Wikipedia:Reference desk/Archives/Science/March 2006
March 1
[edit]Plants and their production of oxygen
[edit]I was wondering if it was possible for future moon colonization for the colony's oxygen supply to be totally relient on plants? Could the colonists provide the plants with their carbon dioxide while the plants supplied them with oxygen? Them
- Yes, so long as the whole thing is in air tight containers. The Earth's moon can not be teraformed because its gravity is too low to maintain an atmosphere. WAS 4.250 02:04, 1 March 2006 (UTC)
In order for a planet/moon to have an atmosphere it has to generate enough gravity to hold the gases to the surface of the planet. Our moon has a very thin atmosphere because of its relatively small mass. There is also the question of sustaining the plants. At the very minimum, plants need water, sugar, and carbon dioxide to live. If the moon cannot provide this, in the right quanities, then plants cannot survive. In actuallity, what you want to do is put plants on the moon to generate oxygen. Though it sounds like a good idea, if it were possible, the moon would be as green as our planet. It would have already gone through this process, which is an interesting read. --Chris 01:03, 1 March 2006 (UTC)
I have another question the moon may not have the gravitational pull strong enough for this process but does Mars? If the temperture could be raised then could plants be grown on the surface, of course this would require the discovery of a water source, but could it be done? The carbon dioxide is already there.
- I think there's already a lot of water (frozen) on Mars. The problem comes in "raising the temperature". I don't think it would be possible to live on Mars in the same way we use Earth because the amount of energy required would be difficult to generate so far from the Sun. Not to mention the lower intensity of natural light required for photosynthesis. Unless there are high quantities of quality radioactive materials available, forget it. --Username132 01:37, 1 March 2006 (UTC)
Mars can be teraformed by crashing into it a large object that has a lot of frozen water. Objects like this are plentiful. There are a lot of interesting details involved, but Mars is do-able, Earth's moon isn't. You do realize we are talking huge amounts of time, right? WAS 4.250 02:04, 1 March 2006 (UTC)
If the caps were melted would the amount of carbon dioxide released be enough to raise the temperture any? Also if the caps were melted then water would be released.
- It's difficult to tell, since to this day we still don't know just how much CO2 there is in the martian polar caps. Modelling planetwide climate change is something we can't even do well on our own planet, let alone on a planet we have never been to.
- Aside: we have a pretty good article on terraforming. — QuantumEleven | (talk) 11:20, 1 March 2006 (UTC)
Degree symbols on Computers
[edit]Anybody know why there is not a key for a "degree symbol" on computer keyboards? I frquently have need of one and I'm sure many other people do too.
- If you need to use the degree symbol "°" regularly, you may be able to configure your word processing program to assign the degree symbol to a keyboard shortcut. --Robert Merkel 03:41, 1 March 2006 (UTC)
- I've got it in my GNOME Character Palette. —Keenan Pepper 04:52, 1 March 2006 (UTC)
There's two things here: 1) computer keyboards evolved from Typewriter keyboards. There was no need for a degree sign with typewriters, because you could simply manually move the platen half a row down and type "o" (in otherwords, put a lower case O in superscript), which is all that was needed given the rudimentary font capabilities of typewriters. 2) most computers use a simple key combination to get a degree symbol. I can't recall offhand what it is with PCs, but with Macs "option-shift-8" gives you "°" in most editing and word-processing programs. Grutness...wha? 05:56, 1 March 2006 (UTC)
- If you are using an Apple Computer, just press option and K. K for Kelvin.
- There is no degree symbol on a standard US QWERTY keyboard. The (IMO much improved, but very rare) US-International layout has the ° accessible by SHIFT-ALTGR-: . The German QWERTZ and French AZERTY layouts also have a ° key (either SHIFT-) or SHIFT-^ ). See keyboard layout - the standard US keyboard is one of the least functional of them all, and useless for languages which use diacritic marks, or for inputting all but the most common of symbols.
- If you're using a French keyboard (probably not, but just in case :)), the ° is on a key just to the right of 0 (press shift + that key). If not, you can get it by using it's ASCII key combination, press and hold down ALT, type 0176 on your numerical keypad, and release ALT. (you can use this method to type pretty much any character, find out the different codes by using the Character Map utility, usually in your start menu under Accessories -> System Tools). You can also install a language pack (e.g. French) and change the language when you need various symbols only available on the other language keyboard, if you know its layout. — QuantumEleven | (talk) 08:36, 1 March 2006 (UTC)
Planet spinning
[edit]Can someone tell me why every thing in the Universe spins? Do all stars, planets etc. spin in the same direction? I can understand that a baseball spins because of the friction between the ball and the throwers hand but I'm not sure this would explain why a star spins. Thanks WSC
- Something started them spinning a long time ago, and they can't stop because angular momentum is conserved. It would be amazing if the angular momentum randomly happened to be exactly zero. —Keenan Pepper 04:49, 1 March 2006 (UTC)
- More specifically, something started them moving a while ago. Then all kinds of gravity happened. Venus and some other orbiting bodies, mainly comets, have retrograde rotations. -LambaJan 07:44, 1 March 2006 (UTC)
- Take a simple analogy - water flowing down a drain. In theory, the water could flow straight down the drain without any sort of spin (all that about the draining water spinning the other way in the southern hemisphere is rubbish). But in practice it almost never does, because any slight imbalance (a bit more water on one side, the bowl not being perfectly symmetrical...) will cause the draining water to start spinning.
- With stars and other objects it works in a similar way - they are formed when clouds of gas and dusk contract under their own gravity. In theory, the cloud could be perfectly uniform in every direction, and contract perfectly symmetrically. In practice, that's never the case, any small variation will cause the resulting object to start spinning, and in space, as there's no friction, something which starts spinning won't stop.
- Because of this, no, not all stars and planets spin the same way, far from it! Their axis and velocity of spin is dependent on how they were formed, and so can be every which way. We have a decent article on axial tilt if you're interested. — QuantumEleven | (talk) 08:27, 1 March 2006 (UTC)
- Consider a good-sized chunk of plasticine hanging from a string attached to the ceiling. This is going to be our model of a planet being formed. Assume that it starts out stationary. If you give the lump a little kick–say, by throwing another little chunk of plasticine at it–our lump will start moving. If you hit it dead center, the lump moves sideways: no rotation. If you're even a little bit off center, there will be some movement sideways and some rotation. If you just graze the edge of the lump with a tangential hit, you'll get essentially pure rotation.
- When any astronomical body forms, billions of little particles smack into it, and very few strike dead center. It's also very unlikely that the off-center impacts will cancel out perfectly. Consequently, everything spins.
- Gravity makes it worse. Like the classic example of a figure skater pulling in her arms, as stars and planets pack their material more densely they spin faster. (Conservation of angular momentum.) TenOfAllTrades(talk) 16:51, 1 March 2006 (UTC)
- It may be that the off-centre hits will not cancel each other out perfectly, but with a large number of relatively small hits they will effectively. And they will in time be small because the surviving celestial bodies will be much much bigger than the stuff that hits them. The amount of energy needed to make a planet spin at the speed at which they do would require either a lot of small ones going off-centre on the same side, which would be statistically impossible or a big one, which would either destroy it or knock it so far out of orbit that it will get destroyed in some other way (or flung out into deep space).
- I have thought about this too and haven't come up with an answer. It makes me think a bit about the question where structure in the Universe comes from. It can not have formed without already being present before the Big Bang. But we can't explain structure there, so we simply have to accept its existence. Jus like matter and time 'just are' and structure 'just is', maybe rotation is something that simply is. Asking about its origin would be like asking why there is matter.
- Now that sounds like a lame answer, so let me try a different one. Maybe it's a matter of starting conditions. Maybe the very first momentum caused by chance will amplify. Chaos theory would probably come in here because we've got am instable system the outcome of which is determined by minute variations in the starting conditions. And I've got a feeling relativity might come in here too, with the initial rotation determining how attracted matter will approach it, but that's no more than a hunch.
- I wonder. If one would add up all the rotations in the Universe, would they cancel each other out? DirkvdM 09:08, 2 March 2006 (UTC)
False Embryo Sketchings?
[edit]I've heard some Christians and creationists say that Ernst Haeckel's drawings of embryoes, which he claimed to be scientific evidence for evolution, are fake or flawed.Is that true?Bowei 06:50, 1 March 2006 (UTC)
- That can be dismissed without addressing the main question. It's a straw man argument, since a drawing isn't scientific evidence of anything, ever. (at least in the context of biology/medicine) --BluePlatypus 07:38, 1 March 2006 (UTC)
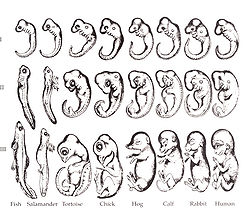
- Yes, Haeckel's sketches are flawed—he gets a bit fanciful with his third row of sketches in the figure at right. Haeckel's Theory of Recapitulation stated that the development of an embryo would follow the evolutionary development of a species. (Often this is condensed down to the catchy slogan 'Ontogeny recapitulates phylogeny'.)
- While Haeckel's theory fails in the strictest sense and his drawings sometimes strayed into wishful thinking, it does provide a useful rule of thumb. In the human embryo, features that evolved early (a backbone, for instance) are formed early in embryogenesis, whereas features that evolved recently (the cerebrum) form last. In whales (which evolved from land mammals) the embryo grows and then loses hair during the course of its development.
- Certainly if Haeckel's sketches were the only evidence in support of evolution, they would be poor proof indeed. However, there is a wealth of other evidence that supports evolutionary theory quite well; an error made by a zoologist in 1866 doesn't render the theory any less reliable. TenOfAllTrades(talk) 16:33, 1 March 2006 (UTC)
- Of course, they are flawed in many ways. Embryos aren't two dimensional, to begin with. deeptrivia (talk) 04:12, 2 March 2006 (UTC)
porn site
[edit]Can anyone give me the best born site in the world?
- What are your criteria? JackofOz 12:21, 1 March 2006 (UTC)
- why are people so weird these days. they dont know that much, that is why we have very smart scienctist.Because there is no reputable ranking site, I don't think you can say. It all boils down to personal preference. For example, you might like http://mary-kateandashley.com or http://www.hairybearmen.com. Proto||type 14:09, 1 March 2006 (UTC)
- As of a couple of years ago Usenet literally contained more FREE porn of EVERY category (including illegal) than you could view in a lifetime. I haven't been there in years, so I don't know its current status. WAS 4.250 18:12, 1 March 2006 (UTC)
- Our article onit says in one place it currently has a daily volume of "2.00 TB" and in another place says " Commonly omitted from such a newsfeed are foreign-language newsgroups and the alt.binaries hierarchy which largely carries software and erotica and, in the 21st century, accounts for over 99 percent of the article data." So does 2 TB of free porn a day qualify as "best"? WAS 4.250 18:22, 1 March 2006 (UTC)
- Sure, but only if you can tell me correctly what the best book ever written and the best movie ever made were (and have *everybody* agree on it). --Robert Merkel 00:25, 2 March 2006 (UTC)
- There is only one book that stands out from all others and that is The Bible. There is no moving picture that stands out above all others so the "best" movie that everyone can agree is the "best" is yet to be made. That's not exactly what you asked, but that's what's available in terms of books and movies. What's available in reality-land is fresh bread with cheese and tomato and sausage topping (pizza) with wine (or beer), and an opposite-sex friend (ask around, find a real life babe, beats anything on the internet); or as someone once put it a loaf of bread, a jug of wine, and thou. WAS 4.250 03:11, 2 March 2006 (UTC)
- To be fair, the question was the best site, not the best porn, it's more like asking what is the best library than what is the best book. I think it's fair to say that the site containing the largest amount and largest range would be most likely to be agreed on by the most people as the best site. Your milage may vary.
- No no no no no! You're saying that 'best' is defined by quantity. That should be quality. Wikipedia is the best site on the Internet because it's structured (and free). The Internet is a huge collection of info, but what was lacking was an easy way to get the right information. Search engines were one solution, but you still usually have to wade through a lot of stuff you're not interrested in. Even though Wikipedia doesn't quite come near the quantity of what these search engines can access (the whole Internet) it is already an equal competitor (I already often search on Wikipedia before I Google a term). When Wikipedia encompasses all information of some importance (a decade from now?) search engines will be out the window. So the best porn site would have all sorts of types of porn, but it would stand out by making it easy to find what you are looking for. And it would be free. That probably doesn't exist yet. So maybe we should start this? Wikiporn anyone? Of course we'd need to have images under gpl, so we'd have to make them ourselves. I suggest you start asking pretty girls (and guys) in your neighbourhood if they'd want to have their photos taken. All in the name of the open source movement of course. It's for a good cause. :) DirkvdM 09:28, 2 March 2006 (UTC)
- It's been suggested already. :) I even proposed the "This page is currently softcore. You can help Wikiporn by hardcoring it" template. ;) ☢ Ҡi∊ff⌇↯ 15:12, 2 March 2006 (UTC)
- So the best porn site would have all sorts of types of porn, but it would stand out by making it easy to find what you are looking for. And it would be free. That probably doesn't exist yet. Obviously you haven't bothered to look at either usenet nor any of the myriad ways of accessing it. Usenet is free, volumous, contains high quality by any standards mixed in with high quality by every standard (meaning whatever you want is in there somewhere), AND it is divided up into categories as precise as "alt.binaries.pictures.olsen-twins" (see [1]). To search and sort more thoroughly, you have to find an accessing method (tool, site, provider) that suits your desires/needs whatever that may be. Just cause you don't check something out doesn't mean it doesn't exist. Ever hear about the economist who said there is no such thing as a quarter on the ground because someone would have picked it up according to economic theory? By any standard except trying to get you to pay money for what you can get elsewhere for free, usenet is the best. Usenet has been for a decade well known as the best web porn site period. On slashdot it's been compared to trying to get a drink of water from a firehose. Porn sites flood the place with free pics trying to get customers to come to their specialized sites. Enthusiasts share entire collections with one another. It's also spam city. examples of tame usenet from[2]:
- alt.binaries.videos.tv.shaggable-babes (sources, sample of binaries)
- alt.binaries.multimedia.japanese (sources, FAQ, sample of binaries)
- finet.binaries.keskustelu (sources, sample of binaries)
- alt.binaries.pictures.autos (sources, sample of binaries)
- alt.binaries.pictures.hannigan (sources, sample of binaries)
- alt.binaries.pictures.ba (sources, sample of binaries)
- alt.binaries.pictures.rail (sources, FAQ, sample of binaries)
- alt.multimedia.mpeg (sources)
- alt.binaries.e-book.flood (sources, sample of binaries)
- alt.binaries.pictures.child.starlets (sources, sample of binaries)
- alt.binaries.pictures.kid (sources)
- alt.binaries.pictures.suntan (sources, sample of binaries)
- alt.binaries.webstars (sources)
- alt.binaries.pictures.chelda (sources)
- alt.binaries.slack (sources, sample of binaries)
- alt.binaries.pictures.woodworking (sources, sample of binaries)
- alt.binaries.pictures.animated.gifs (sources, sample of binaries)
- alt.binaries.sheet-music (sources, sample of binaries)
- alt.binaries.pictures.anime (sources, FAQ, sample of binaries)
- alt.binaries.cracks (sources, sample of binaries)
- alt.binaries.e-book.fantasy (sources, sample of binaries)WAS 4.250 10:32, 2 March 2006 (UTC)
Examples of untame usenet from [3]: alt.binaries.pictures.erotica.boys alt.binaries.pictures.erotica.breasts
Pictures of mammalian protruberances
...pictures.erotica.breasts.large ...pictures.erotica.breasts.natural ...pictures.erotica.breasts.saggy ...pictures.erotica.breasts.small
More than a handful is too much
alt.binaries.pictures.erotica.british
A binaries group devoted to British glamour girls
alt.binaries.pictures.erotica.brunette
Erotic pictures of brunettes
...erotica.brunettes-short-hair.reposts
Reposts of erotic photos of brunettes with short hair (on their head!), corrected
...pictures.erotica.bulgarians.female
Erotic pictures of women of fractional or full Bulgarian ancestry or close enough
...pictures.erotica.buttnuggets alt.binaries.pictures.erotica.butts
Erotic butts come into view
alt.binaries.pictures.erotica.cancel alt.binaries.pictures.erotica.cartoons WAS 4.250 10:57, 2 March 2006 (UTC) p.s. This page is not to be taken seriously :). you people are stupid:p
energy change
[edit]what is the energy change in a pendulum?
- Just take the energy change outside the pendulum and subtract! Seriously, check out pendulum, and work it from there. freshgavinΓΛĿЌ 13:48, 1 March 2006 (UTC)
Where does the EM energy go when the Poynting vector is zero?
[edit]I hope you've guessed that I'm talking about an electromagnetic wave. According to the electromagnetic wave equation, the phase difference between the E and B fields is zero, so the Poynting vector, which is their cross-product, is a cos-squared function of time. This means that the energy in a wavefront oscillates between zero and some value. Where does nature put the energy while the Poynting vector is zero, so that it can magically produce it a quarter-cycle later? I'm not trying to be controversial: I just want to know how I have misunderstood the equations. This stems from a question that someone asked me on my Talk page about why the E and M components in the light-wave.png image are in-phase. --Heron 14:53, 1 March 2006 (UTC)
- It means that the energy of the EM wave is zero at that particular point of the wave - however, the wave must have some spatial extent in the longitudinal direction, and the E & H fields are not zero as you move away from the crossing point. So the answer is, the energy is stored in other parts of the wave, and as it propogates, the zero-energy points move along with the rest of it. If you integrate the energy for the whole wave-packet, you'll see that it is conserved as you expect.
- Note that this isn't just a mathematical fiction - if you create an EM standing wave, you get no effect from the wave at the nodes (since there is no E or H field there), which can cause unwanted effects in lasers. --Bob Mellish 16:27, 1 March 2006 (UTC)
Thanks Bob, that makes sense. --Heron 18:50, 1 March 2006 (UTC)
telephone usage
[edit]Excluding mobiles which country has the most telphones per head of population? 15:48, 1 March 2006 (UTC)~
- You will want an anomoly - extremely small population with high telephone usage. My best guess would be Palau. They have 6,600 wired telephones and 20,000 people. That is 30% of the population with a wired telephone (assuming 1 phone per person and not one person with 6,000 phones). So, look at the micro-nations and look for high telephone use and low population. The Vatican may be a good one. --Kainaw (talk) 16:08, 1 March 2006 (UTC)
Thank you :) much appreciated 19:23, 1 March 2006 (UTC)~
- I'd be willing to bet it's the Vatican City - population about 900, but employing a couple of thousand non-resident workers, and in the heart of Western Europe. I can't see them having less than the 300 phones needed to beat Palau's 30%. Grutness...wha? 01:20, 2 March 2006 (UTC)
- The Netherlands had 8 million connections in 1995 (if that figure includes mobiles it won't be too many). In 1997 that was 8,8 million (different source). Mobiles will have slowed the increase of wired connections, but if we assume 10 million now, on a population of 16 million that would be 60%. That said, the Netherlands is said to be the most 'connected' country in the world in different respects (highest Internet usage, for one). The main causes for that are probably high GDP combined with socialism (everyone gets to share in the wealth) and a high population density (there is no 'outback' in the Netherlands). Scandinavian countries have the former but not the latter (except maybe Denmark). But indeed smaller countries may score better. Maybe Luxemburg, Hong Kong or Singapore? DirkvdM 09:42, 2 March 2006 (UTC)
Well it's for a radio quiz & it's been going for 8 days now & it's not Vatican City because someone tried that last night - before I got chance to ring in. Belgium, Liechtenstein, France, Guernsey, Jersey, Hong Kong, Singapore, Norway, Sweden, England, Luxemburg, Malta, Republic of Ireland, Spain, Andorra, Afghanistan, New Zealand, Australia, US, Canada, Vatican City, Falkland Islands, Taiwan, Holland, Japan & Korea are the answers given so far. It's just driving me nuts now! Tonight I'm hoping to get on there & try Palua which was Kainaw's guess. Failing that it's back to banging the head on the desk! Will keep eveyone informed 82.46.54.254
- If it is a radio contest, the answer they want is most likely wrong. They read obscure news articles that they don't understand, grab a fact that uses words they don't understand, and then turn it into a questions loosely based on the fact. Your best bet is searching Google News for articles about telephone usage as that is where the question came from. --Kainaw (talk) 14:30, 2 March 2006 (UTC)
- Searched Google News... A recent new article claims Monaco has the most telephones per capita (199.4%). --Kainaw (talk) 01:20, 3 March 2006 (UTC)
Thanks Kainaw you've been ever so helpful. I'm new to all this so I do appreciate it :) Only worked out yesterday how to get a user name rather than a number!Devononlyknows
- Have you tried using the CIA The World Factbook? However, it doesn't seem to have per capital figure, and you may have to divide the list of number of telephones with population. The Monaco's figure given above seems difficult to beat. Interestingly, the Pitcairn Islands has only one telephone, i wonder if they complain about long-distance charges. --Vsion 09:13, 3 March 2006 (UTC)
I will check that link now thank you Vsion. I wonder if the Pitcairn Islanders do complain about phone charges! Must be a pain in the neck if they need an engineer because the phone isn't working!Devononlyknows
And the answer is....Monaco! It has 1707 telephones per 1000 people. I didn't get through to give the answer. But well you never know when someone may need this information! :o)Devononlyknows 12:46, 7 March 2006 (UTC)
I've seen and used AAA, AA, A, C, D, 9V, and other sizes of batteries, but why haven't I even seen a "B" battery? Why aren't they made? --Shultz III 18:12, 1 March 2006 (UTC)
- Hmm... it appears that they are made: [4] [5]. Put the second one appears to be "hand-made". KILO-LIMA 18:26, 1 March 2006 (UTC)
- You just weren't looking in the right place. --LarryMac 18:32, 1 March 2006 (UTC)
- Obviously, however, C battery (vacuum tubes) is not the same as the consumer C battery originally referenced, so I wouldn't call B battery (vacuum tubes) "the right place". Duracell only lists yours above as "common" (though I've also seen AAAA), so Bs certainly appear to be long gone. I'll keep poking around. — Lomn Talk 19:00, 1 March 2006 (UTC)
- Well, I did show him a B battery, even if it came from the wrong family. There is amazingly little to be found on the web about the consumer style. The ANSI standard (referenced below) is C18.1, but NEMA wants $79 to see it. --LarryMac 19:49, 1 March 2006 (UTC)
- Here's a quick note on A and B being part of the 1920s ANSI standard for battery sizes (noting simply that those extant today are the ones that caught on commercially) and a chart of standard battery sizes (A is included, but B appears not to be). — Lomn Talk 19:06, 1 March 2006 (UTC)
- Obviously, however, C battery (vacuum tubes) is not the same as the consumer C battery originally referenced, so I wouldn't call B battery (vacuum tubes) "the right place". Duracell only lists yours above as "common" (though I've also seen AAAA), so Bs certainly appear to be long gone. I'll keep poking around. — Lomn Talk 19:00, 1 March 2006 (UTC)
A related question - is it just my imagination, or did Britain used to have the same betteries but with completely different names? ISTR U-11 batteries as a young kid. Grutness...wha? 01:21, 2 March 2006 (UTC)
- They did used to. I can't remember the names either though. I remember figuring out what the equivalents were. 67.40.249.122 03:11, 2 March 2006 (UTC)
- The codes are of the form LR06 = AAA, LR6 = AA, LR20 = D, 6LF22 = 9V, etc., and are still printed on batteries here in the UK. Ojw 20:16, 3 March 2006 (UTC)
- Not the codes I'm thinking of - these were definitely U-number, as in U-11, U-5 (I think) and U-22. Grutness...wha? 00:04, 4 March 2006 (UTC)
- Good quote from Dan's data on the AAAA cells - "no matter whether you're at the bottom of a five-mile cave system, performing extra-vehicular activity on the International Space Station, or lost in the middle of the Gobi Desert, you at least know you're no further away from a place that sells AAAA cells than you'd be if you were standing in your local shopping centre.". Ojw 20:22, 3 March 2006 (UTC)
- I added them as A battery (vacuum tubes), B battery (vacuum tubes), and C battery (vacuum tubes) specifically to avoid confusion with the modern A battery and C battery as the letters used in the vacuum tube batteries refered to usage not specific sizes/packages (all three came in many different sizes/packages). The modern A and C cells are battery sizes/packages. -- RTC 23:46, 4 March 2006 (UTC)
- Ha! Found this, which says that U11 is an alternative code for C batteries. Grutness...wha? 00:55, 7 March 2006 (UTC)
websites
[edit]What is the estimation of current number of WWW. sites?
- By www site, do you mean a unique site or a domain name. Many sites have multiple names all pointing to the same site. Also, some sites have many subsites (like aol.com and all the user pages under the aol.com domain). So, using "site" is very vague. But, an estimate can be made. Assume 5% of the computers in the world are web servers hosting a web site. If you think that is low, then consider that some web servers host multiple sites. If you think that is high, then use a different percentage. Now, take into account that there are currently around 4 billion IP Addresses in use - meaning there are around 4 billions computers connected to the Internet. Many are printers, switches, and the like. But, we only consider 5% of them to be web servers. So, 5% of 4 billion is... 200 million. That may sound high, but I think it is rather low. Google claims to have indexed well over 9 billion pages. They don't even touch all the "Hi. I learned to make my own webpage!" pages. So, either a lot more of the computers on the Internet are web servers or the web servers are hosting a lot more than one site on average. --Kainaw (talk) 01:38, 2 March 2006 (UTC)
- The February 2006 Netcraft survey received responses from 76 184 000 sites, so there are at least 76 million websites out there. --Bowlhover 13:33, 2 March 2006 (UTC)
DRUG HAIR TEST
[edit]WHAT WILL CAUSE HAIR TO TEST POSITIVE FOR COCAINE, IF A PERSON NEVER USED DRUGS BEFORE.
- Little bit of trivia, the mass spectrometers they use for those tests are so sensitive that if you've used cocaine any time in the last 6 months you'll test positive, so if you've haven't done anything recently it doesn't mean that it's a false positive, just a very sensitive spectrometer--205.188.116.74 22:15, 1 March 2006 (UTC)
- Please stop yelling. Turn off your caps lock. The logic of your dillemma there is obviously flawwed. If a person has never used drugs, then the person should pass the test, assuming the test was done correctly. Is there a specific person involved? Is it yourself? Did this actually happen or is it a theoretical question? More information is needed. In the mean time, you can take a look at our article on hair drug testing. It might help you out. --Chris 20:07, 1 March 2006 (UTC)
- In a test, the Mythbusters showed consumption of poppy seeds could cause you to test positive for opiates, but for such things to show up in a hair test, that would require massive amounts of the stuff. - Mgm|(talk) 20:47, 1 March 2006 (UTC)
- Few tests are so good that they have NO false positives - what is the accuracy of this test?
- Actually, there are no false positives, it's the same machine they use to sequence proteins, and is more than capable of telling the difference between cocaine and coca flavored shampoo, LOL--152.163.100.74 05:17, 2 March 2006 (UTC)
- I don't see how, given that coca-flavored shampoo would actually contain cocaine. --Trovatore 05:20, 2 March 2006 (UTC)
- Actually, there are no false positives, it's the same machine they use to sequence proteins, and is more than capable of telling the difference between cocaine and coca flavored shampoo, LOL--152.163.100.74 05:17, 2 March 2006 (UTC)
- Few tests are so good that they have NO false positives - what is the accuracy of this test?
It occurs to me that if you were to use an herbal shampoo containing coca leaf, it could mess you up this way. I've never heard of any such shampoo, but you never know. --Trovatore 03:20, 2 March 2006 (UTC)
The short answer is, it depends. If you have been in the presence of other individuals using cocaine (smoking large amounts of crack, in particular), then it is possible for some cocaine to become attached to your hair. Hair testing protocols call for fairly extensive washing of the hair sample to remove drug that may have been absorbed from the air, but there are studies which suggest that this process may not be 100% effective. Dark-haired individuals may be particularly vulnerable, as there is evidence to suggest that melanin (a pigment in dark hair) can effectively bind cocaine. Modern hair-testing labs should be testing the hair for both cocaine and for its metabolites: compounds like benzoylecgonine. The presence of such compounds is usually taken to be indicative that the drug was ingested, since they are produced as the body processes the drug. (If a lab cuts corners or offers cheap tests, they may not be testing for metabolites.) There is also an off chance that someone screwed up sample handling or labelling somewhere along the line; if someone else's hair was tested in place of yours, then you're going to get incorrect results. I am operating under the assumption that the lab is using a GC/MS (gas chromatography/mass spectrometry) system to run these tests. Such equipment–used properly–is pretty much the gold standard. Other testing protocols may be more prone to false positives. TenOfAllTrades(talk) 05:45, 2 March 2006 (UTC)
- I am actually pretty sure I saw in Scientific American that it is possible to detect certain drug usage, and how much a person has been using after they've had enough time to grow their hair out. I don't remember any details, maybe it was crack they talked about. --
 Mac Davis ☢ ญƛ. 10:18, 2 March 2006 (UTC)
Mac Davis ☢ ญƛ. 10:18, 2 March 2006 (UTC)
You might get a positive result if the lab is sloppy. If they have cocaine around, the dust can contaminate the room so that test samples could pick up cocaine from air or surfaces. The FBI whistleblowers made just that claim about the FBI labs. They had a lot of cocaine around. I have read that money counted out near drugs will pick up particles of drug. As that money gets passed around, it rubs off on other money in the money drawer. Thus I read that a large percentage of money has detectable drug residue, and could be confiscated under USA forfeiture laws, if the police decide to test YOUR money. GangofOne 08:12, 3 March 2006 (UTC)
Why is Schrondinger's Equation The Way It Is?
[edit]This question might be asking something that isn't very answerable by anyone, or at least answerable without recourse to talking about 11 dimensions and Hilbert Spaces, but why is Schrodinger's Equation what it is? I'm in Intro QM at Uni, and was talking to a friend, trying to describe what wave functions are. I was explaining the infinite-square well potential, and she asked why it was that the stationary states were all sinusoidal. The answer, of course, is that time-independent equation becomes a differential equation for a simple harmonic oscillator in the infinite-square well case, and the rest is boundary conditions. But she wanted to know why the time-independent eq. (and by extension the general eq.) worked out to a harmonic oscillator. I had no answer, was wondering if one existed. A simpler question might be, how did Schrodinger come up with the equation in the first place? It hardly seems as intuitive as f=ma and the like.
