Caffeine: Difference between revisions
→Athletic performance: shorter |
→Athletics: Updated with review articles |
||
| Line 99: | Line 99: | ||
===Athletics=== |
===Athletics=== |
||
Caffeine slightly increases both sprint<ref>{{cite journal|last=Bishop|first=D|title=Dietary supplements and team-sport performance.|journal=Sports medicine (Auckland, N.Z.)|date=2010 Dec 1|volume=40|issue=12|pages=995-1017|pmid=21058748}}</ref>,endurance<ref>{{cite journal|last=Conger|first=SA|coauthors=Warren, GL, Hardy, MA, Millard-Stafford, ML|title=Does caffeine added to carbohydrate provide additional ergogenic benefit for endurance?|journal=International journal of sport nutrition and exercise metabolism|date=2011 Feb|volume=21|issue=1|pages=71-84|pmid=21411838}}</ref> and team sports performance.<ref>{{cite journal|last=Astorino|first=TA|coauthors=Roberson, DW|title=Efficacy of acute caffeine ingestion for short-term high-intensity exercise performance: a systematic review.|journal=Journal of strength and conditioning research / National Strength & Conditioning Association|date=2010 Jan|volume=24|issue=1|pages=257-65|pmid=19924012}}</ref> Evidence also shows that it may be helpful at [[high altitude]] contrary to common advice.<ref>{{cite journal|last=Hackett|first=PH|title=Caffeine at high altitude: java at base cAMP.|journal=High altitude medicine & biology|date=2010 Spring|volume=11|issue=1|pages=13-7|pmid=20367483|url=http://www.altitudemedicine.org/publications/Caffeine%20at%20High%20Altitude.pdf}}</ref> |
|||
Elite distance runners who consumed 10 mg of caffeine per kg of body mass immediately before a treadmill run to exhaustion improved performance time compared to placebo and control conditions.<ref>{{cite book|last=McArdle|first=William|title=Physiology. 7th edition|year=2010|publisher=Lippincott Williams and Wilkins|location=Baltimore, MD|isbn=97807818797818|page=557}}</ref> |
|||
| ⚫ | A number of potential mechanisms have been proposed for the performance-enhancing effects of caffeine.<ref>{{cite journal|last=Davis|first=JK|coauthors=Green, JM|title=Caffeine and anaerobic performance: ergogenic value and mechanisms of action.|journal=Sports medicine (Auckland, N.Z.)|date=2009|volume=39|issue=10|pages=813-32|pmid=19757860}}</ref> In the classic, or metabolic theory, caffeine may increase fat utilization and decrease glycogen utilization. Caffeine mobilizes free [[fatty acid]]s from fat and/or intramuscular triglycerides by increasing circulating [[epinephrine]] levels. The increased availability of free fatty acids increases fat oxidation and spares muscle [[glycogen]], thereby enhancing endurance performance. It may also act as a [[central nervous system]] stimulant, caffeine increases alertness and decreases the perception of effort during exercise. Caffeine may reduce the perception of effort by lowering the neuron activation threshold, making it easier to recruit the muscles for exercise.<ref>{{cite book|last=McArdle|first=William|title=Exercise Physiology. 7th edition|year=2010|publisher=Lippincott Williams and Wilkins|location=Baltimore, MD|isbn=9780781797818|page=559}}</ref> |
||
Researchers Kovacs and associates evaluated the effects of different dosages of caffeine (2.1, 3.2 and 4.5 milligrams per kilogram of body mass) added to a 7% carbohydrate electrolyte drink on performance during a one hour cycling time trial. All three caffeine doses improved performance compared with the carbohydrate electrolyte drink alone. The results also demonstrated that ingestion of 2.1, 3.2 and 4.5 mg/kg of caffeine produced the same level of performance enhancement. This study suggests that once the threshold dose of caffeine was reached, there was no further performance benefit from a higher amount of caffeine.<ref>{{cite journal|last=Kovacs|first=Eva|coauthors=Stegen JHCH, Brouns F|title=Effect of caffeinated drinks on substrate metabolism, caffeine excretion, and performance.|journal=Journal of Applied Physiology|year=1998|volume=85|pages=709–715}}</ref> |
|||
| ⚫ | |||
===Other=== |
===Other=== |
||
Revision as of 05:47, 24 September 2011

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione | |||
| Other names
Caffein;[1] 1,3,7-Trimethylxanthine; Trimethylxanthine; Methyltheobromine; 7-Methyltheophylline; Mateine; Guaranine
| |||
| Properties | |||
| C8H10N4O2 | |||
| Molar mass | 194.194 g·mol−1 | ||
| Appearance | Odorless, white needles or powder | ||
| Supplementary data page | |||
| Caffeine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug. It was first isolated from coffee in 1820 by the German chemist Friedlieb Ferdinand Runge, and then independently in 1821 by French chemists Pierre Robiquet, Pierre Pelletier, and Joseph Caventou. Pelletier coined the word "cafeine" from the French word for coffee (café ), and this term became the English word "caffeine".
Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants. It is most commonly consumed by humans in infusions extracted from the bean of the coffee plant and the leaves of the tea bush, as well as from various foods and drinks containing products derived from the kola nut. Other sources include yerba maté, guarana berries, guayusa, and the yaupon holly.
In humans, caffeine acts as a central nervous system stimulant, temporarily warding off drowsiness and restoring alertness. It is the world's most widely consumed psychoactive drug, but, unlike many other psychoactive substances, it is legal and unregulated in nearly all parts of the world. Beverages containing caffeine, such as coffee, tea, soft drinks, and energy drinks, enjoy great popularity; in North America, 90% of adults consume caffeine daily.[2] The U.S. Food and Drug Administration lists caffeine as a "multiple purpose generally recognized as safe food substance".[3]
Caffeine has diuretic properties when administered in sufficient doses to subjects who do not have a tolerance for it.[4] Regular users, however, develop a strong tolerance to this effect,[4] and studies have generally failed to support the common notion that ordinary consumption of caffeinated beverages contributes significantly to dehydration.[5][6][7]
Health effects
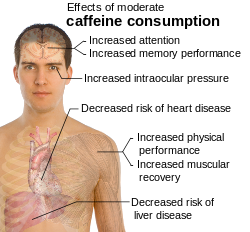
The precise amount of caffeine necessary to produce effects varies from person to person, depending on body size and degree of tolerance. Effects begin less than an hour after consumption, and a mild dose usually wears off in three to four hours.[9]
Caffeine also increases the effectiveness of some drugs. Many over-the-counter headache drugs include caffeine in their formula. It is also used with ergotamine in the treatment of migraine and cluster headaches as well as to overcome the drowsiness caused by antihistamines.
Tolerance and withdrawal
With repetitive use, the stimulatory effects of caffeine are substantially reduced over time, a phenomenon known as a tolerance. Tolerance develops quickly to some (but not all) effects of caffeine, especially among heavy coffee and energy drink consumers. Some coffee drinkers develop tolerance to its sleep-disrupting effects, but others apparently do not.[10] Withdrawal symptoms—including headache, irritability, inability to concentrate, drowsiness, insomnia, and pain in the stomach, upper body, and joints—may appear within 12 to 24 hours after discontinuation of caffeine intake, peak at roughly 48 hours, and usually last from one to five days.[11]
Overuse
Consumption of large amounts of caffeine — usually more than 500 mg per day — especially over extended periods of time, can lead to a condition known as caffeinism.[12] Caffeinism usually combines caffeine dependency with a wide range of unpleasant physical and mental conditions including nervousness, irritability, restlessness, insomnia, headaches, and heart palpitations. In some cases caffeinism can exacerbate other pre-existing problems, including mood disorders or psychosis. Caffeine may also increase the toxicity of certain other drugs, such as paracetamol.[13]
There are four caffeine-induced psychiatric disorders recognized by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: caffeine intoxication, caffeine-induced anxiety disorder, caffeine-induced sleep disorder, and caffeine-related disorder not otherwise specified (NOS).
Caffeine intoxication
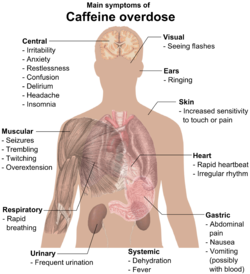
Caffeine overdose can result in a state of central nervous system over-stimulation called caffeine intoxication (DSM-IV 305.90),[15] or colloquially the "caffeine jitters". The symptoms of caffeine intoxication are not unlike overdoses of other stimulants. It may include restlessness, fidgetiness, nervousness, excitement, euphoria, insomnia, flushing of the face, increased urination, gastrointestinal disturbance, muscle twitching, a rambling flow of thought and speech, irritability, irregular or rapid heart beat, and psychomotor agitation.[citation needed] In cases of much larger overdoses, mania, depression, lapses in judgment, disorientation, disinhibition, delusions, hallucinations, and psychosis may occur, and rhabdomyolysis (breakdown of skeletal muscle tissue) can be provoked.[16][17]
Extreme overdose can result in death.[18] The median lethal dose (LD50) given orally, is 192 milligrams per kilogram in rats.[19] The LD50 of caffeine in humans is dependent on weight and individual sensitivity and estimated to be about 150 to 200 milligrams per kilogram of body mass, roughly 80 to 100 cups of coffee for an average adult taken within a limited time frame that is dependent on half-life. Though achieving lethal dose with caffeine would be exceptionally difficult with regular coffee, there have been reported deaths from overdosing on caffeine pills, with serious symptoms of overdose requiring hospitalization occurring from as little as 2 grams of caffeine. An exception to this would be taking a drug such as fluvoxamine or levofloxacin, which blocks the liver enzyme responsible for the metabolism of caffeine, thus increasing the central effects and blood concentrations of caffeine dramatically at 5-fold. It is not contraindicated, but highly advisable to minimize the intake of caffeinated beverages, as drinking one cup of coffee will have the same effect as drinking five under normal conditions.[20][21][22][23] Death typically occurs due to ventricular fibrillation brought about by effects of caffeine on the cardiovascular system.
