Adrenaline
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603002 |
| Pregnancy category |
|
| Routes of administration | IV, IM, endotracheal, IC |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Nil (oral) |
| Metabolism | adrenergic synapse (MAO and COMT) |
| Elimination half-life | 2 minutes |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.090 |
| Chemical and physical data | |
| Formula | C9H13NO3 |
| Molar mass | 183.204 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Epinephrine (also known as adrenaline) is a hormone and a neurotransmitter.[1] It increases heart rate, constricts blood vessels, dilates air passages and participates in the fight-or-flight response of the sympathetic nervous system.[2] Chemically, epinephrine is a catecholamine, a monoamine produced only by the adrenal glands from the amino acids phenylalanine and tyrosine.
The term adrenaline is derived from the Latin roots ad- and renes and literally means "on the kidney", in reference to the adrenal gland's anatomic location on the kidney. The Greek roots epi and nephros have similar meanings and give rise to epinephrine. The term epinephrine is often shortened to epi in medical jargon.[3]
Adrenal extracts containing adrenaline were first obtained by Polish physiologist Napoleon Cybulski in 1895. These extracts, which he called nadnerczyna, contained epinephrine and other catecholamines.[4] Japanese chemist Jokichi Takamine and his assistant Keizo Uenaka independently discovered adrenaline in 1900.[5][6] In 1901, Takamine successfully isolated and purified the hormone from the adrenal glands of sheep and oxen.[7] Adrenaline was first synthesized in the laboratory by Friedrich Stolz and Henry Drysdale Dakin, independently, in 1904.[6]
Medical uses

Epinephrine is used to treat a number of conditions including: cardiac arrest, anaphylaxis,and superficial bleeding.[8] It has been used historically for bronchospasm and hypoglycemia, but better treatments for these, such as salbutamol and dextrose respectively, are now preferred.[8]
Cardiac arrest
Adrenaline is used as a drug to treat cardiac arrest and other cardiac dysrhythmias resulting in diminished or absent cardiac output. Its actions are to increase peripheral resistance via α1receptor-dependent vasoconstriction and to increase cardiac output via its binding to β1 receptors. The usual ACLS concentration for injection is epinephrine 1:10,000.
Anaphylaxis
Due to its vasoconstrictive effects, adrenaline is the drug of choice for treating anaphylaxis. It is also useful in treating sepsis. Allergy[9] patients undergoing immunotherapy may receive an adrenaline rinse before the allergen extract is administered, thus reducing the immune response to the administered allergen. It is also used as a bronchodilator for asthma if specific β2 agonists are unavailable or ineffective.[10]
Because of various expressions of α1 or β2 receptors, depending on the patient, administration of adrenaline may raise or lower blood pressure, depending on whether or not the net increase or decrease in peripheral resistance can balance the positive inotropic and chronotropic effects of adrenaline on the heart, effects which respectively increase the contractility and rate of the heart.[citation needed]
The usual concentration for SQ or IM injection is 1:1,000.
Croup
Racemic epinephrine has historically been used for the treatment of croup.[11][12] Racemic epinephrine is a 1:1 mixture of the dextrorotatory (D) and levorotatory (L) isomers of epinephrine.[13] The L form is the active component.[13] Racemic epinephrine works by stimulation of the α-adrenergic receptors in the airway with resultant mucosal vasoconstriction and decreased subglottic edema and by stimulation of the β-adrenergic receptors with resultant relaxation of the bronchial smooth muscle.[12]
In local anesthetics
Epinephrine is added to injectable forms of a number of local anesthetics, such as bupivacaine and lidocaine, as a vasoconstrictor to retard the absorption and therefore prolong the action of the anesthetic agent. Some of the adverse effects of local anesthetic use, such as apprehension, tachycardia and tremor, may be caused by epinephrine.[14]
Autoinjectors
Epinephrine is available in an autoinjector delivery system. EpiPens, Anapens and Twinjects all use epinephrine as their active ingredient. Twinjects contain a second dose of epinephrine in a separate syringe and needle delivery system contained within the body of the autoinjector.