- A particle in an infinite square potential well wouldn't be a harmonic oscillator, you're confusing two entirely different models, so I'm not sure quite what you're asking, and certianly the number of dimensions is irrelevant, if you're asking why is the Schrondinger Equation sinusoidal then the answer is simple, the linear, or in your case angular momentum can be defined as the sum of two exponential functions (in 1 space), representing momentum in both the positive and negative directions, euler allows us to summarize this as a harmonic function, and as far as where the expoential terms come from, you simply apply your Ĥamiltonian over your wavefunction, which gives you a second order differential equation, which in turn gives you your exponential term, it's not only more intuitive than F=ma, it's more accurate as well, although if you replaced your a term for an expression involving an expression of momentum, and your m term for your reduced mass, you could probably construct some sort of rudimentary Fx operator ;)--205.188.116.74 22:03, 1 March 2006 (UTC)
- Are you asking why the Schrödinger equation is the way it is, or are you asking why the solutions to it are the way they are? The former is physics, the latter is a purely mathematical issue. As for the former, you can't really go by how Schrödinger derived it, because his way was rather ad-hoc. A ground-up approach from basic postulates is given in, for instance, the first three chapters of Landau & Lifschitz book on QM. It's not string theory, but it's not introductory-level stuff either. --BluePlatypus 04:03, 2 March 2006 (UTC)
- This is – approx – how Schrödinger came by his equation (or how we've been told he did): It was at the time that people had quite agreed that particles are waves, and someone asked Schrödinger: "Now, we have waves, shouldn't we have wave equations?" So Schrödinger went searching. In time before QM, fysicians had constructed Lagrangian and Hamiltonian formalisms (you may know them, though you may not) and there's a formula, saying: energy = kinetic energy + potential energy (though it's stated in a slightly more different way, so as to be so general it can be applied to cases that have nothing to do with energy at all; the "real" formula is in fact: H = pq۟ + V). Now he needed waves, and these go like sin(kx - ωt) or cos(kx - ωt). If you derive them with respect to x, you get k (≈impulse) in front of them, and if you derive them to t, you get ω (≈energy). So you add some constants where needed and you get an equation that certainly looks pretty smart and cool, and it even works. Greetings. David
 15:28, 2 March 2006 (UTC)
15:28, 2 March 2006 (UTC)
- This is – approx – how Schrödinger came by his equation (or how we've been told he did): It was at the time that people had quite agreed that particles are waves, and someone asked Schrödinger: "Now, we have waves, shouldn't we have wave equations?" So Schrödinger went searching. In time before QM, fysicians had constructed Lagrangian and Hamiltonian formalisms (you may know them, though you may not) and there's a formula, saying: energy = kinetic energy + potential energy (though it's stated in a slightly more different way, so as to be so general it can be applied to cases that have nothing to do with energy at all; the "real" formula is in fact: H = pq۟ + V). Now he needed waves, and these go like sin(kx - ωt) or cos(kx - ωt). If you derive them with respect to x, you get k (≈impulse) in front of them, and if you derive them to t, you get ω (≈energy). So you add some constants where needed and you get an equation that certainly looks pretty smart and cool, and it even works. Greetings. David
Scientific Journals - where does the money go?
[edit]$20 to download a single article from some journal publishers seems a bit steep (especially for a student). Why do they have to charge so much? Are some of the publishing executives struggling to pay for their second homes? Some day us scientists will raise our conical flasks and petri dishes and revolt! --Username132 16:09, 28 February 2006 (UTC)
- It depends on the journal. Some are published by professional societies; in those cases the money ges back into the cost of production of the journals (which are usually subsidised by members dues). In the case of journals that are published by publishers like Elsevier and others - it's all about profit. You charge a library $1000 for a subscription to a journal, charge authors page charges and get peer reviewers to work for free. Guettarda 16:22, 28 February 2006 (UTC)
- Do you know how the peer review process works? If peer review is free, would it be possible to set up a free/cheap/donation-based online journal? --Username132 20:24, 28 February 2006 (UTC)
- The money certainly isn't paid to the editors: I once applied for a job at a journal produced by a commercial publisher (not a scientific society), but the salary they offered was almost exactly half what I was earning as a university researcher! Physchim62 (talk) 20:33, 28 February 2006 (UTC)
- Is an editor the same as a peer-reviewer? If not, how do they differ? --Username132 23:46, 28 February 2006 (UTC)
- An editor will edit the content for the journal (spell-check, grammar check, introduce incorrect grammar when they simply don't understand the scientific wording...). A peer-reviewer reads and rates submissions. For example, I primarily work in research on hypertension. When we submit articles to journals, the editor most likely knows nothing about hypertension. So, they send it to peers who know a lot about it. Then, they read it and tell the journal if the article is worth publishing. If you are very lucky, you get the article back with suggestions. You fix it up and resubmit it. Sometimes it can be something rather silly. We had to resubmit one because we used "underpriveleged" to mean "lacking in access to healthcare". In this particular journal, "underpriveleged" is a reserved synonym for "African-American". We changed it to "deprived" and everyone was happy. --Kainaw (talk) 01:36, 1 March 2006 (UTC)
- Thanks for the insight. What do peer-reviewers get for their work? Free journal access?
- Gratitude --pom 00:11, 2 March 2006 (UTC)
- Thanks for the insight. What do peer-reviewers get for their work? Free journal access?
- Depends on ethics. Some peer reviewers get into it just to steal ideas. Others are really trying to help. --Kainaw (talk) 01:55, 2 March 2006 (UTC)
- You would be better stealing ideas from reviewing grants not papers. Most people (99%) review papers because others have done it for their own papers. David D. (Talk) 08:34, 3 March 2006 (UTC)
- Most universities have subscriptions to most journals, including the online versions. If you're a university student, you can inquire with your library for what journal subscriptions they have and how to access them. At my university, merely by being on a university machine (or logging in through a proxy server), I can get free copies of articles from any journals I've ever looked for. I don't know if that helps you or not, but it might be something to check into. -- SCZenz 23:51, 28 February 2006 (UTC)
- Yeah we have subscriptions to the most relevant ones. Occasionaly though, I find an article I'd like but can't access. I know people writing some dissertatations are even less well served by the available subscriptions. My main concern is how I keep up between leaving university and getting a research position. --Username132 08:41, 1 March 2006 (UTC)
- The scientific journal business has made a large number of scientists rather unhappy; see open access (which is not the most neutral of articles, but anyway) for an alternative approach. --Robert Merkel 22:17, 28 February 2006 (UTC)
- Thanks, this is great! I'm actually a representative for my university course to our university library. Is there any way our university library can do more to support open access? I'd like to make the most of my position for the remaining couple of months that I still have it.
- The scientific journal business has made a large number of scientists rather unhappy; see open access (which is not the most neutral of articles, but anyway) for an alternative approach. --Robert Merkel 22:17, 28 February 2006 (UTC)
- Also is research funded by a corporation such GlaxoSmithKline more likely to be published in a traditional journal? When they're close to something, they stop publishing at all so they can patent it first, don't they?
- Supposing I had really good eye-site and looked through a GSK window as I passed by and saw the notes jotted by some scientist and then ran to the internet and published "the cure" for HIV before it had been patented... apart from being on GSKs hitlist, would I have comitted a crime, and would people be allowed to use the information published? --Username132 22:56, 28 February 2006 (UTC)
- Patenting depends on where you are. If memory serves me, in Europe, you can publish after the application has been sent in, in the US you have to wait for the patent to be approved. And assuming you weren't trespassing, then no, you haven't done anything illegal. This is all kind of moot though, because in reality Pharma companies patent stuff at a very early stage (pretty much as soon as the idea presents itself), far before the years of development and clinical testing (from candidate to approved drug is a process that takes over a decade). So even if you managed to get hold of a not-yet-patented idea, you wouldn't have the resources to turn it into a drug. And even if you did, less than 1 in 1000 candidates make it through all the way to an approved drug. --BluePlatypus 08:20, 1 March 2006 (UTC)
- What can an individual library do to help support open access? --Username132 08:29, 3 March 2006 (UTC)
Kinetic Energy and Einstein's Theory of Special Relativity
[edit]Firstly, can someone please verify that the equation for kinetic energy, taking into account Einstein's Theory of Special Relativity is:
Secondly, can someone explain how we get that equation from I think I get it now, although it would still be handy if someone could explain it just in case...
Thirdly, can someone please tell me of a computer program/language that will enable me to perform calculations with this with absolute accuracy. All the software I have at the moment doesn't seem to be able to cope with numbers such 299792458 squared (which isn't surprising I suppose). P.S. I think I'll stick to the Key Stage 3 stuff I'm supposed to be doing... 80.229.152.246 21:29, 1 March 2006 (UTC)
- Once you subtract the rest energy from the total energy , you get the energy of motion .
- As for your software, have you looked for a scientific mode? The speed of light should not be stored as an exact integer, but as a floating point value, which usually has a lot more range. —Keenan Pepper 21:41, 1 March 2006 (UTC)
- Thanks for the answer Keenan. As for the software, it doesn't have a scientific mode, although I think I will try it with something else I have found. Thanks. 80.229.152.246 17:30, 2 March 2006 (UTC)
Drug Hair Test
[edit]What causes hair to test positive for cocaine, if one never used it.
- You asked that question already. Scroll up a bit. Optichan 22:03, 1 March 2006 (UTC)
- Ever get really tweaked on some coke, ask a question, and then, just a few minutes later, forget if you asked it or not already? --Kainaw (talk) 01:11, 2 March 2006 (UTC)
- And then the darn test results come in, and you forgot you even took it... --Zeizmic 13:34, 2 March 2006 (UTC)
Drug store?
[edit]Why is a drug store called a drug store instead of a medicine store? (Aidan Age 8)
- I don't know, but my guess is that the word "drug" has less syllables than "medicine". --HappyCamper 23:16, 1 March 2006 (UTC)
- Hi Aidan. The meaning of words changes over time. When the phrase "drug stores" was invented the word "drugs" was a word that people associated with relief from pain and other good things so it was a good name for a type of store. Today "medicine" means something that you won't go to jail for and make you better; while "drugs" means things people say are bad for you. The words mean the same thing, but the connotation is different. You are trained to feel one way about one word and you are trained to feel a different way about the other word even though the two words mean the same thing. WAS 4.250 23:34, 1 March 2006 (UTC)
- When a police officer ask "Are you on medication", such as [6], he is refering to general substance abuse including non-medicinal drug, isn't it? --Vsion 15:05, 2 March 2006 (UTC)
There is a store near me, owned by a person of Middle Eastern descent, whose English leaves quite a bit to be desired. The name of the store ? "The Drug and Party Fair". LOL StuRat 23:55, 1 March 2006 (UTC)
- Out on a limb here, but the Dutch word for 'drug store' is 'drogist', which refers to the word 'drogen', which means 'to dry'. And indeed most medicines have in the past been dried plants. Considering the overlap between the Dutch and English languages there is a good chance the English word has a similar origin. And indeed over time the usage has changed when the word is used by itself, but (possibly) retained its original meaning in the term 'drug store'. DirkvdM 09:56, 2 March 2006 (UTC)
- The OED says drug-store is originally U.S. The archaic English word druggist has the same meaning as drogist in Dutch, and is also usually traced back to a root meaning 'dry'. --Heron 20:56, 2 March 2006 (UTC)
Note that, at least in American English, "drug" still retains the meaning of "pharmaceutical", as well as "recreational substance". (For that matter, the line between the two is not always entirely clear.) --Trovatore 03:30, 3 March 2006 (UTC)
- Well, the definition of the line between the two is in general quite simple; whether it's legal or not. Which, of course, is an upside down way of reasoning; "It is illegal, therefore any use must be recreational". But some drugs are used both pharmaceutically (legally) and recreationally (illegally), such as opium (laudanum) in the past and marihuana (which is literally a dried plant) recently. Interrestingly, heroin was originally devised as a wonder drug against opium addiction (so as a medicinal drug). Now, such a wonder drug is methadon. I wonder how long it will take until that becomes a 'street drug' like heroin. DirkvdM 08:51, 3 March 2006 (UTC)
March 2
[edit]Is it true that the earth wobbles while it spins?
[edit]- How much does it wobble? And at what frequency? --HappyCamper 01:13, 2 March 2006 (UTC)
- Well, he didn't ask that. Anyway, according to axial tilt, "The Earth's axial tilt varies between 21.5° and 24.5° with a 41,000 year periodicity". --Kainaw (talk) 01:41, 2 March 2006 (UTC)
- Oh, I didn't know that was what it was called. Thank you :-) --HappyCamper 01:55, 2 March 2006 (UTC)
- The actual wobble is called a precession, the kind of motion you get if you deliver a sideways prod to a spinning top. --BluePlatypus 03:50, 2 March 2006 (UTC)
- To be clear, there are two overlapping 'wobbles' at work. The axial tilt changes on a 41,000 year cycle, and the axis precesses on a 26,000 year cycle.
- The geophysicist Milutin Milanković looked at the combined effect of these wobbles (as well as a number of other factors) on Earth's climate. He proposed that there would by cyclical variations in climate (resulting in periodic ice ages and the like) on a roughly 100,000 year time scale; these are called Milankovitch cycles. TenOfAllTrades(talk) 05:24, 2 March 2006 (UTC)
- I also found something interesting while searching for this. There's another (much) smaller wobble called the Chandler wobble, which has a period of only 435 days. Interesting read; you learn something new every day. EWS23 | (Leave me a message!) 05:35, 2 March 2006 (UTC)
iTunes and storage
[edit]I have MUCH more music than I want to store on my G4 Powerbook. I have some of it on the PB, and a lot, lot more on an external drive that is accessable when the PB is 'docked' on my desk, but not on the road. I want to have a way to easily choose which music stays and which goes, much as I can with my iPod. Anything?
- Well, it looks like you've actually got three hard drives: the Powerbook's drive, the external drive and the iPod. So how about you store all the music on the external drive, but manage it using iTunes (make sure iTunes on the PB isn't copying the files to the local drive). Set up a playlist in iTunes of the songs you want to take on the road which you can easily alter. Sync the iPod to that playlist only, and then when you go on the road, plug the iPod into the Powerbook and play the music straight off the iPod through the Powerbook speakers. --Canley 05:02, 2 March 2006 (UTC)
Good idea - thanks. The problem is the iPod is tiny, and the PB has a pretty large drive. I'd really like to have a boat load on the PB. Any other ideas?
- I don't understand why you wouldn't just put all the music on the Powerbook. If its too much, just choose what songs go. --
 Mac Davis ☢ ญƛ. 10:07, 2 March 2006 (UTC)
Mac Davis ☢ ญƛ. 10:07, 2 March 2006 (UTC)
The reason that I don't put it all on the PB is that the harddrive is too small for the whole collection. What I am looking for is a way to easily manage what songs go on it, and what stays at home. I want to be able to change what is on the PB and what is not on a fairly regular basis.
- . iPod - 1 gig. Good for jogging, and other small outings.
- . PB - 80 gig. About 20 gig I am willing to use for music. Good for trips out of town.
- . External drive - 300 gig. All my music, about 100 gig filled.
So I want to keep everything on the external. I want to put pretty much a random and changing selection on the iPod. I can do this already. I want to put pretty much a random and changing (but bigger) selection on the PB. I can't figure out how to do that.
Nature red in tooth and claw
[edit]What exactly does the phrase "Nature red in tooth and claw" (from Tennyson's "In Memoriam") mean, particularly as used in evolutionary biology? Dawkins makes a reference in it in The Selfish Gene [7], though he doesn't explain it. --JianLi 04:10, 2 March 2006 (UTC)
It is a reminder that animals eat each other without mercy. It is the reality to which "the lion shall lie down with the lamb" is the imaginary antithesis. alteripse 04:15, 2 March 2006 (UTC)
Earthquakes
[edit]Is it true that earthquakes often occur after a heavy rain during a hot day? --ct
- If you find out, why don't you clean up the article earthquake weather? --Trovatore 06:11, 2 March 2006 (UTC)
- I can assure you there is absolutely no scientific basis behind that. Geothermal mantle currents have nothing to do with how sunny it is or how wet it is. --
 Mac Davis ☢ ญƛ. 10:09, 2 March 2006 (UTC)
Mac Davis ☢ ญƛ. 10:09, 2 March 2006 (UTC)
- That would be a handy indication of wheather or not an earthquake is coming. (Ok, that was lame even by my standards). DirkvdM 10:08, 2 March 2006 (UTC)
The phoney concept of 'earthquake weather' was reinforced by Mark Twain. Here is a hilarious quote. [[8]] --Zeizmic 13:29, 2 March 2006 (UTC)
Perhaps you meant that avalanches or landslides often occur after hot days or heavy rains. --Leah
- Perhaps this is part of where the misconception comes from, as I imagine earthquakes can cause landslides and avalanches as well. EWS23 | (Leave me a message!) 01:46, 3 March 2006 (UTC)
Chewing gum versus bubblegum
[edit]What exactly is it about bubblegum that allows you to blow larger bubbles than is possible with the same amount of regular chewing gum? Is there some chemical that facilitates bubble blowing? —Keenan Pepper 06:00, 2 March 2006 (UTC)
- More gum base (so it holds together better), and softer gum (so it's easier to blow bubbles). It's softened by adding glycerine or vegetable oil. --BluePlatypus 06:28, 2 March 2006 (UTC)
Now that that is answered, if you wish to kick the habit of chewing gum, you might go to a gum producing area. I followed chiclero tracks in Guatemala and saw how the gum is handled. I never touched a chewing gum since (then again I hardly ever did before, so there was no cold turkey :) . DirkvdM 10:15, 2 March 2006 (UTC)
- I thought nowadays it's all made of petroleum products. —Keenan Pepper 14:58, 2 March 2006 (UTC)
- So you're chewing oil. Which is rotten plant material. You know, that stinking black fluid that drips from a garbage bag if you leave it out too long. That sounds a lot tastier. :) DirkvdM 08:59, 3 March 2006 (UTC)
- I thought oil came from dead dinosaurs. User:Zoe|(talk) 16:44, 3 March 2006 (UTC)
- So you're chewing oil. Which is rotten plant material. You know, that stinking black fluid that drips from a garbage bag if you leave it out too long. That sounds a lot tastier. :) DirkvdM 08:59, 3 March 2006 (UTC)
- If you're going to get squeemish about where your food ultimately comes from, you're in big trouble. For great justice. 19:22, 3 March 2006 (UTC)
cure for wet bed
[edit](question moved from Wikipedia:Newcomers help page)
- What is the person's age? potty training, bladder control. - Mgm|(talk) 09:10, 2 March 2006 (UTC)
- Someone makes a diaper with an attached alarm that goes off when it gets wet. This is used to train a person to wake up when they have a full bladder. --Kainaw (talk) 16:47, 2 March 2006 (UTC)
- Or, at least, moments after having a full bladder. :) kmccoy (talk) 03:27, 3 March 2006 (UTC)
old or used systems
[edit]how to use old or used computers as a firewall
- What computers? How old? —The preceding unsigned comment was added by 67.40.249.122 (talk • contribs) .
- You can't use computers as a firewall. A firewall a piece of software. - Mgm|(talk) 09:11, 2 March 2006 (UTC)
- A firewall can be software or hardware. You can use an old computer as a demilitarized zone, but I'm not sure if that's what you want to do.--Commander Keane 09:48, 2 March 2006 (UTC)
- Maybe the user was trying to turn the old computer into a router, presumably with a firewall software running on it. — QuantumEleven | (talk) 11:23, 2 March 2006 (UTC)
- If you have enough old computers, you could weld them together and make a pretty good firewall. It would probably take a good two hours to burn through a wall of old computers. --Kainaw (talk) 16:48, 2 March 2006 (UTC)
Read this for clues. WAS 4.250 11:07, 2 March 2006 (UTC)
Technically, you could put two Ethernet cards in the computer, and run it as a gateway, with some firewall software on it. Thus, using it as a firewall.
if you perform the test for starch and the iodine remains yellow, what does this indicate?
[edit]- It becomes blue if there's starch. So what do you think you proved if there's no reaction? - Mgm|(talk) 09:12, 2 March 2006 (UTC)
- That the raison bread wasn't pregnant! freshgavinΓΛĿЌ 11:15, 2 March 2006 (UTC)
- Simple, that the is no starch present in whatever you tested. If you would like to test for other substances, there's a whole category of tests you can use to find different things Obli (Talk)? 16:23, 2 March 2006 (UTC)
Bacteria in our ears!!
[edit]I have heard that listening to music with headphones for over an hour multiplies the bacteria present in our ears.is this true?? thanks 59.92.36.216 10:03, 2 March 2006 (UTC)sciencefreak!!59.92.36.216 10:03, 2 March 2006 (UTC)
- I think so, it increases the humidity and temperature slightly in our ears. But theoretically, every second bacteria are multiplying, or you could look at it like at all times there are bacteria in your ear being born and dying. Don't worry, the bacteria aren't the bad kind either. They won't make you sick. As gross as bacteria's connotation might seem, don't worry about it when you pump up Green Day on your headphones. --
 Mac Davis ☢ ญƛ. 10:16, 2 March 2006 (UTC)
Mac Davis ☢ ญƛ. 10:16, 2 March 2006 (UTC)
- What music do they prefer then? Wet warm music? DirkvdM 10:18, 2 March 2006 (UTC)
- I've no idea, but I bet if you did a study on it you'd have a good shot for an Ig Nobel prize. —Ilmari Karonen (talk) 10:39, 2 March 2006 (UTC)
- If you're wearing your earbuds for too long I could see a potential risk of it, they'll improve the conditions for bactera substantially (less light, moist, dark, not to mention dirty - earbuds are never washed). Obli (Talk)? 16:13, 2 March 2006 (UTC)
- I read somewhere that bacteria are multiplied in there 700 times! Something to do with anaerobic environments being a major breeding ground. However, your feet are subjected to anaerobic environments for far longer, usually daily, so it multiplies who knows how many times more! I apply Germ-X to my feet at the end of the day to take care of the bacterial build-up. And speaking of ears and bacterial growth, I feel compelled to apply it right now. --Shultz III 00:16, 3 March 2006 (UTC)
- You could also regularly ventilate your feet (and then swap socks) like I do. My feet used to give off a horrible stink. This, plus no more soap, solved the problem. A solution by using less is always preferable to one that uses more, I'd say. DirkvdM 09:03, 3 March 2006 (UTC)
- Ventilate feet often, fresh clean dry socks at LEAST twice a day, let shoes air out for at least 24 hours between uses (IE at least two pairs of shoes, alternated), and using rubbing alcohol on your feet are all good methods to deal with the fungal problems caused by enclosing feet. WAS 4.250 12:21, 3 March 2006 (UTC)
- And which would be that? It often refers to excessive use of soap and it's precisely the fact that I stopped using soap that was probably a major part of my 'cure'. DirkvdM 13:21, 4 March 2006 (UTC)
Head-on auto crash.
[edit]If two autos of equal weight, and each going 50 miles an hour, crash head on, will the impact be 100 miles an hour or just 50 miles an hour? Thank you. Paul Weiss
- Assuming both cars are travelling forward, and the earth isn't spinning at a crazy rate, then yes. Err... I mean 100 miles per hour. It's a 2-dimensional collision so you simply subtract the vectors: . freshgavinΓΛĿЌ 11:17, 2 March 2006 (UTC)
- If you're standing on the road watching the crash, you'll see both autos travelling at 50 mph but in opposite directions. If you're sitting in either auto, you'll see the other as approaching at 100 mph. --Bowlhover 16:57, 2 March 2006 (UTC)
- The question is what you mean by "will the impact by 100 miles an hour". If you imagine an extremely solid wall of heavy steel or something that you could crash a car into without damaging the wall, then the 50+50 mph collision will damage the cars in the same way that a 50 mph collision into this wall would be, because all of the kinetic energy goes into damaging the cars, and colliding with an identical car will stop a car in the same way that the very solid wall would.
- On the other hand, if you imagine the damage from crashing the car into a stationary car of the same kind, then the 50+50 mph collision would be like a crash at
71 mphinto a stationary car.Here 71 is 50 times the square root of 2, and that arises because the energy will be distributed equally between the two cars and energy varies as the square of speed.
- On the other hand, if you imagine the damage from crashing the car into a stationary car of the same kind, then the 50+50 mph collision would be like a crash at
- For some calculations involving collisions you want to consider momentum rather than kinetic energy, but energy is the right measure when you're thinking about the amount of damage.
- --Anonymous, 00:57 UTC, March 3, 2006.
- Nope, I agree with your first para about the wall, but the 50+50 mph collision is like a crash at 100mph into a stationary car. Yes, the 100mph system has twice the total energy, but only half of that energy goes into destroying the cars. The other half is left as kinetic energy after the collision as the two cars, smashed together, are still trundling along the road at 50mph by conservation of momentum. —Blotwell 05:50, 4 March 2006 (UTC)
- Argh dammit. I should have realized that. Sorry. --Anon, 19:30 UTC, March 5.
Cocaine
[edit]A friend of mine claims that rubbing cocaine on one's penis before intercourse makes the sex more pleasurable for the woman, but this doesn't sound right. Is it true?
- Cocaine, when applied topically, acts as an anesthetic and intense vasoconstrictor. I'd say it has the opposite effect... — TheKMantalk 13:21, 2 March 2006 (UTC)
- Rub it on your hair, and you fail the drug test. --Zeizmic 13:36, 2 March 2006 (UTC)
"Because of the extensive processing it undergoes during preparation and its highly addictive nature, cocaine is generally treated as a hard drug, with severe penalties for possession and trafficking." [9]
"After the US helped the Colombian military dismantle the Medellín and Cali cocaine cartels in the '90s, the guerrillas moved in and took over much of the drug trade. By the late '90s, rebels controlled more than a third of the country and had the financial clout to intensify the [civil] war and protect their newfound position as narcotraffickers. It's an extremely lucrative business. The coke habit in the US alone was worth $35 billion in 2000 - about $10 billion more than Microsoft brought in that year." [10]
- Sorry, I couldn't help it. -LambaJan 20:39, 2 March 2006 (UTC)
- I guess an anasthetic, applied to the male, is likely to reduce his stimulation, so things go on longer than the regulation three minutes. This in turn may appeal to the female, if her tastes run that way. Notinasnaid 21:11, 2 March 2006 (UTC)
- I doubt she would appreciate the effect the cocaine would have on her bits =P. — TheKMantalk 21:55, 2 March 2006 (UTC)
- Try powdered ginger mixed with honey. She will never look at another man. (Never use chilli powder!) --Anon.
- Honey? No thank you. She doesn't need a yeast infection. moink 05:27, 7 March 2006 (UTC)
Usenet
[edit]The concept of "Usenet" has confused me (and yes, I read the article). Is it basically just a huge group of "bins" (like alt.binaries.videos, etc) and people post any message or file they want into the suitable "bin", and when you read it with a client it shows everything posted in that "bin" with the most recent first? - unsigned
- Usenet is essentially a newsgroup or forum, however you want to look at it. Its a resource whereby messages very similar to emails are sent into the system and then processed and stored in order, and someone with a reader can come along and read all the stored messages in order. Many people have adapted it for use with files, but its essentially the same strategy as sending files attached to emails. The only thing to remember is that someone has to host those groups, and many places only host the non-space-hogging kind since server disk space isnt exactly cheap. -unsigned
- So what's the deal with the readers? I mean, google groups doesn't let you download files AFAIK, or look very far back. Is there some free way to look at and download usenet files and look atp osts from way back (like months ago?) - unsigned
- Since its a service that is rather resource-intensive, its access is often subscriber based. Most ISPs will run some form of USEnet relay for their customers, although this is going by the wayside as it's use has shifted from the legitimate, to grey filesharing and piracy. —Preceding unsigned comment added by 66.195.232.121 (talk • contribs) 15:44, 2 March 2006
- Google doesn't carry binaries groups, but it does archive old posts (pretty much for ever - the oldest usenet post in Google's archive is from 1981.) Binaries groups are not generally achived and are not carried by as many servers as text groups. -- AJR | Talk 16:50, 2 March 2006 (UTC)
- Go here and try out a temporary free reader called Agent. It's highly recommended. Permanent free versions of Agent exist if you look hard enough. To connect the usenet reader to the usenet, ask your ISP for the net address to type into the reader. Its generally not an additional ISP charge so far as I know. (mine isn't - EarthLink) WAS 4.250 16:53, 2 March 2006 (UTC)
First a terminology correction: the original poster referred to "bins", and those are the newsgroups. Each newsgroup is a forum. Usenet is all of the newsgroups taken collectively. Some newsgroups are intended for messages in text form (that was the original idea, back when most of Usenet was carried on 300 or 1200 baud telephone connections); others are intended for binaries, which are encoded in text form. Most newsgroups are "unmoderated" and anyone can post anything, just as they can here; others have a moderator who screens all postings, or variations on this.
The original poster referred to seeing messages "newest first". The order that you see articles in depends on what newsreader (software ) you use. Historically, the original presentation was oldest first; then came oldest first but grouped by subject line; then came threading, which allows people to read a sequence of followups in a sensible tree-traversal order. Good newsreaders allow you a lot of control over what they see and how they see it, and they keep track of what you've already read, which is essential when you're reading oldest-first. Frankly, it's a much more congenial presentation of messages than the massive concatenation of everything into a single web page that we see in Wikipedia talk pages like this.
If you read newsgroups via Google Groups, then in effect you're using Google's newsreader running on their site (which you access via your web browser), where it accesses the postings locally. This contrasts with the traditional newsreader, which runs on your machine (so you can run whichever one you want to install) and accesses the postings you choose from a remote news server. Historically there was a third approach: all postings in all newsgroups you might want to read were stored on your machine, and your newsreader accessed them as local files. ("Your machine" in that case would likely be a big corporate or institutional one, rather than a personal computer. Postings would stay online for a few days or weeks, depending on disk capacity.) --Anonymous, 01:15 UTC, March 3, 2006.
Gagging, vomiting daily through stress
[edit]My girlfriend has recently started teaching at a public school. Over the first couple months the stress was extremely intense and she thought she was going to quit. Recently, the stress has dies down somewhat, but she is still experiencing stomach problems: each morning she dry heaves or vomits, and can't keep any food down until about lunch.
Obviously the problem is mainly psychological, and ought to go away once the stress starts becoming managable. However, is there anything she can take to help the symptoms? Would antacid tablets help? Any other over-the-counter stomach medicine? What is the physical response that is turning stress into an upset stomach?
Thanks in advance.
- She should probably consult her physician to be sure that there isn't something physically wrong. If necessary, her doctor can also refer her to an appropriate specialist for help in managing her response to stress.