Treatment of severe caffeine intoxication is generally supportive, providing treatment of the immediate symptoms, but if the patient has very high serum levels of caffeine then peritoneal dialysis, hemodialysis, or hemofiltration may be required.
Psychological
Sleep
Two infrequently diagnosed caffeine-induced disorders that are recognized by the American Psychological Association (APA) are caffeine-induced sleep disorder and caffeine-induced anxiety disorder, which can result from long-term excessive caffeine intake.
In the case of caffeine-induced sleep disorder, an individual regularly ingests high doses of caffeine sufficient to induce a significant disturbance in his or her sleep, sufficiently severe to warrant clinical attention.[15]
Caffeine has beneficial effects on reaction time and performance in a variety of tasks, particularly under conditions of low arousal.[24] Consumption of caffeine does not eliminate the need for sleep; it only temporarily reduces the sensation of being tired. It does however lead to fewer mistakes caused by tiredness in shift workers.[25]
Anxiety
In some individuals, large amounts of caffeine can induce anxiety severe enough to necessitate clinical attention. This caffeine-inducedanxiety disorder can take many forms, from generalized anxiety to panic attacks,obsessive-compulsive symptoms, or even phobic symptoms.[15] Because this condition can mimic organic mental disorders, such as panic disorder, generalized anxiety disorder, bipolar disorder, akathisia, or even schizophrenia, a number of medical professionals believe caffeine-intoxicated people are routinely misdiagnosed and unnecessarily medicated when the treatment for caffeine-induced psychosis would simply be to stop further caffeine intake.[26]
ADHD
Though caffeine is not specifically approved for treatment of ADHD, some ADHD sufferers self-medicate with caffeine,[27] reporting a sedative or calmative effect.[28][29] This may be explained by the low arousal theory of ADHD, which suggests that ADHD sufferers have lower than normal levels of dopamine and arousal,[30][31] and are driven to seek more intellectual and emotional stimuli from the surrounding environment than people without ADHD in order to compensate.[32] Because caffeine acts as an antagonist to receptors of adenosine, a neurotransmitter that inhibits arousal, ingestion of caffeine may cause the arousal levels of ADHD sufferers to return to normal and alleviate some of the symptoms of the disorder.[33][34]
Memory
Researchers have found that long-term consumption of low dose caffeine slowed hippocampus-dependent learning and impaired long-term memory in mice. Caffeine consumption for 4 weeks also significantly reduced hippocampal neurogenesis compared to controls during the experiment. The conclusion was that long-term consumption of caffeine could inhibit hippocampus-dependent learning and memory partially through inhibition of hippocampal neurogenesis.[35]
In another study, caffeine was added to rat neurons in vitro. The dendritic spines (a part of the brain cell used in forming connections between neurons) taken from the hippocampus (a part of the brain associated with memory) grew by 33% and new spines formed. After an hour or two, however, these cells returned to their original shape.[36]
Another study showed that human subjects—after receiving 100 milligrams of caffeine—had increased activity in brain regions located in the frontal lobe, where a part of the working memory network is located, and the anterior cingulate cortex, a part of the brain that controls attention. The caffeinated subjects also performed better on the memory tasks.[37]
However, a different study showed that caffeine could impair short-term memory and increase the likelihood of the tip of the tongue phenomenon. The study allowed the researchers to suggest that caffeine could aid short-term memory when the information to be recalled is related to the current train of thought, but also to hypothesize that caffeine hinders short-term memory when the train of thought is unrelated.[38]
Cancer
Coffee consumption is associated with a lower risk of cancer.[39] This protective effect is primarily for hepatocellular and endometrial cancer.[40]
Cardiovascular
According to one study, caffeine in the form of coffee significantly reduces the risk of heart disease in epidemiological studies. However, the protective effect was found only in participants who were not severely hypertensive (i.e., patients that are not suffering from a very high blood pressure). Furthermore, no significant protective effect was found in participants aged less than 65 years or in cerebrovascular disease mortality for those aged equal or more than 65 years.[41] Research also suggests that drinking caffeinated coffee can cause a temporary increase in the stiffening of arterial walls.[42]
During pregnancy
A 2008 study suggested that 200 milligrams or more of caffeine per day "significantly increases the risk of miscarriage" for pregnant women.[43] However, another 2008 study found no correlation between miscarriage and caffeine consumption.[44] The UK Food Standards Agency has recommended that pregnant women should limit their caffeine intake to less than 200 mg of caffeine a day—the equivalent of two cups of instant coffee, or one and a half to two cups of fresh coffee.[45][46] The FSA noted that the design of the studies made it impossible to be certain that the differences were due to caffeine per se, instead of other lifestyle differences possibly associated with high levels of caffeine consumption, but judged the advice to be prudent.
Athletics
Caffeine slightly increases both sprint[47],endurance[48] and team sports performance.[49] Evidence also shows that it may be helpful at high altitude contrary to common advice.[50]
A number of potential mechanisms have been proposed for the performance-enhancing effects of caffeine.[51] In the classic, or metabolic theory, caffeine may increase fat utilization and decrease glycogen utilization. Caffeine mobilizes free fatty acids from fat and/or intramuscular triglycerides by increasing circulating epinephrine levels. The increased availability of free fatty acids increases fat oxidation and spares muscle glycogen, thereby enhancing endurance performance. It may also act as a central nervous system stimulant, caffeine increases alertness and decreases the perception of effort during exercise. Caffeine may reduce the perception of effort by lowering the neuron activation threshold, making it easier to recruit the muscles for exercise.[52]
Other
Caffeine increases intraocular pressure in those with glaucoma but does not appear to affect normal individuals.[53] It may protect people from liver cirrhosis. [54] There is no evidence that coffee stunts a childs growth.[55]
Caffeine is the primary treatment of the breathing disorders apnea of prematurity[56] and may also be effective in preventing bronchopulmonary dysplasia in premature infants.[57] The only short-term risk associated with caffeine citrate treatment is a temporary reduction in weight gain during the therapy,[58] and longer term studies (18 to 21 months) have shown lasting benefits of treatment of premature infants with caffeine.[59]
Detection in biological fluids
Caffeine can be quantified in blood, plasma, or serum to monitor therapy in neonates, confirm a diagnosis of poisoning, or facilitate a medicolegal death investigation. Plasma caffeine levels are usually in the range of 2–10 mg/L in coffee drinkers, 12–36 mg/L in neonates receiving treatment for apnea, and 40–400 mg/L in victims of acute overdosage. Urinary caffeine concentration is frequently measured in competitive sports programs, for which a level in excess of 15 mg/L is usually considered to represent abuse.[60]
Consumption
| Product | Serving size | Caffeine per serving (mg) | Caffeine per liter (mg) |
|---|---|---|---|
| Caffeine tablet (regular-strength) | 1 tablet | 100 | — |
| Caffeine tablet (extra-strength) | 1 tablet | 200 | — |
| Excedrin tablet | 1 tablet | 65 | — |
| Hershey's Special Dark (45% cacao content) | 1 bar (43 g; 1.5 oz) | 31 | — |
| Hershey's Milk Chocolate (11% cacao content) | 1 bar (43 g; 1.5 oz) | 10 | — |
| Percolated coffee | 207 mL (7 U.S. fl oz) | 80–135 | 386–652 |
| Drip coffee | 207 mL (7 U.S. fl oz) | 115–175 | 555–845 |
| Coffee, decaffeinated | 207 mL (7 U.S. fl oz) | 5–15 | 24–72 |
| Coffee, espresso | 44–60 mL (1.5-2 U.S. fl oz) | 100 | 1,691–2254 |
| Black tea | 177 mL (6 U.S. fl oz) | 50 | 282 |
| Green tea | 177 mL (6 U.S. fl oz) | 30 | 170 |
| Guayakí yerba mate (loose leaf) | 6 g (0.2 U.S. oz) | 85[64] | 358 about |
| Coca-Cola Classic | 355 mL (12 U.S. fl oz) | 34 | 96 |
| Barq's Root Beer | 355 mL (12 U.S. fl oz) | 22.5 | 63 |
| Mountain Dew | 355 mL (12 U.S. fl oz) | 54 | 154 |
| Vault | 355 mL (12 U.S. fl oz) | 69 | 194 |
| Guaraná Antarctica | 350 mL (11 U.S. fl oz) | 30 | 100 |
| Monster energy drink | 500 mL (16.4 U.S. fl oz) | 160 | 320 |
| Jolt Cola | 695 mL (23.5 U.S. fl oz) | 280 | 403 |
| Red Bull | 250 mL (8.2 U.S. fl oz) | 80 | 320 |
Caffeine is found in many plant species, where it acts as a natural pesticide, with high caffeine levels being observed in seedlings still developing foliage but lacking mechanical protection;[65] caffeine paralyzes and kills certain insects feeding upon the plant.[66] High caffeine levels have also been found in the surrounding soil of coffee bean seedlings. Therefore, caffeine is understood to have a natural function as both a natural pesticide and an inhibitor of seed germination of other nearby coffee seedlings, thus giving it a better chance of survival.[67]
Common sources of caffeine are coffee, tea, and (to a lesser extent) chocolate derived from cocoa beans.[68] Less commonly used sources of caffeine include the yerba maté, guarana and ilex guayusa plants,[unreliable source?][69] which are sometimes used in the preparation of teas and energy drinks. Two of caffeine's alternative names, mateine and guaranine, are derived from the names of these plants.[70][71] Some yerba mate enthusiasts assert that mateine is a stereoisomer of caffeine, which would make it a different substance altogether.[unreliable source?][69] This is not true because caffeine is an achiral molecule,[72] and therefore has no enantiomers; nor does it have other stereoisomers.[73] The disparity in experience and effects between the various natural caffeine sources could be because plant sources of caffeine also contain widely varying mixtures of other xanthine alkaloids, including the cardiac stimulants theophylline and theobromine, and other substances such as polyphenols that can form insoluble complexes with caffeine.[74]
One of the world's primary sources of caffeine is the coffee "bean" (which is the seed of the coffee plant), from which coffee is brewed. Caffeine content in coffee varies widely depending on the type of coffee bean and the method of preparation used;[75] even beans within a given bush can show variations in concentration. In general, one serving of coffee ranges from 80–100 milligrams, for a single shot (30 milliliters) of arabica-variety espresso, to approximately 100–125 milligrams for a cup (120 milliliters) of drip coffee. In general, dark-roast coffee has very slightly less caffeine than lighter roasts because the roasting process reduces a small amount of the bean's caffeine content.[76][77] Arabica coffee normally contains significantly (+/-50%) less caffeine than the robusta variety.[75] Coffee also contains trace amounts of theophylline, but no theobromine.