Though both EpiPen and Twinject are trademark names, common usage of the terms is drifting toward the generic context of any epinephrine autoinjector.[citation needed]
Adverse effects
Adverse reactions to epinephrine include palpitations, tachycardia, arrhythmia, anxiety, headache, tremor, hypertension, and acute pulmonary edema.[15]
Use is contraindicated in people on nonselective β-blockers, because severe hypertension and even cerebral hemorrhage may result.[16] Although commonly believed that administration of epinephrine may cause heart failure by constricting coronary arteries, this is not the case. Coronary arteries only have β2 receptors, which cause vasodilation in the presence of epinephrine.[17] Even so, administering high-dose epinephrine has not been definitively proven to improve survival or neurologic outcomes in adult victims of cardiac arrest.[18]
Measurement in biological fluids
Epinephrine may be quantitated in blood, plasma or serum as a diagnostic aid, to monitor therapeutic administration or to identify the causative agent in a potential poisoning victim. Endogenous plasma epinephrine concentrations in resting adults are normally less than 10 ng/L, but may increase by 10-fold during exercise and by 50-fold or more during times of stress. Pheochromocytoma patients often have plasma epinephrine levels of 1000-10,000 ng/L. Parenteral administration of epinephrine to acute-care cardiac patients can produce plasma concentrations of 10,000 to 100,000 ng/L.[19][20]
Mechanism of action
As a hormone, epinephrine acts on nearly all body tissues. Its actions vary by tissue type and tissue expression of adrenergic receptors. For example, epinephrine causes smooth muscle relaxation in the airways but causes contraction of the smooth muscle that lines most arterioles.
Epinephrine acts by binding to a variety of adrenergic receptors. Adrenaline is a nonselective agonist of all adrenergic receptors, including α1, α2, β1, β2, and β3 receptors.[16] Epinephrine's binding to these receptors triggers a number of metabolic changes. Binding to α-adrenergic receptors inhibits insulin secretion by the pancreas, stimulates glycogenolysis in the liver and muscle, and stimulates glycolysis in muscle.[21] β-adrenergic receptor binding triggers glucagon secretion in the pancreas, increased adrenocorticotropic hormone (ACTH) secretion by the pituitary gland, and increased lipolysis by adipose tissue. Together, these effects lead to increased blood glucose and fatty acids, providing substrates for energy production within cells throughout the body.[21]
In addition to these metabolic changes, epinephrine also leads to broad alterations throughout all organ systems.
| Organ | Effects |
|---|---|
| Heart | Increases heart rate |
| Lungs | Increases respiratory rate |
| Nearly all tissues | Vasoconstriction or vasodilation |
| Liver | Stimulates glycogenolysis |
| N/A, systemic | Triggers lipolysis |
| N/A, systemic | Muscle contraction |
Biosynthesis and regulation
Adrenaline is synthesized in the medulla of the adrenal gland in an enzymatic pathway that converts the amino acid tyrosine into a series of intermediates and, ultimately, adrenaline. Tyrosine is first oxidized to L-DOPA, which is subsequently decarboxylated to give dopamine. Oxidation gives norepinephrine, which is methylated to give epinephrine.
Adrenaline is synthesized via methylation of the primary distal amine of noradrenaline by phenylethanolamine N-methyltransferase (PNMT) in the cytosol of adrenergic neurons and cells of the adrenal medulla (so-called chromaffin cells). PNMT is only found in the cytosol of cells of adrenal medullary cells. PNMT uses S-adenosylmethionine (SAMe) as a cofactor to donate the methyl group to noradrenaline, creating adrenaline.[citation needed]
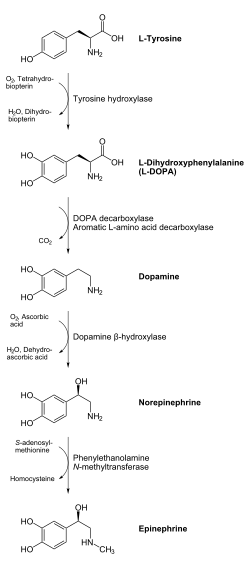
For noradrenaline to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H+ exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized adrenaline from the cytosol back into chromaffin granules in preparation fo release.[citation needed]