- Whether physical or psychological at its root, cranking out gobs of stomach acid and vomiting every morning isn't a good thing, and warrants medical attention. Depending on the precise cause, antacid tablets (calcium carbonate, e.g. TUMS) may provide some relief. More potent blockers of acid production are also available over the counter (ranitidine, sold under the brand name Zantac in North America). TenOfAllTrades(talk) 15:51, 2 March 2006 (UTC)
This sounds like extreme anxiety. Get the doctor to refer to a specialist. I just read about a drug for extreme stage fright. I just get along with common meds, for my anxiety. --Zeizmic 18:00, 2 March 2006 (UTC)
- She needs to go to the doctor (is she pregnant?), but I have noticed ginger and ginger ale help me with nausea. -Ravedave 06:22, 3 March 2006 (UTC)
great pyramids
[edit]It is claimed that even with todays technology the great pyramids could not be built -is this true?
- Since most people agree that they were build even without today's technology, I think one could say with great certainty that they could be built with today's technology. See our Great Pyramid of Giza and Egyptian pyramid construction techniques articles for information on how they were built. — Asbestos | Talk (RFC) 20:20, 2 March 2006 (UTC)
- Of course, it is impossible, with today's technology, to stack rocks. That is why all those buildings and monuments that look like stacked marble, stone, or steel girders are just illusions. It is a four-inch model that appears big based on your frame of reference. In fact, I think Manhattan is only about three feet wide. --Kainaw (talk) 20:25, 2 March 2006 (UTC)
- Actually most buildings that look like large stacked rock slabs are actually illusions, usally they're brick or concrete, and the stone is just a thin outer facade. But that's because it's cheaper of course, not because we can't stack rocks. --BluePlatypus 13:04, 3 March 2006 (UTC)
We couldn't build it the way they did, with today's technology. Just the life insurance on 10,000 expendable slaves would break the bank. --Zeizmic 22:25, 2 March 2006 (UTC)
Whoever is claiming this is trying to prove something; from the way the assertion is worded I suspect that the intent is to demonstrate that extraterrestrials did it. It is of course nonsense; when one considers what can be built with modern construction technology, one realizes that reproducing the Great Pyramid would not be impossible. (Whether anyone today would want to build it is a different question.) —Charles P._(Mirv) 23:47, 2 March 2006 (UTC)
- Sure, it could be done. Just need a nice large quarry, the right machinery, a large dedicated workforce, and plenty of cash and time. And would anyone want to do it? Unlikely, when instead you could make a neat looking hollow one made of glass and put a casino inside. — TheKMantalk 03:17, 3 March 2006 (UTC)
- Asbestos, you basically say that if something could be done in the past of course we can do it too. You seem to assume that all new knowledge is simply added to the old knowledge. But it can also replace it. A simple example is survival skills of hunters/gatherers as they learn western ways and stop hunting and gathering. Or knowledge of old western trades that has been lost because the need has disappeared. Or take cement. The Romans knew about it. Almost 2000 years later it was re-invented. It is quite possible that a technique was used to build the pyramids that we don't need because we have different solutions. Sure, we could build something similar, but with our technology it would be different in many ways. To make an exacts copy would probably be prohibitively expensive, because we would have to first invent the techniques or adapt ours to get the desired effect. Sorry I said this a bit messy, but the thought behind it is not less valid for it. :) DirkvdM 10:38, 3 March 2006 (UTC)
- Another good example of this is Damascus steel (or more specifically Wootz steel.) Reports of weapons made using the technique date back to 30AD, but up until 1980, noone could reproduce the effect. GeeJo (t) (c) • 11:06, 3 March 2006 (UTC)
- We don't know how to make Greek fire, either. User:Zoe|(talk) 16:47, 3 March 2006 (UTC)
- Another good example of this is Damascus steel (or more specifically Wootz steel.) Reports of weapons made using the technique date back to 30AD, but up until 1980, noone could reproduce the effect. GeeJo (t) (c) • 11:06, 3 March 2006 (UTC)
- Right - there are two things going on here 1) whether it would be possible, in the abstract, to build a replica of the pyramids, and 2) whether it would be possible in terms of mobilizing the money, time, manpower and other resources to do it. I think we need to clarify which question we're asking. For great justice. 17:56, 3 March 2006 (UTC)
- Well the answer to 1) is yes. And the answer to 2) is yes as well. Most modern nations have greater economic and human resources than ancient Egypt. Not that it would be needed, we'd no doubt be able to do it with less (human and economic) expense. The main obstacle is political will. Not many countries today would allow their leaders to spend a large part of their national GDP on building a tomb for themselves. Not that it'd be a large part for most countries, but it's still money most people would rather see erecting schools or something useful. --BluePlatypus 18:08, 3 March 2006 (UTC)
- Yes. That's what I meant. For great justice. 19:20, 3 March 2006 (UTC)
March 3
[edit]Dual core CPU questions
[edit]I have an athlon 64 4400 (dual core). I use windows xp.
- 1) Is there some kind of update that I should get for windows to optimize it for using a dual core cpu? If so, what exact update(s) should I get and where?
- 2) I've noticed that when running certain games, it goes extremely fast (not at all playable). Is this because I have a dual core?
- 3) I've also noticed that when playing music and movies and such in windows media player it periodically skips (it sounds similar to how it is when cds skip in old portable cd players) Is this because I have a dual core?
- 4) Is there some program out there that can make my dual core cpu temporarily run on only one core? Flea110 01:16, 3 March 2006 (UTC)
- I don't know all of the answers to your questions, but (1) according to AMD, Windows XP has dual core support; you shouldn't need to do anything special. If you go into Task Manager and look at your CPU usage, you'll see two CPU graphs. (2) It depends on what sort of games you're playing. If they're old DOS games, they were probably designed to run on machines orders of magnitude slower. Look into using something like DOSBox, if that's the case. I don't know about (3), but (4) according to some dude on HardOCP forums, you can disable one core if you add an option with the /onecpu option in boot.ini. If your skippy-media problems go away, then they were probably due to the use of two cores. grendel|khan 15:33, 3 March 2006 (UTC)
- Re your skippy music and games, can you try the same media on a different machine? A different media player? Try to isolate the conditions under which it happens and give some more information. For great justice. 18:14, 3 March 2006 (UTC)
- Re (4), yes, there is (and probably are) programs that support what you are looking for. A slight variation of what you're describing is called CPU Affinity - locking a process to a particular CPU, after so many associations you can achieve dedication of one CPU (core) to a single process. You would have to lock processes to a particular CPU one at a time and eventually you could achieve this dedicated situation such that only 1 process had affinity to 1 cpu, all others to the other (or any number of other cpus). The only program that I know of is called TaskInfo, if my memory serves. I think this feature was available in 'TaskInfo 2000' and may still be in the latest version. You could only confirm that this was occuring by monitoring Windows Taskmanager once you had assigned all of the running processes. Even then it may be challenging to really know for certain. 151.199.150.176 16:35, 4 March 2006 (UTC)
Games running too fast are a result of poor programming. Specifically, display updates should have a wait statement in them (that causes a delay) to make the action proceed at a reasonable pace. Games designed to run on very slow computers may have been too slow already, so the programmers didn't feel the need to include wait statements. It would be relatively easy for a programmer to add these statements to most games, when updating them for new hardware. Another problem is if a form of wait based on CPU cycles is used instead of one based on real time. StuRat 02:52, 10 March 2006 (UTC)
astronomy
[edit]In an elliptical orbit,what happens to the distance between the planet and the sun?
- As compared to? When what happens? Your question is a bit unclear. --Obli (Talk)? 11:18, 3 March 2006 (UTC)
- I think the questioner means how it changes over the course of one orbital period. But since it sounds suspiciously like homework (apols if I am just being hyper-cynical), I'm just going to point 'em at Planetary orbit. --Bth 11:29, 3 March 2006 (UTC)
Cells
[edit]Why are cells usually small? Ana
- The cytoskeleton of a cell is an important, complex, and dynamic cell component made up of microfilaments. It acts to organize and maintain the cell's shape; anchors organelles in place; helps during endocytosis, the uptake of external materials by a cell; and moves parts of the cell in processes of growth and motility. Diffusion does not limit cell size. According to Biology Cell biology Introduction Cell Size which shows cell sizes range from "150-250 nm small bacteria such as Mycoplasma" to "1 mm Diameter of the squid giant nerve cell" the limits to cell size are due to "Prokaryotes - Limited by efficient metabolism Animal Cells (Eukaryotic) - Limited by Surface Area to Volume ratio Plant Cells (Eukaryotic) - Have large sizes due to large central vacuole which is responsible for their growth". Cells have lenghs of meters in some cases, like nerve cells. Multicellualr organisms have out-evolved single cell organisms in the the competive space defined by "large size" because modular structures are a better design. Even things humans build tend to be modular due to the various superiorities of modular over unitary. WAS 4.250 12:57, 3 March 2006 (UTC)
Diffusion is slow. A large spherical cell would not be able to use diffusion to get small molecules into all parts of the cell. Look up Fick's Law of diffusion for more details on the math. David D. (Talk) 08:38, 3 March 2006 (UTC)
- I wanted to say "Define small. Compared to what?" But are you now saying that they couldn't be any bigger because diffusion places a limit? In that case, I'd change the question to "Why are cells so big?" If being smaller makes diffusion easier and still they are as big as that permits, then they must have a reason for being so big. DirkvdM 10:44, 3 March 2006 (UTC)
- I'd like to rephrase the question to "why aren't cells much larger than they are?"
- It's a question of surface area, David D. touched the subject by talking about diffusion. Osmosis (diffusion through a plasma membrane) is not instant, and only a certain amount of osmosis can take place at a give point at the plasma membrane, therefore the area of the plasma membrane limits the volume of the cell, usually to something really small by our standards. Think of the membrane as transport routes in and out of a city, a cell the size of your fist would then be like New York city, with one dirt road connecting it, it just won't work. This problem is remedied in some cells, such as the inner lining of the small intestine by the use of Microvilli.
- I hope I answered your questions, you are welcome to drop me a note on my talk page if you need further clarification. --Obli (Talk)? 11:10, 3 March 2006 (UTC)
ova are often pretty big. Gdr 21:57, 3 March 2006 (UTC)
URGENT! - AutoText in MS Word
[edit]Hey I was using MS Word and would like to insert automatic pagenumber, and that never seems to work. I used "Insert", then "AutoText", "Headers and Footers", and selected "-PAGE-", and the only thing that appeared on the screen is "-1-", and never continued (on the second page, there is no "-2-"). How can that be accomplished?
Also, I accidentally deleted the "-PAGE-" AutoText, how can I restore it? I added one in AutoText Settings "-PAGE-", and when I insert it, it is firstly cannot be found in the headers and footers category, and also, turns out to be "-PAGE-" instead of the expected "-1-". Very urgent! Thanks!
Just Love Science
- Can't you just use "Insert", then "Page numbers" to insert page numbers? - Mgm|(talk) 10:32, 3 March 2006 (UTC)
- I've just had a play with my copy of Word and I think you need to make sure you're in the Header/Footer view. (View->Header and Footer) before you do the insert. The "Header/Footer" section under the Autotext menu is just a collection of things that are useful in headers and footers, it doesn't automatically put it into the footer -- when I followed your description of what you'd done I just got a "-1-" at the location of the cursor. It's only when the cursor is in the footer that it'll propagate properly. HTH. HAND. --Bth 11:18, 3 March 2006 (UTC)
- As to recreating the deleted autotext entry, you need Fields. Ctrl-F9 will bring up a pair of grey-background curly braces {}. Type "PAGE" in between them and you'll have a page number variable. Select it (and any stuff you want to put round it) and save it as a new bit of autotext (Insert->Autotext->New) and you'll have something that should do the job. It won't work quite as neatly as the original -- you may have to switch to print preview or a different view before it kicks in. See site for more detail than you might want. --Bth 13:08, 3 March 2006 (UTC)
- It's possible that you have set it so that the first page has a "different" header and footer than the other pages. This setting should be in document properties or page setup. Skomae 04:14, 4 March 2006 (UTC)
Sharp weapons vs. blunt weapons
[edit]Regarding wounds in pre-modern times, I have two conflicting sources. One tells me that sharp weapons are more lethal because while a broken bone could be set, a cut from a sword might lead to infection, which was a major headache before people learnt to disinfect things. The other source says that broken bones from blunt weapons are more dangerous, because they were difficult at best to treat. Which is correct? 219.93.29.135 11:51, 3 March 2006 (UTC)
- Both statements seem to be correct. A sharp sword will penetrate further and cause more damage to internal organs. A big blunt rock will break more bones. Either can be lethal, depending where they strike and with how much force. --Shantavira 12:28, 3 March 2006 (UTC)
- It depends on the exact nature of the weaponery, the defensive gear, the soldier training, and the battle tactics used. Polybius in The Rise of the Roman Empire recounts a battle between Romans with pointy blades fighting against Gauls/Celts who were armed with sharp edges but non-pointy blades and the Roman tactic was to fight so closely packed that the Gauls couldn't swing their weapons, while the Romans were able to thrust theirs. Romans won. WAS 4.250 13:10, 3 March 2006 (UTC)
- "The Romans are thought to have shown uncommon skill in this battle; the Tribunes instructing the troops how they were to conduct themselves both collectively and individually. They had learned from former engagements that Gallic tribes were always most formidable at the first onslaught, before their courage was at all damped by a check; and that the swords with which they were furnished, as I have mentioned before, could only give one downward cut with any effect, but that after this the edges got so turned and the blade so bent, that unless they had time to straighten them with their foot against the ground, they could not deliver a second blow. The Tribunes accordingly gave out the spears of the Triarii, who are the last of the three ranks, to the first ranks, or Hastati: and ordering the men to use their swords only, after their spears were done with, they charged the Celts full in front. When the Celts had rendered their swords useless by the first blows delivered on the spears, the Romans close with them, and rendered them quite helpless, by preventing them from raising their hands to strike with their swords, which is their peculiar and only stroke, because their blade has no point. The Romans, on the contrary, having excellent points to their swords, used them not to cut but to thrust: and by thus repeatedly hitting the breasts and faces of the enemy, they eventually killed the greater number of them. And this was due to the foresight of the Tribunes: for the Consul Flaminius is thought to have made a strategic mistake in his arrangements for this battle. By drawing up his men along the very brink of the river, he rendered impossible a manœuvre characteristic of Roman tactics, because he left the lines no room for their deliberate retrograde movements; for if, in the course of the battle, the men had been forced ever so little from their ground, they would have been obliged by this blunder of their leader to throw themselves into the river. However, the valour of the soldiers secured them a brilliant victory, as I have said, and they returned to Rome with abundance of booty of every kind, and of trophies stripped from the enemy." [11] WAS 4.250 13:23, 3 March 2006 (UTC)
- Hmm... more complicated than I originally imagined :) Thanks for all your informative replies. 219.93.29.135 05:47, 4 March 2006 (UTC)
if science is about growing and learning
[edit]then my is the MSM so opposed to people who offer new and better theories? Isn't it true that so called "scineces" are much more of a dogma than anything else, yes, some things may be true, but isn't most sceince usually looked back on as stupid after the fact? Whose to say what's a valid scientific theory and what isn't? Isn't the fact that mainstream scinece won't even investigate certian things because they're so steeped in secularist ideolgies, a sign that scinece is, for lack of a better word "broken"?--Name2354325` 14:17, 3 March 2006 (UTC)
- Science is not about growing and learning, it is about performing experiments and doing things that produce results (going to the moon, blowing up cities, fighting H5N1, finding oil in the ground, etc). Science is not a dogma of any kind, it's just "whatever works". Most science is usually looked back on as what worked then, but now we have other additional things that work. "A valid scientific theory" is what works (produces results). Mainstream science investigates everything; it is not broken. Science even has developed to the point that we know what works about theories about what works! The scientific method is all about that. WAS 4.250 14:36, 3 March 2006 (UTC)
- The "mainstream media" (MSM) on the other hand includes whatever media outlets are in harmony with the prevailing direction of influence in the culture at large. In the United States, usage of these terms often depends on the connotations the speaker wants to invoke. The term "corporate media" is often used by leftist media critics to imply that the mainstream media is itself composed of large multinational corporations, and promotes those interests (see e.g., Fairness and Accuracy in Reporting; Noam Chomsky's "propaganda model"). This is countered by right-wingers with the term "MSM", the acronym implying that the majority of mass media sources are dominated by leftist powers which are furthering their own agenda (see Conspiracy theory, Media bias in the United States). WAS 4.250 15:20, 3 March 2006 (UTC)
- I completely agree with the question. I believe that everything science tells is fact - all we see and feel - is just a persistent imagination of a large purple goat. We are in a goat's dream. Some have talked to the goat and called it God. That bothers him and terrible things happen. So, it is our job to be quiet and let the goat sleep soundly. However, no matter how often I send this to all the mainstream media outlets, they never call me in for an interview. They are just secularist mouthpieces for the science fools. --Kainaw (talk) 15:26, 3 March 2006 (UTC)
Who's opposed to "new and better theories"? The media loves new theories; they don't even have to be better! Claim to invent a perpetual motion machine, hold a press conference, and you'll get written up everywhere. You'll even get written up on slashdot, even though they ought to know better.
Science, on the other hand, does tend to insist that new theories actually be better. That may end up looking like dogma, and sometimes there is a certain amount of dogma, but mostly, what's operating is that science demands things like proof and reproducability, and those take time. —Steve Summit (talk) 18:21, 3 March 2006 (UTC)
It should also be noted that historically it has been difficult for new scientific theories to be immediately accepted (for example, ask Galileo). This has improved in recent years a bit, since scientists are less focused on religion and more focused on proving the facts and reproducibility, but it still often takes new theories a relatively long time to gain acceptance, as old theories are so ingrained into our heads as truth. This isn't necessarily a bad thing, as it keeps us from believing every hoax that comes along, but I can see how it would be frustrating to individuals who don't always subscribe to mainstream theories. EWS23 | (Leave me a message!) 19:11, 3 March 2006 (UTC)
- They've hit the high points, so I won't repeat them; how the 'media' is an unreliable way to judge science; how science is only as dogmatic as necessary to prevent hoaxes and mistakes; the fact that most things (electricity, the chemistry that created plastic, sperm) will never be looked back on as stupid; the fact that science's judge is the harsh realities of the natural world; the fact that science investigates more things than you will ever hear of or imagine, and investigates them well... no, I'd like to respond to the message itself. If you ever come back to see the answers to your question, I'd like to ask you a few things, for my own edification:
- Is your post as indignant as it sounds? Because it sounds like a pet theory of yours (probably religious, based on the references to dogma and secularism) is getting what it deserves, and you're pissed off.
- Were you just talking, or were you hoping to learn, or were you hoping we'd see the light?
- Do you have any idea what secular and ideology mean? Do you know what buzzword means?
- What specific things are you thinking of when you talk about stupid past theories? I ask because it's always fun to hear about a new one. Phlogiston is a classic in that area.
- Similarly, what specific things are you thinking of when you talk about things science won't investigate? The paranormal? The existence of Jesus? The existence of Jesus's evil twin brother, Billy? The existence of Juan and Cindy? Black Carrot 00:39, 4 March 2006 (UTC)
- Good questions. I wonder if we'll get any answers, any more than any of the previous N times questions like this have been asked on the various Reference Desks. —Steve Summit (talk) 15:03, 4 March 2006 (UTC)
- There are many texts about whether science is inherently dogmatic or not, whether it is conservative or daring, etc. etc. There's no easy answer to it -- scientists will always claim that they are not dogmatic (unless they feel "outside" the mainstream), while historians, sociologists, and philosophers go back and forth over it depending who you are reading. A good starter into thinking about these sorts of things is Thomas Kuhn's The Structure of Scientific Revolutions. If you are feeling very daring you can try tracking down Pierre Bourdieu's "The Specificity of the Scientific Field and the Social Conditions of the Progress of Reason", which I find to be a bit more to-the-point that Kuhn (and discusses Kuhn quite a bit). There's a copy of it, I think, in The Science Studies Reader, ed. Mario Biagioli. You could also check out Paul Feyerabend's work, which by its titles you can judge is a bit more radical: Against Method and Farewell to Reason. --Fastfission 23:24, 5 March 2006 (UTC)
Sound in water
[edit]When we put our hands inside water and clap we don't hear the sound. Why is this so?
- Because you didn't clap hard enough for the anything to vibrate. Hit the side of the pool with a rock and vibrations will be created that you can hear even underwater. Sound is your perception of vibration that gets to your ears. WAS 4.250 14:43, 3 March 2006 (UTC)
- Because sound waves, at least those in the range audible to human beings, tend to propagate in air, as opposed to water--152.163.100.74 14:29, 3 March 2006 (UTC)
- No, audible sound waves can propagate through water just as well. As WAS 4.250 said, you'd have to clap much harder in water to make an audible sound, because the water is more dense so it's harder to push out of the way. Also, the inside of your ear is filled with air, so the difference in density between the two fluids makes a kind of filter, muffling the sounds. —Keenan Pepper 14:53, 3 March 2006 (UTC)
- Oh, oh, we are getting silly here. Sound waves are perfectly happy in water, the dolphins carry out excellent conversations about silly humans. But you can't get anything to 'clap' underwater because of the viscosity of the thin film of water. --Zeizmic 16:03, 3 March 2006 (UTC)
Scuba divers have developed a number of ways of using sound to communicate under water too - the most common is to band on the air tank with something metal, which produces a sound that travels well (although it is more difficult to locate direction underwater using sound, I don't really know why). More complex systems use pitch shift devices, or wireless radio comms to circumvent difficulties of propogation in water. For great justice. 19:29, 3 March 2006 (UTC)
- Sound does travel really well underwater, and it turns out that's why it's so hard to localize. One way we localize sounds is by (unconsciously) measuring the difference in arrival times between one ear and the other. But since sound travels faster in water, the differences become too quick for our land-evolved brains to measure. —Steve Summit (talk) 20:31, 3 March 2006 (UTC)
- There's a type of lobster or something similar that catches its prey by snapping a claw so fast that it produces a shockwave that stuns (or even kills?) the prey. I don't know if this is audible, but it should be. Because of the viscosity of water clapping hands are slowed down too much. The claws cut through the water more easily. I suspect there will be a cavity in the claw where the water is compressed and then jets out. If this is fast enough it should qualify as sound. (So this is a very Zen creature that can clap with one hand (well, claw).) I'm not sure here, but I suppose most sound is a vibration but it can also be one single shockwave, as with clapping hands.
- A related question: can one snap one's fingers under water? Probably not. DirkvdM 13:42, 4 March 2006 (UTC)
- Actually, yes, you can. A good 'finger snapper' can produce a light sound underwater. Try holding your hand out in front of you and then next to your ear while snapping. Then do it out of water. The volume is greater out of water right next to your ear, and in water it's muffled in both instances and the volume doesn't change much. A good example of the characteristics of sound waves traveling through water vs. air. 151.199.150.176 16:48, 4 March 2006 (UTC)
I think sound travels fine in both air and water, but doesn't pass through the surface from one to another very well. That is, you need a very loud underwater sound to hear it in the air above or a very loud air sound to hear it underwater. StuRat 03:08, 10 March 2006 (UTC)
midwinter
[edit]It's very cold in Birmingham. Why is it that the coldest and hottest weather usually occurs a good two months after the winter and summer solstices, at least in the UK? I'd expect there to be some time lag with the earth/sea having to heat up and cool down, but two months seems a lot. Is this the norm in other parts of the world? --Shantavira 14:32, 3 March 2006 (UTC)
- Yep, this is normal. Where I'm from (Texas) the hottest month is typically August, a good 2 months after the summer solstice. As you suspected this is due to time lag, often referred to as seasonal lag (a page I should probably do some work on). According to this page, the typical lag is about one month for continental areas and about two months for areas near large bodies of water (such as Birmingham and Texas). EWS23 | (Leave me a message!) 19:23, 3 March 2006 (UTC)
- In North America people are taught that the winter solstice is the "official first day of winter" and the spring equinox is the "official last day of winter"; some people in Britain also use this terminology (although of course the solstice is also called "midwinter's day" there). The fact that people generally don't see anything wrong with this terminology shows that people find it natural for temperature changes to lag by something like a month and a half behind the changes in the amount of daylight.
- Remember that during the period near the solstices, the amount of daylight isn't changing very fast. At latitude 45°N, from December 1 to December 21 the reduction in daylight is only about 16 minutes out of 9 hours, and similarly on the other side of the solstice. The continued short days (and the low-angle sunlight that goes along with them) keep the temperature dropping. Of course if the days remained like that permanently then an equilibrium would be reached, but it does take a while.
- --Anonymous, 02:38 UTC, March 4, 2006.
What is Einsteins Theory of Relativity?
[edit]What is Einsteins Theory of Relativity and how do you use it?
What is a black hole?
[edit]What is a black hole (space) and how does it work?
What is the difference between black hole and wormhole in space?
[edit]What is the difference between a black hole and a wormhole in space?
- For one thing, black holes are probably real and wormholes are still only theoretical (at best). For another, if you get sucked into a black hole you'll just get streched out and then crushed into a point. See Spaghettification. —Keenan Pepper 14:55, 3 March 2006 (UTC)
- A black hole is like a huge vacuum cleaner sucking up everything. A wormhole is like a cool subway tunnel between two locations. --Kainaw (talk) 14:59, 3 March 2006 (UTC)
- A wormhole connects two parts of the universe, sort of a shortcut. One way this might exist is as a combination of a black hole and a white hole. See Wormhole#Schwarzschild_wormholes. DirkvdM 13:51, 4 March 2006 (UTC)
transmission
[edit]hi i would like to know 1.why cant we run a vehicle in 2nd or higher gear shifts directly.
2.problems in using diesel in petrol engine and vice virsa
- You can start in second and possibly third if you give it enough gas. Gears are used to optimize the horsepower of the engine. As for diesel and petrol (we call it just "gas" in the U.S.), they burn at different rates. Engines are very particular about timing. Put diesel in a non-diesel engine and it will start, but it will make a knocking sound and you won't get much power out of it. I've never put gas in a diesel engine. I figure it is like a 2-stroke. It will start and then die quickly. --Kainaw (talk) 14:57, 3 March 2006 (UTC)
I had a friend, who's daughter put in diesel unaware. Naturally it went nuts and took it to a mechanic who couldn't figure out what was wrong, and they spent a fortune. I think they even replaced the engine. Finally took it to a second mechanic who had a sniff of the gas tank and immediately knew what happened. --Zeizmic 15:38, 3 March 2006 (UTC)
- Diesel engines and gas engines work in very different manners. Gas/petrol uses a spark and a relatively flammable fuel while diesel use an less flammable fuel (almost non-flammable) and high compression with a glow plug to ignite the fuel. One will certainly not work in the other - diesel may gum up a gas engine and gas may burn up a diesel. See aslo the "Differences in fuel dispensers" in the Filling station article. Rmhermen 17:09, 3 March 2006 (UTC)
- The problem is octane, diesel is much much lower in octane, and much more 'energetic' per volume than typical gasoline (this is my second hand understanding, i am not a chemical engineer). In a normal gasoline engine, diesel fuel will knock due to compression, and the engine will produce very little power and may even become damaged if the operator persists in trying to run it. As for the story about someone doing it on accident, it takes an INCREDIBLY thick-skulled person to do this. Why? The pumps are different, a (larger) diesel nozzle will not fit into the fill hole on a standard gasoline vehicle, which is usually enough of an indication to the operator that something isn't right, since trying to fill it will result in it spilling everywhere.
- The pumps here (South Carolina) and back home (Missouri) are the same size for gas and deisel. So, it is very easy to put deisel in a normal engine. The larger pump was for leaded gasoline back when unleaded gasoline was introduced. --Kainaw (talk) 19:14, 3 March 2006 (UTC)
- All the ones i've seen in OH are sized this way, it's a small difference but it's enough to stop someone from doing something stupid, I know this first hand after watching my friend try it. I just figured this was standard practice as all major brands seem to do it.
- Not literally lower in octane (although probably) - Octane rating is a measurement system for gasoline; for diesel, it is rated by the cetane rating. Rmhermen 00:49, 4 March 2006 (UTC)
- In the US there are petrols with an octane level under 90% (as low as 86% even, I believe). I once put a bit of that in an almost empty tank. The car started making very scary clonking noises, so I quickly filled the tank up with a high quality petrol. Why do such petrols exist in the US? Are there engines that can run well on it? Or is it meant for old cars that are just months away from the scrap heap? DirkvdM 13:59, 4 March 2006 (UTC)
- Octane is not measured in percent - and it doesn't measure the amount of octane either. Most cars work fine on 86 octane, although 87-88 is the most common in the U.S. - but note that this same fuel is labeled as 91 octane in Europe due to a difference in measurement definitions. This may be part of what you are noticing. Rmhermen 15:58, 4 March 2006 (UTC)
- In the US there are petrols with an octane level under 90% (as low as 86% even, I believe). I once put a bit of that in an almost empty tank. The car started making very scary clonking noises, so I quickly filled the tank up with a high quality petrol. Why do such petrols exist in the US? Are there engines that can run well on it? Or is it meant for old cars that are just months away from the scrap heap? DirkvdM 13:59, 4 March 2006 (UTC)
- Ah, that's something I had been wondering about for 10 years now. Given that the octane ratings in Europe are usually in the high nineties, 86 sounded ridiculously low. Could we get all our units standardised please (before another Mars lander crashes)? DirkvdM 10:29, 5 March 2006 (UTC)
- I've seen 85 octane in the US, measured with the (R+M)/2 method, but agree that 87 is the typical minimum. I always use the cheapest gas available and have never had any trouble with engine knocking. Perhaps additives used in the US make this less likely. StuRat 03:13, 10 March 2006 (UTC)
engine
[edit]1. what is the significance of inclination of engine in bikes?
- On an inline motor, or a V?
- Uh... See V engine, straight engine and inline engine? ☢ Ҡi∊ff⌇↯ 18:05, 3 March 2006 (UTC)
- Motorcycles are typically designed to have the lowest possible center of gravity, especially those used for sport riding and racing. This is to allow the bike to turn quicker with less lean angle. To accomplish this, the motor is typically inclined as much as possible, to bring it lower to the ground given that the motor/transmission assembly are the heaviest things on the bike, and by inclining the motor and keeping the transmission in the same spot the overall center of gravity will become lower. --66.195.232.121 19:37, 3 March 2006 (UTC)
- Uh... See V engine, straight engine and inline engine? ☢ Ҡi∊ff⌇↯ 18:05, 3 March 2006 (UTC)
brakes
[edit]why front brakes are stronger than rear in bikes
- The same reason they are on cars. When stopping, the force causes the suspension to compress, because the force vector expressed as the relationship between gravity and acceleration causes the realized weight to shift forward. This means much more downward force is on the front tire. For the exact same reason, bikes and cars (preferrably) power themselves via the rear wheel, the forces involved make it the most ideal wheel for the task.
- Erm, re rear wheel drive, it's a little more complicated than that for cars... For great justice. 19:33, 3 March 2006 (UTC)
- We have pretty good articles on front wheel drive and rear wheel drive. — QuantumEleven | (talk) 12:37, 6 March 2006 (UTC)
Is this monkey a Drill?
