Curcumin: Difference between revisions
m v2.04b - Bot T20 CW#61 - Fix errors for CW project (Reference before punctuation) |
|||
| Line 110: | Line 110: | ||
==Pharmacology== |
==Pharmacology== |
||
[[File:Curcumin fluorescence.jpg|thumb|Curcumin displays green fluorescence under UV light]] |
[[File:Curcumin fluorescence.jpg|thumb|Curcumin displays green fluorescence under UV light]] |
||
Curcumin, which shows positive results in most [[drug discovery]] assays, is regarded as a false [[lead compound|lead]] that [[Medicinal chemistry|medicinal chemists]] include among "[[pan-assay interference compounds]]". This attracts undue experimental attention while failing to advance as viable therapeutic or drug leads |
Curcumin, which shows positive results in most [[drug discovery]] assays, is regarded as a false [[lead compound|lead]] that [[Medicinal chemistry|medicinal chemists]] include among "[[pan-assay interference compounds]]". This attracts undue experimental attention while failing to advance as viable therapeutic or drug leads,<ref name="nelson">{{cite journal | vauthors = Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA | title = The Essential Medicinal Chemistry of Curcumin | journal = Journal of Medicinal Chemistry | volume = 60 | issue = 5 | pages = 1620–1637 | date = March 2017 | pmid = 28074653 | pmc = 5346970 | doi = 10.1021/acs.jmedchem.6b00975 }}<br> See also: {{cite journal | vauthors = Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA | title = Curcumin May (Not) Defy Science | journal = ACS Medicinal Chemistry Letters | volume = 8 | issue = 5 | pages = 467–470 | date = May 2017 | pmid = 28523093 | pmc = 5430405 | doi = 10.1021/acsmedchemlett.7b00139 }}</ref><ref name="baker">{{cite journal | vauthors = Baker M | title = Deceptive curcumin offers cautionary tale for chemists | journal = Nature | volume = 541 | issue = 7636 | pages = 144–145 | date = January 2017 | pmid = 28079090 | doi = 10.1038/541144a | bibcode = 2017Natur.541..144B | doi-access = free }}</ref><ref>{{cite journal | vauthors = Bisson J, McAlpine JB, Friesen JB, Chen SN, Graham J, Pauli GF | title = Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery? | journal = Journal of Medicinal Chemistry | volume = 59 | issue = 5 | pages = 1671–90 | date = March 2016 | pmid = 26505758 | pmc = 4791574 | doi = 10.1021/acs.jmedchem.5b01009 }}</ref> although some derivatives of curcumin such as [[EF-24]] have seen a significant amount of research.<ref>He Y, Li W, Hu G, Sun H, Kong Q. Bioactivities of EF24, a Novel Curcumin Analog: A Review. ''Front Oncol''. 2018 Dec 11;8:614. {{doi|10.3389/fonc.2018.00614}} {{pmid|30619754}}</ref> |
||
Factors that limit the bioactivity of curcumin or its analogs include chemical instability, water insolubility, absence of potent and selective target activity, low bioavailability, limited tissue distribution, and extensive metabolism.<ref name=nelson/> Very little curcumin escapes the [[GI tract]] and most is excreted in feces unchanged.<ref>{{cite journal | vauthors = Metzler M, Pfeiffer E, Schulz SI, Dempe JS | title = Curcumin uptake and metabolism | journal = BioFactors | volume = 39 | issue = 1 | pages = 14–20 | date = 2013 | pmid = 22996406 | doi = 10.1002/biof.1042 | s2cid = 8763463 }}</ref> If curcumin enters plasma in reasonable amounts, there is a high risk of toxicity since it is promiscuous, and interacts with several proteins known to increase the risk of adverse effects, including [[hERG]], [[cytochrome P450]]s, and [[glutathione S-transferase]].<ref name=nelson/> |
