From Wikipedia, the free encyclopedia
Chemical compound
Chemical compound
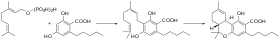
Biosynthesis of tetrahydrocannabinolic acid (THCA). In the first step, geranyl pyrophosphate and olivetolic acid form cannabigerolic acid, which is then enzymatically rearranged to THCA in the second step. Cannabigerolic acid (CBGA) is the acidic form of cannabigerol (CBG). It is a dihydroxybenzoic acid that is olivetolic acid in which the hydrogen at position 3 is substituted by a geranyl group. A biosynthetic precursor to Delta(9)-tetrahydrocannabinol , the principal psychoactive constituent of the Cannabis dihydroxybenzoic acid , a diterpenoid , a polyketide, a member of resorcinols and a phytocannabinoid . It derives from an olivetolic acid. It is a conjugate acid of a cannabigerolate.
In the Cannabis plant, olivetolic acid and geranyl diphosphate are synthesized into CBGA. CBGA is converted in the plant by CBCA synthase, cannabidiolic acid synthase (CBDA synthase) and tetrahydrocannabinolic acid synthase (THCA synthase) into CBCA, CBDA and tetrahydrocannabinolic acid (THCA). THCA can be decarboxylated into THC by drying and heating plant material.
References
Sources
"Compound Summary – cannabigerolic acid" . PubChem United States National Library of Medicine . Retrieved April 7, 2020 .Thomas, Brian F.; ElSohly, Mahmoud A. (2015). The Analytical Chemistry of Cannabis: Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations Elsevier Science . ISBN 978-0-12-804670-8 Degenhardt, F.; Stehle, F.; Kayser, O. (2016). "The biosynthesis of cannabinoids". In Preedy, Victor R. (ed.). Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment . Academic Press . pp. 13–23. ISBN 978-0128008270
Further reading
Phytocannabinoids comparison )
Cannabibutols Cannabichromenes Cannabicyclols Cannabidiols Cannabielsoins Cannabigerols Cannabiphorols Cannabinols Cannabitriols Cannabivarins Delta-8-tetrahydrocannabinols Delta-9-tetrahydrocannabinols Delta-10-Tetrahydrocannabinols Miscellaneous cannabinoids Active metabolites
Endocannabinoids Synthetic
Classical cannabinoids Non-classical Adamantoylindoles Benzimidazoles Benzoylindoles Cyclohexylphenols Eicosanoids Indazole-3- Indole-3-carboxamides Indole-3-carboxylates Naphthoylindazoles Naphthoylindoles Naphthoylpyrroles Naphthylmethylindenes Naphthylmethylindoles Phenylacetylindoles Pyrazolecarboxamides Tetramethylcyclo- Tetramethylcyclo- Others
Allosteric CBR Tooltip Cannabinoid receptor ligands Endocannabinoid (inactivation inhibitors) Anticannabinoids (antagonists/inverse
TRPA
Activators
4-Hydroxynonenal 4-Oxo-2-nonenal 4,5-EET 12S-HpETE 15-Deoxy-Δ12,14 -prostaglandin J2 α-Sanshool (ginger , Sichuan and melegueta peppers )Acrolein Allicin (garlic )Allyl isothiocyanate (mustard , radish , horseradish , wasabi )AM404 ASP-7663 Bradykinin Cannabichromene (cannabis )Cannabidiol (cannabis )Cannabigerol (cannabis )Cinnamaldehyde (cinnamon )CR gas (dibenzoxazepine; DBO) CS gas (2-chlorobenzal malononitrile) Cuminaldehyde (cumin )Curcumin (turmeric )Dehydroligustilide (celery )Diallyl disulfide Dicentrine (Lindera Farnesyl thiosalicylic acid Formalin Gingerols (ginger )Hepoxilin A3 Hepoxilin B3 Hydrogen peroxide Icilin Isothiocyanate JT-010 Ligustilide (celery , Angelica acutiloba Linalool (Sichuan pepper , thyme )Methylglyoxal Methyl salicylate (wintergreen )N-Methylmaleimide Nicotine (tobacco )Oleocanthal (olive oil )Paclitaxel (Pacific yew )Paracetamol (acetaminophen) PF-4840154 Phenacyl chloride Polygodial (Dorrigo pepper )Shogaols (ginger , Sichuan and melegueta peppers )Tear gases Tetrahydrocannabinol (cannabis )Tetrahydrocannabiorcol Thiopropanal S-oxide (onion )Umbellulone (Umbellularia californica )WIN 55,212-2 Blockers
TRPC
TRPM
TRPML
TRPP
TRPV
Activators
2-APB 5',6'-EET 9-HODE 9-oxoODE 12S-HETE 12S-HpETE 13-HODE 13-oxoODE 20-HETE α-Sanshool (ginger , Sichuan and melegueta peppers )Allicin (garlic )AM404 Anandamide Bisandrographolide (Andrographis paniculata Camphor (camphor laurel , rosemary , camphorweed , African blue basil , camphor basil )Cannabidiol (cannabis )Cannabidivarin (cannabis )Capsaicin (chili pepper )Carvacrol (oregano , thyme , pepperwort , wild bergamot , others)DHEA Diacyl glycerol Dihydrocapsaicin (chili pepper )Estradiol Eugenol (basil , clove )Evodiamine (Euodia ruticarpa Gingerols (ginger )GSK1016790A Heat Hepoxilin A3 Hepoxilin B3 Homocapsaicin (chili pepper )Homodihydrocapsaicin (chili pepper )Incensole (incense )Lysophosphatidic acid Low pH (acidic conditions)
Menthol (mint )N-Arachidonoyl dopamine N-Oleoyldopamine N-Oleoylethanolamide Nonivamide (PAVA) (PAVA spray )Nordihydrocapsaicin (chili pepper )Paclitaxel (Pacific yew )Paracetamol (acetaminophen) Phenylacetylrinvanil Phorbol esters (e.g., 4α-PDD )Piperine (black pepper , long pepper )Polygodial (Dorrigo pepper )Probenecid Protons RhTx Rutamarin (Ruta graveolens Resiniferatoxin (RTX) (Euphorbia resinifera /pooissonii Shogaols (ginger , Sichuan and melegueta peppers )Tetrahydrocannabivarin (cannabis )Thymol (thyme , oregano )Tinyatoxin (Euphorbia resinifera /pooissonii Tramadol Vanillin (vanilla )Zucapsaicin Blockers