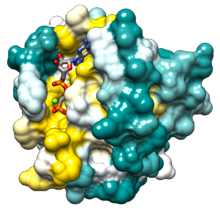
Ras GTPase
 | |||||||||||
| Identifiers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symbol | Ras | ||||||||||
| Pfam | PF00071 | ||||||||||
| InterPro | IPR013753 | ||||||||||
| PROSITE | PDOC00017 | ||||||||||
| SCOP2 | 5p21 / SCOPe / SUPFAM | ||||||||||
| OPM protein | 1uad | ||||||||||
| CDD | cd04138 | ||||||||||
| |||||||||||
Ras is the name given to a family of related proteins found inside cells, including human cells. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells (cellular signal transduction). Ras is the prototypical member of the Ras superfamily of proteins, which are all related in 3D structure and regulate diverse cell behaviours.
The name 'Ras' is an abbreviation of 'Rat sarcoma', reflecting the way the first members of the protein family were discovered. The name ras is also used to refer to the family of genes encoding those proteins.
When Ras is 'switched on' by incoming signals, it subsequently switches on other proteins, which ultimately turn on genes involved in cell growth, differentiation and survival. As a result, mutations in ras genes can lead to the production of permanently activated Ras proteins. This can cause unintended and overactive signalling inside the cell, even in the absence of incoming signals.
Because these signals result in cell growth and division, overactive Ras signaling can ultimately lead to cancer.[1] Ras is the most common oncogene in human cancer - mutations that permanently activate Ras are found in 20-25% of all human tumors and up to 90% in certain types of cancer (e.g. pancreatic cancer).[2] For this reason, Ras inhibitors are being studied as a treatment for cancer, and other diseases with Ras overexpression.
History
The first two ras genes, HRAS and KRAS, were first identified[3] from studies of two cancer-causing viruses, the Harvey sarcoma virus and Kirsten sarcoma virus, by Edward M. Scolnick and colleagues at the National Institutes of Health (NIH).[4] These viruses were discovered originally in rats during the 1960s by Jennifer Harvey[5] and Werner Kirsten,[6] respectively, hence the name Rat sarcoma. In 1982, activated and transforming human ras genes were discovered in human cancer cells by Geoffrey M. Cooper at Harvard,[7] Mariano Barbacid and Stuart A. Aaronson at the NIH[8] and by Robert Weinberg of MIT.[9] A third ras gene was subsequently discovered [10][11] by researchers at the Institute of Cancer Research, funded by the Imperial Cancer Research Fund (now Cancer Research UK), and named NRAS, for its initial identification in human neuroblastoma cells.
The three human ras genes encode extremely similar proteins made up of chains of 188 to 189 amino acids, designated H-Ras, N-Ras and K-Ras4A and K-Ras4B (the two K-Ras proteins arise from alternative splicing).
Structure

Ras contains a six-stranded beta sheet and 5 alpha helices:[12]
- G domain (166 amino acids) which binds guanosine nucleotides, about 20kDa.
- C terminal membrane targeting region (CAAX-COOH, also known as CAAX box) which is lipid-modified by farnesyl transferase, RCE1 and ICMT
The G domain contains five G motifs that bind GDP/GTP directly
- G1 - P-loop binds the beta phosphate of GDP and GTP
- G2 - threonine-35 also switch 1, binds the terminal phosphate of GTP, but makes no contacts with GDP
- G3 - DXXG motif, aspartate-57 is specific for guanine rather than adenine
- G4 - LVGNKxDL motif
- G5 - SAK consensus sequence, the alanine-146 is specific for guanine rather than adenine
and two switches which are the main parts of the protein that move upon activation by GTP.
- switch I includes threonine-35
- switch II glycine-60 in DXXG motif
Ras also binds a magnesium ion which helps to coordinate nucleotide binding.
Function
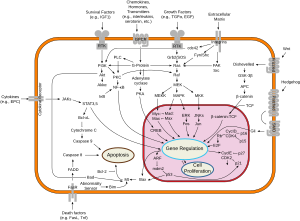
Ras proteins function as binary molecular switches that control intracellular signaling networks. Ras-regulated signal pathways control such processes as actin cytoskeletal integrity, proliferation, differentiation, cell adhesion, apoptosis, and cell migration. Ras and ras-related proteins are often deregulated in cancers, leading to increased invasion and metastasis, and decreased apoptosis.