Factors that limit the bioactivity of curcumin or its analogs include chemical instability, water insolubility, absence of potent and selective target activity, low bioavailability, limited tissue distribution, and extensive metabolism.<ref name=nelson/> Very little curcumin escapes the [[GI tract]] and most is excreted in feces unchanged.<ref>{{cite journal | vauthors = Metzler M, Pfeiffer E, Schulz SI, Dempe JS | title = Curcumin uptake and metabolism | journal = BioFactors | volume = 39 | issue = 1 | pages = 14–20 | date = 2013 | pmid = 22996406 | doi = 10.1002/biof.1042 | s2cid = 8763463 }}</ref> If curcumin enters plasma in reasonable amounts, there is a high risk of toxicity since it is promiscuous, and interacts with several proteins known to increase the risk of adverse effects, including [[hERG]], [[cytochrome P450]]s, and [[glutathione S-transferase]].<ref name=nelson/> |
||
Revision as of 22:50, 22 December 2020
 Enol form
| |
 Keto form
| |

| |
| |
| Names | |
|---|---|
| Pronunciation | /ˈkɜːrkjʊmɪn/ |
| Preferred IUPAC name
(1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione | |
| Other names
(1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
Diferuloylmethane Curcumin I C.I. 75300 Natural Yellow 3 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.619 |
| E number | E100 (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H20O6 | |
| Molar mass | 368.385 g·mol−1 |
| Appearance | Bright yellow-orange powder |
| Melting point | 183 °C (361 °F; 456 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Curcumin is a bright yellow chemical produced by Curcuma longa plants. It is the principal curcuminoid of turmeric (Curcuma longa), a member of the ginger family, Zingiberaceae. It is sold as an herbal supplement, cosmetics ingredient, food flavoring, and food coloring.[1]
Chemically, curcumin is a diarylheptanoid, belonging to the group of curcuminoids, which are natural phenols responsible for turmeric's yellow color. It is a tautomeric compound existing in enolic form in organic solvents and in keto form in water.[2]
Laboratory and clinical research have not confirmed any medical use for curcumin. It is difficult to study because it is both unstable and poorly bioavailable. It is unlikely to produce useful leads for drug development.[3]
History
Curcumin was named in 1815 when Vogel and Pierre Joseph Pelletier reported the first isolation of a "yellow coloring-matter" from the rhizomes of turmeric.[4] Later, it was found to be a mixture of resin and turmeric oil. In 1910, Milobedzka and Lampe reported the chemical structure of curcumin to be as diferuloylmethane.[5] Later in 1913, the same group accomplished the synthesis of the compound.
Although curcumin has been used historically in Ayurvedic medicine,[6] its potential for medicinal properties remains unproven as a therapy when used orally.[3][7][8]
Uses

The most common applications are as an ingredient in dietary supplement, in cosmetics, as flavoring for foods, such as turmeric-flavored beverages in South and Southeast Asia,[1] and as coloring for foods, such as curry powders, mustards, butters, cheeses.[9] As a food additive for orange-yellow coloring in prepared foods, its E number is E 100 in the European Union.[10][11] It is also approved by the U.S. FDA to be used as a food coloring in USA.[12]
Chemistry

Curcumin incorporates a seven carbon linker and three major functional groups: an α,β-unsaturated β-diketone moiety and an aromatic O-methoxy-phenolic group.[5] The aromatic ring systems, which are phenols, are connected by two α,β-unsaturated carbonyl groups.[13] The diketones form stable enols and are readily deprotonated to form enolates; the α,β-unsaturated carbonyl group is a good Michael acceptor and undergoes nucleophilic addition. Because of its hydrophobic nature, curcumin is poorly soluble in water. However, it is easily soluble in organic solvents.[5]
Curcumin is used as a complexometric indicator for boron.[14] It reacts with boric acid to form a red-colored compound, rosocyanine.