[edit]Is this monkey a Drill? I think it is, but I'm not quite certain; I didn't mark down the sign at the zoo at the time. grendel|khan 15:22, 3 March 2006 (UTC)
- I doubt it, the facial features suggests it's a prettier monkey than the Drill (lack of mane, no bald forehead) --Obli (Talk)? 16:54, 3 March 2006 (UTC)
- Maybe Allen's swamp monkey? I think you took the image at Chicago's Lincoln Park Zoo. Here is a list of [mammals] they have. You can google for images of each species. Rmhermen 17:01, 3 March 2006 (UTC)
- Our image in the swamp monkey article doesn't look much like the one on this page though. Rmhermen 17:04, 3 March 2006 (UTC)
- Thanks for the link; that helps tremendously. It looks a bit like a De Brazza's Monkey, I think, but the white beard isn't nearly as pronounced, and it doesn't have the weird brow ridge. Maybe an Allen's Swamp Monkey? That looks pretty similar. grendel|khan 21:38, 3 March 2006 (UTC)
No monkeying around here, THIS IS NOT A DRILL! (sorry, couldn't resist that one.) DirkvdM 14:04, 4 March 2006 (UTC)
It's not a De Brazza's Monkey. If you took it at the Chicago Zoo, then it is certainly Allen's Swamp Monkey. It's much better than the image we have on the article, so please feel free to upload it (to commons.wikipedia.org) and change the image in the taxobox. - UtherSRG (talk) 15:26, 10 April 2006 (UTC)
Right click on words in web textbox
[edit]I'd like to make a website which includes a textbox that users can write in. When the users rightclick on any word in the text box, I want the usual right-click pop-up menu to be replaced by one that allows them to, say, make the text bold or add a link. Is this possible? I know a little JavaScript. Thanks in advance!
- Yes it is. Celcius 02:28, 10 March 2006 (UTC)
Text box that doesn't look like a text box
[edit]How would I be able to make a text box that doesn't look like your standard text box? For instance, I could have a thin line around it, instead of the "indented" look of most text boxes (like this one), or maybe have curved corners. Also, could the font be changed, so it's not courier? Thanks in advance (again)!
- Take a look at cascading style sheets. --Kainaw (talk) 19:10, 3 March 2006 (UTC)
Li-Ion Batteries (specifically for iPod)
[edit]Hey. I have a few questions about iPod battery life. I have a 5G iPod (which I got 2 days ago).
- Is it better to keep the iPod on the charger all the time when not using it, or to take it off when it's charged (even though i'm not using it atm)?
- Apple reccomends not charging inside a case, however my case has little holes around the corners that allow the iPod to breathe. Also, it's been on the charger and in the case for at least 12 hours and is still cool to the touch on the outside. On the other hand, the case is made of leather. I am paranoid of leaving it out of its case though because i have a REALLY dusty house. What should I do?
— Ilyanep (Talk) 18:41, 3 March 2006 (UTC)
- iPod batteries last an optimal amount of time if they maintain a 40% charge. So take it off once its charged. And yeah, I know you are paranoid (I was too) but it is better to charge without the case. They do warm during charging, and its best if the heat can dissapate. pschemp | talk 00:54, 4 March 2006 (UTC)
But I can't stand taking it out :P It gets completely covered in dust within 10 seconds :( Oh well. Thanks for the website — Ilyanep (Talk) 01:34, 4 March 2006 (UTC)
- I've never understood this...why is it important to keep dust off the iPod? Isn't it completely closed? And isn't it meant to be used, ie, scratched and dropped? Isn't it designed to be robust against such things? --HappyCamper 14:16, 7 March 2006 (UTC)
Can viruses invade a host and creat a zombie?
[edit]Yes. HHV Latency Associated Transcript. WAS 4.250 20:50, 3 March 2006 (UTC)
Viruses invade a host by attaching to a cell and injecting DNA or RND into the cell. This DNA/RND info tells the cell to stop making the product it was and to start making protein coat and viral genetic info. This however will eventually kill the host.
But what would happed if a virus attached to a cell and injected DNA or RND into the cell which did not coda for making protein coat and viral genetic info, instead, made the cell duplicate its self with the new DNA/RND being replicated too. Eventually (7 years or so) the entire body would be a cross breed of the virus and a human… a Zombie! Many problems of reality must be over come such as the inability of the Brain cells to divide … yet this may be untrue as brain tumours exist.
My question is could this be a reality and has anything like it ever been reported?
- Um, so you're asking what would happen if a tiny fraction of a viral genome were replicated by the cell and unintentionally incorporated into the host genome?? See Junk DNA for ref, about 60% or so of the human genome doesn't code for anything at all, just tiny bits of viral DNA that replicate themselves, and resplice over and over again, and ultimatly don't code for anything, non-coding DNA, over a few million years these tend to pile up, and it's kind of neat, because if you examine the genomes of several distantly related species, you can find paterns of non-coding DNA vs coding DNA, and you can see where they diverged, but no, no zombies--64.12.116.74 20:23, 3 March 2006 (UTC)
- Ok, I just read the articles on Junk DNA and non-coding DNA, and it's prompted me to ask a sub-question:
- Why is it that...
- ...all the science related articles on wikipedia contain creationist counter arguments against whatever the article is about?--64.12.116.74 20:48, 3 March 2006 (UTC)
- Because Wikipedia is a Wiki. What did you expect when everyone is allowed to edit something? I'm more concerned with why every page about pop culture includes a list of where it is parodied in The Simpsons. DJ Clayworth 22:51, 7 March 2006 (UTC)
- It actually would happen much faster, occuring in 3-4 hours or so. See Shaun of the Dead for more information. --Sam Pointon United FC 18:47, 3 March 2006 (UTC)
- No, a virus could not cause a person to become a zombie. Because viruses are so small, they can contain very little genetic information and code only for a few different proteins. There is no way for them to control a person's movements or do anything like you see on TV. I virus especially, cannot re-animate someone who is already dead.
- Making cells divide (for which they must be alive to begin with) does not create zombies. If they divide frequently enough, out of control, it creates a cancer.
- Furthermore, only certain cells can replicate to form other cells and there is not ONE cell in you body that can replicate and form any differrent type of cell. Some cells, [[stem cells], can be induced to form many different types of cell, but not ANY type of cell. This of course means that you have many different types of stem cell in your body.
- Also, you mean RNA, not RND... --Username132 19:42, 3 March 2006 (UTC)
- Not true, if I was a virus, I'd want to do some serious Research and Development before turning anyone into a zombie!--64.12.116.74 20:23, 3 March 2006 (UTC)
- Hahaha. Kudos. — Ilyanep (Talk) 20:46, 3 March 2006 (UTC)
- Not true, if I was a virus, I'd want to do some serious Research and Development before turning anyone into a zombie!--64.12.116.74 20:23, 3 March 2006 (UTC)
As mentioned above, it is likely that every cell in your body has bits of DNA from various viruses, and you will pass this amalgam of genetic material on to your children. In fact, it has even been theorized (but not widely accepted by any means) that the eukaryotic cell nucleus arose through incorporation of a DNA virus (a la the endosymbiotic theory). Under most circumstances, neurons cannot divide. Tumors in the brain are due to unrestrained growth of the “helper” cells such as astrocytes or glial cells, not neurons. — Knowledge Seeker দ 02:00, 4 March 2006 (UTC)
A certain virus(I think tobacco mosaic virus), does what is described above in plants. It can actually bring dead cells back to life by inserting DNA that codes for plant proteins . This can help the virus reproduce itself. However there haven't been any documented cases of this happening in humans.
Long-term Effects of Medication for Attention-Deficit (Stimulants)
[edit]I have searched and come across very little about what science knows about the longe-range effects of stimulant medications used to treat Attention Deficit Disorder, such as Adderall and Ritalin. Is it fair to thus compare the changes amphetamine (which Adderall is, I believe) and cocaine create in the brain to such medications? The studies I read were linked to in the Wiki ADHD article; yet they were only two and themselves admitted further research is necessary. Are such long-term studies being done? - C
- Probably. I might be able to hook you up if you can be more specific about what studies you're after (how about; "Review of ritalin (methylphenidate) medication use in children, in British Columbia, Canada"?). What level of study are you at? I've got access to a lot of journal articles, but you have to be well versed in the area to understand them. What year were the studies that you read conducted? --Username132 19:53, 3 March 2006 (UTC)
Anything relating to evidence for/against/concerning permanent changes in the brain as a result of amphetamine or methylphenidate, as prescribed for Attention Deficit. I have no medical training to speak of. The studies were both from 2005, one June, the other November. I confess only a vague awareness of what they are discussing. They can be found here and here. - C
occuring in 3-4 hours or so? What ?
[edit]occuring in 3-4 hours or so? What ?
- Come again? --Obli (Talk)? 18:59, 3 March 2006 (UTC)
- In 3-4 hours from now, I'll be ending my workday, headed out to run some errands, maybe stop for a beer. --LarryMac 19:05, 3 March 2006 (UTC)
- Hopefully in 3-4 hours I'll be done with homework. — Ilyanep (Talk) 19:13, 3 March 2006 (UTC)
- I think this is referring to the virus/zombie question above. — Ilyanep (Talk) 19:26, 3 March 2006 (UTC)
Doesn't this refer to the zombie virus question above? For great justice. 19:34, 3 March 2006 (UTC)
Gues what? I think this refers to the zombie virus question above. DirkvdM 14:19, 4 March 2006 (UTC)
Are you sure it refers to the zombie virus question above? Kilo-Lima Vous pouvez parler 14:43, 5 March 2006 (UTC)
As one who has been coughing for about a week now, and is just now starting to feel better, what component of phlegm makes it green? User:Zoe|(talk) 19:57, 3 March 2006 (UTC)
- I could have gone all day without reading that... --Username132 20:17, 3 March 2006 (UTC)
- I know that green phlegm is a sign of bacterial infection, but I couldn't tell you precisely why--might be that the bacterial mass itself is green. grendel|khan 21:53, 3 March 2006 (UTC)
- Yes, but what pigment is responsible? It couldn't be chlorophyll, could it? —Keenan Pepper 23:06, 3 March 2006 (UTC)
- Thanks, Keenan, that was really what I was trying to ask. Is there any copper involved? User:Zoe|(talk) 23:32, 3 March 2006 (UTC)
- Yes, but what pigment is responsible? It couldn't be chlorophyll, could it? —Keenan Pepper 23:06, 3 March 2006 (UTC)
- I doubt there would be copper, but I'm no biologist or doctor. I think of Cu2+ ions, which are blue. I suppose it would look green if some copper compounds were somehow mixed in with some yellow pigments in the phlegm, but I really have no idea. --HappyCamper 13:31, 4 March 2006 (UTC)
- Iron, not copper. Myeloperoxidase is the culprit, which has a ferric (Fe(II)) heme iron, which makes it green. --BluePlatypus 13:54, 4 March 2006 (UTC)
- <phew> Thank God it is iron! --HappyCamper 13:56, 4 March 2006 (UTC)
- Wow. Thanks! I'd really been wondering about that. Color me impressed. And green. grendel|khan 14:49, 4 March 2006 (UTC)
- Awesome! Thanks, Blue. User:Zoe|(talk) 19:43, 4 March 2006 (UTC)
Titanium dioxide as a sunscreen in cosmetics
[edit]I know that titanium dioxide is a sunscreen and is currently used as such in products. I make my own lip balm and recently some people who like to use my product asked if I could make it with sunscreen properties. I have titanium doixed in powder form, but as stated in its fact sheet, it is insoluable. How do go about using titanium dioxed in my lip balm so that it will act as a sunscreen without the product feeling gritty? Thanks for your help. Debbie Bliss
- In suntan creams it is a suspenion of tiny particles, not a solution. You need to grind the powder more finely if it feels gritty. For great justice. 22:18, 3 March 2006 (UTC)
- Titanium dioxide is in sunscreens because of how immensely white it is, in fact either zinc or titanium di/oxide is the whitest thing known to man. Black as you probably know is hotter to wear on a sunny day than a bright color, because light is absorbed into black, whereas white reflects most of the light. Long and medium wave ultraviolet rays from the sun are what cause sunburn, more of these are reflected off with the addition of zinc or titanium di/oxide You're not going to be able to add the titanium in, unless you can make it finer than a dust, the kind of dust you see in the light floating around in the air. --
 Mac Davis] ☢ ญƛ. 04:31, 6 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 04:31, 6 March 2006 (UTC)
There seems to be another issue here: any lip balm with sufficient titanium dioxide to have sunscreen effects will be white. Do people really want white lips ? If not, perhaps you are considering angel dusting ? StuRat 03:29, 10 March 2006 (UTC)
March 4
[edit]Maple program
[edit]How does one factor polynomials in Maple?
- Have you tried using factor(whatever)? expand() and factor() should do what you need. grendel|khan 01:45, 4 March 2006 (UTC)
Moved from my user talk page. grendel|khan 14:55, 4 March 2006 (UTC)
Thanks, but how do I factor equations in the form ax+bx+c. It doesn't work out well in Maple.
- You mean ? Like quadratic equations? You should be able to enter, for instance, factor(x^2-5*x+6) and have it return to you . I've never actually used Maple; I Googled up an instructional page about it, which might be of more help to you. grendel|khan 14:55, 4 March 2006 (UTC)
- If you enter "factor(x^2+5*x+6);" then you will get the answer "(x+3)*(x+2)". However if you try to enter something that doesn't factorize then you will just get the original expression as the answer. If you don't get the answer I recommend asking it again here. DJ Clayworth 22:36, 7 March 2006 (UTC)
Detecting the "days-without-a-shower" smell
[edit]In a club meeting one night, when we were all asked to tell the club some of our greatest pet peeves, one of the leaders of the club said that hers was the "days-without-a-shower" smell, and that she could detect the difference between the "after-a-heavy-workout" smell, and the "days-without-a-shower" smell. (Apparently, a lot of smelly people she's encountered tried the "after-a-heavy-workout" excuse if their days-without-a-shower smell was brought to their attention.) Can most (if not all) girls detect a difference between the after-a-heavy-exercise smell and the days-without-a-shower smell, or can only some?
I read in a Maxim magazine a long time ago that a female's sense of smell is 100x (yes, one-hundred times) more sensitive than a male's. I do have my doubts, however. How much more sensitive is the female sense of smell really? Also, how much sooner can a female detect a negative odor than a male?
What hints would typical college girls give, directly or indirectly, that she's detecting this kind of smell from you? (Or otherwise in each other's vicinity, if not you?) I know that in Elementary, Middle, and sometimes High School, people were more direct about what odors they sensed. In college, we're more polite and tactful, so oftentimes hints are given so subtly we may not even decipher them when we need to.
Finally, when a guy who hasn't had a shower in a few days decides to apply deodorant more thoroughly (beyond the armpits; onto the torso, back, legs, arms, et al.) and spray himself some cologne, can it successfully "camouflage" the bad smell or can one still sense the odor "underneath" the deodorant and cologne? --Shultz III 00:39, 4 March 2006 (UTC)
- From personal experience, I've had the days-without-a-shower smell mistaken for the just-worked-out smell. Do you live on Arrakis? All this seems a bit much work to avoid washing. (Though I shouldn't really throw stones here.) The method I preferred for the thirty second scrub at work was to take a paper towel in the work restroom, wet it, throw some soap on it, scrub under the armpits and down the shorts. Worked quite well, much better than throwing on a ton of cheap cologne, which, trust me, even guys can detect. As for the women's sense of smell being better than men's, that sounds way fishy to me. If that were true, wine tasters and perfume testers would all be women, because their performance would be so superior to men's that they'd take over the trade. Which they haven't. grendel|khan 01:35, 4 March 2006 (UTC)
- I guess this heightened sense of smell only applies to some women, based on what you say about wine and perfume testing. --Shultz III 01:58, 4 March 2006 (UTC)
- According to http://news.bbc.co.uk/1/hi/health/1796447.stm, "A US study showed women of reproductive age are far better at identifying odours than men after repeated exposure to the source of the smell". Females were able to improve their sensitivity to the smell of benzaldehyde after smelling it multiple times, while males did not improve. This means that there is no significant difference in the sensitivity of males and females to smell, if the test subjects are only allowed to smell something for an instant.
- There was no significant gender differences in people older than 45 years. There was also no significant difference in "young" boys/girls. (I think "young" means younger than the reproductive age, i.e. before puberty begins.)
- Nobody can detect a bad smell "underneath" deodorant, if the deodorant is 100% effective. A person smells bad after a few days without a shower because of bacteria, and if a deodorant can kill all the bacteria, the smell will disappear. Cologne (Eau de Cologne?) is just a perfume, and perfumes do nothing but provide a pleasant smell, so it is possible to smell bad odour underneath cologne.
- Finally, for the question "Can most (if not all) girls detect a difference between the after-a-heavy-exercise smell and the days-without-a-shower smell, or can only some?" Obviously some people are more sensitive to smell than others. Those with anosmia cannot smell anything. My guess is that if most girls you know can detect a difference between a specific after-a-heavy-exercise smell and a specific days-without-a-shower smell, than most girls can detect that difference. --Bowlhover 02:17, 4 March 2006 (UTC)
- I have anosmia and it suck! I had to learn the hard way exactly how often you need to shower and how much deodorant to use without killing your houseplants. Ye, when you can't smell it's not immediately obvious that you can be dirty without looking dirty. In my now-paranoid state of mind I shower minimum once a day. As for people letting you know you stink - well, they don't. Trust me on this - even your best life long friends won't do it. They'll make fun of your receding harline, mismatching clothes and weight gains - but they'll certainly never risk hurting your feelings by commenting on your oder. Don't worry though, girls tend to let you know right before intimacy "Honey - could you... go disinfect yourself?" It's mostly a drag but it's not all bad. I never tasted another persons gassified feces for instance - I guess there's some bright spots to everything. Celcius 02:52, 10 March 2006 (UTC)
What material is best to make something leap over 20m?
[edit]What sort of material will work best for designing an A4 sized object to leap (or jump) over 20m from the ground? The object must not expel gas/liquid, and not require any other external devise to make it leap. Another thing is that all energy must come from the person supplying the force for the leap. Thank you for your help.
- Designing? Or making? A4 is a two-dimensional size, so how tall can it be?
Slumgum 01:48, 4 March 2006 (UTC) - I am not completely sure that this is the best material for this application, but the Titanium-Nickel alloy used in braces is exceptionally springy and generally only retains shapes that it is formed in when heated. Skomae 04:30, 4 March 2006 (UTC)
- How about a big rubber band? GangofOne 04:58, 4 March 2006 (UTC)
- Making an A4 sized object with a height limit of say 1m. Are springs and rubber bands the only materials possible? Thanks.
- Does the entire object need to get to 20m or can it propel an object into the air? Celcius 03:00, 10 March 2006 (UTC)
Affectedness
[edit]I was reading the Klinefelter's Syndrome article and noticed that one of the symptoms mentioned was "affectedness." I'm no doctor, but I'm pretty sure that "affectedness" is not a medical condition. Does anyone know more about this? --JianLi 01:04, 4 March 2006 (UTC)
- I think it's just "creative" writing. What they mean by "signs of affectedness"(!) is "symptom". JackofOz 01:41, 4 March 2006 (UTC)
- Oh, I see. --JianLi 02:47, 4 March 2006 (UTC)
Microphone Query
[edit]My microphone will make a low hum when recording unless I blow loudly into it. The hum is recorded as unchanging. Why does this happen? What can I do? Thanks. M@$+@ Ju ~ ♠ 01:42, 4 March 2006 (UTC)
- Are you near a fluorescent tube? Does the hum get louder or quieter if you move it closer or further from a piece of equipment? If someone is running a microwave or a fluorescent light (among other things), it can emit radio interference that the mic will pick up. grendel|khan 01:49, 4 March 2006 (UTC)
- Nah, it goes away only if I blow loudly into it, like overloading then it resets. M@$+@ Ju ~ ♠ 03:48, 4 March 2006 (UTC)
- A constant low hum is most likely (IME) to be either: (1) interference from mains electricity (50Hz/60Hz depending on what country you're in) which will be at its worst if your mic cable is running alongside mains power cabling, or (2) a ground loop, which can happen if you are using an unbalanced line (if you don't know, you probably are) between two powered devices. I've heard examples of the latter where the hum is unnoticable when there's a signal on the line but imensely distracting when the source is muted. -- AJR | Talk 20:41, 7 March 2006 (UTC)
- I can attest to the fact of a hum from mains power in a PA system, which was resolved by using an adapter to remove the ground connection on all the electronics (amplifiers, mixers, etc.). -- WhiteDragon 15:23, 10 March 2006 (UTC)
Your microphone may also be too close to your speakers.--172.162.83.248 03:27, 11 March 2006 (UTC)
Type of thermoplastic polyurethane fro making soles/shoes
[edit]The soles of the shoes are made of thermoplastic polyurethane or NON thermoplastic polyurethane.
- The P... article tells us that there are different kinds ; some foams can be moulded.
- Thermoplastic means "mouldable with the action of heat". If a substance is not thermoplastic, its use is quite different : varnishes are layered rather than moulded. Moulding leaves sometimes marks and allows to design textures that you would not want to draw mechanically. Also, my shoes' soles are leather. Can you decide by yourself now ? --DLL 21:49, 4 March 2006 (UTC)
mysql password
[edit]I've just installed mysql on my computer, and following some online post-installation configuration instructions, I guess I accidentally set the password to something I don't know. Can you tell me how to recover it? -lethe talk + 05:47, 4 March 2006 (UTC)
- I found some instructions, which tells you to start mysql with an initfile containing instructions to change the passowrd. However, it didn't work, I got the error
ERROR: 1133 Can't find any matching row in the user table
- So I guess I need to also tell the init file to add a user named root? How do I do that? -lethe talk + 06:26, 4 March 2006 (UTC)
OK, so I've started mysql with
/usr/local/mysql/bin/mysqld_safe --init-file=~/mysqlpass
and the file contains this:
use mysql;
INSERT INTO user (host, user, password, select_priv, insert_priv, update_ priv) VALUES ('localhost', 'root',
PASSWORD('somepassword'), 'Y', 'Y', 'Y');
FLUSH PRIVILEGES;
which I believe should create a root user with a specified password. Nevertheless, when I try to connect using
/usr/local/mysql/bin/mysql -u root -p
I get the delightfully uninformative error:
ERROR 1045 (28000): Access denied for user 'root'@'localhost' (using password: YES)
So I guess what I really need to know is: is there a way I can see which accounts are defined without being able to connect? -lethe talk + 07:13, 4 March 2006 (UTC)
- OK, well I figured it out. If you read the file data/mysql/user.MYD, it's a binary file, but you can still see some text in there with all the crud, and I saw the names of all the accounts. So now I can connect and should be able to fix. -lethe talk + 07:21, 4 March 2006 (UTC)
- When you first install MySQL, root has no password. You start it without using any password option so you can set a password for root. --Kainaw (talk) 01:22, 5 March 2006 (UTC)
- That's true. But maybe other stuff also happens. -lethe talk + 15:03, 5 March 2006 (UTC)
- When you first install MySQL, root has no password. You start it without using any password option so you can set a password for root. --Kainaw (talk) 01:22, 5 March 2006 (UTC)
Shortcut keys
[edit]I use Windows XP. I know that I can type Alt+0147, for instance, to type a left double quotation mark (“). Is there any way I can assign a keyboard shortcut to type characters like this (and for instance, different dashes)? I'd like to be able to do this in all programs, not just Microsoft Word. I know there are some programs available to download that let you create complex macros, but is there any way within Windows XP to do this? Or alternately, does anyone have a suggestion for a simple, light program that I can use to accomplish this? I'd rather not have a memory hog running all the time, if possible. Thanks for any assistance! — Knowledge Seeker দ 06:22, 4 March 2006 (UTC)
- Try AutoIt (website). It's a scripting language that can do almost anything, including Window recognisation and manipulation, mouse moving, clicking, keyboard typing, and even complex actions like COM object manipulation and ability to call DLL functions. It should be simple to do what you want to do in AutoIt; it's easy to create a keyboard shortcut to do anything. For example, you could assign "Ctrl-Alt-Q" to insert a quotation mark. AutoIt can send keys from ASCII codes, by something like "{ASC 0147}". If you'd like me to make the program for you, just ask. -- Daverocks (talk) 10:37, 8 March 2006 (UTC)
I need help for my science assignment.
[edit]I'm doing my assignment on ALLOYS. I just want to know who invented the idea. PLease answer as soon as possible or maybe you can give me some advice about where to look it up. Thank you guys.
I'm really desperate.
Gemmy
- For general information, alloy is a good place to start. However, as for the first creation of alloys, this page seems like a good jumping-off point. It doesn't say who or what civilization started alloys, but it says that bronze was the first intentional alloy created by man. It also mentions brass which, according to our article on it, has been known to man since prehistoric times, so there seems to be slightly conflicting reports on this. I really don't know much about the topic, but hopefully this gets the ball rolling, or hopefully someone more knowledgable will come along to help. EWS23 | (Leave me a message!) 07:14, 4 March 2006 (UTC)
- I suppose it largely depends on your definition of "prehistoric" whether that's conflicting information. After all, the discovery of the alloy bronze is the reason for the age known as the "Bronze Age" Prior to that Iron was the principal metal in use (hence "Iron Age"). Bronze Age culture is often (perhaps questionably) described as prehistoric, simply because so little of its history was preserved. As far as the initial question is concerned, although the first alloys may be difficult to track down the original creators of, some later innovators of processes for creating alloys are far easier to trace (one of the most famous is Henry Bessemer, who revolutionised the production of steel). Actually, the article on steel might give you several pointers, since that is still one of the most widely used alloys and historically has been around for a long time. Grutness...wha? 13:26, 4 March 2006 (UTC)
- Asking who invented alloys is like asking who invented salt water. It predates life itself. WAS 4.250 16:02, 4 March 2006 (UTC)
- True? Do alloys occur in nature with no human involvement? JackofOz 07:53, 5 March 2006 (UTC)
- Depends on your definition; 16 Psyche if apparently one enormous lump of iron, nickel, and probably a collection of various platinum group metals. But it gets into whether you assert that by definition an alloy must be artificial. --Robert Merkel 09:20, 5 March 2006 (UTC)
- True? Do alloys occur in nature with no human involvement? JackofOz 07:53, 5 March 2006 (UTC)
- Asking who invented alloys is like asking who invented salt water. It predates life itself. WAS 4.250 16:02, 4 March 2006 (UTC)
- There exist very few metals (and they're all the very unreactive ones) in the Earth's crust which exist as the metals themselves, rather than as compounds, or ores. But there is no reason, I suppose, why two such metals, eg silver and gold, could not alloy themselves "with no human involvement".G N Frykman 09:19, 5 March 2006 (UTC)
bending
[edit]Light waves bend when they move from one medium to other. So does sound or radio waves ben when they move from one medium to other?why?