This section needs expansion. You can help by adding to it. (April 2009) |
Ras activates several pathways, of which the mitogen-activated protein (MAP) kinase cascade has been well-studied. This cascade transmits signals downstream and results in the transcription of genes involved in cell growth and division.[13] There is a separate AKT pathway that inhibits apoptosis.
Activation and deactivation
Ras is a G protein, or a guanosine-nucleotide-binding protein. Specifically, it is a single-subunit small GTPase, which is related in structure to the Gα subunit of heterotrimeric G proteins (large GTPases). G proteins function as binary signaling switches with "on" and "off" states. In the "off" state it is bound to the nucleotide guanosine diphosphate (GDP), while in the "on" state, Ras is bound to guanosine triphosphate (GTP), which has an extra phosphate group as compared to GDP. This extra phosphate holds the two switch regions in a "loaded-spring" configuration (specifically the Thr-35 and Gly-60). When released, the switch regions relax which causes a conformational change into the inactivate state. Hence, activation and deactivation of Ras and other small G proteins are controlled by cycling between the active GTP-bound and inactive GDP-bound forms.
The process of exchanging the bound nucleotide is facilitated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). As per its classification, Ras has an intrinsic GTPase activity, which means that the protein on its own will hydrolyze a bound GTP molecule into GDP. However this process is too slow for efficient function, and hence the GAP for Ras, RasGAP, may bind to and stabilize the catalytic machinery of Ras, supplying additional catalytic residues ("arginine finger") such that a water molecule is optimally positioned for nucleophilic attack on the gamma-phosphate of GTP. An inorganic phosphate is released and the Ras molecule is now bound to a GDP. Since the GDP-bound form is "off" or "inactive" for signaling, GTPase Activating Protein inactivates Ras by activating its GTPase activity. Thus, GAPs accelerate Ras inactivation.
GEFs catalyze a "push and pull" reaction which releases GDP from Ras. They insert close to the P-loop and magnesium cation binding site and inhibit the interaction of these with the gamma phosphate anion. Acidic (negative) residues in switch II "pull" a lysine in the P-loop away from the GDP which "pushes" switch I away from the guanine. The contacts holding GDP in place are broken and it is released into the cytoplasm. Because intracellular GTP is abundant relative to GDP (approximately 10 fold more[13]) GTP predominantly re-enters the nucleotide binding pocket of Ras and reloads the spring. Thus GEFs facilitate Ras activation.[12] Well known GEFs include Son of Sevenless (Sos) and cdc25 which include the RasGEF domain.
The balance between GEF and GAP activity determines the guanine nucleotide status of Ras, thereby regulating Ras activity.
In the GTP-bound conformation, Ras has high affinity for numerous effectors which allow it to carry out its functions. These include PI3K. Other small GTPases may bind adaptors such as arfaptin or second messenger systems such as adenylyl cyclase. The Ras binding domain is found in many effectors and invariably binds to one of the switch regions, because these change conformation between the active and inactive forms. However, they may also bind to the rest of the protein surface.
Other proteins exist which may augment the activity of Ras family proteins. One example is GDI (GDP Disassociation Inhibitor); These function by slowing the exchange of GDP for GTP and thus, prolonging the inactive state of Ras family members. Other proteins that further augment this cycle may exist.
Membrane attachment
Ras is attached to the cell membrane owing to its prenylation and palmitoylation (HRAS and NRAS) or the combination of prenylation and a polybasic sequence adjacent to the prenylation site (KRAS). The C-terminal CaaX box of Ras first gets farnesylated at its Cys residue in the cytosol, allowing Ras to loosely insert into the membrane of the endoplasmatic reticulum and other cellular membranes. The Tripeptide (aaX) is then cleaved from the C-terminus by a specific prenyl-protein specific endoprotease and the new C-terminus is methylated by a methyltransferase. K-Ras procession is completed at this stage. Dynamic electrostatic interactions between its positively charged basic sequence with negative charges at the inner leaflet of the plasma membrane account for its predominant localization at the cell surface at steady-state. NRAS and HRAS are further processed on the surface of the Golgi apparatus by palmitoylation of one or two Cys residues, respectively, adjacent to the CaaX box. The proteins thereby become stably membrane anchored and are transported to the plasma membrane on vesicles of the secretory pathway. Depalmitoylation eventually releases the proteins from the membrane, allowing them to enter another cycle of palmitoylation and depalmitoylation.[14] This cycle is believed to prevent the leakage of NRAS and HRAS to other membranes over time and to maintain their steady-state localization along the Golgi apparatus, secretory pathway, plasma membrane and inter-linked endocytosis pathway.