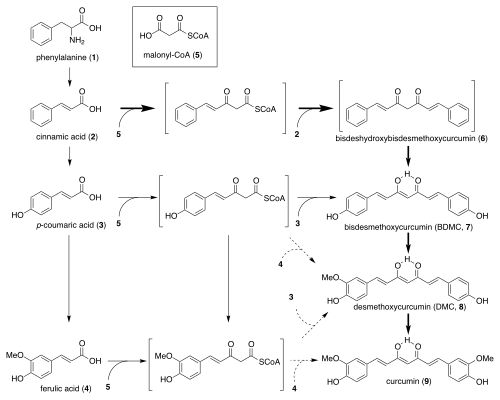
Biosynthesis
The biosynthetic route of curcumin is uncertain. In 1973, Peter J. Roughley and Donald A. Whiting proposed two mechanisms for curcumin biosynthesis. The first mechanism involves a chain extension reaction by cinnamic acid and 5 malonyl-CoA molecules that eventually arylize into a curcuminoid. The second mechanism involves two cinnamate units coupled together by malonyl-CoA. Both use cinnamic acid as their starting point, which is derived from the amino acid phenylalanine.[15]
Plant biosynthesis starting with cinnamic acid is rare compared to the more common p-coumaric acid.[15] Only a few identified compounds, such as anigorufone and pinosylvin, build from cinnamic acid.[16][17]
Biosynthetic pathway of curcumin in Curcuma longa.[15]
|
Pharmacology

Curcumin, which shows positive results in most drug discovery assays, is regarded as a false lead that medicinal chemists include among "pan-assay interference compounds". This attracts undue experimental attention while failing to advance as viable therapeutic or drug leads,[3][7][18] although some derivatives of curcumin such as EF-24 have seen a significant amount of research.[19]
Factors that limit the bioactivity of curcumin or its analogs include chemical instability, water insolubility, absence of potent and selective target activity, low bioavailability, limited tissue distribution, and extensive metabolism.[3] Very little curcumin escapes the GI tract and most is excreted in feces unchanged.[20] If curcumin enters plasma in reasonable amounts, there is a high risk of toxicity since it is promiscuous, and interacts with several proteins known to increase the risk of adverse effects, including hERG, cytochrome P450s, and glutathione S-transferase.[3]
Safety
As a component of turmeric, curcumin may interact with prescription drugs and dietary supplements.[21] In high amounts, it may be unsafe for women during pregnancy.[21] It may cause side effects, such as nausea, diarrhea, hives, or dizziness.[21]
Two preliminary clinical studies in cancer patients consuming high doses of curcumin (up to 8 grams per day for 3–4 months) showed no toxicity, though some subjects reported mild nausea or diarrhea.[22]
The intended use of curcumin as a food additive is generally recognized as safe by the U.S. Food and Drug Administration.[23]
Research
In vitro, curcumin exhibits numerous interference properties which may lead to misinterpretation of results.[3][7][24] Although curcumin has been assessed in numerous laboratory and clinical studies, it has no medical uses established by well-designed clinical research.[25] According to a 2017 review of more than 120 studies, curcumin has not been successful in any clinical trial, leading the authors to conclude that "curcumin is an unstable, reactive, non-bioavailable compound and, therefore, a highly improbable lead".[3]
The US government has supported US$150 million in research into curcumin through the National Center for Complementary and Integrative Health, and no support has been found for curcumin as a medical treatment.[3][26] Curcumin has been identified by the U.S. Food and Drug Administration as a "fake cancer 'cure'".[27]
Research fraud
Bharat Aggarwal was a cancer researcher at the University of Texas MD Anderson Cancer Center, who as of April 2018[update] had 19 papers retracted for research fraud.[28][29] Aggarwal's research had focused on potential anti-cancer properties of herbs and spices, particularly curcumin, and according to a March 2016 article in the Houston Chronicle, "attracted national media interest and laid the groundwork for ongoing clinical trials".[30][31][32] Aggarwal co-founded a company in 2004 called Curry Pharmaceuticals, based in Research Triangle Park, North Carolina, which was seeking to develop drugs based on synthetic analogs of curcumin.[31][33] SignPath Pharma, a company seeking to develop liposomal formulations of curcumin, licensed three patents invented by Aggarwal related to that approach from MD Anderson in 2013.[34]
Alternative medicine
Though there is no evidence for the safety or efficacy of using curcumin as a therapy,[3][7] some alternative medicine practitioners give it intravenously, supposedly as a treatment for numerous diseases.[35][36][37] In 2017, there were two serious cases of adverse events reported from curcumin or turmeric products — one severe allergic reaction and one death[35] — that were caused by administration of a curcumin-polyethylene glycol (PEG40) emulsion product by a naturopath.[37] One treatment caused anaphylaxis leading to death.[35][37]
References
- ^ a b Majeed S (28 December 2015). "The State of the Curcumin Market". Natural Products Insider.