- Yes, they do. --HappyCamper 08:23, 4 March 2006 (UTC)
- As do water waves as they go from one depth to another. It's because they change thier speed. Theresa Knott | Taste the Korn 08:41, 4 March 2006 (UTC)
- You might find our article on refraction interesting. --HappyCamper 08:43, 4 March 2006 (UTC)
- Also, light waves and radio waves are both forms of electromagnetic radiation. Light waves are electromagnetic waves with a wavelength range of 400 nm to 700 nm, whilst radio waves are electromagnetic waves with a wavelength of 1mm to 100 000km. So they behave in the same way. - Akamad 13:35, 4 March 2006 (UTC)
- Sound waves refract by changing their speed. Electromagnetic waves can't do this, since their speed is fixed at the value c. So they change their wavelength.G N Frykman 09:22, 5 March 2006 (UTC)
- That's not true. When an electromagnetic wave is refracted, both its speed and its wavelength changes (but the frequency doesn't change), just like with sound waves. – b_jonas 22:09, 6 March 2006 (UTC)
- Yeah, c is just the speed of light in vacuum. When it's traveling through a medium, its speed is reduced. Black Carrot 23:43, 6 March 2006 (UTC)
- Actually, the reason that light (and radio, etc. see Electromagnetic Radiation) bends is because its speed of propagation changes. The index of refraction links these two ideas.Jburt1 23:05, 8 March 2006 (UTC)
Genes
[edit]Is it possible for genes to overlap one another? --HappyCamper 08:46, 4 March 2006 (UTC)
- It is called "alternate open reading frame".Google for details, we lack an article on it. H5N1 has one one these. Our article on that states: "PB1 codes for the PB1 protein and the PB1-F2 protein.* The PB1 protein is a critical component of the viral polymerase.* The PB1-F2 protein is encoded by an alternative open reading frame of the PB1 RNA segment and "interacts with 2 components of the mitochondrial permeability transition pore complex, ANT3 and VDCA1, [sensitizing] cells to apoptosis. [...] PB1-F2 likely contributes to viral pathogenicity and might have an important role in determining the severity of pandemic influenza."[22] This was discovered by Chen et. al. and reported in Nature[23]." WAS 4.250 15:46, 4 March 2006 (UTC)
- In a way, yes. See Alternative splicing. Basically if several proteins share parts of their amino-acid sequence, that part can be encoded in a single exon and both proteins can be created by the same gene as splice variants. --BluePlatypus 09:47, 4 March 2006 (UTC)
In that way yes, but not in the sense that you can imagine two meaningful encyclopedia articles that overlap, with the last page of one constituting the first page of the next-- for exactly the reasons the metaphor implies: starts and stops of genes are defined by special signals, and it is hard to imagine an instance where it would be useful to have the tail of one protein constitute the tail of another in reversed attachment. alteripse 12:55, 4 March 2006 (UTC)
- Oh, I see. How about in smaller examples, like plasmids? Are there examples where the overlapping is much more significant? --HappyCamper 13:26, 4 March 2006 (UTC)
Language question about anencephaly
[edit]It's a question about norms of english language: how may the notion "anencephalic person" be expressed by a single word? In russian it's "anacephal" -- a person born without brains. Does anything similar exist in English? Thank you. ellol 10:03, 4 March 2006 (UTC)
- I suppose if it had to be shortened to one word, you'd use anencephalic, in the same way that diabetic can be used as a noun. Though strictly speaking a person with the condition is only missing the forebrain, not the whole brain.GeeJo (t) (c) • 10:17, 4 March 2006 (UTC)
- Thank you, GeeJo! ellol 12:04, 4 March 2006 (UTC)
Many doctors avoid using a disease adjective as a noun, especially in formal or public speech. Person with diabetes is preferred over diabetic. One never wants to forget that a person is not a disease. This distinction was introduced publicly in the 1970s in an era of PC speech that often degraded accuracy and enshrined ignorance, and much of it (fortunately) has not survived, but this distinction is both functional and accurate, as well as sensitive. The people who use the term diabetic as a noun most freely are people with diabetes. Anencephaly and anencephalic is a special case, however, as you may be aware, because there has long been debate about the personhood of a body born without a brain. Anencephalic is sometimes used as a noun in a medical context. alteripse 12:50, 4 March 2006 (UTC)
- Thank you. It's interesting. Actually, i wondered why does the word that must exist seem to be non-existing. The concept of political correctness is not common here. ellol 17:46, 4 March 2006 (UTC)
- Is it likely that AA will become Persons With Alcoholism Anonymous? JackofOz 07:50, 5 March 2006 (UTC)
water potential
[edit]hello, I'm doing biology coursework on osmosis in potato tissue and there is one criteria i'm slightly confused on how to achieve. I have to say why is it better to use water potential instead of molarity.can you help? thank you
- There is a hint in the article water potential which you might find useful. Also, check out molarity (and possibly molality). Note how the former takes something very important into account... --HappyCamper 14:21, 4 March 2006 (UTC)
ssh -X and ssh -Y
[edit]What is the difference between the -X and -Y options for ssh? I've read the man pages over and over, and I still don't understand what it means. Thanks for your help! --HappyCamper 14:49, 4 March 2006 (UTC)
- Judging by this mailing list message, it's to do with whether or not you trust the applications running using X forwarding (ie what permissions they have on your machine you're SSHing from). -X runs them as untrusted, which can cause some things to break. -Y runs them as trusted. But you shouldn't notice any difference if you're not using an application sensitive to whether or not it's trusted. --Bth 15:20, 4 March 2006 (UTC)
Plugged Ears feeling after running in cold weather
[edit]Why do my ears feel like they're all plugged up during and after I go running/jogging in the cold weather (see your breath kind of cold)? It seems I have to 'pop' my ears after each breath once I go indoors. It's horrible if I have a stuffy nose. 151.199.150.176 17:04, 4 March 2006 (UTC)
- If your Eustachian tubes get plugged up with a bit of mucous, the pressure on the inside of your eardrum will be higher than that outside. Your hearing will be distorted, and 'popping' your ears will clear it. TenOfAllTrades(talk) 21:47, 4 March 2006 (UTC)
Its possible that you have an Ear Infection. I have had it on multiple occasions and its symptoms include plugging on the ears and that popping of the ears is the ears trying to adjust the the pressure changes within your ear to the outisde pressure but somehting is blocking it making it more noticeable. Ear infection is caused by fluid entering the Eusachan tube in the ear and bacteria getting in there and infecting it. See a physician for more of an explanation. This is usually remided with common medicines. I hope this helps. :-) 5aret 00:55, 5 March 2006 (UTC)
Perhaps I just have overproductive mucous membranes in the area of my eustachian tubes since it occurs everytime I exercise in the cold and feel quite healthy. Thanks for the info. But is there a reason why these membranes would produce mucous while Im exercising and not when Im just sitting around out in the cold? 70.106.18.50 16:38, 5 March 2006 (UTC)
OpenID
[edit]Does OpenID provide a way to link different identities? For example, let's say I have a Livejournal OpenID and a DeadJournal (or experimental MediaWiki) OpenID. Is there any way I can tell the servers that these are one and the same person? — Ilyanep (Talk) 19:48, 4 March 2006 (UTC)
- If you have full control over one of the URLs you can delegate it to the other URL, but if it's LiveJournal or MediaWiki, then no. Ashibaka tock 06:09, 5 March 2006 (UTC)
- You'll probably have do decide which one you prefer more and just stick with that one. Use your favorite OpenID to log into sites that support it. Why did you create more than one OpenID anyway? --Optichan 19:48, 6 March 2006 (UTC)
Java loading code
[edit]Does anyone know how I could make a program that reads in java code from a text file and executes it. I think I can do it by reading in a class, using the "load"[13] command and doing somthing similar to the code on this [14] page. I'm lost though. Could someone give me an overview of what has to be done? I know I'll have to cast the class after reading it... but how do I get it in the first place? Is this even possible. BrokenSegue 22:11, 4 March 2006 (UTC)
- By "java code" I'm assuming you mean "compiled bytecode stored in a .class" file (and not "java sourcecode"). The easy way is to make sure the class file is in the classpath, and then invoke Class.forName (an example is here). If it isn't, you have to create your own classloader (i.e a class that extends ClassLoader), which is what that example you quote does. The important line is the call to defineClass (which is in ClassLoader) which takes a byte array (containing the same stuff as a .class file) and loads it. That will give you a java.lang.Class object. Now, if memory serves, you can't just cast that to something (like an interface) and just go using it (unless they've added some clever stuff to do that after I stopped doing so much java). Instead you have to call the Class object's newInstance method. Note that if your constructor (for that class) has arguments, you'll have to use java.lang.reflect to build the arglist for newInstance (discussed here). One last thing (it's obvious, probably): your compiled class has to implement an interface which your hosting code knows about (and was compiled with) so that you can have something meaningful to cast the result of newinstance to. Here is a rather basic AppletClassLoader (the Sun one is rather more complete) which uses a classloader that comes with the system (NetworkClassLoader). Don't worry if this all seems rather excessive - this is probably the hardest, yet most infomative, part of Java. Once you get this you'll understand how security really works, how reflection works, and how stuff like JINI and Javabeans work. -- Finlay McWalter | Talk 22:37, 4 March 2006 (UTC)
- Thanks man. BrokenSegue 12:50, 5 March 2006 (UTC)
mac OS finder windows
[edit]Since this seems to have become the computing reference desk....
I'm running Mac OS 10.4.4. I like to apple-tab between applications. But something very frustrating happens with the Finder. When I apple-tab to the finder, it doesn't bring the last finder window I was working with to the front. The focus seems to go there, because I can apparently navigate directory structures with the arrow keys, but the window itself doesn't come to the front, so I usually can't see what's going on. I have to either somehow find it with the mouse, or apple-tilde until the right window shows up. Extremely annoying. I don't think it behaved like this on older OSes. Does anyone know how to fix this? Thanks... Dmharvey 22:18, 4 March 2006 (UTC)
- Not really a fix, but I tend to use Exposé (F9 or F10) to pick the Finder window I want. Sum0 22:52, 4 March 2006 (UTC)
- I think it's always been for computing. Its description is "To ask questions about science, medicine, computing, and technology". — Knowledge Seeker দ 23:29, 4 March 2006 (UTC)
- I think it might be worthwhile to split off into a Reference desk/Technology. — Ilyanep (Talk) 23:30, 4 March 2006 (UTC)
- I don't think you can get what you want, you're going to need to use the exposé feature, F9 or F10.
- Apple-backtick cycles between windows in the same application, e.g. between different finder windows. Ojw 12:21, 5 March 2006 (UTC)
- (Oops, when I said apple-tilde I meant apple-backtick.) Well, it's very strange, after behaving like this for several months, as soon as I ask a question on the reference desk, the problem goes away. Now it does what it's supposed to do. I don't think I changed any settings. Very mysterious. Thanks guys! (I guess!) Dmharvey 12:31, 5 March 2006 (UTC)
Science fiction weapon
[edit]I had an idea for a fancy futuristic weapon: Ordinary rifled barrel, magnetic/magnetised bullet. Behind the bullet is a powerful (as powerful as necessary/possible) electromagnet with an opposite charge to the bullet. When the electromagnet switches on, the bullet is repelled straight out of the gun at a high speed. So, sort of like a coilgun but with no coil. Plausible, or fatally flawed? Thanks y'all. Sum0 23:04, 4 March 2006 (UTC)
- Doesn't sound very likely to me. By the way you don't really mean "charge"; no one has been able to prove experimentally the existence of magnetic charge, though there is theoretical work about how it would behave if it did exist. If it did exist, you wouldn't want opposite magnetic charge, but rather the same (opposites attract, like charges repel). So what you're really talking about is a magnetic dipole. Dipole forces fall off very quickly with distance. My guess is you'd be lucky to get the bullet to go ten feet. --Trovatore 23:16, 4 March 2006 (UTC)
- Oh yeah, I meant same charge. Also, I must have not explained it very well: the idea isn't for the magnetic force to keep "pushing" the bullet as it goes along, but to accelerate it very quickly to a high speed before it leaves the magnetic field. Would that work, or does physics get in the way? Thanks for the replies, anyhow. Sum0 23:32, 4 March 2006 (UTC)
- It'd make a nice pistol-like weapon. But what real advantage would it have? — Ilyanep (Talk) 23:26, 4 March 2006 (UTC)
- I think the magnet-at-the-back design is just wrong. Why don't you want the coil along the barrel? I haven't worked the numbers or anything, but my intuition is you'll get a much higher muzzle velocity with the latter design. --Trovatore 23:36, 4 March 2006 (UTC)
- This is called a rail gun. It isn't science fiction. Working models have been made. The problem is that the power supply is about the size of a VW Bug - so it isn't very portable. --Kainaw (talk) 01:18, 5 March 2006 (UTC)
- Some fellow pupils made one of these (under supervision) when I was at school in Oxford in the 1970s. They used the properties of the 3-phase supply to induce huge eddy currents in a metal bolt and to shoot it out of a tube. When it was first tried out, it was just as well that sand-bags had been placed at both ends of the tube, because the connections had been made the wrong way round, and the bolt shot out backwards with huge force... Do not try this at home, is the motto!G N Frykman 09:31, 5 March 2006 (UTC)
- One problem is that the bullet will want to turn around, which will cause a lot of friction and make it come out of the barrel at a slight angle. Btw, I came up with the rail gun principle as a kid and asked the physics teacher about it and he hadn't a clue what I meant. Only later did I find out that some trains use this principle. Someone else stole my idea again. :( DirkvdM 10:38, 5 March 2006 (UTC)
- The most successful one I've seen (Texas A&M) used a little caddy to carry the bullet down the rails. It was a little bullet-sized bucket with two wings that rode along the rails. This meant that the bullet could be uncharged - instead the wings of the caddy were charged. Then, you can set it up so they try to turn into each other - cancelling out that problem. The new problem is that the wings will easily weld themselves to the rails due to the high amount of electricity travelling through the system. When that happens, you get big sparks and a very expensive paper weight. --Kainaw (talk) 17:58, 5 March 2006 (UTC)
- But from what I can tell, this isn't a railgun. There are no rails, for a start, and it's just one magnet behind the bullet. Sum0 20:37, 5 March 2006 (UTC)
- Sounds very much like a Gauss gun, but with only a single electromagnet. BTW, you can make a handheld gaussian gun with 5 (magnetic) ball bearings and some similiarly sized neodymium magnets. Connect 4 bearings and the magnets in series, then let the fifth bearing roll towards the exposed magnets. The impact velocity is transferred to the farthest bearing, which by not being in as strong a magnetic field, is propelled away at high velocity. It's more of a toy, since neodymium magnets are too brittle for large-scale versions. PS - I credit MechWarrior 2 for introducing the topic to me - the Gauss Cannon rocked faces. Tzarius 07:54, 6 March 2006 (UTC)
- Basically what everyone's saying, Sum0, is that it's not really plausible. A single magnet behind the bullet wouldn't have nearly enough power to project the bullet at any great distance (because the magnetic repulsion would only be effective for a very short distance), something that a rail gun does much more elegantly. freshgavinΓΛĿЌ 04:52, 8 March 2006 (UTC)
March 5
[edit]Thermostat 7 to 5 wire
[edit]I have a 7-wire thermostat. I went through every thermostat at Lowes and Home Depot. The most connections any have is 5. I tried to use one by connecting the 5 matching colors and leaving the two odd colors (Cyan and Orange) disconnected. The heater runs non-stop. I've been searching and searching on the Internet for information, but I can't find anything about how to connect a 7-wire system to a 5-wire thermostat. Are there any AC experts here? --Kainaw (talk) 00:47, 5 March 2006 (UTC)
- Well, I'm not an expert, but I have here the instruction sheet for the Lux TX 1500 thermostat I installed in my house a couple of weeks ago, and it accepts up to 7 wires. (I can't imagine what they're all for; my heating system has only two.) —Steve Summit (talk) 04:35, 5 March 2006 (UTC)
- Stopped by an AC supply shop today. They were closed (Sunday), but some guys were there putting in extra hours. It turns out that my "7-wire" is technically called a "C-wire". It is considered commercial, not residential, so I had to get a commercial thermostat. The only ones they carry are from Rite-Temp, which are cheap, but function. They showed me why it is was impossible to use a C-wire heat pump with a 5-wire thermostat. The relay for the fan and heater gets voltage on one side, but not the other. So, no matter what the thermostat says, it always thinks it is supposed to be running the heat. --Kainaw (talk) 17:54, 5 March 2006 (UTC)
some compound
[edit]I mixt (chlorine) bleach with (household) ammonia and put a 1989 canadian 1 cent into it. 2 days later, the mixture is blue. i'm wondering what it's chemical formula is and why it turned blue. --152.163.100.74 04:01, 5 March 2006 (UTC)
- Gah! Please, don't try this again, and nobody try this at home. Mixing chlorine bleach and aqueous ammonia will result in the formation of chloramine, a highly toxic compound that is being used in many cities as a substitute for chlorine in water purification. Nasty stuff. TenOfAllTrades(talk) 04:26, 5 March 2006 (UTC)
Did you actually do it or is it just a nicely phrased homework question? deeptrivia (talk) 04:41, 5 March 2006 (UTC)
- I'm kind of hoping that a homework question wouldn't encourage a student to do something so blindingly dumb, actually. There's a reason why household bleach and ammonia bottles both have dire warnings on their labels not to mix the two of them together. *grumbles* What are they teaching kids in school these days? *grumbles*
- To answer your question, Canadian pennies before 1997 were almost (98%) pure copper. Taking stock of what's present in your beaker, you have copper, sodium hypochlorite (from the bleach) and ammonia (from the ammonia). Now just do some research and find out which combination of those is vividly blue. TenOfAllTrades(talk) 04:57, 5 March 2006 (UTC)
- According to a very old book I read, old coins can be cleaned by putting them in ammonia, which will leave the coin clean and the liquid blue. Given the age of the book, I'd assume they presumed most coins are made from copper, which would suggest that ammonia was the only ingredient doing the cleaning in the experiment you described. --Aramգուտանգ 20:23, 5 March 2006 (UTC)
As mentioned above on this page (in some discussion about phlegm) Cu2+ ions are blue. So if you can get some copper to ionize, for instance by putting it in an acid, you'll end up with a blue solution. I think. My memory of high school chemistry is poor. moink 23:40, 5 March 2006 (UTC)
- Unless the ammonia is redoxed, the only negative ion available would be hypochlorite, ClO-. Seahen 22:36, 7 March 2006 (UTC)
Okay! Thanks. It's not homework, I was just bored so i wanted to see what would happen if i mixt stuff that it says i shouldn't mix. I really dont care what the compound is called anymore, i just wanted to know why it's blue. PS: I realize it would produce poisonous gas, so i first poured some bleach into a plastic cola bottle and then pushed in a water balloon filled with ammonia, and then popped the balloon while it was inside. Now...is there anyway i can make the mixture blow up, or fizzle or some cool reaction by adding more household chemicals? --152.163.100.74
- Look, just mixing random stuff together is just dumb alchemy. Go find a book on chemistry experiments you can do at home and try some of the stuff in that. Some of it can involve explosions, fizzing, and so on, you may actually learn some interesting stuff in the process, and you run a far lesser chance of killing or injuring yourself and your family in the process. --Robert Merkel 03:39, 6 March 2006 (UTC)
Incidentally, the blue may well be the complex "cuprammonium" ion, which is a deeper blue than copper. Hyperman 42 00:29, 6 March 2006 (UTC)
Australia's First Triple Transplant
[edit]I'm requesting information on the details of Jason Grey's transplant, which was Australia's first triple transplant (heart, lung and liver).
I would specifically like to know the date that the 14 hour operation was done on.
(I have tried using Google, but am not getting much luck.)
Thanks for the help.
05:40, 5 March 2006 (UTC)
- I'm not having much luck with Google either. I can't get an exact date, but I've narrowed it down to the last week of July or the first week of August 2003. According to [15] Jason's surgery was performed sometime during the week prior to August 8. This is confirmed by [16] [17], which gives Jason's post-op condition as of August 5. --David Iberri (talk) 16:28, 5 March 2006 (UTC)
viruses
[edit]i bought some software from e bay about 3 month ago and ive looked at it a few times with no trouble. today i done a avg test on my pc and this software had a virus in it. the virus was
Trojan horse psw. Banker. wzp ive looked up a few types of virus but could not find this one
is it very dangerous for my pc and files or not --Titanicful 08:56, 5 March 2006 (UTC)
- What software did you buy? Some anti virus programs label stuff as trojan by accident, but you can't be too careful. I would look up the trojan's name on symantec's website www.symantec.com and download a patch to get rid of it. - Mgm|(talk) 14:01, 5 March 2006 (UTC)
- What antivirus software are you using?
- The question says AVG.
Slumgum 20:18, 5 March 2006 (UTC)- Oh you're quite right... Are you sure the virus defs are up to date?
Hello everyone, it happened to me too. AVG found some time ago the Trojan Horse PSW.Banker.WZP in 2 files I downloaded during Christmas promotions. The rest of the files were ok, now, after some weeks, another AVG scan found some more files infected with this PSW.Banker.WZP. These files had been scanned several times before and they were ok. I update the definitions every day. I have scanned the same files with other antiviruses: Norton on my pc, and online: f-secure, Panda and BitDefender, they found no problems. Really strange. Kind regards, Irene
Is it possible to change a Yahoo username?
[edit]Is it possible to change a Yahoo username? —Masatran 12:28, 5 March 2006 (UTC)
- Did you try asking it at Yahoo first? They are the people who are supposed to know this. - Mgm|(talk) 14:02, 5 March 2006 (UTC)
- No. But, you can just create a new Yahoo account and stop using the old one. --Kainaw (talk) 15:05, 5 March 2006 (UTC)
Undeletion
[edit]Can anyone recommend good free software to recover files from my old NTFS hard drive? I'm running Windows XP. The freeware I've found has found and recovered some of the deleted files I need, but not all. A shareware program I've found (called WinUndelete [18]) detects the remaining files and tells me they're in "good" condition, but the shareware version won't actually recover them. Seahen 14:12, 5 March 2006 (UTC)
- What is the freeware you have used already?
- It is found at http://www.officerecovery.com/freeundelete/ and http://home.nexgo.de/christian_grau/rescue/index.html. The latter always gives me an error message. 22:29, 7 March 2006 (UTC)
- There isn't a lot of free data-recovery software, but Directory Snoop is a shareware program that you can try for free. It can recover files instead of just telling you they're in good condition. I used it back in May/June 2005, but unfortunately it couldn't help me out.
- Good luck! --Bowlhover 20:59, 5 March 2006 (UTC)
- Works, but won't recover a folder all at once if it has subfolders?! Seahen 23:28, 9 March 2006 (UTC)
- Good luck! --Bowlhover 20:59, 5 March 2006 (UTC)
- I've had good results using "PC Inspector". It's free, but not Free. - mako 05:08, 8 March 2006 (UTC)
- Try Microsoft's recover from the command line. Don't know how well it works, but I know it's there. :) -- Daverocks (talk) 02:35, 11 March 2006 (UTC)
Food allergy?
[edit]I've noticed repeatedly and quite predictably that these two situations will cause 1 episode of diarrhea for me (almost without fail) within 30 minutes after finishing: 1) having large quantities (1/4 teaspoon) of nutmeg in 1 8-12 oz cappuccino, 2) an Italian dinner from a respectable American-Italian restaraunt (e.g. The Olive Garden, Romano's Macaroni Grill). Obviously, I know what the cure is, but i was wondering if anyone knows why this happens? Is this a food allergy? Male, I have an unusually high metabolism, have weighed 150 lbs for more than the last 5 years, and am generally healthy. 70.106.18.50 17:04, 5 March 2006 (UTC)
- Nutmeg is a common cure for diarrhea, not a cause of it. Also, a person with a high metabolism usually has a very low weight as a high metabolism requires more calories to maintain muscle and fat than a normal metabolism. 150-180 pounds is an average weight for a normal male. In our modern obese time, 200-250 is considered normal, but that is normally obese, not normal. So, if you are maintaining 150 pounds without trouble, you probably have a normal metabolism. --Kainaw (talk) 17:50, 5 March 2006 (UTC)
- Bloody hell, over 100 kg normal? For someone 2,5 m tall maybe. DirkvdM 07:11, 6 March 2006 (UTC)
- Interesting point about my metabolism - you're probably right. 71.241.8.77 05:14, 6 March 2006 (UTC)
- When I and my friends were, how you say, "experimenting" with nutmeg, we had problems with nausea, but not diarrhea. —Keenan Pepper 17:59, 5 March 2006 (UTC)
- I once 'experimented' with nutmeg, but remembered incorrectly and put a tablespoon of cinnamon in my mouth. Which instantly sucked my mouth dry and made it almost impossible to spit it out. That cured me from any desire for further experimentation. :) DirkvdM 07:16, 6 March 2006 (UTC)
Could it be coffee? That does it for me in large enough quantities.
- Nutmeg is extremely poisonous if inject instantaneously! Kilo-Lima Vous pouvez parler 16:31, 6 March 2006 (UTC)
- Inject? You mean intravenously? Don't start giving people ideas. :) But wouldn't the same go for any solid (spice or not)? DirkvdM 11:24, 7 March 2006 (UTC)
Don't know about the nutmeg, but you may have lactose intolerance. - Taxman Talk 19:41, 6 March 2006 (UTC)
Why do objects appear smaller with distance?
[edit]Why do objects appear smaller as the distance between the object and the eye increases? Consider a square object that is smaller than the eye lens and is lined up perfectly with the center of the lens some distance away. Now the parallel rays of photons/lightwaves coming from the top edge and the bottom edge of the object will hit the eye lens at exactly the same spot no matter how far the object is. So why should the object appear smaller with distance? I once brought this up with my physics teacher back when I was still in high school and he wasn't able to give a satisfactory explanation, and suggested that the post-processing of the optical signal in the brain is what makes the object appear smaller, referring to the Moon illusion as an example. Is that explanation valid? Thanks, this has been bothering me for ages. --Aramգուտանգ 20:37, 5 March 2006 (UTC)
- Objects do not appear smaller because of the "post-processing of the optical signal in the brain". If you measure the Sun's angular diameter at 1 AU, and measure it again at 50 million kilometres, you'll find that it is much larger at 50 million kilometres than at 1 AU. But if you measure the Moon's angular diameter when it's high in the sky, and measure it again just before it sets, you'll notice that the angular diameter doesn't change.
- Let's say you have an object that's bigger than your eyes. As you move farther away from the object, light rays from the top and bottom of the object will gradually become more parallel. If you measure the angle they form when they intersect (at your eyes), you'll get the angular distance between the top and bottom of the object. The angular distance is what determines how big or small an object appears to be, and as the light rays become more parallel to each other, the angular distance will shrink. This is why objects appear smaller when you move farther away from them--it's not because of an optical illusion like the Moon illusion is. --Bowlhover 21:23, 5 March 2006 (UTC)
- Well said, Bowlhover. Objects subtend a smaller angle on your visual sphere the farther away they are. —Keenan Pepper 21:36, 5 March 2006 (UTC)
- To address your specific query about parallel rays, you're right that those will not change as the object moves away; however, they are not the only relevant ones (otherwise, you wouldn't be able to see anything larger than the diameter of your lenses. Image:Lens3.png gives an illustration of what's involved in focusing a point of the image. One could imagine moving the object father back. The top ray would remain the same, as it is parallel to the axis of the lens, as you have noted. But the slope of the middle one would decrease and it is easy to see that it would now intersect the top ray further to the left of the original intersection; calculations will show that the other rays will do the same. Of course, the new image is also more ot the left of the original and wouldn't be focused on the retina, but even as the lens adjusted the new image would still be smaller. If you'd like to go through the specific calculations, let me know. Post-processing is not involved in the phenomenon as you have described it. — Knowledge Seeker দ 21:55, 5 March 2006 (UTC)
- Ah, thanks a lot Knowlegde Seeker, the Image:Lens3.png diagram really got the point through. I'm surprised at the incompetency of my old teacher now, I recall he wasn't able to explain why mass increases with velocity in general relativity either, but that's not a question that bothers me a lot. Oh and thanks to Bowlhover too, although I was really looking for an exlanation that would go into ray bending in the eye lens. --Aramգուտանգ 05:16, 6 March 2006 (UTC)
Vinyl Cancer
[edit]Now that they have whimped out in using the killer plasticizers in Polyvinyl chloride, something very bizarre has happened. I have a vinyl insulated cover on my hot-tub (I use it all year up in Canada). It's santized with Bromine. The last couple of months, the underside started bubbling in the middle, and quickly spread. I finally had to rip it off today. It was as hard and as brittle as original PVC. Has anybody seen this happening. Is this a new thing? Many thanks. --Zeizmic 21:37, 5 March 2006 (UTC)
- It *sounds* like the plasticizer which is used to keep the plastic flexible has simply left the polymer. How old is it? --HappyCamper 05:16, 6 March 2006 (UTC)
- Sounds like it could be water causing seperation of layers, which is something that could happen if the thing is made from a laminate. --BluePlatypus 09:51, 6 March 2006 (UTC)
Only about 2 years old. It was a funny vinyl, without the usual cloth backing. It happened so fast, and now the bottom is tissue paper. I just ripped it out, and there's another type of plastic underneath. Maybe that was the original backing. --Zeizmic 12:54, 6 March 2006 (UTC)
How much do we cost?
[edit]If we were to deconstruct our body to elementary segments, and then convert the cost of each quantity to monetary units, how much would we cost? Now, we all know we are priceless, but...just for the sake of this question.
- It all depends on what you mean by "elementary segments". If that means atoms, we're almost worthless. —Keenan Pepper 22:43, 5 March 2006 (UTC)
- Here's our percentage makeup of the elements, I guess you'll have to do the maths and some stoichiometry from there, find a chemicals supplier and see how much they charge for the elements, I didn't manage to find any such supplier, though. --Obli (Talk)? 22:56, 5 March 2006 (UTC)
If we were to deconstruct a canon DSLR camera EOS 1Ds mark II body to elementary segments, and then convert the cost of each quantity to monetary units, how much would the canon DSLR camera cost? Now, we all know Canon Camera are expensive, but...just for the sake of this question.
You see the problem. A canon camera cost more than the sum of its parts. A human being cost more than the sum of the chemical components. What about the cost of the skills in a human being if that human is a olympic gold medalist? Ohanian 03:14, 6 March 2006 (UTC)
- It would be interesting to try to make a breakdown of how much value is added at each level of complexity. The raw elements are worth some small amount of money; the molecules are worth a different, higher amount, the living cells even more, and so on. —Keenan Pepper 03:29, 6 March 2006 (UTC)
- But value depends on supply, which means the means of production, which vary greatly. Producing 1 gram of a single protein in pure form is a *lot* more expensive than producing 1 gram of cultivatable cells. Producing 1 gram of copies of a single DNA is rather cheap, thanks to PCR. The cost of synthesizing organic substances varies enormously depending on what precursors you need, and what reactions yuo can use. And so on. --BluePlatypus 19:32, 6 March 2006 (UTC)
- Also, I guess the cost of the deconstruction would probably be larger than the price of the atoms you get. – b_jonas 21:53, 6 March 2006 (UTC)
- It depends on what you would count as an 'elementary' segment. If you separate someone down into their constituent atoms and sell those for their bulk market prices, you probably don't get very much—erhaps as much as ten or fifteen dollars worth of raw material.
- On the other hand, certain parts have a much higher value when not divided down quite so small. You could probably sell a kidney for five or ten thousand dollars—at least if it weren't illegal to do so. (Some other organs would fetch even higher prices.) Women could sell eggs for quite a chunk of money, too.
- Then there's all the useful hormones. You're full of steroids and growth hormones and cell signalling factors and all kinds of other handy biomolecules. If you could collect and purify all of those, then you'd probably be worth hundreds of thousands of dollars. TenOfAllTrades(talk) 00:42, 7 March 2006 (UTC)
- I'd estimate about $847.63 --JianLi 05:30, 7 March 2006 (UTC)
- Why don't you put yourself on ebay as "spares only", and see how much you get? --Heron 20:57, 7 March 2006 (UTC)
- Question is - should he post a picture with it or would that ruin the experiment? Celcius 03:07, 10 March 2006 (UTC)
- "Includes bonus Keratin (long hair and nails, extra body and facial hair), Perfumes (may not be suitable for human olfactory centres) and now with 25% extra body mass!" Tzarius 07:16, 10 March 2006 (UTC)
histogram equalization stretch
[edit]when modifying a satellite image, in what situation should the histogram equalization stretch be used, should it be used to enhance a rare feature on an image?
