin.[127][128][better source needed] Umifenovir (Arbidol) and darunavir were proposed by the National Health Commission.[129][better source needed]
Remdesivir and chloroquine effectively inhibit the coronavirus in vitro.[122]
Preliminary results from a multicentric trial, announced in a press conference and described by Gao, Tian, and Yang, suggested that chloroquine is effective and safe in treating COVID-19 associated pneumonia, "improving lung imaging findings, promoting a virus-negative conversion, and shortening the disease course".[84]
Terminology
The process of naming the disease has been called "chaotic".[130]
The World Health Organization announced on 11 February 2020 that "COVID-19" will be the official name of the disease. World Health Organization chief Tedros Adhanom Ghebreyesus said "co" stands for "corona", "vi" for "virus" and "d" for "disease", while "19" was for the year, as the outbreak was first identified on 31 December 2019. Tedros said the name had been chosen to avoid references to a specific geographical location (i.e. China), animal species, or group of people in line with international recommendations for naming aimed at preventing stigmatization.[131][132]
See also
- Coronavirus diseases, a group of closely related syndromes
- Li Wenliang, a doctor at Wuhan Central Hospital and first to describe the syndrome
References
- ^ "国家卫生健康委关于新型冠状病毒肺炎暂命名事宜的通知". 7 February 2020. Archived from the original on 28 February 2020. Retrieved 9 February 2020.
- ^ Belluz, Julia (20 January 2020). "Wuhan pneumonia outbreak: What we know and don't know". Vox. Archived from the original on 13 January 2020. Retrieved 27 February 2020.
- ^ Cheung, Elizabeth (17 January 2020). "Wuhan pneumonia: Hong Kong widens net for suspected cases but medical workers fear already overstretched hospitals will suffer". South China Morning Post. Archived from the original on 21 January 2020. Retrieved 27 February 2020.
- ^ Chan, Jasper Fuk-Woo; Yuan, Shuofeng; Kok, Kin-Hang; To, Kelvin Kai-Wang; Chu, Hin; Yang, Jin; Xing, Fanfan; Liu, Jieling; Yip, Cyril Chik-Yan; Poon, Rosana Wing-Shan; Tsoi, Hoi-Wah; Lo, Simon Kam-Fai; Chan, Kwok-Hung; Poon, Vincent Kwok-Man; Chan, Wan-Mui; Ip, Jonathan Daniel; Cai, Jian-Piao; Cheng, Vincent Chi-Chung; Chen, Honglin; Hui, Christopher Kim-Ming; Yuen, Kwok-Yung (15 February 2020). "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster". The Lancet. 395 (10223) (published 24 January 2020): 514–523. doi:10.1016/S0140-6736(20)30154-9. ISSN 0140-6736. PMID 31986261.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ "Wuhan designates hospitals for viral pneumonia treatment as cases rise". The Straits Times. 21 January 2020. Archived from the original on 21 January 2020. Retrieved 27 February 2020.
- ^ See SARS-CoV-2 for more.
- ^ a b c "Coronavirus Disease 2019 (COVID-19) Symptoms". Centers for Disease Control and Prevention. United States. 10 February 2020. Archived from the original on 30 January 2020.
- ^ Cite error: The named reference
JHMAPwas invoked but never defined (see the help page). - ^ a b c "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)". ArcGIS. Johns Hopkins University. Retrieved 10 March 2023.
- ^ "Naming the coronavirus disease (COVID-19) and the virus that causes it". www.who.int. World Health Organization. Archived from the original on 28 February 2020. Retrieved 28 February 2020.
- ^ World Health Organization (11 February 2020). Novel Coronavirus (2019-nCoV): situation report, 22 (PDF) (Report). World Health Organization.
{{cite report}}: Cite has empty unknown parameter:|1=(help) - ^ a b Gorbalenya, Alexander E. (11 February 2020). "Severe acute respiratory syndrome-related coronavirus – The species and its viruses, a statement of the Coronavirus Study Group". bioRxiv: 2020.02.07.937862. doi:10.1101/2020.02.07.937862. Archived from the original on 11 February 2020. Retrieved 11 February 2020.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Coronavirus disease named Covid-19". BBC News. 11 February 2020. Archived from the original on 11 February 2020. Retrieved 11 February 2020.
- ^ a b c d Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020 Jan 14;91:264–266. doi:10.1016/j.ijid.2020.01.009. PMID 31953166.