Tea is another common source of caffeine. Although tea contains more caffeine than coffee (by dry weight), a typical serving contains much less, as tea is normally brewed much weaker. Besides strength of the brew, growing conditions, processing techniques and other variables also affect caffeine content. Certain types of tea may contain somewhat more caffeine than other teas. Tea contains small amounts of theobromine and slightly higher levels of theophylline than coffee. Preparation and many other factors have a significant impact on tea, and color is a very poor indicator of caffeine content.[unreliable source?][78] Teas like the pale Japanese green tea, gyokuro, for example, contain far more caffeine than much darker teas like lapsang souchong, which has very little.
Caffeine is also a common ingredient of soft drinks, such as cola, originally prepared from kola nuts. Soft drinks typically contain about 10 to 50 milligrams of caffeine per serving. By contrast, energy drinks, such as Red Bull, can start at 80 milligrams of caffeine per serving. The caffeine in these drinks either originates from the ingredients used or is an additive derived from the product of decaffeination or from chemical synthesis. Guarana, a prime ingredient of energy drinks, contains large amounts of caffeine with small amounts of theobromine and theophylline in a naturally occurring slow-release excipient.[79]
Chocolate derived from cocoa beans contains a small amount of caffeine. The weak stimulant effect of chocolate may be due to a combination of theobromine and theophylline, as well as caffeine.[80] A typical 28-gram serving of a milk chocolate bar has about as much caffeine as a cup of decaffeinated coffee, although some dark chocolate currently in production contains as much as 160 mg per 100g.
Various manufacturers market caffeine tablets, claiming that using caffeine of pharmaceutical quality improves mental alertness. These effects have been borne out by research that shows caffeine use (whether in tablet form or not) results in decreased fatigue and increased attentiveness.[9] These tablets are commonly used by students studying for their exams and by people who work or drive for long hours.[81] One U.S. company is also marketing dissolving caffeine strips as an alternative to energy drinks.[82]
Caffeine is also used pharmacologically to treat apnea in premature newborns and, as such, is one of the 10 drugs most commonly given in neonatal intensive care,[83] though questions are now raised based on experimental animal research whether it might have subtle harmful side-effects.[83]
A 2011 analysis published by PLoS Genetics reviewed 5 studies covering more than 47,000 subjects of European descent. Researchers determined that habitual caffeine intake is associated with variations in 2 genes that regulate how quickly the body processes caffeine. Subjects who had a high-intake mutation of either gene on both chromosomes consumed 40 mg more caffeine per day (equivalent to a can of soda) than people who did not.[84][85]
Synthesis and chemical properties
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.329 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H10N4O2 | |||
| Molar mass | 194.194 g·mol−1 | ||
| Appearance | Odorless, white needles or powder | ||
| Density | 1.23 g/cm3, solid[86] | ||
| Melting point | 227–228 °C, 500-501 K (anhydrous) 234–235 °C, 507-508 K (monohydrate) | ||
| Boiling point | 178 °C (352 °F; 451 K) | ||
| 2.17 g/100 mL (25 °C) 18.0 g/100 mL (80 °C) 67.0 g/100 mL (100 °C) | |||
| Acidity (pKa) | −0.13–1.22[87] | ||
| 3.64 D (calculated) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
192 mg/kg (rat, oral)[19] | ||
| Supplementary data page | |||
| Caffeine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
In 1819, the German chemist Friedlieb Ferdinand Runge isolated relatively pure caffeine for the first time.[88] In 1821, caffeine was isolated both by French chemist Pierre Jean Robiquet and by a pair of French chemists, Pierre-Joseph Pelletier and Joseph Bienaimé Caventou, according to Swedish chemist Jöns Jacob Berzelius in his yearly journal.[89] Furthermore, Berzelius stated the French chemists had made their discoveries independently of any knowledge of Runge's or each other's work. Berzelius states on page 180: "Cafein ist eine Materie im Kaffee, die zu gleicher Zeit, 1821, von Robiquet und [von] Pelletier und Caventou entdekt wurde, von denen aber keine etwas darüber im Drucke bekannt machte." (Caffeine is a substance in coffee, which simultaneously, in 1821, was discovered by Robiquet and by Pelletier and Caventou, by whom however nothing was made known about it in print.)
Pelletier's article on caffeine was the first to use the term in print.[90] It corroborates Berzelius's account: "Caféine, s. f. Principe cristallisable découvert dans le café en 1821 par M. Robiquet. A la même époque, cherchant la quinine dans le café, parce que le café, considéré par plusieurs médecins comme fébrifuge, est d'ailleurs de la même famille que le quinquina, MM. Pelletier et Caventou obtenaient de leur côté la caféine; mais leurs recherches n'ayant qu'un but indirect, et n'ayant pas été terminées, laissent à M. Robiquet la priorité sur cet objet. Nous ignorons pourquoi M. Robiquet n'a pas publié l'analyse du café qu'il a lue à la société de pharmacie. Sa publication nous aurait permis de mieux faire connaître la caféine, et de donner des idées exactes sur la composition du café...." (Caffeine, noun (feminine). Crystallizable substance discovered in coffee in 1821 by Mr. Robiquet. During the same period – while they were searching for quinine in coffee because coffee is considered by several doctors to be a medicine that reduces fevers and because coffee belongs to the same family as the cinchona [quinine] tree – on their part, Mssrs. Pelletier and Caventou obtained caffeine; but because their research had a different goal and because their research had not been finished, they left priority on this subject to Mr. Robiquet. We do not know why Mr. Robiquet has not published the analysis of coffee which he read to the Pharmacy Society. Its publication would have allowed us to make caffeine better known and give us accurate ideas of coffee's composition .... )
Robiquet's article on coffee[91] gives an account of his research on coffee on pages 54–56, detailing the extraction of caffeine and its properties on pages 55–56.
Pelletier's elemental analysis of caffeine appears on pages 182–183 of the article: Dumas and Pelletier (1823) "Recherches sur la composition élémentaire et sur quelques propriétés caractéristiques des bases salifiables organiques" (Studies into the elemental composition and some characteristic properties of organic bases), Annales de Chimie et de Physique, vol. 24, pages 163–191.