- Try it and see if it works for you. GangofOne 02:36, 6 March 2006 (UTC)
- What does 'histogram equalisation stretch' mean? Is that some preset for a program? If so, which one and could you describe it's function? I suppose it means reducing the overall contrast. What effect this has on a specific feature depends on that feature. You should increase the contrast between the important parts of the image (and thus 'stretch' the rest?). In Photoshop or the Gimp, play around with 'curves' (don't know what that's called in other programs). DirkvdM 07:33, 6 March 2006 (UTC)
- Check your textbook. If you don't have one, go to the library and find one on satellite image processing. --Robert Merkel 00:42, 9 March 2006 (UTC)
Christmas lights fiasco
[edit]I did something stupid the other day: I used a string of Christmas lights as an extension cord for a vaccum cleaner. (Don't ask why. Yes, it was stupid. Yes, I probably could have guessed what would happen.) The lights of course went off and won't come back on, and electricity no longer seems to pass through the cord at all. What's the probable extent of the damage -- is it a light or two that blew needs to be replaced, or is the whole string dead? --Fastfission 23:16, 5 March 2006 (UTC)
- Do you happen to know if the lights are strung in series or in parallel? When were they made approximately (and in what country)? moink 23:22, 5 March 2006 (UTC)
- From looking at User:Fastfission/About me, you're in the US and youngish. So unless you inherited these from your parents when they were young, they're most likely in parallel. If none of them are working, replacing one won't cause the rest to work. I don't know what's wrong with them. moink 23:29, 5 March 2006 (UTC)
- If I am not mistaken, most strings of Christmas lights produced for use in the US in the recent past have a fuse within the plug. (see the fourth major paragraph here); check to see if there seems to be a latch or cover of some kind in the plug. And yeah, don't try that again! --LarryMac 23:40, 5 March 2006 (UTC)
I had a recent set of those tiny lights, and the cord broke. No problem, I thought, I would just solder it together. Well, they are soooo cheap that there is no wire! It just looks like thread wound into string! I think it must be silvered string, or somehow they get it to conduct the little bit that is necessary. This stuff cannot be rejoined, and if you put a bolt through it, I'm sure you get a lot of burnt string! Puts new meaning to a 'string of lights'. --Zeizmic 02:27, 6 March 2006 (UTC)
- The tiny lights are actually in series, but if one goes out the whole string doesn't go out because the blown light is designed to short itself. These are low voltage blubs, the voltage of the mains is divided among the either string. The wire is small, you probably burnout the wire somewhere due to too much current. But maybe the bulbs can be used as replacements on your next string. GangofOne 02:41, 6 March 2006 (UTC)
March 6
[edit]March 6? But can you be more specific in your question? For great justice. 05:24, 6 March 2006 (UTC)
- You made my day! I think it was referring to the actual date of questions to be asked :) --Ali K 05:29, 6 March 2006 (UTC)
- Hey, if you go up a bit, there's another header saying 'March 5' and a bit further up there's a 'March 4'. And so on. Maybe these are not questions at all. Now what might they be for? DirkvdM 07:37, 6 March 2006 (UTC)
- It's the Wikipedia fitness regime. Yesterday we were supposed to march five miles and today it's six. --Bth 08:15, 6 March 2006 (UTC)
- Actually, I think it's a typo. Today we are supposed to answer questions at Mach 6. - EWS23 | (Leave me a message!) 16:58, 6 March 2006 (UTC)
- Aren't you US'ers a bit too tough on yourselves? Switch to kilometres and you only need to march 2/3 the distance. DirkvdM 11:30, 7 March 2006 (UTC)
- It's the Wikipedia fitness regime. Yesterday we were supposed to march five miles and today it's six. --Bth 08:15, 6 March 2006 (UTC)
- To whoever forgets: Remember Wikipedia uses UTC time and may switch to the next day, when you're still a few hours behind. - Mgm|(talk) 09:32, 6 March 2006 (UTC)
- Thanks - it was actually just a silly joke, but the Mach comment made me wonder - if Mach is the ratio of the speed of a thing to the speed of sound in the same medium, what happens as a plane gets higher into the atmosphere and eventually into the vacuum of space, where the speed of sound is, presumably, zero, because there is nothing for it to transmit through in that medium? For great justice. 19:21, 6 March 2006 (UTC)
- If the speed of an object in space is refereed to as a Mach number, then the speed of sound at sea level at room temperature was used. Also, airplanes cannot get into the vacuum of space, because they need air to provide lift to counter gravity, and because fuel for the engines cannot burn without oxygen. --Bowlhover 21:00, 6 March 2006 (UTC)
- Thanks - it was actually just a silly joke, but the Mach comment made me wonder - if Mach is the ratio of the speed of a thing to the speed of sound in the same medium, what happens as a plane gets higher into the atmosphere and eventually into the vacuum of space, where the speed of sound is, presumably, zero, because there is nothing for it to transmit through in that medium? For great justice. 19:21, 6 March 2006 (UTC)
- I believe you are incorrect - the Mach number of, say, a plane, is always relative to the local speed of sound (ie the speed of sound at the altitude at which the plane is flying), which, are you pointed out, does change with height. The reason for this is that Mach number is a critical design factor in aerodynamics - air does very funny things when you get to high, subsonic Mach numbers (above about 0.8), everything goes haywire close to Mach 1 (speed of sound), and many things suddenly work the wrong way around above Mach 1 (for instance, above Mach 1, the flow speed in an expanding duct actually increases, which is counter-intuitive and not how things work below Mach 1, see de Laval nozzle). This is why flying at high altitude is a difficult design challenge, as unless you are specifically designing a supersonic airplane, you have to be careful not to fly at too high a Mach number - but, at altitude, the absolute (relative to the Earth's surface) speed of sound decreases, so you can't fly as fast at altitude without getting to the 'dangerous' regions around Mach 1. See compressibility and Mach number. — QuantumEleven | (talk) 07:14, 7 March 2006 (UTC)
- Yes, the Mach number is the speed relative to the speed of sound. It's also true that space isn't really a vacuum, just something close to it. But aren't the particles in space so far apart (compared to their size) that no sound can propagate? --Bowlhover 04:49, 8 March 2006 (UTC)
- Space isn't actually a vacuum... it's just very low density. The speed of sound up there is therefore very low (has to do with the large mean free path of a molecule) so it's easy to get to a very high Mach number without actually going very fast. moink
- Thnaks - yes, I realise that airplanes can't go into space, and that space isn't a perfect vacuum, I was just doing the thought experiment of what happens to the Mach number as a useful indicator of speed as you approach a vacuum. I guess for any sort of real world case you just wouldn't use it? For great justice. 21:54, 6 March 2006 (UTC)
- It is used for very high altitude flight occasionally - the re-entry speed of spacecraft such as the Space Shuttle is occasionally given in terms of Mach number (something around Mach 30, IIRC). However, Monik is right, because of the very rarified air, Mach 30 up there is a lot slower than Mach 30 at sea level (it's still ludicrously fast, though!). I would say that Mach number is used whenever there is aerodynamics involved, and therefore starts to be less meaningful at very high altitude, where aerodynamics are less and less involved in controlling a vehicle as there just isn't enough air around to make a difference! — QuantumEleven | (talk) 07:14, 7 March 2006 (UTC)
Pang tree?
[edit]Please see Pang (color). What is a pang tree? User:Zoe|(talk) 18:19, 6 March 2006 (UTC)
- Hmm, good question. That article has only been edited by one person, who hasn't edited anything else.. --BluePlatypus 19:26, 6 March 2006 (UTC)
- I'm not altogether convinced that there is such a thing... For great justice. 19:28, 6 March 2006 (UTC)
- I don't think it exists. --User:Mac_Davis
- This is a fairly typical article on a color. That is, it has no references. If Wikipedia standards were applied properly, almost all color articles would be deleted tomorrow. Notinasnaid 23:24, 6 March 2006 (UTC)
- Google Image gives a result for "pang tree": a sculpture. For -pang tree- without quotes, it leads to the pangkuna tree, which as far as I can tell doesn't have flowers, but there's not much about it. It could just be a tree most people haven't heard of. Black Carrot 23:34, 6 March 2006 (UTC)
rotational equilibrium
[edit]Is "rotational equilibrium with constant angular velocity" dynamic rotational equilibrium?, analogous to the dynamic equilibrium with constant linear velocity
- I think you're confusing dynamic equilibrium with mechanical equilibrium. They are substantially different. If you need more help, you'll have to clarify your question. —Keenan Pepper 18:12, 7 March 2006 (UTC)
Dripping Faucets
[edit]This has been bugging be for quite some time now. Turn on your faucet so that it produces a continuous stream of water. Then slowly nudge it closed until the stream is as thin as it can be without turning into drops. Now place your finger under the stream and move it up towards the faucet. As your finger approaches it, the stream turns into drops. But — and this is the weird part — the faucet stays dripping even after you remove your finger. You would think that it would go back to being a continuous stream, considering that the conditions are exactly the same as before and you have not adjusted the faucet. Does anyone know why this happens? --BrainInAVat 20:35, 6 March 2006 (UTC)
- It's to do with surface tension on the water stream. The surface tension on the stream keeps it as a stream. When you interupt it, it colapses, causing a drip to form - the surface tension on the drip 'sucks' more water into it, causing it to fall, etc. You could coax it back into a stream if you were careful. For great justice. 20:47, 6 March 2006 (UTC)
- Wow, that was fast :-). So do you mean each drop causes another drop to form? --BrainInAVat 21:01, 6 March 2006 (UTC)
- I think it does because when the first drop forms, its a shorter stream, and it is easier to collapse the stream. -- User:Mac_Davis Now, 6, March
- Wow, that was fast :-). So do you mean each drop causes another drop to form? --BrainInAVat 21:01, 6 March 2006 (UTC)
- Yes, water 'wants' to form into a shape that has the lowest energy. Depending on the volume and surface tension issues that might be a stream, or it might be a drop. For great justice. 21:54, 6 March 2006 (UTC)
- You've found a bistable configuration. —Keenan Pepper 23:01, 6 March 2006 (UTC)
Interesting. Thanks people. --BrainInAVat 01:32, 7 March 2006 (UTC)
- There's even more; the dripping of a leaky faucet exhibits chaotic behavior under the right circumstances, including period-doubling cascades (with respect to flow rate) and boundary crises. Physicists have studied these phenomena with Ford carburetor valves, among more advanced components. Check out this link and don't forget to click on "Citations to the Article (39)"! Melchoir 06:38, 7 March 2006 (UTC)
- Everyone loves the dripping faucet. :) --Optichan 14:16, 7 March 2006 (UTC)
- My first two science fair projects were on that. Black Carrot 23:41, 7 March 2006 (UTC)
- Yea, you gotta love a strobe light on a dripping faucet (that appears to make the water drip upwards). StuRat 04:12, 10 March 2006 (UTC)
Weight
[edit]Is it possible to estimate the weight of the earth? and does the weight of the earth change through the burning of materials and what effect will this have on the earth?
- The mass of the Earth is about 6×1024 kilograms, so its weight in the gravitational field of the Sun is about 3.5×1022 newtons. Burning materials does not change the mass or weight of the Earth because the mass of the ash and smoke is equal to the mass of the burned material and oxygen. That's called conservation of mass. —Keenan Pepper 22:47, 6 March 2006 (UTC)
- Burn anything : won't some gases leave the atmosphere after an unpredictable period of time ? --DLL 22:51, 6 March 2006 (UTC)
- On the other hand, meteors tend to add their mass to that of the Earth (either as atmospheric debris or physical bolides). — Lomn Talk 23:28, 6 March 2006 (UTC)
- Burn anything : won't some gases leave the atmosphere after an unpredictable period of time ? --DLL 22:51, 6 March 2006 (UTC)
There is some seapage of gas into space, but for practical purposes it's not a big deal. For great justice. 22:54, 6 March 2006 (UTC)
- Seepage is the wrong word. The atmosphere is completely open to the vacuum of space, so why doesn't space suck all the air out? Answer: Gravity. To overcome gravity, anything, such as any molecule, has to exceed the escape velocity to leave the earth. At the top of te atmosphere, the lightest molecules, H2 and He, have velocites that sometimes exceed the escape velocity, and they indeed escape. That's why Helium doesn't accumulate in the atmosphere, although it is constantly being produced due to alpha decays in the earth. GangofOne 03:29, 8 March 2006 (UTC)
- Yes, according to A Shite History of Nearly Everything (ISBN 1-84317-138-4 ; printed 2005), the Earth is 6.6 sextillion tons. And this makes me want to write more; here are nine other facts about planet earth:
- Our planet was formed from a ball of dust and hot gases about 4,5000,000,000 years ago (if you believe in the Big Bang).
- The Earth is not flat, nor is it round - it's an ellipsoid.
- Nearly one-eigth of the land on Earth is desert, while almost one-fifth is mountaneous.
- About one-tenth opf the Earth's surface is permanently covered with ice.
- The circumference of the Earth is 40,000km (25,000 miles).
- Oxygen is the most abundant element on the Earth's crust, waters and atmosphere (about 19.5%).
- The Earth travels at around 107,000 km (66,500 miles) per hour. Luckily there are no speed camers in space. Yet.
- This fact is the one I wrote above.
- It is estimated that sunlight takes eight minutes and 20 seconds to reach the Earth at 299,792km/sec (186,282 miles/sec).
- Hope this helps! Kilo-Lima Vous pouvez parler 21:20, 8 March 2006 (UTC)
- Yes, according to A Shite History of Nearly Everything (ISBN 1-84317-138-4 ; printed 2005), the Earth is 6.6 sextillion tons. And this makes me want to write more; here are nine other facts about planet earth:
- About you first pointyou don't have to believe in the Big Bang for that. That our solar system, and the Earth along with it, was created about 5 million years ago is pretty much accepted (I don't think anyone questions that), but the Big Bang is really just a theory to explain certain observations (most notably the expanding universe). But there are big holes in that theory, so that is indeed rather like a belief. Earth's age isn't. (For my alternative to the Big Bang theory see User:DirkvdM#Alternative to the Big Bang theory.)
- About your sixth point: I assume that's volume-percentage, not weight. And one should realise that the crust is just a minute fraction of the total size of the Earth (both in weight and volume). DirkvdM 13:15, 9 March 2006 (UTC)
- There are a lot of people who don't think Earth and the solar system formed 4.5 billion years ago. See young earth creationism. --Bowlhover 16:56, 9 March 2006 (UTC)
- And a lot of people think the earth is flat - a lot of people believe a lot of things. Celcius 03:18, 10 March 2006 (UTC)
Weight of the Earth
[edit]I have a number of points/questions related to the previous post, about the \"weight of the Earth\". They turned out to be so long that I decided to make them a separate post. I\'d be grateful is anybody could answer them:
- I guess \"weight\" could mean attraction in relation to whatever gravitational field an object is in (so, like Keenan Pepper said, the attraction between the Earth and the Sun would suffice).
- However, in general usage \"weight\" means \"weight in relation to the Earth.\" The weight of a rock is calculated GMM/r-squared, where the first M is the mass of the rock and the second M is the mass of the Earth \'\'minus the weight of the rock\'\', because the rock is not attracted to itself. But usually we do not minus the latter value, because the difference is negligible. However, if we were to measure the Earth in relation to itself, the latter value would be zero, since the Earth is not attracted to itself, so \'\'\'\'\'the weight of the Earth relative to itself would be zero\'\'\'\'\'. Am I right?
- But wait, the Earth \'\'is\'\' attracted to itself, as in, there is attraction between the different layers of the Earth. Isn\'t this how a black hole is formed, when a star has a gravitational collapse caused by self-attraction? So how would one calculate self-attraction for the Earth?
- Googling \"weight of the Earth,\" I got [20]. It says that the Earth \"weighs in at 5.972 sextillion (5,972,000,000,000,000,000,000) metric tons,\" but this is just the \'\'mass\'\' of the Earth (6E21 metric tons = 6E24 kilograms). Isn\'t this a misuse of \"weight,\" incorrectly substituted for \"mass\"?
- --JianLi 00:57, 7 March 2006 (UTC)
- 2. I would say this is closer to being undefined than zero. Don\'t ask why I say that though, just my intuition.
- 3. I would argue that there is no single value for that. The outer crust layer for example would weigh in relation to the earth, but the mantle would weigh less.
- 4. This is an incorrect use of the word weight. But this is a common occurrance, for example, people always say I weigh 80kg, when they meant to say I have a mass of 80kg.
- - Akamad 02:17, 7 March 2006 (UTC)
- 2. That formula only works when the two masses do not overlap. It is meaningless when they are the same object.
- 3. The particles of the Earth attract each other, just like any other particles, but you\'ll have to define more clearly what you mean by \"self-attraction for the Earth\". —Keenan Pepper 03:31, 7 March 2006 (UTC)
- 2. Right, Keenan, that is exactly my point. That\'s why I have to subtract the mass of the rock from the mass of the earth, so that the rock and the earth (as it is now defined) don\'t overlap --JianLi 05:20, 7 March 2006 (UTC)
We can easily state the mass of the Earth because it is an absolute quantity. It is true that in general usage \"weight\" means \"weight in relation to the Earth\", but when the thing you\'re weighing is the Earth itself, that breaks down. You cannot use the Earth as its own reference point, but would need to choose a celestial body with a known gravitational effect on the Earth. And the answer would be different depending on which body you chose (eg. the Moon, the Sun, a planet, or a distant star). Dividing the Earth up into the crust and other parts is no longer talking about the Earth per se, so this is getting into a different question altogether. JackofOz 04:24, 7 March 2006 (UTC)
- The fact is that outside of physics courses, the word \"weight\" very often means \"mass\". It\'s normal for people to interpret the word that way when mass is what makes sense, and when you ask for the weight of the Earth, \"mass\" is what makes sense. --Anonymous, 06:13 UTC, March 7, 2006.
I guess an easy way to answer this question is to think of one particle of matter, which can\'t have any weight because there is nothing attracting it/nothing to attract to. Add in a second particle and you have double the mass, but the weight stays the same (zero) because the weight of each particle relative to the other is the same (only in a negative direction) and they cancel out. Add a gazillion more particles and you have the earth, and it still weighs nothing. freshgavinΓΛĿЌ 06:50, 7 March 2006 (UTC)
You might want to look at shell theorem, which states that a spherically symmetric object acts like a point mass at its centre to objects outside the sphere, and has zero total gravity at any point within the sphere. moink 18:54, 7 March 2006 (UTC)
- Which means that, if want to calculate how much a hunk of rock within the Earth weighs in Earth gravity, it\'s F = GMm/r^2 - 0, where r is the distance from the center to the rock, M is the amount of Earth\'s mass that lies within that radius, m is the mass of the rock, and 0 is the net effect that all mass beyond that radius has. If the earth is a sphere, which it isn\'t quite, and if its mass is evenly distributed, which it isn\'t quite. Black Carrot 23:34, 7 March 2006 (UTC)
- So is there a way to integrate the attraction of the Earth (taking into account the shell theorem, of course) on each bit of the Earth? Would the result have any significance? --JianLi 01:20, 8 March 2006 (UTC)
I guess I should better explain my second point, to explain my reasoning that the weight of the Earth would be zero. If you were to calculate the weight of a mountain using GMM/r-squared, the first M would be the Mountain as expected, but the second M would be the mass of the Earth \'\'minus the mass of the mountain\'\' since the attraction is between the mountain the the \'\'rest\'\' of the Earth. So if you were to \"weigh\" a third of the Earth, the first M would equal 1/3 the mass of the Earth, while the second M would equal the remainder, 2/3 the mass of the Earth. And if you proceed along this path, by the time that you get weighing the entire Earth, the second \"M\" in GMM/r-squared would be equal to zero, making the \"weight of the Earth\" equal to zero. --JianLi 01:36, 8 March 2006 (UTC)
- I like that process better than mine. Easier to write on paper. freshgavinΓΛĿЌ 02:05, 8 March 2006 (UTC)
- I don\'t think that\'s quite right. It seems like a more in-depth answer would be more helpful than a two-body formula where, by the time you\'re done, one of the bodies doesn\'t exist. I mean, essentially what you\'re saying is that if the earth\'s all there is, there\'s nothing else to pull on it.
- The main problem is that your question just doesn\'t mean anything. Now, if it\'s \"how much inward force, in pounds, is exerted on a layer of the earth by the rest of the earth,\" that has an answer. If it\'s \"what\'s the sum of all those values moving outwards from the center to the surface and including the atmosphere,\" once again, that has an answer, and that is probably the closest thing to what you mean that you\'ll find. To give you an idea what that is, it would take just a bit more than that amount of force, outward in all directions from the center, to form a small bubble in the earth\'s core. And it would have to be a sustained force, because of course everything would fall back down as soon as you stopped pushing. Black Carrot 22:27, 8 March 2006 (UTC)
- I think that would be ( being density), assuming a perfect sphere with evenly distributed mass. I don't think that would be very accurate, though, since the rock at the core is a whole lot denser, and therefore a whole lot more massive, than the rock at the crust, which is again a whole lot denser than air or water. Black Carrot 23:20, 8 March 2006 (UTC)
- I fixed the math symbols above, by taking out the extra slashes. Did I get it right ? StuRat 04:51, 10 March 2006 (UTC)
Vegans and meat
[edit]I've heard that after going without meat for so long, vegans (and vegetarians) can acquire a sensitivity to meat that is so great that they become nauseous after unknowingly consuming food with meat. Is there any scientific name for this acquired "distaste"? (And since it involves unknowingly consuming it, we can rule out a psychosomatic cause.) --JianLi 23:44, 6 March 2006 (UTC)
- I've heard that vegetarians stop producing certain enzymes required to digest meat, so that if we were to eat it, our bodies wouldn't be able to break it down and we'd throw it up. But I don't know if it's true or not. moink 04:11, 7 March 2006 (UTC)
- hmm, interesting. can anyone confirm that? --JianLi 05:34, 7 March 2006 (UTC)
- As a vegetarian of 30 years, I think the above suggestions are unlikely. I used to fancy seafood for a while after I gave up meat, but now I no longer like it. I don't know of a word for this disacquisition of the taste for meat; will that one do? No doubt the many links from the vegetarianism article would provide more info. Some vegetarians do need to eat a small amount of meat for medical reasons, but I don't know the details. --Shantavira 09:38, 7 March 2006 (UTC)
- I've been vegan for 12 years, and I tried meat a couple of times during that period. I can say that after a while, it does seem really thick, chewy and heavy, and after eating it you really do feel lethargic and slow. It seems to be that after a while on veganism, your mind gets lighter and you feel more energetic in general, mentally and physically.
- I've been vegetarian all my life, and any dead meat within a radius of 100m makes me nauseate. deeptrivia (talk) 01:29, 12 March 2006 (UTC)
Abilify VS Risperdal
[edit]I'm bipolar. I'm being treated for bipolar mania.Right now I'm switching over from Risperdal to Abilify. Now, I'm pretty well versed on these two medications. I know that I needed to be on Risperdal first before they switched me over to Ablify because Abilify would have probably made me worse if I started it first. Also, Risperdal acts fast and pretty much numbs your mind by blocking all the dopamine. I noticed that, while on Risperdal, my creativity was not what it used to be. I could barely come up with new ideas for stories, and the ones I did come up with aren't great, and my drawings now suck. They, really, really suck. Before my drawings were awesome. And it wasn't because I was delusional, everyone told me they were. My question is, once I'm on Abilify, will my creativity and drawing skills return? Or is it gone forever now?
- If requesting medical advice, please consider asking a doctor instead. --Canley 02:12, 7 March 2006 (UTC)
- Doesn't really seem like they're requesting medical advice, but rather information on the lasting side-effects of the drugs they're taking. First of all, check out the articles on Aripiprazole (Abilify) and Risperidone (Risperdal) if you haven't already. Although the articles don't seem to be that comprehensive, they do give the impression that Risperdal has a lot more side-effects than Abilify, though there is no mention of a high-risk of long-term effects.
- I would like to assume that a return of your creativity would merely be a return of your motivation and will, something that may be being affected by your consumption of Risperdal. It's not very productive to think of Abilify as the drug that gave you your skills, when I'm sure it had really nothing to do with Abilify in the first place. I don't see any reason why some change in your lifestyle (or medication) could bring back your prior creativity, unless Risperdal is known to cause long-term (permanent) mental deficiencies. freshgavinΓΛĿЌ 06:46, 7 March 2006 (UTC)
- Risperdal blocks (antagonizes) all of the dopamine, like you said. On the other hand, aripiprazole is a partial agonist of the dopamine receptor. A partial agonist will block dopamine where there is too much of it, but it will also act as dopamine where there is too little of it.
- Anyways, back to your concern about whether your creative skills will return after switching from Risperdal to Abilify: I haven't been able to find studies done on the long-term cognitive effects. As a pharmacy student, I'd be interested in hearing your experience switching between the two drugs. Feel free to leave me a message at my talk page. --Uthbrian (talk) 12:50, 7 March 2006 (UTC)
- Being marginal bipolar, and from my experience, great creative bouts are a symptom of the disease. The other side being descent into hell. Many famous bipolars were unmedicated or on drugs and booze. They had short lives. If you are stable, and feel good, you have to work harder for creativity. Try lucid dreams, etc. --Zeizmic 14:22, 7 March 2006 (UTC)
- Agreed. The meds may, unfortunately, diminish these positive side effects as well as the main condition. StuRat 10:52, 10 March 2006 (UTC)
Goethe's Theory of Colours
[edit]What exactly is the verdict on Goethe's Theory of Colours? The article on Goethe says it is his "weakest work," while the article on the Theory of Colours itself is considerably more sympathetic. --JianLi 01:36, 7 March 2006 (UTC)
- I think it's safest to defer to the most specialized article on a given topic; i.e., Johann Wolfgang von Goethe should be changed to reflect Theory of Colours. In fact, Johann Wolfgang von Goethe cites the description of a book that is considerably more sympathetic than the article suggests. You may want to raise this issue on the talk pages... Melchoir 06:27, 7 March 2006 (UTC)
- Thanks, I just did. --JianLi 01:15, 8 March 2006 (UTC)
March 7
[edit]annoying keystroke shortcut in Outlook
[edit]I'm a poor typist. Too often in Outlook, while composing a message, my left fingers slip on the shift & ctrl keys while hitting another key with my right hand, and the message is immediately sent automatically. What keys am I hitting? If I knew, maybe I could avoid the problem. BTW, the Fn key is also in that area on my laptop. I believe that CRTL is the key (no pun intended) to the problem, the gateway to many unintended destinations. But in combination with what? --Halcatalyst 03:29, 7 March 2006 (UTC)
- Are you sure the message is only sent, and nothing else is done? According to http://office.microsoft.com/en-us/assistance/HP030842231033.aspx, the keyboard shortcut for sending emails is Alt+S. One way to fix the problem is to leave out the "To:" field until you're ready to send. --Bowlhover 05:17, 7 March 2006 (UTC)
- I don't use Microsoft products, but isn't Ctrl-W the MS shortcut for "Close everything right away without giving me any sort of warning of any kind." I get a lot of people who apparently hit it while in Word, Excel, IE, and Outlook and complain that the window just closes. For me, it is Nano's "where is" shortcut. --Kainaw (talk) 13:29, 7 March 2006 (UTC)
- For mine it's "ask if you want to save, then close". It could be irritating if hit accidentally but it shouldn't be disastrous except for users who don't believe in reading the boxes they click on. I realize that's a large segment of the population, but I can't find much sympathy. — Lomn Talk 14:32, 7 March 2006 (UTC)
- Ctrl+Enter in Outlook means "instantly send this message that I haven't finished typing". Bowlhover's suggestion (leaving the To: field empty until the last minute) is the only defence I know of. --Heron 20:49, 7 March 2006 (UTC)
- I know of another defence, at least on Thunderbird: tell it to encrypt emails by default, and it will complain when trying to send if you don't have the public key for the person you are sending to. At the last moment, turn off encryption for that particular message. I don't know if it works on Outlook, since I never used it, but I do know it has encryption support. --cesarb 21:17, 7 March 2006 (UTC)
- I found a registry hack that disables this shortcut. See Outlook Daily Tips, Tip 333: Prevent Accidental Sending. It worked for me on Outlook 2002/XP. --Heron 11:16, 25 May 2006 (UTC)
- Ctrl+Enter in Outlook means "instantly send this message that I haven't finished typing". Bowlhover's suggestion (leaving the To: field empty until the last minute) is the only defence I know of. --Heron 20:49, 7 March 2006 (UTC)
- For mine it's "ask if you want to save, then close". It could be irritating if hit accidentally but it shouldn't be disastrous except for users who don't believe in reading the boxes they click on. I realize that's a large segment of the population, but I can't find much sympathy. — Lomn Talk 14:32, 7 March 2006 (UTC)
- I don't use Microsoft products, but isn't Ctrl-W the MS shortcut for "Close everything right away without giving me any sort of warning of any kind." I get a lot of people who apparently hit it while in Word, Excel, IE, and Outlook and complain that the window just closes. For me, it is Nano's "where is" shortcut. --Kainaw (talk) 13:29, 7 March 2006 (UTC)
Did any of those awful non-consensual experiments make any significant or lasting contributions to medical knowledge? moink 04:13, 7 March 2006 (UTC)
- This question is addressed at Talk:Nazi human experimentation. Short answer, no. Melchoir 06:19, 7 March 2006 (UTC)
- The U.S. made a secret deal with Shiro Ishii, Unit 731, and Unit Ei 1644 leaders that germ warfare data based on human experimentation would be offered in exchange for immunity from war-crimes prosecution in 1948. The US also made secret deals with Nazis. How many have not been since unclassified is unknown. WAS 4.250 16:02, 7 March 2006 (UTC)
- Untrue, I recall reading in Hans Rudel's book, Stuka Pilot, there was a discovery about how pilots could survive in freezing water. This was discovered using human experimentation. —The preceding unsigned comment was added by 12.41.204.3 (talk • contribs) 15:00, 7 March 2006.
- Ah. That deserves checking on, if anyone has access to the book. I'll copy this discussion to the talk page. Melchoir 02:14, 8 March 2006 (UTC)
- Does our hypothermia article mention it ? StuRat 02:17, 10 March 2006 (UTC)
- I heard this mentioned several times as the one single piece of useful knowledge that came out of the experiments. Celcius 03:23, 10 March 2006 (UTC)
Development of Artificial Chemical Expert system
[edit]Sirs, I am doing research on data mining. My application area is the development of artificial expert based systems based on quantum mechanics. If anybody is doing research in that area, please provide me with information concerning the latest developments in this area. Thank you. I am an Assistant Professor in Computer Science in ISTAR, Anand, India.
- Interesting topic, but this is the wrong place to look for such information. Try something like a scientific journal instead. —Keenan Pepper 18:07, 7 March 2006 (UTC)
- I think you meant scientific journal. Black Carrot 23:37, 7 March 2006 (UTC)
Lorenzo's oil
[edit]I was searching the internet to purchase Lorenzo's oil and your website came up, although I couldn't find anywhere to purchase it. Any suggestions? Thanks (Email removed to avoid @spam) 65.78.72.157 06:01, 7 March 2006 (UTC)
- I'll say it again, it's not my website, and there is NO cabal. I found this on the first link that google gave me:
- In the U.S., Lorenzo’s Oil is currently only available to patients taking part in a clinical trial under the direction of Dr. Hugo Moser of the Kennedy Krieger Institute. He can be reached at (800) 873-3377. We are working with Dr. Moser and the FDA to obtain approval of Lorenzo's Oil as a drug, so that it can be widely available. The oil is jointly manufactured by Croda International of Britain and SHS International (Scientific Hospital Supply). SHS is also the worldwide distributor of the oil; for a list of international offices, please refer to their website. SHS North America can be reached at (800) 365-7354.
- In the U.S., Lorenzo's Oil can be obtained through prescription only by Kennedy Krieger authorized physicians. A 500ml bottle costs $56.00. Some insurance companies will provide coverage for the oil, but others do not because it is still considered an experimental drug by the FDA. Parties residing outside the U.S. should contact Scientific Hospital Supply directly at + 1 (301) 795-2300, to obtain pricing and shipping information.