- ^ Manolova Y, Deneva V, Antonov L, Drakalska E, Momekova D, Lambov N (November 2014). "The effect of the water on the curcumin tautomerism: a quantitative approach" (PDF). Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 132: 815–20. Bibcode:2014AcSpA.132..815M. doi:10.1016/j.saa.2014.05.096. PMID 24973669.
- ^ a b c d e f g h i Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA (March 2017). "The Essential Medicinal Chemistry of Curcumin". Journal of Medicinal Chemistry. 60 (5): 1620–1637. doi:10.1021/acs.jmedchem.6b00975. PMC 5346970. PMID 28074653.
See also: Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA (May 2017). "Curcumin May (Not) Defy Science". ACS Medicinal Chemistry Letters. 8 (5): 467–470. doi:10.1021/acsmedchemlett.7b00139. PMC 5430405. PMID 28523093. - ^ Vogel H, Pelletier J (1815). "Curcumin –biological and medicinal properties". Journal de Pharmacie. 1: 289.
- ^ a b c Farooqui, Tahira; Farooqui, Akhlaq A. (2019). "Curcumin: Historical Background, Chemistry, Pharmacological Action, and Potential Therapeutic Value". Curcumin for Neurological and Psychiatric Disorders: 23–44. doi:10.1016/B978-0-12-815461-8.00002-5.
- ^ Wilken R, Veena MS, Wang MB, Srivatsan ES (February 2011). "Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma". Molecular Cancer. 10: 12. doi:10.1186/1476-4598-10-12. PMC 3055228. PMID 21299897.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Baker M (January 2017). "Deceptive curcumin offers cautionary tale for chemists". Nature. 541 (7636): 144–145. Bibcode:2017Natur.541..144B. doi:10.1038/541144a. PMID 28079090.
- ^ "Turmeric". US National Center for Complementary and Integrative Health, National Institutes of Health. May 31, 2016. Retrieved June 15, 2016.
- ^ "Turmeric". www.webmd.com.
- ^ European Commission. "Food Additives". Retrieved February 15, 2014.
- ^ "Curcumin, E 100, page 9". Specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament. March 9, 2012. Retrieved July 24, 2019.
- ^ "CFR - Code of Federal Regulations Title 21". www.accessdata.fda.gov.
- ^ Miłobȩdzka J, van Kostanecki S, Lampe V (1910). "Zur Kenntnis des Curcumins". Berichte der Deutschen Chemischen Gesellschaft. 43 (2): 2163–2170. doi:10.1002/cber.191004302168.
- ^ "EPA Method 212.3: Boron (Colorimetric, Curcumin)" (PDF).
- ^ a b c Kita T, Imai S, Sawada H, Kumagai H, Seto H (July 2008). "The biosynthetic pathway of curcuminoid in turmeric (Curcuma longa) as revealed by 13C-labeled precursors". Bioscience, Biotechnology, and Biochemistry. 72 (7): 1789–98. doi:10.1271/bbb.80075. PMID 18603793.
- ^ Schmitt B, Hölscher D, Schneider B (February 2000). "Variability of phenylpropanoid precursors in the biosynthesis of phenylphenalenones in Anigozanthos preissii". Phytochemistry. 53 (3): 331–7. doi:10.1016/S0031-9422(99)00544-0. PMID 10703053.