Berzelius later acknowledged Runge's priority in the extraction of caffeine, stating:[92] "Es darf indessen hierbei nicht unerwähnt bleiben, dass Runge (in seinen phytochemischen Entdeckungen 1820, p.146-7.) dieselben Methode angegeben, und das Caffein unter dem Namen Caffeebase ein Jahr eher beschrieben hat, als Robiquet, dem die Entdeckung dieser Substanz gewöhnlich zugeschrieben wird, in einer Zussamenkunft der Societé de Pharmacie in Paris die erste mündliche Mittheilung darüber gab." (However, at this point, it should not remain unmentioned that Runge (in his Phytochemical Discoveries, 1820, pages 146–147) specified the same method and described caffeine under the name Caffeebase a year earlier than Robiquet, to whom the discovery of this substance is usually attributed, having made the first oral announcement about it at a meeting of the Pharmacy Society in Paris.) According to Runge, he did this at the behest of Johann Wolfgang von Goethe.[93] In 1827, Oudry isolated "theine" from tea,[94] but it was later proved by Mulder[95] and by Jobst[96] that theine was the same as caffeine.[93] The structure of caffeine was elucidated near the end of the 19th century by Hermann Emil Fischer, who was also the first to achieve its total synthesis.[97] This was part of the work for which Fischer was awarded the Nobel Prize in 1902. The nitrogen atoms are all essentially planar (in sp2 orbital hybridization), resulting in the caffeine molecule's having aromatic character. Being readily available as a byproduct of decaffeination, caffeine is not usually synthesized.[98] If desired, it may be synthesized from dimethylurea and malonic acid.[99]
Pharmacology

Global consumption of caffeine has been estimated at 120,000 tonnes per year,[unreliable source?][100] making it the world's most popular psychoactive substance. This amounts to one serving of a caffeinated beverage for every person every day. Caffeine is a central nervous system and metabolic stimulant,[101] and is used both recreationally and medically to reduce physical fatigue and restore mental alertness when unusual weakness or drowsiness occurs. Caffeine and other methylxanthine derivatives are also used on newborns to treat apnea and correct irregular heartbeats. Caffeine stimulates the central nervous system first at the higher levels, resulting in increased alertness and wakefulness, faster and clearer flow of thought, increased focus, and better general body coordination, and later at the spinal cord level at higher doses.[9] Once inside the body, it has a complex chemistry, and acts through several mechanisms as described below.
Mechanism of action
Adenosine

Caffeine readily crosses the blood–brain barrier that separates the bloodstream from the interior of the brain. Once in the brain, the principal mode of action is as a nonselective antagonist of adenosine receptors.[102][103] The caffeine molecule is structurally similar to the aglycone of adenosine, adenine, and is capable of binding the adenosine receptors on the surface of cells without activating them (an "antagonist" mechanism of action), thereby acting as a competitive inhibitor.
Adenosine is found in every part of the body, because it plays a role in the fundamental ATP-related energy metabolism and is necessary for RNA synthesis, but it has special functions in the brain. There is a great deal of evidence that concentrations of brain adenosine are increased by various types of metabolic stress including anoxia and ischemia. The evidence also indicates that brain adenosine acts to protect the brain by suppressing neural activity and also by increasing blood flow through A2A and A2B receptors located on vascular smooth muscle.[104] By counteracting adenosine, caffeine reduces resting cerebral blood flow between 22% and 30%.[105] Caffeine also has a generally disinhibitory effect on neural activity. It has not been shown, however, how these effects cause increases in arousal and alertness.
Adenosine is released in the brain through a complex mechanism.[104] There is evidence that adenosine functions as a synaptically released neurotransmitter in some cases, but stress-related adenosine increases appear to be produced mainly by extracellular metabolism of ATP. It is not likely that adenosine is the primary neurotransmitter for any group of neurons, but rather that it is released together with other transmitters by a number of neuron types. Unlike most neurotransmitters, adenosine does not seem to be packaged into vesicles that are released in a voltage-controlled manner, but the possibility of such a mechanism has not been completely ruled out.
Several classes of adenosine receptors have been described, with different anatomical distributions. A1 receptors are widely distributed, and act to inhibit calcium uptake. A2A receptors are heavily concentrated in the basal ganglia, an area that plays a critical role in behavior control, but can be found in other parts of the brain as well, in lower densities. There is evidence that A 2A receptors interact with the dopamine system, which is involved in reward and arousal. (A2A receptors can also be found on arterial walls and blood cell membranes.)
Beyond its general neuroprotective effects, there are reasons to believe that adenosine may be more specifically involved in control of the sleep-wake cycle. Robert McCarley and his colleagues have argued that accumulation of adenosine may be a primary cause of the sensation of sleepiness that follows prolonged mental activity, and that the effects may be mediated both by inhibition of wake-promoting neurons via A1 receptors, and activation of sleep-promoting neurons via indirect effects on A2A receptors.[106] More recent studies have provided additional evidence for the importance of A2A, but not A1, receptors.[107]
Phosphodiesterase inhibitors
Some of the secondary effects of caffeine are probably caused by actions unrelated to adenosine. Like other methylated xanthines, caffeine is a competitive nonselective phosphodiesterase inhibitor[108] which raises intracellular Cyclic adenosine monophosphate (cAMP), activates PKA, inhibits TNF-alpha[109][110] and leukotriene[111] synthesis, and reduces inflammation and innate immunity.[111] Phosphodiesterase inhibitors like caffeine increase cAMP levels because they inhibit cAMP-phosphodiesterase (cAMP-PDE) enzymes, which convert cAMP (containing a phosphodiester bond) to its noncyclic form, thus allowing cAMP to build up. Cyclic AMP can increase activity of the funny current, which directly increases heart rate. Additionally, cAMP participates in the activation of protein kinase A (PKA) which will phosphorylate specific enzymes used in glucose synthesis. Activated PKA also increases the activation of H+/K+ ATPase, resulting in increased gastric acid secretion by the cell. As a result, caffeine's effect of increasing cAMP intensifies and prolongs the effects of epinephrine and epinephrine-like drugs such as amphetamine, methamphetamine, and methylphenidate.
Glycine receptors
Caffeine is also a structural analogue of strychnine and, like it (though much less potent), a competitive antagonist at ionotropic glycine receptors.[112]
Caffeine metabolites
Metabolites of caffeine also contribute to caffeine's effects. Paraxanthine is responsible for an increase in the lipolysis process, which releases glycerol and fatty acids into the blood to be used as a source of fuel by the muscles. Theobromine is a vasodilator that increases the amount of oxygen and nutrient flow to the brain and muscles. Theophylline acts as a smooth muscle relaxant that chiefly affects bronchioles and acts as a chronotrope and inotrope that increases heart rate and efficiency.[113]
Metabolism

Caffeine from coffee or other beverages is absorbed by the stomach and small intestine within 45 minutes of ingestion and then distributed throughout all tissues of the body.[114] Peak blood concentration is reached within one hour.[115] It is eliminated by first-order kinetics.[116] Caffeine can also be ingested rectally, evidenced by the formulation of suppositories of ergotamine tartrate and caffeine (for the relief of migraine)[117] and chlorobutanol and caffeine (for the treatment of hyperemesis).[118]
The biological half-life of caffeine—the time required for the body to eliminate one-half of the total amount of caffeine—varies widely among individuals according to such factors as age, liver function, pregnancy, some concurrent medications, and the level of enzymes in the liver needed for caffeine metabolism. In healthy adults, caffeine's half-life is approximately 4.9 hours.[119] In women taking oral contraceptives, this is increased to 5–10 hours,[120] and in pregnant women the half-life is roughly 9–11 hours.[121]
Caffeine can accumulate in individuals with severe liver disease, increasing its half-life.[122] In infants and young children, the half-life may be longer than in adults; half-life in a newborn baby may be as long as 30 hours. Other factors such as smoking can shorten caffeine's half-life.[123] Fluvoxamine (Luvox) reduced the clearance of caffeine by 91.3%, and prolonged its elimination half-life by 11.4-fold; from 4.9 hours to 56 hours.[119]
Caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme system (to be specific, the 1A2 isozyme) into three metabolic dimethylxanthines,[124] each of which has its own effects on the body:
- Paraxanthine (84%): Has the effect of increasing lipolysis, leading to elevated glycerol and free fatty acid levels in the blood plasma.
- Theobromine (12%): Dilates blood vessels and increases urine volume. Theobromine is also the principal alkaloid in the cocoa bean, and therefore chocolate.
- Theophylline (4%): Relaxes smooth muscles of the bronchi, and is used to treat asthma. The therapeutic dose of theophylline, however, is many times greater than the levels attained from caffeine metabolism.
Each of these metabolites is further metabolized and then excreted in the urine.
Some quinolones, including ciprofloxacin, exert an inhibitory effect on the cytochrome P-450 enzyme CYP1A2, thereby reducing clearance, and thus increasing blood levels of tizanidine and methylxanthines (e.g.caffeine).[125][126]
There is also research which suggests that alcohol inhibits the metabolism of caffeine in the liver, especially by influencing its demethylation to other dimethyl- and monomethylxanthines.[127]
Decaffeination

Extraction of caffeine from coffee, to produce decaffeinated coffee and caffeine, is an important industrial process and can be performed using a number of different solvents. Benzene, chloroform, trichloroethylene, and dichloromethane have all been used over the years but for reasons of safety, environmental impact, cost, and flavor, they have been superseded by the following main methods:
Water extraction
Coffee beans are soaked in water. The water, which contains many other compounds in addition to caffeine and contributes to the flavor of coffee, is then passed through activated charcoal, which removes the caffeine. The water can then be put back with the beans and evaporated dry, leaving decaffeinated coffee with its original flavor.[128] Coffee manufacturers recover the caffeine and resell it for use in soft drinks and over-the-counter caffeine tablets.