Members
The clinically most notable members of the Ras subfamily are HRAS, KRAS and NRAS, mainly for being implicated in many types of cancer.[15]
However, there are many other members of this subfamily as well:[16] DIRAS1; DIRAS2; DIRAS3; ERAS; GEM; MRAS; NKIRAS1; NKIRAS2; NRAS; RALA; RALB; RAP1A; RAP1B; RAP2A; RAP2B; RAP2C; RASD1; RASD2; RASL10A; RASL10B; RASL11A; RASL11B; RASL12; REM1; REM2; RERG; RERGL; RRAD; RRAS; RRAS2
Ras in cancer
Mutations in the Ras family of proto-oncogenes (comprising H-Ras, N-Ras and K-Ras) are very common, being found in 20% to 30% of all human tumours.[15] it is reasonable to speculate that a pharmacological approach that curtails Ras activity may represent a possible method to inhibit certain cancer types. Ras point mutations are the single most common abnormality of human proto-oncogenes.[17] Ras inhibitor trans-farnesylthiosalicylic acid (FTS, Salirasib) exhibits profound anti-oncogenic effects in many cancer cell lines.[18][19]
Inappropriate activation
Inappropriate activation of the gene has been shown to play a key role in signal transduction, proliferation and malignant transformation.[13]
Mutations in a number of different genes as well as RAS itself can have this effect. Oncogenes such as p210BCR-ABL or the growth receptor erbB are upstream of Ras, so if they are constitutively activated their signals will transduce through Ras.
The tumour suppressor gene NF1 encodes a Ras-GAP – its mutation in neurofibromatosis will mean that Ras is less likely to be inactivated. Ras can also be amplified, although this only occurs occasionally in tumours.
Finally, Ras oncogenes can be activated by point mutations so that the GTPase reaction can no longer be stimulated by GAP – this increases the half life of active Ras-GTP mutants.[20]
Constitutively active Ras
Constitutively active Ras (RasD) is one which contains mutations that prevent GTP hydrolysis, thus locking Ras in a permanently 'On' state.
The most common mutations are found at residue G12 in the P-loop and the catalytic residue Q61.
- The glycine to valine mutation at residue 12 renders the GTPase domain of Ras insensitive to inactivation by GAP and thus stuck in the "on state". Ras requires a GAP for inactivation as it is a relatively poor catalyst on its own, as opposed to other G-domain-containing proteins such as the alpha subunit of heterotrimeric G proteins.
- Residue 61[21] is responsible for stabilizing the transition state for GTP hydrolysis. Because enzyme catalysis in general is achieved by lowering the energy barrier between substrate and product, mutation of Q61 to K necessarily reduces the rate of intrinsic Ras GTP hydrolysis to physiologically meaningless levels.
See also "dominant negative" mutants such as S17N and D119N.
Ras-targeted cancer treatments
Reovirus was noted to be a potential cancer therapeutic when early studies on reovirus suggested it reproduces well in certain cancer cell lines. It has since been shown to replicate specifically in cells that have an activated Ras pathway (a cellular signaling pathway that is involved in cell growth and differentiation).[22] Reovirus replicates in and eventually kills Ras-activated tumour cells and as cell death occurs, progeny virus particles are free to infect surrounding cancer cells. This cycle of infection, replication and cell death is believed to be repeated until all tumour cells carrying an activated Ras pathway are destroyed. Activating mutations of the Ras protein and upstream elements of the Ras protein may play a role in more than two thirds of all human cancers, including most metastatic disease. Reolysin, a formulation of reovirus, is currently in clinical trials for the treatment of various cancers.[23]
References
- ^ Goodsell DS (1999). "The molecular perspective: the ras oncogene". Oncologist. 4 (3): 263–4. PMID 10394594.