- ^ a b c "Q&A on coronaviruses". World Health Organization (WHO). Archived from the original on 20 January 2020. Retrieved 27 January 2020.
- ^ a b Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. (February 2020). "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study". Lancet. 395 (10223): 507–513. doi:10.1016/S0140-6736(20)30211-7. PMID 32007143.
- ^ a b Hessen, Margaret Trexler (27 January 2020). "Novel Coronavirus Information Center: Expert guidance and commentary". Elsevier Connect. Archived from the original on 30 January 2020. Retrieved 31 January 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (February 2020). "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". Lancet. 395 (10223): 497–506. doi:10.1016/S0140-6736(20)30183-5. PMID 31986264.
- ^ https://www.nytimes.com/2020/02/27/world/asia/coronavirus-treament-recovery.html
- ^ "Q&A on coronaviruses". World Health Organization (WHO). 11 February 2020. Archived from the original on 20 January 2020. Retrieved 24 February 2020.
- ^ "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020. Archived from the original on 23 February 2020. Retrieved 24 February 2020.
- ^ "Symptoms of Novel Coronavirus (2019-nCoV) | CDC". www.cdc.gov. 10 February 2020. Archived from the original on 30 January 2020. Retrieved 11 February 2020.
- ^ Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR (February 2020). "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges". International Journal of Antimicrobial Agents: 105924. doi:10.1016/j.ijantimicag.2020.105924. PMID 32081636.
- ^ Velavan, Thirumalaisamy P.; Meyer, Christian G. (2020). "The COVID-19 epidemic". Tropical Medicine & International Health. n/a (n/a): 278–280. doi:10.1111/tmi.13383. ISSN 1365-3156. PMID 32052514.
- ^ a b c d Normile, Dennis; 2020; Pm, 4:30 (27 February 2020). "Singapore claims first use of antibody test to track coronavirus infections". Science | AAAS. Retrieved 2 March 2020.
{{cite web}}:|last2=has numeric name (help)CS1 maint: numeric names: authors list (link) - ^ a b Jin, Ying-Hui; Cai, Lin; Cheng, Zhen-Shun; Cheng, Hong; Deng, Tong; Fan, Yi-Pin; Fang, Cheng; Huang, Di; Huang, Lu-Qi; Huang, Qiao; Han, Yong; Hu, Bo; Hu, Fen; Li, Bing-Hui; Li, Yi-Rong; Liang, Ke; Lin, Li-Kai; Luo, Li-Sha; Ma, Jing; Ma, Lin-Lu; Peng, Zhi-Yong; Pan, Yun-Bao; Pan, Zhen-Yu; Ren, Xue-Qun; Sun, Hui-Min; Wang, Ying; Wang, Yun-Yun; Weng, Hong; Wei, Chao-Jie; Wu, Dong-Fang; Xia, Jian; Xiong, Yong; Xu, Hai-Bo; Yao, Xiao-Mei; Yuan, Yu-Feng; Ye, Tai-Sheng; Zhang, Xiao-Chun; Zhang, Ying-Wen; Zhang, Yin-Gao; Zhang, Hua-Min; Zhao, Yan; Zhao, Ming-Juan; Zi, Hao; Zeng, Xian-Tao; Wang, Yong-Yan; Wang, Xing-Huan (6 February 2020). "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)". Military Medical Research. 7 (1): 4. doi:10.1186/s40779-020-0233-6. ISSN 2095-7467. PMC 7003341. PMID 32029004.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b "CT provides best diagnosis for COVID-19". ScienceDaily. 26 February 2020. Retrieved 2 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ a b "Advice for public". www.who.int. Archived from the original on 26 January 2020. Retrieved 25 February 2020.
- ^ CDC (11 February 2020). "2019 Novel Coronavirus (2019-nCoV)". Centers for Disease Control and Prevention. Archived from the original on 14 February 2020. Retrieved 15 February 2020.
- ^ "Advice for public". www.who.int. Archived from the original on 26 January 2020. Retrieved 15 February 2020.
- ^ "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention (CDC). 15 February 2020. Archived from the original on 26 February 2020. Retrieved 20 February 2020.
- ^ a b "Wuhan Coronavirus Death Rate - Worldometer". www.worldometers.info. Archived from the original on 31 January 2020. Retrieved 2 February 2020.