Supercritical carbon dioxide extraction
Supercritical carbon dioxide is an excellent nonpolar solvent for caffeine, and is safer than the organic solvents that are otherwise used. The extraction process is simple: CO2 is forced through the green coffee beans at temperatures above 31.1 °C and pressures above 73 atm. Under these conditions, CO2 is in a "supercritical" state: It has gaslike properties that allow it to penetrate deep into the beans but also liquid-like properties that dissolve 97–99% of the caffeine. The caffeine-laden CO2 is then sprayed with high pressure water to remove the caffeine. The caffeine can then be isolated by charcoal adsorption (as above) or by distillation, recrystallization, or reverse osmosis.[128]
Extraction by organic solvents
Certain organic solvents such as ethyl acetate present much less health and environmental hazard than previously used chlorinated and aromatic organic solvents. Another method is to use triglyceride oils obtained from spent coffee grounds.
History

Humans have consumed caffeine since the Stone Age.[129] Early peoples found chewing the seeds, bark, or leaves of certain plants had the effects of easing fatigue, stimulating awareness, and elevating one's mood. Only much later was it found that the effect of caffeine was increased by steeping such plants in hot water. Many cultures have legends that attribute the discovery of such plants to people living many thousands of years ago.
According to one popular Chinese legend, the Chinese emperor Shennong, reputed to have reigned in about 3000 BCE, accidentally discovered that when some leaves fell into boiling water, a fragrant and restorative drink resulted.[130][131][132] Shennong is also mentioned in Lu Yu's Cha Jing, a famous early work on the subject of tea.[133] The history of coffee has been recorded as far back as the ninth century. During that time, coffee beans were available only in their native habitat,Ethiopia. A popular legend traces its discovery to a goatherder named Kaldi, who apparently observed goats became elated and sleepless at night after grazing on coffee shrubs and, upon trying the berries the goats had been eating, experienced the same vitality. The earliest literary mention of coffee may be a reference to Bunchum in the works of the 9th-century Persian physician al-Razi. In 1587, Malaye Jaziri compiled a work tracing the history and legal controversies of coffee, entitled ʕUmdat aṣ-Ṣafwa Fī Ḥill al-Qahwah. In this work, Jaziri recorded that one Sheikh, Jamal-al-Din al-Dhabhani, mufti of Aden, was the first to adopt the use of coffee in 1454, and in the 15th century, the Sufis of Yemen routinely used coffee to stay awake during prayers.
Towards the close of the 16th century, the use of coffee was recorded by a European resident in Egypt, and about this time it came into general use in the Near East. The appreciation of coffee as a beverage in Europe, where it was first known as "Arabian wine", dates from the 17th century. A legend states that, after the Ottoman Turks retreated from the walls of Vienna after losing a battle for the city, many sacks of coffee beans were found among their baggage. Europeans did not know what to do with all the coffee beans, being unfamiliar with them. So Franz George Kolschitzky, a Pole who had actually worked for the Turks, offered to take them. He subsequently taught the Viennese how to make coffee, and the first coffee house in the Western world was opened in Vienna, thus starting a long tradition of coffee appreciation.[134] In Britain, the first coffee houses were opened in London in 1652, at St Michael's Alley, Cornhill. They soon became popular throughout Western Europe, and played a significant role in social relations in the 17th and 18th centuries.[135]
Use of the kola nut, like the coffee berry and tea leaf, appears to have ancient origins. It is chewed in many West African cultures, individually or in a social setting, to restore vitality and ease hunger pangs. In 1911, kola became the focus of one of the earliest documented health scares, when the US government seized 40 barrels and 20 kegs of Coca-Cola syrup in Chattanooga, Tennessee, alleging the caffeine in its drink was "injurious to health".[136] On March 13, 1911, the government initiated United States v. Forty Barrels and Twenty Kegs of Coca-Cola, hoping to force Coca-Cola to remove caffeine from its formula by making claims the product was adulterated and misbranded. The allegation of adulteration was, in substance, that the product contained an added poisonous or added deleterious ingredient: caffeine, which might render the product injurious to health. It was alleged to be misbranded in that the name 'Coca Cola' was a representation of the presence of the substances coca and cola; that the product 'contained no coca and little if any cola' and thus was an 'imitation' of these substances and was offered for sale under their 'distinctive name.'[137] Although the judge ruled in favor of Coca-Cola, two bills were introduced to the U.S. House of Representatives in 1912 to amend the Pure Food and Drug Act, adding caffeine to the list of "habit-forming" and "deleterious" substances, which must be listed on a product's label.
The earliest evidence of cocoa bean use comes from residue found in an ancient Mayan pot dated to 600 BCE. In the New World, chocolate was consumed in a bitter and spicy drink called xocolatl, often seasoned with vanilla, chile pepper, and achiote.Xocolatl was believed to fight fatigue, a belief probably attributable to the theobromine and caffeine content. Chocolate was an important luxury good throughout pre-Columbian Mesoamerica, and cocoa beans were often used as currency.
Xocolatl was introduced to Europe by the Spaniards, and became a popular beverage by 1700. The Spaniards also introduced thecacao tree into the West Indies and the Philippines. It was used in alchemical processes, where it was known as "black bean".
The leaves and stems of the yaupon holly (Ilex vomitoria) were used by Native Americans to brew atea called asi or the "black drink".[138] Archaeologists have found evidence of this use stretch back far into antiquity, possibly dating to Late Archaic times.
Religion
Some Latter-day Saints (Mormons), Seventh-day Adventists, Church of God (Restoration) adherents, and Christian Scientists[139] do not consume caffeine. Some from these religions believe that one is not supposed to consume a non-medical, psychoactive substance, or believe that one is not supposed to consume a substance that is addictive.
The Church of Jesus Christ of Latter-day Saints has said the following with regard to caffeinated beverages: “With reference to cola drinks, the Church has never officially taken a position on this matter, but the leaders of the Church have advised, and we do now specifically advise, against the use of any drink containing harmful drugs under circumstances that would result in acquiring the habit. Any beverage that contains ingredients harmful to the body should be avoided.”[140]
Gaudiya Vaishnavas generally also abstain from caffeine, as it is alleged to cloud the mind and over-stimulate the senses. To be initiated under a guru, one must have had no caffeine (along with alcohol, nicotine and other drugs) for at least a year.
In Islam the main rule on caffeine is that it is permissible. With regard to the caffeine in coffee, Imam Shihab al-Din said: 'it is halal (lawful) to drink, because all things are halal (lawful) except that which God has made haraam (unlawful)'.[141]
In other animals

While relatively safe for humans, caffeine is considerably more toxic to some other animals such as dogs and birds[142][143] due to a much poorer ability to metabolize this compound. Caffeine also has a pronounced effect on mollusks and various insects as well as spiders.[144]
References
- ^ "caffeine or caffein". Collins English Dictionary (10th ed.). HarperCollins Publishers. 2009. Retrieved 2010-11-08.
- ^ Lovett, Richard (24 September 2005). "Coffee: The demon drink?". New Scientist (2518). Retrieved 2009-08-03.(subscription required)
- ^ "21 CFR 182.1180". U.S. Code of Federal Regulations. U.S. Office of the Federal Register. 2003-04-01. p. 462. Retrieved 2009-08-03.
- ^ a b Maughan, R. J.; Griffin, J. (2003). "Caffeine ingestion and fluid balance: a review". Journal of Human Nutrition and Dietetics. 16 (6): 411–20. doi:10.1046/j.1365-277X.2003.00477.x. PMID 19774754.
- ^ O'connor, Anahad (2008-03-04). "Really? The Claim: Caffeine Causes Dehydration". The New York Times. Retrieved 2009-08-03.
- ^ Armstrong, Lawrence E.; Casa, Douglas J.; Maresh, Carl M.; Ganio, Matthew S. (2007). "Caffeine, Fluid-Electrolyte Balance, Temperature Regulation, and Exercise-Heat Tolerance". Exercise and Sport Sciences Reviews. 35 (3): 135–140. doi:10.1097/jes.0b013e3180a02cc1. PMID 17620932.
- ^ Armstrong, LE; Pumerantz, AC; Roti, MW; Judelson, DA; Watson, G; Dias, JC; Sokmen, B; Casa, DJ; Maresh, CM (2005). "Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption". International journal of sport nutrition and exercise metabolism. 15 (3): 252–65. PMID 16131696.
- ^ References are found on image description page in Wikimedia Commons.
- ^ a b c Bolton, Sanford (1981). "Caffeine: Psychological Effects, Use and Abuse" (PDF). Orthomolecular Psychiatry. 10 (3): 202–211.