In liver cells, adrenaline binds to the β-adrenergic receptor, which changes conformation and helps Gs, a G protein, exchange GDP to GTP. This trimeric G protein dissociates to Gs alpha and Gs beta/gamma subunits. Gs alpha binds to adenyl cyclase, thus converting ATP into cyclic AMP. Cyclic AMP binds to the regulatory subunit of protein kinase A: Protein kinase A phosphorylates phosphorylase kinase. Meanwhile, Gs beta/gamma binds to the calcium channel and allows calcium ions to enter the cytoplasm. Calcium ions bind to calmodulin proteins, a protein present in all eukaryotic cells, which then binds to phosphorylase kinase and finishes its activation. Phosphorylase kinase phosphorylates glycogen phosphorylase, which then phosphorylates glycogen and converts it to glucose-6-phosphate. [citation needed]
Regulation
The major physiologic triggers of adrenaline release center upon stresses, such as physical threat, excitement, noise, bright lights, and high ambient temperature. All of these stimuli are processed in the central nervous system.[22]
Adrenocorticotropic hormone (ACTH) and the sympathetic nervous system stimulate the synthesis of adrenaline precursors by enhancing the activity of tyrosine hydroxylase and dopamine-β-hydroxylase, two key enzymes involved in catecholamine synthesis.[citation needed] ACTH also stimulates the adrenal cortex to release cortisol, which increases the expression of PNMT in chromaffin cells, enhancing adrenaline synthesis. This is most often done in response to stress.[citation needed] The sympathetic nervous system, acting via splanchnic nerves to the adrenal medulla, stimulates the release of adrenaline. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of adrenaline (and noradrenaline) into the bloodstream.[citation needed]
Adrenaline (as with noradrenaline) does exert negative feedback to down-regulate its own synthesis at the presynaptic alpha-2 adrenergic receptor.[citation needed] Abnormally elevated levels of adrenaline can occur in a variety of conditions, such as surreptitious epinephrine administration, pheochromocytoma, and other tumors of the sympathetic ganglia.
Its action is terminated with reuptake into nerve terminal endings, some minute dilution, and metabolism by monoamine oxidase and catechol-O-methyl transferase.
Chemical synthesis
Epinephrine may be synthesized by the reaction of catechol with chloroacetyl chloride, followed by the reaction with methylamine to give the ketone, which is reduced to the desired hydroxy compound. The racemic mixture may be separated using tartaric acid.

For isolation from the adrenal glands tissue of livestock:
- J. Takamine, J. Soc. Chem. Ind., 20, 746 (1901).
- J. B. Aldrich, Am. J. Physiol., 5, 457 (1901).
Synthetic production:
- A. F. Stolz, Chem. Ber., 37, 4149 (1904).
- K. R. Payne, Ind. Chem. Chem. Manuf., 37, 523 (1961).
- H. Loewe, Arzneimittel-Forsch., 4, 583 (1954).
- Farbenwerke Meister Lucins & Bruning in Hochst a.M., DE 152814 (1903).
- Farbenwerke Meister Lucins & Bruning in Hochst a.M., DE 157300 (1903).
- Farbenwerke Meister Lucins & Bruning in Hochst a.M., DE 222451 (1908).
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja01186a024, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja01186a024instead. - D. Flacher, Z. Physiol. Chem., 58, 189 (1908).
Adrenaline junkie
Adrenaline junkie is a term used to describe somebody who appears to be addicted to epinephrine (endogenous), and such a person is sometimes described as getting a "high" from life. The term adrenaline junkie was popularly used in the 1991 movie Point Break to describe individuals who enjoyed dangerous activities (such as extreme sports, e.g. BASE jumping) for the adrenaline "rush". Adrenaline junkies appear to favour stressful activities for the release of epinephrine as a stress response. Whether or not the positive response is caused specifically by epinephrine is difficult to determine, as endorphins are also released during the fight-or-flight response to such activities.[23][24]
Terminology
This chemical is widely referred to as "adrenaline" outside of the United States; however, its United States Adopted Name and International Nonproprietary Name is epinephrine. Epinephrine was chosen because adrenaline bore too much similarity to the Parke, Davis & Co trademark Adrenalin (without the e), which was registered in the United States. The British Approved Name and European Pharmacopoeia term for this chemical is adrenaline and is indeed now one of the few differences between the INN and BAN systems of names.[25]
Amongst American health professionals and scientists, the term epinephrine is used over adrenaline. However, it should be noted that pharmaceuticals that mimic the effects of epinephrine are often called adrenergics, and receptors for epinephrine are called adrenergic receptors or adrenoceptors.