- ^ "Report 4: Severity of 2019-novel coronavirus (nCoV)" (PDF). Archived (PDF) from the original on 10 February 2020. Retrieved 10 February 2020.
- ^ "Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)". www.who.int. Archived from the original on 31 January 2020. Retrieved 11 February 2020.
- ^ Mahtani S, Berger M, O'Grady S, Iati M (6 February 2020). "Hundreds of evacuees to be held on bases in California; Hong Kong and Taiwan restrict travel from mainland China". The Washington Post. Archived from the original on 7 February 2020. Retrieved 11 February 2020.
- ^ "Areas with presumed ongoing community transmission of 2019-nCoV". European Centre for Disease Prevention and Control. Archived from the original on 28 February 2020. Retrieved 28 February 2020.
{{cite web}}:|archive-date=/|archive-url=timestamp mismatch; 19 February 2020 suggested (help) - ^ Christina Maxouris; Dakin Andone (29 February 2020). "First death from coronavirus in the United States confirmed in Washington state, officials say". CNN - Health. Retrieved 29 February 2020.
- ^ "WHO COVID-19 situation report 29" (PDF). World Health Organization. 19 February 2020. Archived (PDF) from the original on 24 February 2020. Retrieved 26 February 2020.
- ^ "Q&A on coronaviruses (COVID-19): How long is the incubation period for COVID-19?". www.who.int. Archived from the original on 20 January 2020. Retrieved 26 February 2020.
- ^ "Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)" (PDF). WHO. pp. 11–12. Retrieved 29 February 2020.
- ^ Guan, Wei-jie; Ni, Zheng-yi; Hu, Yu; Liang, Wen-hua; Ou, Chun-quan; He, Jian-xing; Liu, Lei; Shan, Hong; Lei, Chun-liang; Hui, David S.C.; Du, Bin; Li, Lan-juan; Zeng, Guang; Yuen, Kwok-Yung; Chen, Ru-chong; Tang, Chun-li; Wang, Tao; Chen, Ping-yan; Xiang, Jie; Li, Shi-yue; Wang, Jin-lin; Liang, Zi-jing; Peng, Yi-xiang; Wei, Li; Liu, Yong; Hu, Ya-hua; Peng, Peng; Wang, Jian-ming; Liu, Ji-yang; Chen, Zhong; Li, Gang; Zheng, Zhi-jian; Qiu, Shao-qin; Luo, Jie; Ye, Chang-jiang; Zhu, Shao-yong; Zhong, Nan-shan (28 February 2020). "Clinical Characteristics of Coronavirus Disease 2019 in China". New England Journal of Medicine. Massachusetts Medical Society. doi:10.1056/nejmoa2002032. ISSN 0028-4793. PMID 32109013.
- ^ Bernheim, Adam; Mei, Xueyan; Huang, Mingqian; Yang, Yang; Fayad, Zahi A.; Zhang, Ning; Diao, Kaiyue; Lin, Bin; Zhu, Xiqi; Li, Kunwei; Li, Shaolin; Shan, Hong; Jacobi, Adam; Chung, Michael (20 February 2020). "Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection". Radiology. Radiological Society of North America (RSNA): 200463. doi:10.1148/radiol.2020200463. ISSN 0033-8419. PMID 32077789.
- ^ CDC (11 February 2020). "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. Retrieved 2 March 2020.
- ^ "New Images of Novel Coronavirus SARS-CoV-2 Now Available | NIH: National Institute of Allergy and Infectious Diseases". www.niaid.nih.gov. Retrieved 1 March 2020.
- ^ "2019 Novel Coronavirus (2019-nCoV)". Centers for Disease Control and Prevention. 11 February 2020. Archived from the original on 22 February 2020. Retrieved 18 February 2020.
- ^ Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. (23 January 2020). "Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin". bioRxiv: 2020.01.22.914952. doi:10.1101/2020.01.22.914952. Archived from the original on 24 January 2020. Retrieved 5 February 2020.
- ^ "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) - China, 2020" (PDF). China CDC Weekly. 2. 20 February 2020. Archived (PDF) from the original on 18 February 2020. Retrieved 19 February 2020 – via unpublished master.
- ^ Heymann, David L; Shindo, Nahoko (22 February 2020). "COVID-19: what is next for public health?". The Lancet. 395 (10224): 542–545. doi:10.1016/S0140-6736(20)30374-3. ISSN 0140-6736. PMID 32061313. Retrieved 2 March 2020.