- ^ "Actions of caffeine in the brain with special reference to factors that contribute to its widespread use". Pharmacol Rev. 51: 83–133. 1999. PMID 10049999.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Juliano, Laura M.; Griffiths, Roland R. (2004). "A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features". Psychopharmacology. 176 (1): 1–29. doi:10.1007/s00213-004-2000-x. PMID 15448977.
- ^ Smith BD, Gupta U, Gupta BS, ed. (2007). "Caffeinism: History, clinical features, diagnosis, and treatment". Caffeine and activation theory: effects on health and behavior. CRC Press. pp. 331-344. ISBN 9780849371028.
{{cite book}}: Cite uses deprecated parameter|authors=(help)CS1 maint: multiple names: editors list (link) - ^ Acetaminophen & Caffeine: Bad Combo for Your Liver. Hepatitis Central 2009-02-02. Retrieved 2010-11-03.
- ^ "Caffeine (Systemic)". MedlinePlus. 2000-05-25. Archived from the original on 2007-02-23. Retrieved 2009-08-03.
- ^ a b c American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). American Psychiatric Association. ISBN 0-89042-062-9.
- ^ "Caffeine overdose". MedlinePlus. 2006-04-04. Retrieved 2009-08-03.
- ^ Verkhratsky, A. (2005). "Physiology and Pathophysiology of the Calcium Store in the Endoplasmic Reticulum of Neurons". Physiological Reviews. 85: 381–3. doi:10.1152/physrev.00004.2004.
- ^ "Man Dies From Caffeine Overdose". Toronto Sun. Quebecor Media Inc. October 30, 2010.
- ^ a b Peters, Josef M. (1967). "Factors Affecting Caffeine Toxicity: A Review of the Literature". The Journal of Clinical Pharmacology and the Journal of New Drugs (7): 131–141.
- ^ Kerrigan, S; Lindsey, T (2005). "Fatal caffeine overdose: Two case reports". Forensic Science International. 153 (1): 67–69. doi:10.1016/j.forsciint.2005.04.016. PMID 15935584.
- ^ Holmgren, P; Nordén-Pettersson, L; Ahlner, J (2004). "Caffeine fatalities—four case reports". Forensic Science International. 139 (1): 71–3. doi:10.1016/j.forsciint.2003.09.019. PMID 14687776.
- ^ Verkhratsky, A. (2005). "Physiology and Pathophysiology of the Calcium Store in the Endoplasmic Reticulum of Neurons". Physiological Reviews. 85: 244–9. doi:10.1152/physrev.00004.2004.
- ^ Verkhratsky, A. (2005). "Physiology and Pathophysiology of the Calcium Store in the Endoplasmic Reticulum of Neurons". Physiological Reviews. 85: 571–2. doi:10.1152/physrev.00004.2004.
- ^ Smith A (2002). "Effects of caffeine on human behavior". Food Chem Toxicol. 40: 1243–1255. PMID 12204388.
- ^ Ker K, Edwards PJ, Felix LM, Blackhall K, Roberts I (2010). "Caffeine for the prevention of injuries and errors in shift workers". Cochrane Database of Systematic Reviews (5): CD008508. doi:10.1002/14651858.CD008508. PMID 20464765.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shannon, MW (1998). Clinical Management of Poisoning and Drug Overdose (3rd ed.). Philadelphia: Saunders. ISBN 0-7216-6409-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Caffeine’s Effect on ADHD Symptoms | Psych Central
- ^ The ADHD and Sleep Conundrum: A Review : Journal of Developmental & Behavioral Pediatrics
- ^ Attention Deficit Disorder (ADD): Ritalin, Caffeine, and ADHD, cause insomnia, sleepyness
- ^ "ADHD". David A. Sandberg, Ph.D. Retrieved 2009-05-25.
- ^ "Attention Deficit Hyperactivity Disorder is a neurologically based disorder". Incrediblehorizons.com. Retrieved 2009-05-25.
- ^ "Sleep in Mental and Behavioural Disorders" (PDF). Nina Lindberg (Institute of Clinical Medicine, Department of Psychiatry, and Institute of Biomedicine, Department of Physiology, University of Helsinki. 2003. Retrieved 2009-08-05.
- ^ Cambridge Journals Online - Abstract
- ^ ScienceDirect - Behavioural Brain Research : The effect of caffeine to increase reaction time in the rat during a test of attention is mediated through antagonism of adenosine...
- ^ Han, M; Park, K; Baek, S; Kim, B; Kim, J; Kim, H; Oh, S (2007). "Inhibitory effects of caffeine on hippocampal neurogenesis and function". Biochemical and Biophysical Research Communications. 356 (4): 976–80. doi:10.1016/j.bbrc.2007.03.086. PMID 17400186.
- ^ "Caffeine clue to better memory". BBC News. 1999-10-12. Retrieved 2009-08-03.
- ^ "Caffeine Boosts Short-Time Memory". Retrieved 2009-08-03.
- ^ Lesk, Valerie E.; Womble, Stephen P. (2004). "Caffeine, Priming, and Tip of the Tongue: Evidence for Plasticity in the Phonological System". Behavioral Neuroscience. 118: 453–6. doi:10.1037/0735-7044.118.3.453. PMID 15174922.
- ^ Nkondjock, A (2009 May 18). "Coffee consumption and the risk of cancer: an overview". Cancer letters. 277 (2): 121–5. PMID 18834663.
{{cite journal}}: Check date values in:|date=(help) - ^ Arab, L (2010). "Epidemiologic evidence on coffee and cancer". Nutrition and cancer. 62 (3): 271–83. PMID 20358464.
- ^ Greenberg, J.A.; Dunbar, CC; Schnoll, R; Kokolis, R; Kokolis, S; Kassotis, J (2007). "Caffeinated beverage intake and the risk of heart disease mortality in the elderly: a prospective analysis". Am J Clin Nutr. 85 (2): 392–8. PMID 17284734.
- ^ Mahmud, A; Feely, J (2001). "Acute effect of caffeine on arterial stiffness and aortic pressure waveform". Hypertension. 38 (2): 227–31. PMID 11509481.
- ^ "Kaiser Permanente Study Shows Newer, Stronger Evidence that Caffeine During Pregnancy Increases Miscarriage Risk". Retrieved 2009-08-03.
- ^ David A. Savitz, as reported in: Rubin, Rita (2008-01-20). "New studies, different outcomes on caffeine, pregnancy". USA TODAY. Retrieved 2009-08-03.
- ^ "Food Standards Agency publishes new caffeine advice for pregnant women". Retrieved 2009-08-03.
- ^ Danielle Dellorto (January 21, 2008). "Study: Caffeine may boost miscarriage risk". CNN. Retrieved 2009-08-03.
- ^ Bishop, D (2010 Dec 1). "Dietary supplements and team-sport performance". Sports medicine (Auckland, N.Z.). 40 (12): 995–1017. PMID 21058748.
{{cite journal}}: Check date values in:|date=(help) - ^ Conger, SA (2011 Feb). "Does caffeine added to carbohydrate provide additional ergogenic benefit for endurance?". International journal of sport nutrition and exercise metabolism. 21 (1): 71–84. PMID 21411838.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Astorino, TA (2010 Jan). "Efficacy of acute caffeine ingestion for short-term high-intensity exercise performance: a systematic review". Journal of strength and conditioning research / National Strength & Conditioning Association. 24 (1): 257–65. PMID 19924012.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hackett, PH (2010 Spring). "Caffeine at high altitude: java at base cAMP" (PDF). High altitude medicine & biology. 11 (1): 13–7. PMID 20367483.
{{cite journal}}: Check date values in:|date=(help) - ^ Davis, JK (2009). "Caffeine and anaerobic performance: ergogenic value and mechanisms of action". Sports medicine (Auckland, N.Z.). 39 (10): 813–32. PMID 19757860.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ McArdle, William (2010). Exercise Physiology. 7th edition. Baltimore, MD: Lippincott Williams and Wilkins. p. 559. ISBN 9780781797818.
- ^ Li, M (2011 Mar). "The effect of caffeine on intraocular pressure: a systematic review and meta-analysis". Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 249 (3): 435–42. PMID 20706731.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Muriel, P (2010 Jul). "Coffee and liver diseases". Fitoterapia. 81 (5): 297–305. PMID 19825397.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ O'Connor, Anahad (2007). Never shower in a thunderstorm : surprising facts and misleading myths about our health and the world we live in (1st ed. ed.). New York: Times Books. p. 144. ISBN 9780805083125.
{{cite book}}:|edition=has extra text (help) - ^ Mathew, OP (2011 May). "Apnea of prematurity: pathogenesis and management strategies". Journal of perinatology : official journal of the California Perinatal Association. 31 (5): 302–10. PMID 21127467.
{{cite journal}}: Check date values in:|date=(help) - ^ Kugelman, A (2011 Aug 3). "A comprehensive approach to the prevention of bronchopulmonary dysplasia". Pediatric pulmonology. PMID 21815280.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Schmidt, Barbara; Roberts, Robin S.; Davis, Peter; Doyle, Lex W.; Barrington, Keith J.; Ohlsson, Arne; Solimano, Alfonso; Tin, Win; Caffeine for Apnea of Prematurity Trial Group (2006). "Caffeine Therapy for Apnea of Prematurity". New England Journal of Medicine. 354 (20): 2112–21. doi:10.1056/NEJMoa054065. PMID 16707748.
