GYKI 52895
 | |
 | |
| Clinical data | |
|---|---|
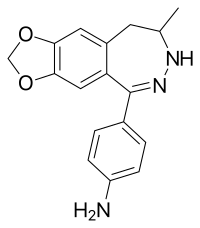
| Other names | 4-(8,9-Dihydro-8-methyl-7H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)benzenamine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H17N3O2 |
| Molar mass | 295.342 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
GYKI 52895 is a drug which is a 2,3-benzodiazepine derivative that also shares the 3,4-methylenedioxyphenethylamine pharmacophore. Unlike other similar drugs, GYKI 52895 is a selective dopamine reuptake inhibitor (DARI),[1][2] which appears to have an atypical mode of action compared to other DARIs.[3] Its DRI activity is shared by numerous addictive drugs including amphetamine and its derivatives (e.g. dextromethamphetamine), cocaine, and methylphenidate and its derivatives (e.g. ethylphenidate). However, dopaminergic drugs are also prone to producing emetic effects such as in the case of apomorphine.
Egis Pharmaceuticals began clinical development of the drug in 1997 for major depressive disorder and Parkinson's disease, but it was discontinued in 2001.[4]
See also
- GYKI 52466, another 2,3-benzodiazepine with other than GABAergic function
- Tifluadom
- Lufuradom
- Benzodiazepine
- Substituted methylenedioxyphenethylamine
References
- ^ Horváth K, Szabó H, Pátfalusi M, Berzsenyi P, Andrási F (1990). "Pharmacological Effects of GYKI 52895, a New Selective Dopamine Uptake Inhibitor". European Journal of Pharmacology. 183 (4): 1416–1417. doi:10.1016/0014-2999(90)94548-C.
- ^ Huang CL, Chen HC, Huang NK, Yang DM, Kao LS, Chen JC, et al. (June 1999). "Modulation of dopamine transporter activity by nicotinic acetylcholine receptors and membrane depolarization in rat pheochromocytoma PC12 cells". Journal of Neurochemistry. 72 (6): 2437–44. doi:10.1046/j.1471-4159.1999.0722437.x. PMID 10349853.
- ^ Vaarmann A, Gandhi S, Gourine AV, Abramov AY (2010). "Novel pathway for an old neurotransmitter: dopamine-induced neuronal calcium signalling via receptor-independent mechanisms". Cell Calcium. 48 (2–3): 176–82. doi:10.1016/j.ceca.2010.08.008. PMID 20846720.
- ^ "GYKI 52895". Adis Insight.