- ^ Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). World Health Organization (WHO), 16-24 February 2020
- ^ CDC (5 February 2020). "CDC Tests for 2019-nCoV". Centers for Disease Control and Prevention. Archived from the original on 14 February 2020. Retrieved 12 February 2020.
- ^ Schirring, Lisa (16 January 2020). "Japan has 1st novel coronavirus case; China reports another death". CIDRAP. Archived from the original on 20 January 2020. Retrieved 16 January 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: Interim guidance". World Health Organization. Archived from the original on 20 January 2020. Retrieved 28 January 2020.
- ^ "2019 Novel Coronavirus (2019-nCoV) Situation Summary". Centers for Disease Control and Prevention. 30 January 2020. Archived from the original on 26 January 2020. Retrieved 30 January 2020.
- ^ "Real-Time RT-PCR Panel for Detection 2019-nCoV". Centers for Disease Control and Prevention. 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- ^ "Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe". GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
- ^ Brueck, Hilary (30 January 2020). "There's only one way to know if you have the coronavirus, and it involves machines full of spit and mucus". Business Insider. Archived from the original on 1 February 2020. Retrieved 1 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". Archived from the original on 21 February 2020. Retrieved 26 February 2020.
- ^ "Undiagnosed pneumonia – China (HU) (01): wildlife sales, market closed, RFI Archive Number: 20200102.6866757". Pro-MED-mail. International Society for Infectious Diseases. Archived from the original on 22 January 2020. Retrieved 13 January 2020.
- ^ Cohen J, Normile D (January 2020). "New SARS-like virus in China triggers alarm" (PDF). Science. 367 (6475): 234–235. doi:10.1126/science.367.6475.234. PMID 31949058. Archived (PDF) from the original on 11 February 2020. Retrieved 11 February 2020.
- ^ "Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome". NCBI. Nature. 11 February 2020. Archived from the original on 21 January 2020. Retrieved 25 February 2020.
- ^ "Severe acute respiratory syndrome coronavirus 2 data hub". NCBI. Retrieved 4 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2) Sequences". NCBI. Retrieved 4 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "Genomic epidemiology of SARS-CoV2". GISAID. Retrieved 5 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "China Makes Over 1.7 Million Covid-19 Testing Kits per Day, Official Says". Yicai Global.
- ^ Zhang, Wei; Du, Rong-Hui; Li, Bei; Zheng, Xiao-Shuang; Yang, Xing-Lou; Hu, Ben; Wang, Yan-Yi; Xiao, Geng-Fu; Yan, Bing; Shi, Zheng-Li; Zhou, Peng (1 January 2020). "Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes". Emerging Microbes & Infections. 9 (1): 386–389. doi:10.1080/22221751.2020.1729071. PMID 32065057.
- ^ "Duke-NUS used COVID-19 antibody tests to establish link between church clusters in a world-first". CNA. Retrieved 2 March 2020.
- ^ "Coronavirus | About | Prevention and Treatment | CDC". www.cdc.gov. 3 February 2020. Archived from the original on 15 December 2019. Retrieved 10 February 2020.
- ^ "Advice for public". www.who.int. Archived from the original on 26 January 2020. Retrieved 10 February 2020.
- ^ "Coronavirus (COVID-19)". Australian Government Department of Health. 21 January 2020. Retrieved 15 February 2020.
{{cite web}}: Unknown parameter|authors=ignored (help) - ^ "MOH | Updates on 2019 Novel Coronavirus (2019-nCoV) Local Situation". www.moh.gov.sg. Retrieved 11 February 2020.
- ^ "Novel coronavirus (2019-nCoV)". Australian Government Department of Health. 21 January 2020. Retrieved 11 February 2020.
{{cite web}}: Unknown parameter|authors=ignored (help) - ^ CDC (11 February 2020). "What to do if you are sick with 2019 Novel Coronavirus (2019-nCoV)". Centers for Disease Control and Prevention. Archived from the original on 14 February 2020. Retrieved 13 February 2020.
- ^ CDC (11 February 2020). "Coronavirus Disease 2019 Prevention & Treatment". Centers for Disease Control and Prevention. Retrieved 5 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "Advice for public". www.who.int. Retrieved 5 March 2020.