- ^ Schmidt, Barbara (2005). "Methylxanthine Therapy for Apnea of Prematurity: Evaluation of Treatment Benefits and Risks at Age 5 Years in the International Caffeine for Apnea of Prematurity (CAP) Trial". Biology of the Neonate. 88 (3): 208–213. doi:10.1159/000087584. PMID 16210843.
- ^ Baselt, R. (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, CA: Biomedical Publications. pp. 236–9. ISBN 0-931890-08-X.
- ^ "Caffeine Content of Food and Drugs". Nutrition Action Health Newsletter. Center for Science in the Public Interest. 1996. Archived from the original on 2007-06-14. Retrieved 2009-08-03.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Caffeine Content of Beverages, Foods, & Medications". The Vaults of Erowid. July 7, 2006. Retrieved 2009-08-03.
- ^ "Caffeine Content of Drinks". Energy Fiend. Retrieved 2011-03-04.
- ^ "Traditional Yerba Mate in Biodegradable Bag". Guayaki Yerba Mate. Retrieved 2010-07-17.
- ^ Frischknecht, Peter M.; Ulmer-Dufek, Jindra; Baumann, Thomas W. (1986). "Purine alkaloid formation in buds and developing leaflets of Coffea arabica: Expression of an optimal defence strategy?". Phytochemistry. 25: 613–6. doi:10.1016/0031-9422(86)88009-8.
- ^ Nathanson, J. (1984). "Caffeine and related methylxanthines: possible naturally occurring pesticides". Science. 226 (4671): 184–7. doi:10.1126/science.6207592. PMID 6207592.
- ^ Baumann, T. W. (1984). "Metabolism and excretion of caffeine during germination of Coffea arabica L". Plant and Cell Physiology. 25 (8): 1431–6.
- ^ Matissek, R (1997). "Evaluation of xanthine derivatives in chocolate: nutritional and chemical aspects". European Food Research and Technology. 205 (3): 175–84.
- ^ a b [unreliable source?]"Does Yerba Maté Contain Caffeine or Mateine?". The Vaults of Erowid. 2003. Retrieved 2009-08-03.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "PubChem: mateina". National Library of Medicine. Retrieved 2009-08-03.. Generally translated as mateine in articles written in English
- ^ "PubChem: guaranine". National Library of Medicine. Retrieved 2009-08-16.
- ^ Thomas Vallombroso (2001). Organic Chemistry: Pearls of Wisdom. Boston Medical Publishing Corp. p. 48. ISBN 978-1-58409-016-8.
- ^ Marshall Cavendish (2006). The Facts About Caffeine. Marshall Cavendish. p. 43. ISBN 978-0-7614-2242-6.
- ^ Balentine D. A., Harbowy M. E. and Graham H. N. (1998). G Spiller (ed.). Tea: the Plant and its Manufacture; Chemistry and Consumption of the Beverage.
{{cite book}}:|journal=ignored (help) - ^ a b "Caffeine". International Coffee Organization. Retrieved 2009-08-01.
- ^ "Coffee and Caffeine FAQ: Does dark roast coffee have less caffeine than light roast?". Retrieved 2009-08-02.
- ^ "All About Coffee: Caffeine Level". Jeremiah’s Pick Coffee Co. Retrieved 2009-08-03.[dead link]
- ^ "Caffeine in tea vs. steeping time". 1996. Retrieved 2009-08-02.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Haskell, C. F.; Kennedy, D. O.; Wesnes, K. A.; Milne, A. L.; Scholey, A. B. (2006). "A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guarana in humans". Journal of Psychopharmacology. 21 (1): 65–70. doi:10.1177/0269881106063815. PMID 16533867.
- ^ Smit, Hendrik J.; Gaffan, Elizabeth A.; Rogers, Peter J. (2004). "Methylxanthines are the psycho-pharmacologically active constituents of chocolate". Psychopharmacology. 176 (3–4): 412–9. doi:10.1007/s00213-004-1898-3. PMID 15549276.
- ^ Bennett Alan Weinberg, Bonnie K. Bealer (2001). The world of caffeine. Routledge. p. 195. ISBN 0-415-92722-6.
- ^ LeBron James Shills for Sheets Caffeine Strips, a Bad Idea for Teens, Experts Say - ABC News
- ^ a b Funk, G. D. (2009). "Losing sleep over the caffeination of prematurity". The Journal of Physiology. 587 (Pt 22): 5299–300. doi:10.1113/jphysiol.2009.182303. PMC 2793860. PMID 19915211.
- ^ "Genome-Wide Meta-Analysis Identifies Regions on 7p21 (AHR) and 15q24 (CYP1A2) As Determinants of Habitual Caffeine Consumption". PLoS Genetics. 7 (4): e1002033. 2011. doi:10.1371/journal.pgen.1002033. ISSN 1553-7404.
{{cite journal}}: Cite uses deprecated parameter|authors=(help)CS1 maint: unflagged free DOI (link) - ^ Melnick, Meredith. "Can't Get Enough Coffee? It Might Be In Your Genes." TIME, April 8, 2011.
- ^ Caffeine, International Occupational Safety and Health Information Centre (CIS)
- ^ This is the pKa for protonated caffeine, given as a range of values included in Harry G. Brittain, Richard J. Prankerd (2007). Profiles of Drug Substances, Excipients and Related Methodology, volume 33: Critical Compilation of pKa Values for Pharmaceutical Substances. Academic Press. ISBN 0-12-260833-X.
- ^ Runge, Friedlieb Ferdinand (1820). "6". Neueste phytochemische Entdeckungen zur Begründung einer wissenschaftlichen Phytochemie. Berlin: G. Reimer. pp. 144–159.
{{cite book}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) Details Runge's (partial) isolation of caffeine, which he calls "Kaffebase" (i.e., a base that exists in coffee). - ^ "Jahres-Bericht über die Fortschritte der physischen Wissenschaften von Jacob Berzelius" (in German). 4. 1825: 180.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) Dr. F. Wöhler, trans. - ^ Pelletier, Pierre Joseph (April 1822). "Cafeine". Dictionnaire de Médecine (in French). Vol. 4. Paris: Béchet Jeune. pp. 35–36. Retrieved 2011-03-03.
{{cite encyclopedia}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Robiquet, Pierre Jean (1823). "Cafe". Dictionnaire Technologique, ou Nouveau Dictionnaire Universel des Arts et Métiers (in French). Vol. 4. Paris: Thomine et Fortic. pp. 50–61. Retrieved 2011-03-03.
{{cite encyclopedia}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Jahres-Bericht über die Fortschritte der physischen Wissenschaften von Jacob Berzelius (in German). Vol. 7. 1828. p. 270.
- ^ a b Weinberg, BA (2001). The World of Caffeine. Routledge. ISBN 0-415-92722-6.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Oudry (March 1827) "Note sur la Théine" (Note on theine), Nouvelle Bibliothèque médicale..., vol. 1, page 477-479. See also: Oudry (1827) "Theïn, eine organische Salzbase im Thee" (Theïn, an organic base in tea), Geiger's Magazin für Pharmacie, vol. 19, pages 49–50.
- ^ Gerardus Johannes Mulder (1838) "Über Kaffein und Thein" (On caffeine and theine), Journal für praktische Chemie, vol. 15, pages 280–284.
- ^ Carl Jobst (1838) "Thein identisch mit Caffein" (Theine is identical to caffeine), Liebig's Annalen der Chemie und Pharmacie, vol. 25, pages 63–66.
- ^ "Nobel Prize Presentation Speech by Professor Hj. Théel, President of the Swedish Royal Academy of Sciences". December 10, 1902. Retrieved 2009-08-03.
- ^ Simon Tilling. "Crystalline Caffeine". Bristol University. Retrieved 2009-08-03.
- ^ Ted Wilson, Norman J. Temple (2004). Beverages in Nutrition and Health. Humana Press. p. 172. ISBN 1-58829-173-1.
- ^ "What's your poison: caffeine". Australian Broadcasting Corporation. 1997. Retrieved 2009-08-03.
- ^ Nehlig, A; Daval, JL; Debry, G (1992). "Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects". Brain Research Reviews. 17 (2): 139–70. doi:10.1016/0165-0173(92)90012-B. PMID 1356551.
- ^ Fisone, G.; Borgkvist, A.; Usiello, A. (2004). "Caffeine as a psychomotor stimulant: mechanism of action". Cellular and Molecular Life Sciences (CMLS). 61: 857–72. doi:10.1007/s00018-003-3269-3.
- ^ Daly, JW; Jacobson, KA; Ukena, D (1987). "Adenosine receptors: development of selective agonists and antagonists". Progress in clinical and biological research. 230: 41–63. PMID 3588607.
- ^ a b Latini, Serena; Pedata, Felicita (2008). "Adenosine in the central nervous system: release mechanisms and extracellular concentrations". Journal of Neurochemistry. 79 (3): 463–84. doi:10.1046/j.1471-4159.2001.00607.x. PMID 11701750.
