Cypenamine: Difference between revisions
m Stub sorting and placement of stub template(s): nervous-system-drug-stub. See approval. Report errors and suggestions at User talk:PotatoBot. |
Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoB |
||
| Line 1: | Line 1: | ||
{{Refimprove|date=August 2009}} |
{{Refimprove|date=August 2009}} |
||
{{Drugbox |
{{Drugbox |
||
| ⚫ | |||
| Watchedfields = changed |
|||
| ⚫ | |||
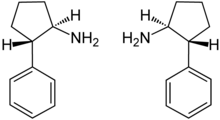
| IUPAC_name = (±)-''trans''-2-phenylcyclopentan-1-amine |
| IUPAC_name = (±)-''trans''-2-phenylcyclopentan-1-amine |
||
| image = Trans-(±)-Cypenamine Enantiomers Structural Formulae.png |
| image = Trans-(±)-Cypenamine Enantiomers Structural Formulae.png |
||
Revision as of 18:48, 10 September 2011
This article needs additional citations for verification. (August 2009) |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15N |
| Molar mass | 161.2435 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cypenamine, or phenylcyclopentamine, is a stimulant drug. It is currently known only in scientific research and has never been developed for market use. The trans- isomer is reported to be more active than the racemate.[1] Cypenamine is currently legal throughout the entire world, and though its chemical structure has a vague similarity to certain controlled stimulants like fencamfamine (Glucoenergan, Reactivan), it is likely that it is too distant for it to be considered an illicit analogue under say the United States (U.S.) Federal Analogue Act (FAA) of the Controlled Substances Act (CSA).
Stereochemistry
2-Phenylcyclopentan-1-amine is a compound with two stereocenters. Thus, the following four following stereoisomers may exist:
- (1R,2S)-trans-2-phenylcyclopentan-1-amine
- (1S,2R)-trans-2-phenylcyclopentan-1-amine
- (1R,2R)-cis-2-phenylcyclopentan-1-amine
- (1S,2S)-cis-2-phenylcyclopentan-1-amine
The racemate (±)-trans-2-phenylcyclopentan-1-amine [1:1 mixture of (1R,2S)-trans-2-phenylcyclopentan-1-amine (box, left) and (1S,2R)-trans-2-phenylcyclopentan-1-amine (box, right)] is the active ingredient of cypenamine.[2] Furthermore, the kinetic resolution of (±)-trans-2-phenylcyclopentan-1-amine by lipase B from Candida antarctica may effectivily performed by an aminolysis reaction.[2]
Racemic cis-2-phenylcyclopentan-1-amine [1:1 mixture of (1R,2R)-cis-2-phenylcyclopentan-1-amine and (1S,2S)-cis-2-phenylcyclopentan-1-amine] has found no pharmacological application.
Homology
Cypenamine is an obvious homologue, containing an expanded alicyclic ring that is two -CH2- units larger than the highly strained/reactive cyclopropane of tranylcypromine. Based on this logic, one would have to contend that the cyclobutane[citation needed] homologue is yet another possibility. The cyclohexane homologue is known to have been reported, although the LD50's were all less than for plain amphetamine, it was still a functional stimulant.
See also
References
- ^ Burgos A, Kamenka JM, Rousseau B. Use of tritiated methylborane in the synthesis of labelled primary amines. Journal of Labelled Compounds and Radiopharmaceuticals. 1991;29(12):1347–1350. doi:10.1002/jlcr.2580291212.
- ^ a b González-Sabín J, Gotor V, Rebolledo F. Kinetic resolution of (±)-trans- und (±)-cis-phenylcyclopentanamine by CALB-catalyzed aminolysis of esters: the key role of the leaving group. Tetrahedron:Asymmetry. 2004;15(3):481–488. doi:10.1016/j.tetasy.2003.11.013.