- ^ Grenfell, Rob; Drew, Trevor (17 February 2020). "Here's Why It's Taking So Long to Develop a Vaccine For The New Coronavirus". Science Alert. Archived from the original on 28 February 2020. Retrieved 26 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Kui L, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. (February 2020). "Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province". Chinese Medical Journal: 1. doi:10.1097/CM9.0000000000000744. PMID 32044814.
- ^ Cheng ZJ, Shan J (February 2020). "2019 Novel coronavirus: where we are and what we know" (PDF). Infection. doi:10.1007/s15010-020-01401-y. PMID 32072569. Archived (PDF) from the original on 19 February 2020. Retrieved 26 February 2020.
- ^ "Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected". www.who.int. Archived from the original on 31 January 2020. Retrieved 13 February 2020.
- ^ a b Vetter, Pauline; Eckerle, Isabella; Kaiser, Laurent (19 February 2020). "Covid-19: a puzzle with many missing pieces". BMJ. 368: m627. doi:10.1136/bmj.m627. ISSN 1756-1833. PMID 32075791. Retrieved 29 February 2020.
- ^ Nebehay, Stephanie; Kelland, Kate; Liu, Roxanne (5 February 2020). "WHO: 'no known effective' treatments for new coronavirus". Thomson Reuters. Archived from the original on 5 February 2020. Retrieved 5 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ La Monica, Paul R. (25 February 2020). "Gilead Sciences drug remdesivir may help treat coronavirus symptoms, according to WHO". CNN. Archived from the original on 24 February 2020. Retrieved 25 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "China names HIV drugs as part of treatment plan for Wuhan virus". The Japan Times Online. 26 January 2020. ISSN 0447-5763. Archived from the original on 15 February 2020. Retrieved 14 February 2020.
- ^ "Interferon, Kaletra to get insurance benefit for new coronavirus treatment - Korea Biomedical Review". www.koreabiomed.com (in Korean). 4 February 2020. Retrieved 14 February 2020.
- ^ a b c Gao J, Tian Z, Yang X (February 2020). "Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies". Bioscience Trends. doi:10.5582/bst.2020.01047. PMID 32074550.
- ^ Xiang YT, Yang Y, Li W, Zhang L, Zhang Q, Cheung T, Ng CH (March 2020). "Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed". The Lancet. Psychiatry. 7 (3): 228–229. doi:10.1016/S2215-0366(20)30046-8. PMID 32032543.
- ^ Kang L, Li Y, Hu S, Chen M, Yang C, Yang BX, et al. (March 2020). "The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus". The Lancet. Psychiatry. 7 (3): e14. doi:10.1016/S2215-0366(20)30047-X. PMID 32035030.
- ^ "新冠肺炎治疗:讲究实证的西医和自我定位的中药" [Treating the novel conoravirus: the empirical Western medicine and the self-positioning Chinese medicine]. BBC News (in Simplified Chinese). 14 February 2020.
- ^ "中医来了!8个防治"协定方" 辅助治疗新型冠状病毒感染肺炎" [Here comes Chinese medicine! 8 "agreed-on prescriptions" help prevent and treat the new coronavirus pneumonia]. CCTV News. Archived from the original on 15 February 2020. Retrieved 15 February 2020.
- ^ "武汉要求所有患者必须吃中药 网民质疑" [Wuhan wants all patients put on TCM; Netizens question]. Epoch Times (in Chinese (China)). 4 February 2020. Archived from the original on 15 February 2020. Retrieved 15 February 2020.
- ^ "Virus-hit Wuhan opens first TCM-oriented temporary hospital". Xinhua. Archived from the original on 15 February 2020. Retrieved 15 February 2020.
- ^ "Dispelling the myths around the new coronavirus outbreak". www.aljazeera.com. Archived from the original on 6 February 2020. Retrieved 8 February 2020.
- ^ Wee, Sui-Lee (5 February 2020). "In Coronavirus, China Weighs Benefits of Buffalo Horn and Other Remedies". The New York Times. Archived from the original on 6 February 2020. Retrieved 15 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. China CDC Weekly, 2020, 2(8): 113-122.
- ^ "WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus". who.int.
- ^ Wang W, Tang J, Wei F (April 2020). "Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China". Journal of Medical Virology. 92 (4): 441–447. doi:10.1002/jmv.25689. PMID 31994742.