- ^ Gehlert R, Schoeppner A, Kindl H (1990). "Stilbene Synthase from Seedlings of Pinus sylvestris: Purification and Induction in Response to Fungal Infection" (PDF). Molecular Plant-Microbe Interactions. 3 (6): 444–449. doi:10.1094/MPMI-3-444.
- ^ Bisson J, McAlpine JB, Friesen JB, Chen SN, Graham J, Pauli GF (March 2016). "Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery?". Journal of Medicinal Chemistry. 59 (5): 1671–90. doi:10.1021/acs.jmedchem.5b01009. PMC 4791574. PMID 26505758.
- ^ He Y, Li W, Hu G, Sun H, Kong Q. Bioactivities of EF24, a Novel Curcumin Analog: A Review. Front Oncol. 2018 Dec 11;8:614. doi:10.3389/fonc.2018.00614 PMID 30619754
- ^ Metzler M, Pfeiffer E, Schulz SI, Dempe JS (2013). "Curcumin uptake and metabolism". BioFactors. 39 (1): 14–20. doi:10.1002/biof.1042. PMID 22996406. S2CID 8763463.
- ^ a b c "Turmeric". Drugs.com. December 6, 2017. Retrieved November 28, 2018.
- ^ Hsu CH, Cheng AL (2007). Clinical studies with curcumin. Vol. 595. pp. 471–80. doi:10.1007/978-0-387-46401-5_21. ISBN 978-0-387-46400-8. PMID 17569225.
{{cite book}}:|journal=ignored (help) - ^ "GRAS Notice (GRN) No. 822". U.S. Food & Drug Administration. GRAS Notice Inventory.
- ^ Lowe D (January 12, 2017). "Curcumin Will Waste Your Time". In the Pipeline. Archived from the original on April 6, 2019. Retrieved June 19, 2017.
- ^ "Curcumin". Micronutrient Information Center; Phytochemicals. Linus Pauling Institute, Oregon State University, Corvallis. 2016. Retrieved June 18, 2016.
- ^ Lemonick S (January 19, 2017). "Everybody Needs To Stop With This Turmeric Molecule". Forbes. Retrieved May 27, 2018.
- ^ "187 Fake Cancer 'Cures' Consumers Should Avoid". U.S. Food and Drug Administration. Archived from the original on May 2, 2017. Retrieved May 20, 2020.
- ^ Ackerman T (February 29, 2012). "M.D. Anderson professor under fraud probe". Houston Chronicle. Retrieved March 8, 2016.
- ^ "Caught Our Notice: Researcher who once threatened to sue Retraction Watch now up to 19 retractions". Retraction Watch. April 10, 2018.
- ^ Ackerman T (March 2, 2016). "M.D. Anderson scientist, accused of manipulating data, retires". Houston Chronicle.
- ^ a b Stix G (February 2007). "Spice Healer". Scientific American. 296 (2): 66–9. Bibcode:2007SciAm.296b..66S. doi:10.1038/scientificamerican0207-66. PMID 17367023.
- ^ Ackerman T (July 11, 2005). "In cancer fight, a spice brings hope to the table". Houston Chronicle. Retrieved March 24, 2015.
- ^ Singh S (September 2007). "From exotic spice to modern drug?". Cell. 130 (5): 765–8. doi:10.1016/j.cell.2007.08.024. PMID 17803897. S2CID 16044143.
- ^ Baum S (March 26, 2013). "Biotech startup raises $1M for lung cancer treatment using component of tumeric". Med City News.
- ^ a b c "FDA investigates two serious adverse events associated with ImprimisRx's compounded curcumin emulsion product for injection". Food and Drug Administration. August 4, 2017.
- ^ Hermes BM (March 27, 2017). "Naturopathic Doctors Look Bad After California Woman Dies From Turmeric Injection". Forbes. Retrieved May 12, 2017.
- ^ a b c Hermes BM (April 10, 2017). "Confirmed: Licensed Naturopathic Doctor Gave Lethal 'Turmeric' Injection". Forbes. Retrieved December 9, 2017.