Notes
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 6278965, please use {{cite journal}} with
|pmid=6278965instead. - ^ Cannon, W. B. (1929). American Journal of Physiology. 89: 84–107.
{{cite journal}}: Missing or empty|title=(help)[full citation needed] - ^ Gail Askew and Marilyn Smith-Stoner. (2001). The Pharmacy Assistant (Clinical Allied Heathcare Series). Clifton Park, NY: Thomson Delmar Learning. pp. 4–6. ISBN 0-89262-438-8.
- ^ "Polish Thread in the History of Circulatory Physiology". Retrieved 2011-04-24.
- ^ Yamashima T (2003). "Jokichi Takamine (1854–1922), the samurai chemist, and his work on adrenalin". J Med Biogr. 11 (2): 95–102. PMID 12717538.
- ^ a b Bennett M (1999). "One hundred years of adrenaline: the discovery of autoreceptors". Clin Auton Res. 9 (3): 145–59. doi:10.1007/BF02281628. PMID 10454061.
- ^ Takamine J (1901). The isolation of the active principle of the suprarenal gland. Great Britain: Cambridge University Press. pp. xxix–xxx.
{{cite book}}:|work=ignored (help) - ^ a b "Epinephrine". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ Sicherer, Scott H. (2006). Understanding and Managing Your Child's Food Allergy. Baltimore: The Johns Hopkins University Press. ISBN 0801884918.
- ^ "Asthma Causes, Types, Symptoms, Treatment, Medication, Facts and the Link to Allergies by MedicineNet.com".
- ^ Bjornson CL, Johnson DW (2008). "Croup". The Lancet. 371 (9609): 329–339. doi:10.1016/S0140-6736(08)60170-1. PMID 18295000.
- ^ a b Thomas LP, Friedland LR (1998). "The cost-effective use of nebulized racemic epinephrine in the treatment of croup". American Journal of Emergency Medicine. 16 (1): 87–89. doi:10.1016/S0735-6757(98)90073-0. PMID 9451322.
- ^ a b Malhotra A, Krilov LR (2001). "Viral Croup". Pediatrics in Review. 22 (1): 5–12. doi:10.1542/pir.22-1-5. PMID 11139641.
- ^ R. Rahn and B. Ball. Local Anesthesia in Dentistry, 3M ESPE AG, ESPE Platz, Seefeld, Germany, 2001, 44 pp.
- ^ About.com - "The Definition of Epinephrine"
- ^ a b Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 4. ISBN 1-59541-101-1.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12147535, please use {{cite journal}} with
|pmid=12147535instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 1522840, please use {{cite journal}} with
|pmid=1522840instead. - ^ Raymondos, K.; Panning, B.; Leuwer, M.; Brechelt, G.; Korte, T.; Niehaus, M.; Tebbenjohanns, J.; Piepenbrock, S. (2000). "Absorption and hemodynamic effects of airway administration of adrenaline in patients with severe cardiac disease". Ann. Intern. Med. 132 (10): 800–803. PMID 10819703.
- ^ Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 545–547. ISBN 0962652377.
- ^ a b Sabyasachi Sircar (2007). Medical Physiology. Thieme Publishing Group. p. 536. ISBN 3-13-144061-9.
{{cite book}}:|access-date=requires|url=(help) - ^ Nelson, L.; Cox, M. (2004). Lehninger Principles of Biochemstry (4th ed.). New York: Freeman. p. 908. ISBN 0716743396.
- ^ What Is An Adrenaline Junkie? What Can You Do If You Are One? by Elizabeth Scott, M.S. (updated: November 1, 2007) About.com Health's Disease and Condition content is reviewed by the Medical Review Board
- ^ Fight-or-flight reaction - Explanations - Brain - ChangingMinds.org
- ^ http://www.mhra.gov.uk/Howweregulate/Medicines/Namingofmedicines/ChangestomedicinesnamesBANstorINNs/index.htm
References
- Boron WF, Boulpaep EL (2005). Medical Physiology: A Cellular And Molecular Approach. Philadelphia, PA: Elsevier/Saunders. ISBN 1-4160-2328-3. OCLC 56191776.
- Voet D, Voet J (2004). Biochemistry (3rd ed.). USA: Wiley. ISBN 0-471-19350-x. OCLC 154657578.
{{cite book}}: Check|isbn=value: invalid character (help)