- freshgavinΓΛĿЌ 06:33, 7 March 2006 (UTC)
- Silly question, but we are sure that 65.78.72.157 isn't just looking for a DVD of the movie, right? Grutness...wha? 12:59, 7 March 2006 (UTC)
- Actually, I would think that more likely. I'm not advocating any single seller, but typing "Lorenzo's Oil" into Amazon.com and eBay seem to get enough results for the 1993 VHS release and the 2004 DVD release. EWS23 | (Leave me a message!) 18:04, 7 March 2006 (UTC)
- How silly of me. But still I doubt that. This is the science page and there are quite a few people asking where to buy difficult to find industrial and medical substances here. freshgavinΓΛĿЌ 02:09, 8 March 2006 (UTC)
- Actually, I would think that more likely. I'm not advocating any single seller, but typing "Lorenzo's Oil" into Amazon.com and eBay seem to get enough results for the 1993 VHS release and the 2004 DVD release. EWS23 | (Leave me a message!) 18:04, 7 March 2006 (UTC)
External fertilization
[edit]Is this possible in humans? If yes how and if no why?--Suraj vas
- In vitro fertilisation? freshgavinΓΛĿЌ 06:35, 7 March 2006 (UTC)
- Its not possible, sperm has to get to the egg, probably only possible through the vagina. --
 Mac Davis] ☢ ญƛ. 04:28, 8 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 04:28, 8 March 2006 (UTC)
- I take it you haven't heard of in vitro fertilisation either then. freshgavinΓΛĿЌ 04:34, 8 March 2006 (UTC)
- Ask Louise Brown - she may have an opinion on the matter. Grutness...wha? 06:51, 8 March 2006 (UTC)
Portable document readers
[edit]I am curious: are there devices on the market whose function is primarily to act as a portable document reader? I was envisaging something about A5 sized, which could store and read documents, perhaps annotate them with a stylus. I know PDAs currently perform these functions, but I was thinking of a more purpose-built device, essentially a form of electronic paper. I know these things were talked about over ten years ago, but I don't think I have really seen them available yet. Or what have I missed? Does anyone know more about such things? — QuantumEleven | (talk) 13:30, 7 March 2006 (UTC)
- Sony Reader is one. It uses e-paper, a few megabytes of storage, and can handle special books and PDFs. -- Ec5618 13:34, 7 March 2006 (UTC)
- Thanks, Ec - yes, I had seen the Sony reader, it just surprises me that someone has only come up with an item like this recently, or have there been other examples in the past (but which failed commercially, for whatever reason?) — QuantumEleven | (talk) 14:19, 7 March 2006 (UTC)
- Weight / battery life / ease of use / reasonable cost have been a difficult combo to achieve. I don't think it's so much been a case of past failure as past lack of a feasible design. — Lomn Talk 14:35, 7 March 2006 (UTC)
- Thanks, Ec - yes, I had seen the Sony reader, it just surprises me that someone has only come up with an item like this recently, or have there been other examples in the past (but which failed commercially, for whatever reason?) — QuantumEleven | (talk) 14:19, 7 March 2006 (UTC)
- I have one called Paper. Features: Inexpensive. Requires no batteries. Lightweight. Durable. Disposable. Environmentally-friendly. Handles all text formats. Easier to read than LCD displays. (does require an external light source). Supports annotation and underlining. Etc. I'm serious here.. there aren't many benefits that justify spending $300 on a e-book reader (or even much less) when you can get a used paperback for $0.50. Looking at Amazon, it appears a new paperback is cheaper than the e-book. And that's if it's available as an e-book. I don't have to worry about dropping a paperback either. It's the classical case of a solution in search of a problem. --BluePlatypus 15:36, 7 March 2006 (UTC)
- It depends a lot on what you what to read, and where. I do scientific research for a living. I'd love to have a lightweight, portable device that I could store, read, and annotate papers in. Something light, easy on batteries, maybe solid state storage, durable, touch screen for annotation...it would be sweet. I can imagine that someone travelling to a non-English-speaking country might want someplace to keep his novels—putting a month of reading in a backpack would be far too heavy. University students would be thrilled not to have to cart textbooks around.
- Though the solution doesn't solve any of your problems, it's a bit hasty to suggest that it's useless. Presumably since the questioner is asking, he has a purpose in mind. TenOfAllTrades(talk) 16:19, 7 March 2006 (UTC)
- The question was "Why aren't there any e-book readers?" or "Why aren't e-book readers more common?", and my answer was that they're simply not useful for most people and purposes. Especially compared to the hype they were getting 10 years ago. I didn't say they were useless. --BluePlatypus 16:58, 7 March 2006 (UTC)
- When I have to wait for something, and I'm totally bored, I read classic novels on my Blackberry. It's good enough for reading and it's convenient. Proper e-books will never sell if the publishers want to price it the same as paper books. This is the same thing with the record collusions. --Zeizmic 18:13, 7 March 2006 (UTC)
How to categorize inventions?
[edit]There are branches of industries such as light/heavy industry, chemical, biological, information, medical, aerospace industries. How do I categorize patents using their U.S. class numbers or internaptional class numbers (IPC)? In my opinion, the U.S. class system is quite unstructuralized. Its class codes are not grouped by their technological features. Here are some U.S. class codes:
- http://www.uspto.gov/go/classification/selectnumwithtitle.htm
- 002 Apparel
- 003 - UNUSED -
- 004 Baths, closets, sinks, and spittoons
- 005 Beds
- 006 - UNUSED -
- 007 Compound tools
- 008 Bleaching and dyeing; fluid treatment and chemical modification of textiles and fibers
Are there tools that enanles us to quickly categorize U.S. or other countries' patents? -- Toytoy 16:02, 7 March 2006 (UTC)
Banned Gases
[edit]Hi there, can anyone remember the banned gases becuase it deterioated the Ozone layer. I was think carbon dioxide, but that seems too extreme as we breathe it out. Wasn't it that one that was originally put in fridges? Thanks. Kilo-Lima Vous pouvez parler 17:49, 7 March 2006 (UTC)
- It was CFC. --cesarb 17:54, 7 March 2006 (UTC)
- You're probably thinking of Freon, which is in the article on haloalkanes. —Keenan Pepper 18:00, 7 March 2006 (UTC)
- That'd suck if they banned carbon dioxide. freshgavinΓΛĿЌ 02:12, 8 March 2006 (UTC)
While carbon dioxide does not damage the ozone layer, it is a greenhouse gas, and, as such, excess levels of may contribute to global warming. Thus, there have been attempts, such as the Kyoto Protocol, at limiting releases. Ironically, ozone itself is also a greenhouse gas. StuRat 01:00, 10 March 2006 (UTC)
Health question
[edit]I'm trying to find out and a rash on my sons face, stomach,arms and legs, and he looks a little pale in his face.I want to know if maybe allergic or something? Moved by me from the Newcomers help page. --Chachu207 18:14, 7 March 2006 (UTC)
- If benadryl clears up the problem, then it is probably an allergy. --Kainaw (talk) 18:43, 7 March 2006 (UTC)
- Or a self-limited problem. alteripse 01:04, 8 March 2006 (UTC)
- This sounds like a question that should be answered with: You should probably go to a family doctor and have your son checked out, especially if he is very young. freshgavinΓΛĿЌ 02:15, 8 March 2006 (UTC)
- Well, do that then, if you are so inclined. I, however, prefer not to bother people with the blatantly obvious. (This, of course, should itself have been a blatantly obvious remark, but given the amount of people who blurt out these disclaimers, apparently it is not.) DirkvdM 13:24, 9 March 2006 (UTC)
- I don't know, I didn't think that giving a child benadryl was an obvious solution. I've actually never seen benadryl before, and I don't know (yet) how powerful or safe it is, though I'll surely check it up now. I also said that because it seemed like a pretty serious rash (spreading over the face, stomach, arms, and legs) and I thought it might be an indication of something else. freshgavinΓΛĿЌ 07:32, 10 March 2006 (UTC)
- Well, do that then, if you are so inclined. I, however, prefer not to bother people with the blatantly obvious. (This, of course, should itself have been a blatantly obvious remark, but given the amount of people who blurt out these disclaimers, apparently it is not.) DirkvdM 13:24, 9 March 2006 (UTC)
- A rash could be a zillion things - of course it's a case for a doctor! If he had a headache, fine, aspirin, but a rash over the kids entire body isn't something to just diagnose yourself or over an internet "forum"!! Do people in America have to pay to see a GP? --Username132 17:12, 12 March 2006 (UTC)
drug screen
[edit]Two weeks ago I took a random drug screen at work, no problems with that but I was wondering what the intials of the drugs that they tested for were and what they meant. The letters were,"THC,COC,PCP,OPI,AMP". Any idea?
- I'm at a loss on the first, but I'd go with cocaine, PCP, opiates like heroin, and amphetamines. — Lomn Talk 18:29, 7 March 2006 (UTC)
- Upon searching "THC drug" here at WP, it's tetrahydrocannabinol, the active chemical in marijuana. — Lomn Talk 18:32, 7 March 2006 (UTC)
- I believe one tests positive on such tests for THC for years after the last usage, which makes the tests rather useless, I suppose. Depending on what one wants to know of course, but who would want to know you once had a joint as a kid? DirkvdM 13:27, 9 March 2006 (UTC)
- THC (marijuana), COCaine, PCP, OPIates (heroin), AMPhetamines (ecstacy).
You were tested for the NIDA Five. NIDA is the U.S. National Institute on Drug Abuse. The five drugs tested are marijuana, cocaine, PCP, or phencyclidine (angel dust, Love Boat, "embalming fluid"), opiates (heroin, morphine, and other painkillers derived from Opium), and amphetamine. Methyldioxymethamphetamine (MDMA) or Extasy, is covered under the amphetamine test. There's also a more comprehensive panel of ten drugs including the former drugs plus barbiturates, benzodiazepines, propoxyphene (Darvocet) and methadone which are similar chemically, lysergic acid diethylamide (LSD), and methaqualone (Quaalude).
Marijuana is not detectable in blood or urine after 7 to 37 days. Over-the-counter decongestants can cause a positive result for amphetamines, and poppy seeds can cause a false positive for opiates. Brian G. Crawford 19:47, 14 March 2006 (UTC)
Impacted wisdom tooth
[edit]Here's one for the dentists out there: If you've got a wisdom tooth impacted against your rearmost molar, why remove the wisdom tooth? Wouldn't it be possible (and perhaps easier) to remove the molar and let the wisdom tooth replace it? Seems a bit of a waste to throw out a brand new tooth just because it doesn't fit. --BluePlatypus 20:04, 7 March 2006 (UTC)
i'm not a denstist, but common sense says that you should remove the tooth that is causing trouble and leave the tooth that works. why would you want a crooked, troublesome tooth to be left in there? --Chris 00:28, 8 March 2006 (UTC)
Did you see the recent discussion of this very topic on the Wisdom teeth talk page? At least one of the participants seems to be a dental student. --LarryMac 00:50, 8 March 2006 (UTC)
- A wisdom tooth should optimally be extracted when doing so confers some benefit to the patient (I won't discount the possibility that it is sometimes done for the benefit of the dentist, but that is the stuff of another discussion). In other words, the extraction of a wisdom tooth should address some problem without causing a worse problem. And there are many situations where the presentation of the wisdom tooth or the health profile of the patient makes an extraction too risky to justify. At the same time, an impacted wisdom tooth will often happily co-exist with the other teeth without any harm to its owner. As is the case in all diagnosis and treatment planning, a dentist must assess the current and future risk posed by the impacted tooth, and compare it to the risk of intervention, which usually takes the form of extraction of the wisdom tooth. In the situation posed by the original poster, the removal of the second molar may indeed be justified if it is in poor repair and there is reasonable expectation, based on the position and orientation of the wisdom tooth, that it will erupt into the space left by the extraction. However, wisdom teeth only occasionally meet the criteria for this expectation. Also, wisdom teeth often have aberrant shapes that may detract from their functionality. Each situation must be considered on its own individual merits.--Mark Bornfeld DDS 18:01, 8 March 2006 (UTC)
- Interesting! Thanks for the reply. --BluePlatypus 19:47, 8 March 2006 (UTC)
Survey Question (Curiosity)
[edit]What kind of thing have you bought on eBay recently? Black Carrot 23:22, 7 March 2006 (UTC)
- Records, a mouse, a New Folder icon (just kidding). freshgavinΓΛĿЌ 02:19, 8 March 2006 (UTC)
- I\'ve only bought ferrofluid, neodynium magnets, and planning to buy 205x4 foot sheet of mylar. —Preceding unsigned comment added by Mac Davis (talk • contribs)
- A New England Patriots shirt, camo trousers, and a pair of Doc Martens. Not all at once, obviously. Sum0 16:44, 8 March 2006 (UTC)
- Books, and some Frankoma pottery. User:Zoe|(talk) 16:45, 8 March 2006 (UTC)
- Nothing. Does that count? DirkvdM 13:30, 9 March 2006 (UTC)
- Not really. I\'m trying to learn the market. You may have heard of the misspelling thing on eBay? Well, I\'m trying to see if I can make any money off that, but it requires knowing what will sell in the first place. Black Carrot 20:31, 9 March 2006 (UTC)
- I have a total hatred for "spoof" sites (that use misspellings of common web URLs) which will prevent me from ever buying anything from them. From eBay I've bought a board game (King Oil) and a book ("North By Northwest" - The Alfred Hitchcock movie). StuRat 00:53, 10 March 2006 (UTC)
World's largest desert...
[edit]The current revisions of Antarctica and Sahara both state that each is the world's largest desert. Which is correct? --Robert Harrisontalk contrib 23:56, 7 March 2006 (UTC)
- Antarctica appears to be bigger than the Sahara. [21] The Sahara is only the biggest (at least in its category) if you separate it like so: [22]. And another source with a nice map: [23]. EWS23 | (Leave me a message!) 00:34, 8 March 2006 (UTC)
- Answer - it depends. If you define a desert as having less than a certain amount of precipitation per year, antarctica (which actually gets very little rain or snow) qualifies as a desert and thus is the largest desert in the world. If you define desert in terms of present ambient water, Antarctica (which contains a lot of permanently frozen water) does not qualify and thus the sahara is the largest. Raul654 03:15, 8 March 2006 (UTC)
- Yes, it depends on what answer you want. For the largest dry desert, it is Antarctica, for the largest hot desert, it is the Sahara.
- You appear to be rather proud you have an answer on that, that you advertise it so boldly. :) DirkvdM 13:32, 9 March 2006 (UTC)
Kind of similar, the article for Easter Island claims it's the most isolated inhabited island, while some reliable sources (namely Bill Bryson) give the honour to Tristan da Cunha. I've left a comment on the Easter Island page but there's been no response yet. freshgavinΓΛĿЌ 02:22, 8 March 2006 (UTC)
- Hm. That honour (?) often goes to Pitcairn Island. As to deserts, it's a little known fact that the Sahara contains so much sand that - if it was spread out - it could cover all of North Africa. Grutness...wha? 06:57, 8 March 2006 (UTC)
- Yes, Antarctica is definitely a bigger desert. The problem is that a lot of people don't define desert correctly. Hopefully, desert states correctly a desert is an extreme environment (not just a hot and/or dry one), but adjusting Sahara to say "hot desert" and Antarctica to say something else would fix this inaccuracy. - Mgm|(talk) 09:39, 8 March 2006 (UTC)
Scientific Misconduct
[edit]Earlier this year, there were news stories Boston Globe NYTimes about how the editors of scientific journals, most notably The Journal of Cell Biology, have had to become more vigilant in response to photo manipulation by researchers. The editor of that journal is quoted, "In 1 percent of the cases we find authors have engaged in fraud." Who exactly are the kinds of scientists who engage in this? I'm not familiar at all with how scientific research is conducted, so I would like to know: Is there be some kind of mechanism for penalizing these scientists, similar to how lawyers are disbarred or doctors lose their license? It seems that these scientists get off scot-free. In fact, according to the Boston Globe Article, some just re-submit their fraudulent papers in other magazines and get published. --JianLi 01:03, 8 March 2006 (UTC)
- In the US, the method mentioned for penalizing lawyers by disbarrment is mostly theoretical. An attorney can violate law (eg, in knowingly misrepresenting the identity and criminal status of a witness in a criminal trial) and suffer no penalty whatsoever. Not from employer (local district attorny's office), not from the courts, not from any agency charged to police crime -- perjury and subornation of justice would seem to be crimes to which attention ought to be paid by police, ... Even when the conduct is admitted under oath in another coart and in an affidavit given under penalty of perjury. The police are not interested, the FBI is not, and the courts ruled that no further filings would be accepted in matters relateing to the trial and alleged perjury during it.
- The local Bar Association took nearly a year to even appoint an investigating attorney, he took several years to 'gather' evidence, and in the end the Bar Association Disciplinary Committee decided there was nothing to discipline. Actually happened in the 80s in King County Washington, believe it or not. Justice is not a word I'd apply with a straight face to such a system. An attempt at it perhaps. The theory's good, though. ww 12:15, 8 March 2006 (UTC)
- Yes, there usually is one or even two ways of policing research fraud. First is that most (reputable) universities will appoint, if an accusation of fraud occurs, a review board to scrutinize in detail the research in question. If it's found to be fraudulent the researcher can face suspension or termination from their position. And there will be notices of this in the journals who published the papers. Secondly, the people issuing grants almost always have independent reviewers and auditors of the research conducted for their grant money (They don't just give the money away without checking that you're doing what you said!). For serious research fraud, it almost certainly means your career as a scientist is over, because you'll have little hope of getting a new job, or getting grants.
- As for what kinds of scientists get involved in it.. I think it depends on the fraud. Ideally, a scientist is supposed to have an open mind, and be prepared to drop any idea as soon as there's convincing evidence for the opposite. In practice, this can be harder to do. How do you not get emotional about something you've worked hard on for years? Also, there's an entire spectrum of dishonest behaviour, and it's not always clear when you're in the wrong. Some of the main things reviewers encounter are whether you've investigated alternative explanations enough, and whether your conclusions are supported by the actual results. It's pretty much accepted that people will often draw conclusions as large as can possibly be supported. Then you've got leaving out things which may cast doubt on your results. It could be something insignificant, but it could also be something which you're fooled yourself into believing is insignificant. At the far end, you've got folks who systematically fabricate results without even doing the experiments. I guess they're motivated by careers. I wouldn't really know, though. --BluePlatypus 19:19, 8 March 2006 (UTC)
March 8
[edit]Composite Spacecraft
[edit]I was wondering how much a cubed foot of spacecraft worthy composite material would wiegh?
- I would have to say it would depend on the specific composite material. Have anything specific in mind? — TheKMantalk 02:18, 8 March 2006 (UTC)
Would about kevlar?
The Sea as the Largest Desert
[edit]I think the sea is the largest desert on earth if desert only means a place unhabitable to life.
Let's say if I capture a salt water fish from its natural habitat, I think I can easily find a place in the high seas of similar temperature but without any food. The fish will starve to death before seeing any edible thing. Not even a tuna or a mackrel.
Am I wrong? -- Toytoy 02:13, 8 March 2006 (UTC)
- If you capture an American from its natural habitat and displace it to someplace faraway (let's say, Northern China) there's a good chance it'd die of starvation before seeing anything considered "edible" to an American. freshgavinΓΛĿЌ 02:26, 8 March 2006 (UTC)
- According to Chambers
desert: noun; an arid area of land where rainfall is less than potential evaporation, vegetation is scarce or non-existent, and which is characterized by extremely high or low temperatures. Also as adj.
And I agree.
Slumgum 02:45, 8 March 2006 (UTC)
- According to Chambers
- And Wikipedia's desert. As for habitability, the sea is absolutely filthy with life, historically and presently. Melchoir 02:47, 8 March 2006 (UTC)
As to microscopic life, I think the ocean surface (plenty of sunshine, water, CO2, ...) can support planktons. However, I have heard a theory stating that most sea surface lacks iron so lives cannot grow. You need wind over land to carry dust (rich in Fe2O3) to the sea so lives can grow. -- Toytoy 02:57, 8 March 2006 (UTC)
- Basically, in many ways the upper regions of deep seas are very similar to a desert, but because it's not land, it's not a real desert. If you were doing a report on it you could coin the term "sea desert". freshgavinΓΛĿЌ 04:28, 8 March 2006 (UTC)
Is natural selection inherently "intelligent?"
[edit]There's been a lot of nonsense recently regarding "Intelligent Design," so to start off, just to set the record straight, I am not advocating any theological explanations.
Here is what I am saying: Scientifically speaking, humans are merely very complex machines, made of the same types of elements as anything else. However, their organization leads to extraordinary emergent properties such as intelligence. So if humans, who are merely machines, can be intelligent, can't the process of natural selection itself, by some definitions of "intelligent," be considered that?
Natural selection in effect "distinguishes" between good and bad traits, and then allows the good ones to continue.Take trees for example. "Within any one species, the taller the tree, the relatively larger the buttresses. It is widely accepted that the shape and size of these buttresses are close to the economic optimum for keeping the tree errect, although an engineer would require quite sophisticated mathematics to demonstrate this." (Dawkins, Selfish Gene) So, in effect, even though it does not do any mathematical calculations, natural selection is solving an optimization problem.
The difference between natural selection and the engineer is that the engineer uses mathematical calculations, while natural selection relies exclusively on trial-and-error (not to mention the considerable difference in time taken to solve the problem).
So, just as the human body is a complex piece of machinery whose emergent properties make it intelligent, why can't the biosphere, which is the complex machinery engaged in natural selection, have emergent properties which give it "intelligence?" --JianLi 02:17, 8 March 2006 (UTC)
- I think you first gotta define what intelligence is. What do you consider to be intelligence here? One could argue it's about conciousness. You think the biosphere is self-aware? ☢ Ҡi∊ff⌇↯ 02:23, 8 March 2006 (UTC)
- If intelligence is the ability to come to conclusions without resorting to trial-and-error, then I don't see why this wouldn't be a significant benefit in terms of evolutionary gain. While it is often said that intelligence is not expected from evolution, it is certainly probably because of the possible benifits towards survival that it allows. If an ancient ancestor of a tree (a pre-tree organism) had been able to estimate an efficient size-ratio for a tree through the use of basic mathematics, they would have saved a lot of evolutionary time that could be spent evolving on other benificial things (like walking trees, talking trees, cocktails manufactured for tree-like sensibilities). POV: I don't believe evolution constitutes intelligent design because it doesn't have this ability to make a logical choice without significant stimulus. By some definitions of intelligence, though, it could be interpreted that way. You'd be pushing it though. freshgavinΓΛĿЌ 02:37, 8 March 2006 (UTC)
- Regarding the definition of intelligence: I agree that the biosphere doesn't fulfill the requirement of self-awareness or consciousness, but why should that be a prerequisite of intelligence? Consciousness is "the most profound mystery facing modern biology" (also Dawkins). If we do not even understand it fully, I think this allows us some latitude in defining intelligence. I think it's unfair (as in anthropocentric) to have such a narrow definition of intelligence as the one commonly accepted, with the restrictions imposed on it by both Kieff (conciousness) and freshgavin (a non-trial-and-error thought process). It seems as if we define intelligence for the express purpose of excluding all non-human entities from having it! --JianLi 02:40, 8 March 2006 (UTC)
- If you use the definition "The capacity to acquire and apply knowledge" and you fudge a bit by considering encoded information to be "knowledge", then you could say that a biological system exhibits intelligence through the use of DNA and natural selection. If, however, you use the definition "The faculty of thought and reason", I don't think it would apply. Even in the first case, though, it's a bit of a stretch, semantically. Kaldari 02:53, 8 March 2006 (UTC)
- I didn't mean to imply that a "non-trial-and-error thought process" equals intelligence, but merely that it's probably a subset of a few processes that when combined form a complete definition of intelligence. I agree that stating intelligence is conciousness or self-awareness is very presumptuous given our understanding, and it's inherent difficulty to justify, and I think it's more useful to define intelligence as a combination of ... abilities? which can each be (relatively) easily defined. You could liken it to Wikipedia, which is much easier to define accurately in WP:NOT than in Wikipedia:What Wikipedia is. freshgavinΓΛĿЌ 04:23, 8 March 2006 (UTC)
- If you use the definition "The capacity to acquire and apply knowledge" and you fudge a bit by considering encoded information to be "knowledge", then you could say that a biological system exhibits intelligence through the use of DNA and natural selection. If, however, you use the definition "The faculty of thought and reason", I don't think it would apply. Even in the first case, though, it's a bit of a stretch, semantically. Kaldari 02:53, 8 March 2006 (UTC)
- Regarding the definition of intelligence: I agree that the biosphere doesn't fulfill the requirement of self-awareness or consciousness, but why should that be a prerequisite of intelligence? Consciousness is "the most profound mystery facing modern biology" (also Dawkins). If we do not even understand it fully, I think this allows us some latitude in defining intelligence. I think it's unfair (as in anthropocentric) to have such a narrow definition of intelligence as the one commonly accepted, with the restrictions imposed on it by both Kieff (conciousness) and freshgavin (a non-trial-and-error thought process). It seems as if we define intelligence for the express purpose of excluding all non-human entities from having it! --JianLi 02:40, 8 March 2006 (UTC)
- I am not quite certain that humans have a non-trial-and-error though process. Certainly, we do not manifest every erroneous creation. But it is quite possible that we making leaps of imagination, we are simulating trial and error in our heads, and combining it with a processing engine that processes the consequences of each assumption - something really fairly identical to natural selection. -User:Fangz (not logged in)
One of the significant ways in which evolution is not intelligent is that while it's very good at blindly finding its way to a local optimum or maximum, it's very bad at stepping back, getting the "big picture", and trying a completely different approach that would let it reach some other, and much higher, optimum or maximum. (Although on the other hand, us supposedly intelligent humans aren't always so hot at doing this, either.) —Steve Summit (talk) 05:51, 8 March 2006 (UTC)
- Very true, and a point raised too seldom in these discussions. There's an huge amount of stuff which simply isn't 'intelligent' at all. For instance, vestigial traits like wisdom teeth and appendixes (appendices?). Another point not often raised is how evolution isn't just macroscopic traits. It's something which is visible on every single level of structure, down to the molecular level. So while we don't have very many vestigial organs, we've got plenty of vestigial genes that are never expressed. --BluePlatypus 13:03, 8 March 2006 (UTC)
The universe is a computer running an Evolutionary computation program. If humans running this program call it intelligent, why not call nature running it intelligent? WAS 4.250 07:10, 8 March 2006 (UTC)
- You've asked "why not" without first considering "why". freshgavinΓΛĿЌ 07:27, 8 March 2006 (UTC)
- BluePlatypus, I don't think vestigial organs or genes immediately disqualify the process that created it from being intelligent. Intelligent animals do many irrational things (for example, things which are done more out of habit than because of utility, and which may actually detract from utility). Yet, in the sense that I am talking about, people who are highly irrational are still "intelligent beings." Of course, you may call them "unintelligent" or "stupid" in the sense of "what a stupid/unintelligent person!", but of course that's a different usage of "intelligence." JianLi 23:26, 8 March 2006 (UTC)
- 'Intelligent' is a subjective term, and it's pretty useless to discuss what constitutes an 'intelligent' process or not. Do you want a semantic discussion or a biological one? My point is/was that it obviously costs energy to produce and maintain those vestigal traits and since they provide no benefit to the organism, they're a burden. That doesn't mean they're 'mistakes'. Vestigal traits are an unavoidable consequence of how evolution works. - It's an illustration of Steve's point, that evolution does not work towards a 'global' optimum, but towards the best it can do "working with what it has". --BluePlatypus 12:37, 9 March 2006 (UTC)
- I feel I should add that even though the question won't be resolved here, it's a really beautiful idea, JianLi, and lots of fun to think about. Thanks for bringing it up.Adambrowne666 02:54, 14 March 2006 (UTC)
- thanks :) JianLi 03:47, 14 March 2006 (UTC)
Big Bang
[edit]If the big bang theory states that the universe began in an enormous explosion, and a law of science states that "you can't get something from nothing", then how could such an explosion occur?
- The question assumes facts that are not in evidence (and which are, frankly, false). All the matter/energy present in our current universe was, to the bset of our knowledge, present at the moment of the big bang some 13 billion years ago. So it is not a case of "something from nothing", it's a case of something from itself. Raul654 03:13, 8 March 2006 (UTC)
Antimatter? --JianLi 03:07, 8 March 2006 (UTC)
- It is quite possible to create "something from nothing" - ie, ex nihilo, viz. quantum field fluctuations and such. Of course, this in itself raises a lot of peculiar issues. As for the original question, I think it is too general for it to be answered with any precision. I would like to see someday, someone publish a paper linking evolution and nonextensivity. Now that would be most wonderful. --HappyCamper 04:47, 8 March 2006 (UTC)
- While it is quite possible to "create something from nothing," due to quantum effects, a common misconception of what the big bang theory presents is that all matter today came out of one point in time in a huge explosion. This is incorrect. The big bang theory presents that at some point billions of years ago, spacetime suddenly began expanding, greatening the distance between things, but without moving them. The big bang theory breaks no laws of thermodynamics.
- A recent Gallop poll showed roughly 50% of Americans believe in creationism over evolution. People don’t generally reject evolution because of little or no evidence in support of it, they reject it because of their religious convictions, and because it’s not obviously intuitive, there is an illusion of design which people find hard to comprehend, so why bother with scientific evidence showing evolution to be wrong when you can simply refuse to believe in it out of a rigid mindset which rejects the possibility of accepting design without a creator? Evolution's illusion of design misleads even very smart, educated people. Lots of engineers, for example, will quote you the eye as something that is "obviously designed". Werner von Braun, father of both Hitler's and Kennedy's rocket programs, was of this opinion and was not shy about saying so. --
 Mac Davis] ☢ ญƛ. 04:54, 8 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 04:54, 8 March 2006 (UTC)
- Since the nazis believed they were a "master race" of eveolved supermen, wouldn't that actually make them histories strongest supporters of darwin?--Somebodye6436346 05:15, 8 March 2006 (UTC)
- Now that this topic has been Godwinned, can we pretend it's gone? Melchoir 05:23, 8 March 2006 (UTC)
- I think so, but read my answer first. --
 Mac Davis] ☢ ญƛ. 06:43, 8 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 06:43, 8 March 2006 (UTC)
- I think so, but read my answer first. --
- Now that this topic has been Godwinned, can we pretend it's gone? Melchoir 05:23, 8 March 2006 (UTC)
- Re the Nazi's, I think that would merely classify them as people who have incorrectly applied Darwin's theory. Evolution has no "goal" (but if it did, I guess that would prove my argument ;-) ), so the supposed "Master Race" is just an accident of evolution. In addition, humans aren't the epitome of evolution anyway. Just the fact that both humans and cockroaches exist today puts us on about equal footing, I think. And if there were a nuclear holocaust, cockroaches would probably have a better chance of surviving. So, in that case, the "master race" would more likely be cockroaches than Aryans. JianLi 23:41, 8 March 2006 (UTC)
- Or if you read Kafka, it could be both ;)64.12.116.74 23:47, 8 March 2006 (UTC)
- Since the nazis believed they were a "master race" of eveolved supermen, wouldn't that actually make them histories strongest supporters of darwin?--Somebodye6436346 05:15, 8 March 2006 (UTC)
- Some points of clarification since we seem to have gone off track:
- The Big Bang is not an explosion. It is an inflation of space. Space itself expanded - not just a ball of matter within it.