- ^ Downward J (2003). "Targeting RAS signalling pathways in cancer therapy". Nat. Rev. Cancer. 3 (1): 11–22. doi:10.1038/nrc969. PMID 12509763.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Malumbres M, Barbacid M (2003). "RAS oncogenes: the first 30 years". Nat. Rev. Cancer. 3 (6): 459–65. doi:10.1038/nrc1097. PMID 12778136.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chang EH, Gonda MA, Ellis RW, Scolnick EM, Lowy DR (1982). "Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses". Proc. Natl. Acad. Sci. U.S.A. 79 (16): 4848–52. doi:10.1073/pnas.79.16.4848. PMC 346782. PMID 6289320.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Harvey JJ (1964). "An unidentified virus which causes the rapid production of tumours in mice". Nature. 204 (4963): 1104–5. doi:10.1038/2041104b0. PMID 14243400.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kirsten WH, Schauf V, McCoy J (1970). "Properties of a murine sarcoma virus". Bibl Haematol (36): 246–9. PMID 5538357.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cooper GM (1982). "Cellular transforming genes". Science. 217 (4562): 801–6. doi:10.1126/science.6285471. PMID 6285471.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Santos E, Tronick SR, Aaronson SA, Pulciani S, Barbacid M (1982). "T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes". Nature. 298 (5872): 343–7. doi:10.1038/298343a0. PMID 6283384.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Parada LF, Tabin CJ, Shih C, Weinberg RA (1982). "Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene". Nature. 297 (5866): 474–8. doi:10.1038/297474a0. PMID 6283357.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Marshall, C. J.; Hall, A.; Weiss, R. A. (1982). "A transforming gene present in human sarcoma cell lines". Nature. 299 (5879): 171–173. doi:10.1038/299171a0. ISSN 0028-0836.
- ^ Hall, Alan; Marshall, Christopher J.; Spurr, Nigel K.; Weiss, Robin A. (1983). "Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1". Nature. 303 (5916): 396–400. doi:10.1038/303396a0. ISSN 0028-0836.
- ^ a b Vetter IR, Wittinghofer A (2001). "The guanine nucleotide-binding switch in three dimensions". Science. 294 (5545): 1299–304. doi:10.1126/science.1062023. PMID 11701921.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000). "Chapter 25, Cancer". Molecular cell biology (4th ed.). San Francisco: W.H. Freeman. ISBN 0-7167-3706-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Lodish" was defined multiple times with different content (see the help page). - ^ Rocks O, Peyker A, Bastiaens PI (2006). "Spatio-temporal segregation of Ras signals: one ship, three anchors, many harbors". Current Opinion in Cell Biology. 18 (4): 351–7. doi:10.1016/j.ceb.2006.06.007. PMID 16781855.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bos J (1989). "ras oncogenes in human cancer: a review". Cancer Res. 49 (17): 4682–9. PMID 2547513.
- ^ Wennerberg K, Rossman KL, Der CJ (2005). "The Ras superfamily at a glance". J. Cell. Sci. 118 (Pt 5): 843–6. doi:10.1242/jcs.01660. PMID 15731001.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pathologic Basis of Disease 8th ed. 2010. p. 282.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rotblat B, Ehrlich M, Haklai R, Kloog Y (2008). "The Ras inhibitor farnesylthiosalicylic acid (Salirasib) disrupts the spatiotemporal localization of active Ras: a potential treatment for cancer". Methods Enzymol. 439: 467–89. doi:10.1016/S0076-6879(07)00432-6. PMID 18374183.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Roy Blum, yoel kloog (2005). "Ras Inhibition in Glioblastoma Down-regulates Hypoxia-Inducible Factor-1, Causing Glycolysis Shutdown and Cell Death". Cancer Research. 65 (3): 999–1006. PMID 15705901.
- ^ Reuter C, Morgan M, Bergmann L (2000). "Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies?". Blood. 96 (5): 1655–69. PMID 10961860.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Omim - Neuroblastoma Ras Viral Oncogene Homolog; Nras
- ^ Lal R, Harris D, Postel-Vinay S, de Bono J (2009). "Reovirus: Rationale and clinical trial update". Curr. Opin. Mol. Ther. 11 (5): 532–9. PMID 19806501.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thirukkumaran C, Morris DG (2009). "Oncolytic viral therapy using reovirus". Methods Mol. Biol. 542: 607–34. doi:10.1007/978-1-59745-561-9_31. PMID 19565924.
- Agrawal, Anuj G.; Somani, Rakesh R. (2009)Farnesyltransferase Inhibitor as Anticancer Agent, 9 (6): pp. 638–652(15)[1]
- Anuj G. Agrawal and Rakesh R. Somani (2011). Farnesyltransferase Inhibitor in Cancer Treatment, Current Cancer Treatment - Novel Beyond Conventional Approaches, Öner Özdemir (Ed.), ISBN 978-953-307-397-2, InTech, Available from: http://www.intechopen.com/articles/show/title/farnesyltransferase-inhibitor-in-cancer-treatment
External links
- "Brain tumour findings offer hope of new strategy Canadian Cancer Society says" at ncic.cancer.ca
- "Novel cancer treatment gets NCI support" at arstechnica.com
- ras+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ras+Genes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Drosophila Ras oncogene at 85D - The Interactive Fly