- ^ a b c "Coronavirus Age, Sex, Demographics (COVID-19) - Worldometer". www.worldometers.info. Archived from the original on 27 February 2020. Retrieved 26 February 2020.
- ^ "Limited data on coronavirus may be skewing assumptions about severity". STAT. 30 January 2020. Archived from the original on 1 February 2020. Retrieved 1 February 2020.
- ^ Sparrow, Annie. "How China's Coronavirus Is Spreading—and How to Stop It". Foreign Policy. Archived from the original on 31 January 2020. Retrieved 2 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "WHOが"致死率3%程度" 専門家「今後 注意が必要」". NHK. 24 January 2020. Archived from the original on 26 January 2020. Retrieved 3 February 2020.
- ^ Boseley, Sarah (17 February 2020). "Coronavirus causes mild disease in four in five patients, says WHO". The Guardian. Archived from the original on 18 February 2020. Retrieved 18 February 2020.
- ^ Diao, Ying; Liu, Xiaoyun; Wang, Tao; Zeng, Xiaofei; Dong, Chen; Zhou, Changlong; Zhang, Yuanming; She, Xuan; Liu, Dingfu; Hu, Zhongli (20 February 2020). "Estimating the cure rate and case fatality rate of the ongoing epidemic COVID-19". doi:10.1101/2020.02.18.20024513.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b "2019-nCoV: preliminary estimates of the confirmed-case-fatality-ratio and infection-fatality-ratio, and initial pandemic risk assessment". institutefordiseasemodeling.github.io. Retrieved 1 March 2020.
- ^ "Report 4: Severity of 2019-novel coronavirus (nCoV)" (PDF). Archived (PDF) from the original on 10 February 2020. Retrieved 10 February 2020.
- ^ Jung, Sung-mok; Akhmetzhanov, Andrei R.; Hayashi, Katsuma; Linton, Natalie M.; Yang, Yichi; Yuan, Baoyin; Kobayashi, Tetsuro; Kinoshita, Ryo; Nishiura, Hiroshi (14 February 2020). "Real-Time Estimation of the Risk of Death from Novel Coronavirus (COVID-19) Infection: Inference Using Exported Cases". Journal of Clinical Medicine. 9 (2): 523. doi:10.3390/jcm9020523. ISSN 2077-0383. PMID 32075152.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Chen, Huijun; Guo, Juanjuan; Wang, Chen; Luo, Fan; Yu, Xuechen; Zhang, Wei; Li, Jiafu; Zhao, Dongchi; Xu, Dan; Gong, Qing; Liao, Jing; Yang, Huixia; Hou, Wei; Zhang, Yuanzhen (12 February 2020). "Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records". The Lancet. doi:10.1016/S0140-6736(20)30360-3. ISSN 0140-6736. Retrieved 1 March 2020.
- ^ Cui, Pengfei; Chen, Zhe; Wang, Tian; Dai, Jun; Zhang, Jinjin; Ding, Ting; Jiang, Jingjing; Liu, Jia; Zhang, Cong; Shan, Wanying; Wang, Sheng; Rong, Yueguang; Chang, Jiang; Miao, Xiaoping; Ma, Xiangyi; Wang, Shixuan (27 February 2020), Clinical features and sexual transmission potential of SARS-CoV-2 infected female patients: a descriptive study in Wuhan, China, Cold Spring Harbor Laboratory, doi:10.1101/2020.02.26.20028225
- ^ a b c Steenhuysen, Julie; Kelland, Kate (24 January 2020). "With Wuhan virus genetic code in hand, scientists begin work on a vaccine". Reuters. Archived from the original on 25 January 2020. Retrieved 25 January 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c Praveen Duddu. Coronavirus outbreak: Vaccines/drugs in the pipeline for Covid-19 Archived 19 February 2020 at the Wayback Machine. clinicaltrialsarena.com 19 February 2020.
- ^ "China CDC developing novel coronavirus vaccine". Xinhua. 26 January 2020. Archived from the original on 26 January 2020. Retrieved 28 January 2020.
- ^ "Chinese scientists race to develop vaccine as coronavirus death toll jumps". South China Morning Post. 26 January 2020. Archived from the original on 26 January 2020. Retrieved 28 January 2020.