- ^ Addicott, Merideth A.; Yang, Lucie L.; Peiffer, Ann M.; Burnett, Luke R.; Burdette, Jonathan H.; Chen, Michael Y.; Hayasaka, Satoru; Kraft, Robert A.; Maldjian, Joseph A. (2009). "The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate?". Human Brain Mapping. 30 (10): 3102–14. doi:10.1002/hbm.20732. PMC 2748160. PMID 19219847.
- ^ Basheer, R; Strecker, R; Thakkar, M; McCarley, R (2004). "Adenosine and sleep?wake regulation". Progress in Neurobiology. 73 (6): 379–96. doi:10.1016/j.pneurobio.2004.06.004. PMID 15313333.
- ^ Huang, Zhi-Li; Qu, Wei-Min; Eguchi, Naomi; Chen, Jiang-Fan; Schwarzschild, Michael A; Fredholm, Bertil B; Urade, Yoshihiro; Hayaishi, Osamu (2005). "Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine". Nature Neuroscience. 8 (7): 858–9. doi:10.1038/nn1491. PMID 15965471.
- ^ Essayan, David M. (2001). "Cyclic nucleotide phosphodiesterases". Journal of Allergy and Clinical Immunology. 108 (5): 671–80. doi:10.1067/mai.2001.119555. PMID 11692087.
- ^ Deree, Jessica; Martins, Joilson O.; Melbostad, Heidi; Loomis, William H.; Coimbra, Raul (2008). "Insights into the regulation of TNF-a production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition". Clinics. 63 (3): 321–8. doi:10.1590/S1807-59322008000300006. PMC 2664230. PMID 18568240.
- ^ Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U (1999). "Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages". Am. J. Respir. Crit. Care Med. 159 (2): 508–11. PMID 9927365.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. (2005). "Leukotrienes: underappreciated mediators of innate immune responses". J Immunol. 174 (2): 589–94. PMID 15634873.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Duan, L.; Yang, J.; Slaughter, M. M. (2009). "Caffeine inhibition of ionotropic glycine receptors". The Journal of Physiology. 587 (Pt 16): 4063–75. doi:10.1113/jphysiol.2009.174797. PMC 2756438. PMID 19564396.
- ^ Dews, P.B. (1984). Caffeine: Perspectives from Recent Research. Berlin: Springer-Valerag. ISBN 978-0-387-13532-8.
- ^ Liguori, A (1997). "Absorption and Subjective Effects of Caffeine from Coffee, Cola and Capsules". Pharmacology Biochemistry and Behavior. 58: 721–6. doi:10.1016/S0091-3057(97)00003-8.
- ^ Caffeine component of Koffazon], taken from Fass.se (Swedish Drug Catalog). Last updated 2010-02-10
- ^ Newton, R.; Broughton, L. J.; Lind, M. J.; Morrison, P. J.; Rogers, H. J.; Bradbrook, I. D. (1981). "Plasma and salivary pharmacokinetics of caffeine in man". European Journal of Clinical Pharmacology. 21 (1): 45–52. doi:10.1007/BF00609587. PMID 7333346.
- ^ Graham, John R. (1954). "Rectal Use of Ergotamine Tartrate and Caffeine Alkaloid for the Relief of Migraine". New England Journal of Medicine. 250 (22): 936–8. doi:10.1056/NEJM195406032502203. PMID 13165929.
- ^ Brødbaek HB, Damkier P (2007). "[The treatment of hyperemesis gravidarum with chlorobutanol-caffeine rectal suppositories in Denmark: practice and evidence]". Ugeskr. Laeg. (in Danish). 169 (22): 2122–3. PMID 17553397.
- ^ a b Drug Interaction: Caffeine Oral and Fluvoxamine Oral Medscape Multi-Drug Interaction Checker
- ^ Meyer, FP; Canzler, E; Giers, H; Walther, H (1991). "Time course of inhibition of caffeine elimination in response to the oral depot contraceptive agent Deposiston. Hormonal contraceptives and caffeine elimination". Zentralbl Gynakol. 113 (6): 297–302. PMID 2058339.
- ^ Ortweiler, W; Simon, HU; Splinter, FK; Peiker, G; Siegert, C; Traeger, A (1985). "Determination of caffeine and metamizole elimination in pregnancy and after delivery as an in vivo method for characterization of various cytochrome p-450 dependent biotransformation reactions". Biomed Biochim Acta. 44 (7–8): 1189–99. PMID 4084271.
- ^ Verbeeck RK (2008 Dec). "Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction". Eur J Clin Pharmacol. 64 (12): 1147-61. PMID 18762933.
{{cite journal}}: Check date values in:|date=(help) - ^ Springhouse (January 1, 2005). Physician's Drug Handbook; 11th edition. Lippincott Williams & Wilkins. ISBN 1-58255-396-3.
{{cite book}}: More than one of|author=and|last=specified (help) - ^ "Caffeine". The Pharmacogenetics and Pharmacogenomics Knowledge Base. Retrieved 2010-10-25.
- ^ Harder S, Fuhr U, Staib AH, Wolff T, S (1989). "Ciprofloxacin-caffeine: a drug interaction established using in vivo and in vitro investigations". Am. J. Med. 87 (5A): 89S–91S. doi:10.1016/0002-9343(89)90031-4. ISSN 0002-9343. PMID 2589393.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|format=requires|url=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Janknegt R, R (1990). "Drug interactions with quinolones". J. Antimicrob. Chemother. 26 Suppl D: 7–29. ISSN 0305-7453. PMID 2286594.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hartmann, M.; Czok, G. (1980). "Untersuchungen an Mäusen zur Pharmakokinetik von Coffein und deren Beeinflussung durch Äthanol". Zeitschrift für Ernährungswissenschaft (in German). 19 (3): 215–227. doi:10.1007/BF02018787. ISSN 0044-264X. PMID 7445577.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: extra punctuation (link) - ^ a b Senese, Fred (2005-09-20). "How is coffee decaffeinated?". General Chemistry Online. Retrieved 2009-08-03.
- ^ Escohotado, Antonio (1999). A Brief History of Drugs: From the Stone Age to the Stoned Age. Park Street Press. ISBN 0-89281-826-3.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Všechny čaje Číny (in Czech). Michal Synek (translator). Prague: DharmaGaia Praha. 1998. pp. 19–20. ISBN 80-85905-48-5.
{{cite book}}: CS1 maint: others (link) Translation of Kit Chow, Ione Kramer (1990). All the Tea in China. San Francisco: China Books & Periodicals Inc. ISBN 0-8351-2194-1. - ^ Jana Arcimovičová, Pavel Valíček (1998). Vůně čaje (in Czech). Benešov: Start. p. 9. ISBN 80-902005-9-1.
{{cite book}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ John C. Evans (1992). Tea in China: The History of China's National Drink. Greenwood Press. p. 2. ISBN 0-313-28049-5.
- ^ Yu, Lu (1995). The Classic of Tea: Origins & Rituals. Ecco Pr; Reissue edition. ISBN 0-88001-416-4.
- ^ Ukers, W.H. (1922). All About Coffee. New York: The Tea and Coffee Trade Journal Company. p. 40. ISBN 0-8103-4092-5.
- ^ "Coffee". Encyclopædia Britannica. 1911.
- ^ Benjamin, Ludy T.; Rogers, Anne M.; Rosenbaum, Angela (1991). "Coca-Cola, caffeine, and mental deficiency: Harry Hollingworth and the Chattanooga trial of 1911". Journal of the History of the Behavioral Sciences. 27 (1): 42–55. doi:10.1002/1520-6696(199101)27:1<42::AID-JHBS2300270105>3.0.CO;2-1. PMID 2010614.
- ^ U.S. v. Forty Barrels and Twenty Kegs of Coca Cola, 241 U.S. 265 (SCOTUS May 22, 1916).
- ^ Fairbanks, Charles H. (2004). "The function of black drink among the Creeks". In Hudson, Charles M. (ed.). Black Drink. University of Georgia Press. p. 123. ISBN 9780820326962.
- ^ "Voices of Faith: April 12, 2008". Retrieved 2009-08-03.
- ^ (Priesthood Bulletin, Feb. 1972, p. 4.) See also Word of Wisdom.[page needed]
- ^ "Drinking drinks with caffeine". Retrieved 2009-08-03.
- ^ Caffeine Poisoning in Dogs | Pet Information
- ^ Why Caffeine is Toxic to Birds | Hotspot for Birds
- ^ Noever, R., J. Cronise, and R. A. Relwani. 1995. Using spider-web patterns to determine toxicity. NASA Tech Briefs 19(4):82. Published inNew Scientist magazine, 29 April 1995
External links
- The Consumers Union Report on Licit and Illicit Drugs, Caffeine-Part 1 Part 2
- Caffeine: ChemSub Online
- Mayo Clinic staff (October 3, 2009). "Caffeine content for coffee, tea, soda and more". Mayo Clinic. Retrieved 2010-11-08.
- eMedicine Caffeine-Related Psychiatric Disorders