- ^ a b Cheung, Elizabeth (28 January 2020). "Hong Kong researchers have developed coronavirus vaccine, expert reveals". South China Morning Post. Archived from the original on 28 January 2020. Retrieved 28 January 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Business, Hanna Ziady, CNN. "Biotech company Moderna says its coronavirus vaccine is ready for first tests". CNN. Retrieved 2 March 2020.
{{cite web}}:|last=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b Devlin, Hannah (24 January 2020). "Lessons from SARS outbreak help in race for coronavirus vaccine". The Guardian. Archived from the original on 25 January 2020. Retrieved 25 January 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Mazumdar, Tulip (30 January 2020). "Coronavirus: Scientists race to develop a vaccine". BBC News Online. Archived from the original on 30 January 2020. Retrieved 3 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b "Saskatchewan lab joins global effort to develop coronavirus vaccine". Canadian Broadcasting Corporation. 24 January 2020. Archived from the original on 25 January 2020. Retrieved 25 January 2020.
- ^ Mishra, Manas (29 January 2020). "Johnson & Johnson working on vaccine for deadly coronavirus". Reuters. Archived from the original on 29 January 2020. Retrieved 19 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Branswell, Helen (18 February 2020). "Sanofi announces it will work with HHS to develop coronavirus vaccine". STAT. Archived from the original on 19 February 2020. Retrieved 19 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "HHS Engages Sanofi's Recombinant Technology for 2019 Novel Coronavirus Vaccine". U.S. Department of Health & Human Services (HHS) (Press release). 14 February 2020. Archived from the original on 18 February 2020. Retrieved 19 February 2020.
- ^ Deese, Kaelan (26 February 2020). "Health official says coronavirus vaccine will take 'at least a year to a year and a half' to develop". TheHill. Retrieved 26 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c Li, Guangdi; De Clercq, Erik (2020). "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)". Nature Reviews Drug Discovery. 19 (3): 149–150. doi:10.1038/d41573-020-00016-0.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 28 Jan 2020. doi:10.5582/bst.2020.01020
- ^ a b c Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. (February 2020). "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro". Cell Research. 30 (3): 269–271. doi:10.1038/s41422-020-0282-0. PMID 32020029.
- ^ Lin S, Shen R, He J, Li X, Guo X (January 2020). "Molecular Modeling Evaluation of the Binding Effect of Ritonavir, Lopinavir and Darunavir to Severe Acute Respiratory Syndrome Coronavirus 2 Proteases" (PDF). bioRxiv. doi:10.1101/2020.01.31.929695.
- ^ a b Paules CI, Marston HD, Fauci AS (January 2020). "Coronavirus Infections-More Than Just the Common Cold". JAMA. 323 (8): 707. doi:10.1001/jama.2020.0757. PMID 31971553.
- ^ Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. (January 2020). "First Case of 2019 Novel Coronavirus in the United States". The New England Journal of Medicine: NEJMoa2001191. doi:10.1056/NEJMoa2001191. PMID 32004427.
- ^ Xu, Zhijian; Peng, Cheng; Shi, Yulong; Zhu, Zhengdan; Mu, Kaijie; Wang, Xiaoyu; Zhu, Weiliang (28 January 2020). "Nelfinavir was predicted to be a potential inhibitor of 2019 nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation". bioRxiv: 2020.01.27.921627. doi:10.1101/2020.01.27.921627 – via www.biorxiv.org.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "China to test Russian antiviral for battle against coronavirus". Washington post. Archived from the original on 18 February 2020. Retrieved 20 February 2020.
- ^ Keulemans[, Maarten (20 February 2020). "Het middel tegen het coronavirus bestaat misschien al lang" [The remedy for the corona virus may have existed for a long time]. de Volkskrant (in Dutch). Retrieved 2 March 2020.
- ^ "Are cocktail therapies for flu and HIV the magic cure for coronavirus?". South China Morning Post. 4 February 2020. Archived from the original on 6 February 2020. Retrieved 22 February 2020.
- ^ Enserink, Martin (12 February 2020). "Update: 'A bit chaotic.' Christening of new coronavirus and its disease name create confusion". American Associaton for the Advancement of Science. Science Magazine. Archived from the original on 20 February 2020. Retrieved 27 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Novel coronavirus named 'Covid-19': WHO". TODAYonline. Retrieved 11 February 2020.
- ^ "The coronavirus spreads racism against—and among—ethnic Chinese". The Economist. 17 February 2020. Archived from the original on 17 February 2020. Retrieved 17 February 2020.