- We don't know what there was before the big bang. In fact, it is fairly absurd to talk about such a notion. Theories about the origin of the big bang are currently really only just speculations, because we have no measurements whatsoever from such a time. In particular, it cannot be asserted that there was nothing before the big bang.
- Nothing itself is hard to define. Empty space is filled with all sorts of quantum weirdness. And even without that, we have the curvature of space to consider and so on. The big bang may simply break all laws of science, because these laws only came into existence with the big bang.
- -User:Fangz (not logged in)
Sand ridges
[edit]I noticed in the article Sand that the whenever the ridges split, it always splits in twos, not threes or fours. Why is this? Is there a reason why this is the case? --HappyCamper 03:43, 8 March 2006 (UTC)
- The answer is antimatter, it makes sand split in twos, because everyone knows antimatter doesn't split into fours--205.188.116.74 03:52, 8 March 2006 (UTC)
- Well, a 3-split would quickly become two 2-splits when one of the three daughter ridges shifts a little. Then there's probably an effective repulsive force between ridges, which would in turn move the 2-splits away from each other until they don't coincide visually. Of course, I'm just making this up. Melchoir 04:05, 8 March 2006 (UTC)
- I'm looking for an answer possibly related to fluid dynamics and criticality... --HappyCamper 04:32, 8 March 2006 (UTC)
- Perhaps it's the other way 'round - two ridges approach each other and meld into one. hydnjo talk 04:48, 8 March 2006 (UTC)
- Okay, I've done some research (try searching for "aeolian" on arXiv), and the ripples do tend to merge over time. There is little known about the two-dimensional dynamics, particularly the evolution of defects, but I found at least one reference:
- See "Two-dimensional ripple fields" towads the bottom. Melchoir 05:10, 8 March 2006 (UTC)
- How did you know to search "aeolian"? This was a key word that I was not aware of. --HappyCamper 17:20, 8 March 2006 (UTC)
- Oh, I tried searching arXiv for more obvious words like "sand" first, which led me to the magic jargon. Melchoir 20:14, 8 March 2006 (UTC)
Chemical Engineering
[edit]Give the binary as well as ternary azeotropic data for the following system>
Ethanol - Water - Ethyl Acrylate
If possible then also give the phase diagram and residue curve map data
- If you're going to post your homework, could you at least not phrase it as a command? Melchoir 07:45, 8 March 2006 (UTC)
- People who aren't creative enough to do their own homework also aren't creative enough to rephrase it when they type it into a reference desk service like this and "ask" someone else to do it for them. But don't complain; the blatant problem-set diction is a useful marker: it reminds us not to answer. —Steve Summit (talk) 15:49, 8 March 2006 (UTC)
- Indeed. Melchoir 20:18, 8 March 2006 (UTC)
- Good point JianLi 00:14, 9 March 2006 (UTC)
- People who aren't creative enough to do their own homework also aren't creative enough to rephrase it when they type it into a reference desk service like this and "ask" someone else to do it for them. But don't complain; the blatant problem-set diction is a useful marker: it reminds us not to answer. —Steve Summit (talk) 15:49, 8 March 2006 (UTC)
- I'm sure they asked you the question with the intention of teaching you how to look it up yourself. Either try the course book, or use a library reference like the "Handbook of Chemistry and Physics" or some "Physical chemistry" book. - Mgm|(talk) 09:44, 8 March 2006 (UTC)
nuclear reaction
[edit]E=mc2,in this equation m stands for mass lost. But the law of conservation of mass states that mass cannot be created nor distroyed. why is this so? Suraj ThanksSuraj
- Our article on conservation of mass is a bit short, but might be of help to you. In brief, the principle of conservation of mass is an approximation - in chemical reactions, some mass is lost/gained, but the quantities are so infinitessimally small that they can be safely ignored, leading to the "mass is constant" rule, which for these works perfectly well. For nuclear reactions (such as nuclear fission or nuclear fusion), the mass gain/loss starts to become measurable, and so must be taken into account. The extreme case is matter-antimatter annihilation, where all mass is converted to energy, and so "mass is conserved" is obviously incorrect.
- More accurately, energy is always conserved, if, by using E=mc² , you equate mass to its equivalent in energy. — QuantumEleven | (talk) 08:36, 8 March 2006 (UTC)
- With nuclear reactions the mass isn't lost either. It's just converted into energy. - Mgm|(talk) 09:45, 8 March 2006 (UTC)
- The conservation of mass and conservation of energy laws must just be combined to say "the total of the mass and energy of a system is preserved, with respect to the equation E=mc²". This can be described by the following equation:
- Where:
- initial energy of the system
- final energy of the system
- initial mass of the system
- final mass of the system
- speed of light
- StuRat, your terminology is... shall we say, nonstandard. What you call "energy" is really kinetic energy; what you call "total of mass and energy" is really energy. Melchoir 01:07, 10 March 2006 (UTC)
- "Energy" would not just be kinetic energy, but would also include other forms of energy such as heat and gravitational potential energy. The equation I listed does convert everything (including mass) into energy for a quantity comparison. However, you could just as well divide by to convert everything into mass for a quantity comparison:
- Okay, yes, if you want to include those kinds of energy without calling them kinetic; I was thinking of simpler systems. My point is that in relativistic contexts, the symbol E and the word "energy" already include mass. So either you're using symbols in a way contrary to everyone else, or you're counting the mass twice. Melchoir 02:03, 10 March 2006 (UTC)
- Well, I doubt if most people, including the person posing the question, are thinking in those "relativistic terms", thus the need for my explanation. StuRat 02:08, 10 March 2006 (UTC)
- If most people think of the speed of light and mass-energy equivalence in nonrelativistic terms, then they're wrong, and we do them a disservice to perpetuate their ignorance. Energy is conserved, period. Melchoir 02:18, 10 March 2006 (UTC)
- Using the term "energy" to include mass is just a linguistic choice, thus there is no right or wrong answer, only "more common" and "less common" usages. What exactly do you call energy excluding mass, then ? StuRat 02:28, 10 March 2006 (UTC)
- It is, in fact, a linguistic choice that was made decades ago. For a single particle, energy minus mass is the definition of kinetic energy; for a complex system it's more complex. Melchoir 02:41, 10 March 2006 (UTC)
- There is no body that "decides" what a word will mean in the English language, it means whatever people use it to mean. Dictionaries only report what it means, they don't chose what it will mean. StuRat 04:35, 10 March 2006 (UTC)
Why is there no footage of moon astronauts jumping really high?
[edit]We\\\'re always told that on the Moon your gravity is something like 1/6th of its weight on Earth and you could effortlessly jump three metres high (or something like that). So why is it that in existing moon footage all the astronauts do is pussyfoot around with those tiny skip-hops? If I ever landed on the moon the FIRST thing I\\\'d do is jump around like I was on a trampoline. So why didn\\\'t they? Professional discipline? Risk of damaging the suits? Or is it proof the landings were faked? —Preceding unsigned comment added by 202.72.148.102 (talk • contribs)
- Some reasons include:
- the suit had a mass of 180 pounds [24]
- the soles of the boots were (I think entirely) stiff, depriving the astronaut of all of the jumping power of his foot and ankle - try putting on a pair of snowboarding boots and see how high you can jump.
- I guess they\\\'d also be worried that, if they fell, they couldn\\\'t get up.
- --Finlay McWalter | Talk 12:05, 8 March 2006 (UTC)
- I\\\'d assume they\\\'d also be worried about landing badly and ripping the suit on a rock or something, which would ruin one\\\'s day rather. Sum0 16:40, 8 March 2006 (UTC)
- Also, the legs of the spacesuits were rather stiff - they would have trouble bending their knees very far. You try jumping very high if you\\\'re only allowed to bend your knees a little way (and barely using your arms for balance and momentum). — QuantumEleven | (talk) 12:40, 8 March 2006 (UTC)
- You may also be interested in reading our article on the moon landing hoax. In a nutshell, most \\\"hoax\\\" arguments are the result of drastic simplifications or sheer ignorance. — Lomn Talk 15:08, 8 March 2006 (UTC)
- I read once that in addition to all the other hinderances of the suit, it was nearly impossible to bend at the waist considering the suit stiffness and pressures involved, so they had to use a pulley mechanism just to lean over. Think of it this way, you are bound in nearly every way except the ability to wiggle your arms and legs a minimal amount. The fact that they got around as much as they did is amazing in itself.
I\\\'d certainly be a lot more worried about the air-less space of death 5 centimeters from my skin than I would be about jumping around like an idiot, but that\\\'s just me. freshgavinΓΛĿЌ
- LOLZ!!1!1 onely noobs r \\\'fraid of xpl0siV d-comPr3ssi0n!1!1!! NOOB! rofl Black Carrot 20:00, 9 March 2006 (UTC)
- They would have needed thicker wires in the studio for higher jumps. For great justice. 21:58, 9 March 2006 (UTC)
Why are computers so flaky?
[edit]Most people I know want reliability rather than extra bells and whistles in their computers, so why are the things still so prone to self-destruction? It is not technically possible to create hard drives which won't crash, and circuit boards which won't burn out? Or is it because we're too stupid to buy a safe, reliable computer rather than a sexy one with new features? Markyour words 13:00, 8 March 2006 (UTC)
- I blame the parents.
Slumgum 13:03, 8 March 2006 (UTC) - Did you buy a cheap computer, or look for a highly reliable one? For example, most hard disk manufacturers sell premium lines that go into servers and have a much higher MTBF. In fact, hard disks are the only thing I've seen much of failing, and they inherently will fail one day: reliable computers are designed to accept this and make sure it does not matter. But people don't look for reliable computers, by and large: they look for the cheapest one with the list of features that they think they need. Notinasnaid 13:07, 8 March 2006 (UTC)
- Computers are seen as consumer products, and are sold to the masses who, by and large, have absolutely no idea what they're buying. They look at a few of the numbers in the spec (speed, HD space, RAM...), then buy the cheapest one for a given spec. This drives the PC manufacturers to build cheaper and cheaper components - they can't sacrifice performance, so they sacrifice quality, especially knowing as computers become obsolete so fast anyway that they often don't even reach component failure.
- It's certainly possible to build computers that are tough and durable - look at the computers on deep-space probes, those things are designed to run for decades in a radiation-filled environment, extreme cold with no maintenance whatsoever. They're ridiculously tough. And, of course, ridiculously expensive, for the same reason.
- Bottom line - if you know what you're buying, you can certainly buy a computer which is much less likely to die (for whatever reason) than that $899 super-deal PC at the local supermarket. But you'll pay for it. — QuantumEleven | (talk) 15:22, 8 March 2006 (UTC)
Basically, you're right: we're too stupid. For one thing, even though we say we don't care about bells and whistles, we can almost always be goaded (by salesmen and advertising) into buying something fancier over something plain. In other words, the bells and whistles sell, so we get them even though we don't need them.
More significantly, we're suckers for bargains. By and large, in the mass market, cheaper sells better -- very few people are actually willing to pay significantly more for something that's incrementally better. Disk drives are a perfect example: they're ridiculously cheap, but this economy comes at a cost: they're not nearly as reliable as they used to be. A few years ago, many if not most disk drives came with 3-year warranties; now they're all 1-year (or 90 day). Venerable manufacturers like IBM have gotten out of the disk drive market because they can't make money in it; price competition is too fierce.
- At the risk of sounding like an ad, Seagate hard drives still all have 5 year warranties WhiteDragon 17:08, 10 March 2006 (UTC)
Price competition tends to bifurcate a market in a very cruel way. You end up with stuff that's cheap but lower-quality, but it's very cheap, because it enjoys the economies of scale of highly optimized mass production. High-quality but more expensive stuff gets marginalized, and though you can usually find it somewhere, it tends to be a niche, luxury market, and you can end up paying a lot more for it. Also, since it's a niche and "expensive" market, it tends to end up pandering to people with more money than they know what to do with, who like to spend money on "prestigious" items. So if you resolve not to be a bargain hunter, if you decide to put your money where your mouth is and pay more for higher-quality items, you often find that the widget that's (say) twice as reliable costs five times as much, because it's also leather-padded and diamond-studded with gold-plated mounting hardware and a designer name on the front.
—Steve Summit (talk) 15:43, 8 March 2006 (UTC)
I gather Apple computers are robust both in terms of hardware and software. They cost a little more but they look nice and don't crash so often. --Username132 18:29, 8 March 2006 (UTC)
- Righty-o. I've never EVER had any hardware problems with my last three Apple computers. Well, one time I dropped my Apple Pro mouse on the tile floor too many times. :| --
 Mac Davis] ☢ ญƛ. 07:35, 10 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 07:35, 10 March 2006 (UTC)
- Personal computers are consumer products, and as such they're made to be as cheap as possible, even if that means occassional hardware failures. For applications where failure is more important there are correspondingly Workstation machines and at the top end, there are Mainframes. Mainframes are almost invunerable to hardware failure, because there are backup systems for just about every component. --BluePlatypus 19:54, 8 March 2006 (UTC)
So if I were trying to find a (not ridiculously expensive) reliable hard drive, for example, how could I tell which one is least likely to die on me? Would it be reasonable to assume that the more expensive it is, the more reliable? Markyour words 20:43, 8 March 2006 (UTC)
- I think this question suggests the wrong approach. Hard drives will fail, and unless you prepare for it, then it will be a nuisance in a week, or a nuisance in 2 years: still a nuisance. You should be looking for ways to avoid hard drive failure being a big deal. Backups, of course, but restoring can still be an annoying waste of a day, so why not add mirroring. Not a hugely expensive option, and I tend to specify it on any computer I value. Notinasnaid 21:09, 8 March 2006 (UTC)
- Hmm, what is a mirroring option? hydnjo talk 21:59, 8 March 2006 (UTC)
- Mirroring is basically installing two identical hard drives, and setting up either a hardware or software solution that makes sure that every disk write goes immediately to both drives. If one drive fails, the other drive is used - nominally automatically, but especially with IDE drives a failed drive may lock up the PC so you might still need to disconnect it. Then you put in a replacement as quickly as possible and resynchronise. Key thing to watch out for: if you don't have a system for checking whether one mirror has failed, you might as well not have bothered! I use a hardware solution from Promise, preinstalled by Dell. Monitoring options are poor: you have to do it manually. Notinasnaid 22:13, 8 March 2006 (UTC)
- Emphasize what Notinasnaid said about monitoring! There is nothing so embarrassing as losing all your data because your backup drive fails also (i.e. when a second drive fails after you failed to notice that the first had failed). It's like discovering that your spare tire, when you need it, is flat, or that your fire extinguisher is empty, or that your UPS doesn't work. —Steve Summit (talk) 03:51, 9 March 2006 (UTC)
- I'd say the basic answer to this question is that computers are based on principles that were invented 100 years ago. The basic structure of a computer has hardly changed at all in the last 30 years (or even 50). We just keep on inventing cooler parts, and adding them to a very old model (that works pretty well). The computer industry would benefit hugely from a completely re-thought computer model, one that didn't have you navigating through things like the BIOS, valence/integrity checking, memory permission errors, priority issues in IDA ports, and peanut butter on your CD-ROM tray. They've got a lot of new principles (like true plug-and-play and modular systems), but since they're all based on really old structures that weren't initially intended for such fancy shmancy gidjits, there's always going to be weird errors popping up, backdoors, and headaches. freshgavinΓΛĿЌ 01:42, 9 March 2006 (UTC)
I can't believe this hasn't been mentioned yet. A major difference between computers and just about any other product is that the technology develops at a very rapid pace; double the capacity and speed every year and a half (for decades now). So if you buy a computer at half the price that lasts half as long then after it's 'dead' you buy another and after that's 'dead' too you'll have spent the same amount of money but half the time you've been working on a much bigger and faster computer. But what's more, excepting hard disks, people don't even 'use up' their computer. I've got three computers, all three functional, except that I only use one of them. I might use the middle one as a dedicated machine for simple stuff, but the oldest one is a 80286 - software for that hasn't even been written for a decade now. So there's no point in making them more robust - even at the 'low quality' we get now, they're obsolete before they die.
Another thing that matters, though, is software. Now if they'd do a decent job of making robust software (ie Linux in stead of ms bloody Windows) that would make me a much happier person. Having said that, neither OS uses my graphics card/monitor combination well (the monitor is a wide-screen and the card is pretty non-standard too), but when a friend connected a Mac (a mini-Mac!) to it, it used the full width after a few simple clicks, without any special drivers needed. So if you're willing to spend a little more for quality, go for a Mac.
About the mirroring thing, is that the same as RAID? DirkvdM 18:54, 9 March 2006 (UTC)
- Yes, mirroring is one particular type of RAID, specifically RAID 1. --WhiteDragon 17:14, 10 March 2006 (UTC)
- Going back to the discussion of software, that is where I find most of the trouble. I rarely have hardware fail but on a daily basis run into an "illegal operation" type error. The basic concept of a PC, that many programs will run simultaneously, some originating locally and some from over the Internet, and that they will each work together nicely, is flawed. This allows one program, whether maliciously or accidentally, to mess up all the other programs and bring the computer down. Each program should run in total isolation, with no ability to affect the others. I envision a parallel computer with, say 32, old, dirt-cheap processors, each with it's own old, dirt cheap hard drive. These programs could run independently so that even the most virulent could not possibly affect anything beyond it's processor and hard disk. One of the 32 processors would run a master control program which would kick off the others and control which programs have control of the monitor and accept input from the keyboard and mouse. Too bad we can't convince Bill Gates to come out with something like this (he prefers coming out with "new and improved" versions of windows with "new and improved" bugs, since each new O/S of his is based on the same flawed model of programs "cooperating nicely"). StuRat 20:26, 9 March 2006 (UTC)
- This would be possible if somebody went back and thought about how to build a computer from scratch, and build it in a way that programmers wouldn't have to worry about system specs and fidgities. I don't even think we need to bother with the concept of a conventional "processor". freshgavinΓΛĿЌ 07:25, 10 March 2006 (UTC)
- To answer your questoin - yes, for people who are willing to pay for it, you can get a computer that has extra reliability. A blade server, for example - you can literally rip pieces out of it while it's running and it will keep on humming fine. On the other hand, it's going to cost you a pretty penny. If you want to insulate yourself from hard drive crashes, you can pay for full drive mirroring (Raid level 1, I think -- two hard drives doing exactly the same actions in paralllel - if one of them fails, you have a perfect copy already handy without worrying about data loss).
- As to your more general - why are they prone to destructoin question - it depends on what you mean. Hardware destructon is relatively rare - certainly much less so than an automobile, for example. Software screwups are quite a bit more common, owing to the huge general purposes for which computers are applied, and the number of possible bad interactions increases in proportion to the factorial of the number of tasks a computer is running. Raul654 07:31, 10 March 2006 (UTC)
- BTW if using mirroring or raid it may be worth mixing the makes/models. You will like lose in performance and waste some capacity (as the drives will never be exactly the same size) but you will protect yourself from batch faults. Plugwash 01:34, 14 March 2006 (UTC)
Searching For Research Groups
[edit]I'm hoping to go into research when I leave uni, in the feild of engineered zinc finger protein transcription factors (preferably mammalian) - I was wondering how I could find all the relevant research groups in the UK and Netherlands? Is there some kind of central database I could use? --Username132 18:25, 8 March 2006 (UTC)
- Nope. I'd suggest you either A) Just ask someone in the field. They should know. B) Check some review papers on the subject and check the reference list for such groups or C) Look for representatives from those places in conference schedules on the subject. --BluePlatypus 18:53, 8 March 2006 (UTC)
- As BluePlatypus said, check existing papers on the subject. Most will be written by professors at other universities. One problem I've found about working in research for the past fifteen years is that it is very hard to get into if you aren't either a professor or a student. So, I decided to get my PhD and become a professor so I can keep doing research easily. --Kainaw (talk) 19:03, 8 March 2006 (UTC)
Conservation Of Momentum In Time-Demension
[edit]Does the conservation of momentum apply to the dimension of time? --Username132 18:26, 8 March 2006 (UTC)
- It depends, what does that question mean??--64.12.116.74 20:17, 8 March 2006 (UTC)
- Oh, you're going to love this. Check out Noether's theorem. Symmetry under translations in space leads to conservation of momentum, and symmetry under translations in time leads to conservation of energy. —Keenan Pepper 20:22, 8 March 2006 (UTC)
- Dang, beaten to it! Well, also check out the article Four-momentum. Melchoir 20:25, 8 March 2006 (UTC)
- well, 2 edit conflicts later, all I have left to offer is fourth dimension, I feel so inadequate );64.12.116.74 20:30, 8 March 2006 (UTC)
- Thirteen minutes to post a sub-adequate reply, that must be a record! freshgavinΓΛĿЌ 01:36, 9 March 2006 (UTC)
- well, 2 edit conflicts later, all I have left to offer is fourth dimension, I feel so inadequate );64.12.116.74 20:30, 8 March 2006 (UTC)
- Dang, beaten to it! Well, also check out the article Four-momentum. Melchoir 20:25, 8 March 2006 (UTC)
- So if something explodes, the products of the masses and the amounts they are accelerated through time will equal those of the particles deccelerated and/or sent back in time? --Username132 02:31, 9 March 2006 (UTC)
- If you interpret "acceleration through time" as a change in the Lorentz factor of a body, and you carefully also take into account changes in mass, then yes: you can make this into a meaningful, true statement. Ultimately it's still just conservation of energy. Melchoir 02:44, 9 March 2006 (UTC)
Cold shock and free radicals
[edit]Why does cold shock cause free radical release (in potatoes if possible)? Thanks --Nick123 (t/c) 22:51, 8 March 2006 (UTC)
- It's not just cold shock. *Any sort of stress causes cells to release free radicals. Reactive oxygen species should have more info on the issue and a lot of those radicals come from the mitochondrion. - Mgm|(talk) 10:11, 9 March 2006 (UTC)
- To elaborate just a bit, freezing a food product such as a potato can cause the formation of ice crystals that puncture cell membranes, leading to stress. Edgar181 22:12, 9 March 2006 (UTC)
- Thanks for your help! --Nick123 (t/c) 21:35, 11 March 2006 (UTC)
March 9
[edit]animals that are consumers and producers
[edit]Is a grey seal and lion considered a consumer or predator and why
- A grey seal and a grey lion? Or a lion and a grey seal? Or possibly a grey seal and a sea lion? freshgavinΓΛĿЌ 01:33, 9 March 2006 (UTC)
- It depends. If they are middle class, they are consumers. If they are upper class, they are predators. If they are lower class, they are a safety net for the middle class and the source of all society\'s problems for the upper class. Wait... isn\'t this your sociology homework? --Kainaw (talk) 01:44, 9 March 2006 (UTC)
- Following the link in Consumer to the biological form of the word, I get Heterotroph, which links to Autotroph, the scientific word for producers. Those go into comfortable detail about what each is and isn\'t. In case you actually meant to include the word \"predator\" and it wasn\'t a typo, you might try Predator and Prey as well. Black Carrot 13:05, 9 March 2006 (UTC)
Chewing Gum (CareFree)
[edit]What is florason?
- She's the Roman goddess of Spring dad. Grutness...wha? 07:17, 9 March 2006 (UTC)
- Floraison is the French word for "flowering." My guess is that it is artificial fragrance in the gum. --
 Mac Davis] ☢ ญƛ. 10:10, 9 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 10:10, 9 March 2006 (UTC)
The Moon
[edit]Why is the flag waving if theirs no gravity.
- Cuz they obviously faked the moon landing, and you've discovered the one long lost bit of proof that we've been looking for ever since 1894 when the aliens first landed in the hills of MT.BOGATAWOGASFEILD, at last we can expose their secret, moon-landing-faking, alien agenda, and the MT.BOGATAWOGASFEILD aliens will be defeated forever!--64.12.116.74 00:38, 9 March 2006 (UTC)
- See moon landing hoax. Also, the reason why the flag is waving is because there's an atmosphere in the sound stage where they faked the landing. Both Earth and the Moon have gravity—which is necessary for the flag to hang properly. TenOfAllTrades(talk) 00:40, 9 March 2006 (UTC)
- There is gravity on the Moon, but little wind. If you're talking about Image:Buzz Aldrin with U.S. flag.jpg, I can only assume that when you jostle a hanging piece of cloth in the absence of air resistance, it doesn't settle down until its internal friction absorbs the energy, which will take a while.
- Also, it's a fake. Melchoir 00:44, 9 March 2006 (UTC)
- Dang, TenOfAllTrades' link kicks my ass. Melchoir 00:47, 9 March 2006 (UTC)
- Has anyone considered that the Moon Landing hoax goes even deeper? What if the Moon itself is a hoax? The Moon Landing was just there to reinforce the propoganda that there is a Moon and not a huge spy satellite floating around above our heads. --Kainaw (talk) 01:41, 9 March 2006 (UTC)
- No, don't be crazy, it was the aliens--64.12.116.74 01:44, 9 March 2006 (UTC)
- The 'Moon Landing Hoax' was itself a hoax. They wanted to prove to the Russians that they could do a really good hoax, and have them quiver in their vodka. Unfortunately, they couldn't make convincing enough and actually had to go to the moon for the pictures, while still saying it was done in a sound studio. --Zeizmic 02:40, 9 March 2006 (UTC)
There is no real moon, we all just have this huge cataract on one of our eyes and wen we look into the sky its visible.--Im_in_ur_house 01:30, 9 March 2006 (UTC)
- Actually, there is no Earth, and those who live on the moon discuss the Earth landing hoax. EWS23 | (Leave me a message!) 06:35, 9 March 2006 (UTC)
Sigh. The flag had wire sewn into it along the top. It was deliberately put there because it was known that - with no wind - the flag would otherwise have just hung limply and made for lousy photographs. Grutness...wha? 07:17, 9 March 2006 (UTC)
- BTW, have you ever wondered who creates all these conspiracy theories? Have they a motive for doing so? There must be a reason for it... perhaps they're trying to divert our intention from the truth... Perhaps the Moon landing was fake - as fake as Christ of the Andes! Grutness...wha? 07:22, 9 March 2006 (UTC)
Right. Stop this. This section has gotten entirely too silly. The whole premise is silly and the answers are badly written. Move along. --The Colonel 08:47, 9 March 2006 (UTC)
- Gravity doesn't make the flag wave, wind does (and it doesn't wave), it is rigid. --
 Mac Davis] ☢ ญƛ. 10:12, 9 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 10:12, 9 March 2006 (UTC)
- Here's why the flag was moving:
- There is gravity on the moon, just less than on earth.
- There was something sewn in the top to keep it up.
- No gravity doesn't mean no wind.
- Why people are so hell-bent on claiming anything to be a hoax, I'll never know. - Mgm|(talk) 10:18, 9 March 2006 (UTC)
- Well one reason they keep on huffing about is because a lot of the explanations given are a little bit weak. There's some flaws in the way you worded it. a) The flag was moving because there's gravity on the moon. If you think of from the perspective of a human being, gravity is the thing that causes everything to STOP moving (when it hits the ground). An absense of gravity causes things to coninue moving forever (from a relative position) and so this doesn't make sense as an argument. b) It moves because there's something on the top to keep it up. Again, something stiff on the top was put there to keep it from moving (e.g. keep it up) so it sounds strange to use this as a reason. c) No gravity doesn't mean no wind. Actually, it does. Without any gravity, particles will not gather together in one place (let's say, the atmosphere) and thus there will be no substance around to create any wind, just empty space. What I think you meant to say was: 1) The gravity on the moon caused the flag to ruffle as it was being set. 2) There is a bar sewn at the top of the flag to keep it from falling down. #3 doesn't really need to be said. freshgavinΓΛĿЌ 07:18, 10 March 2006 (UTC)
- OK, since it seems people want every T dotted and every eye crossed, let's put it this way. The wire at the top of the flag kept the top rigid. The flag would therefore have been stretched to its full length from hoist to fly, rather than hanging limply. Since the flag would need to have been moved into position in order for it to have been erected on the lunar surface, it would have been in motion to get it to that position. The bottom edge of the flag was not wired, and was hanging freely. Due to inertia, it would not have automatically been in direct position below the upper edge of the flag the second the flag was erected. Thus, the bottom edge would have acted very much like a pendulum - it would have moved backwards and forwards until it came to rest due to gravity at its lowest possible point. Due to the lower gravity of the moon (as compared to the Earth) and the lack of air resistance to the flag, this slight motion would have taken quite a long time to subside. What was seen as the "flapping" of the flag was nothing more than this - the bottom edge of the flag acting as a pendulum beneath the rigid upper edge. Happy? Grutness...wha? 10:09, 10 March 2006 (UTC)
- Well one reason they keep on huffing about is because a lot of the explanations given are a little bit weak. There's some flaws in the way you worded it. a) The flag was moving because there's gravity on the moon. If you think of from the perspective of a human being, gravity is the thing that causes everything to STOP moving (when it hits the ground). An absense of gravity causes things to coninue moving forever (from a relative position) and so this doesn't make sense as an argument. b) It moves because there's something on the top to keep it up. Again, something stiff on the top was put there to keep it from moving (e.g. keep it up) so it sounds strange to use this as a reason. c) No gravity doesn't mean no wind. Actually, it does. Without any gravity, particles will not gather together in one place (let's say, the atmosphere) and thus there will be no substance around to create any wind, just empty space. What I think you meant to say was: 1) The gravity on the moon caused the flag to ruffle as it was being set. 2) There is a bar sewn at the top of the flag to keep it from falling down. #3 doesn't really need to be said. freshgavinΓΛĿЌ 07:18, 10 March 2006 (UTC)
For a very detailed debunking of this, see the article on Clavius. And thank you for all the sarcasm above - it certainly made me grin! — QuantumEleven | (talk) 11:12, 9 March 2006 (UTC)
Very simply put: See Newton's laws of motion. Whenever the flag fluttered, an astronaut had placed, fixed, or bumped it. Prove me wrong. Here7ic 20:55, 10 March 2006 (UTC)
- Not the flag, not the moon—mind is waving. —Blotwell 06:14, 14 March 2006 (UTC)
Evolution
[edit]How can an environment affect a phenotype of an organism so that that organism is able to pass on altered traits to its offspring? If that isn't even part of evolution and I'm on the complete wrong track tell me. RENTASTRAWBERRY FOR LET? röck 02:06, 9 March 2006 (UTC)
See Lamarckianism and Lysenkoism for the simple versions, now refuted. alteripse 02:31, 9 March 2006 (UTC)
The phenotype of an organism is determined basicly by the genotype. The genotype has variations within a population (which produce variations in the traits of the organism including variatins in phenotype). Some of these genotypes produce more offspring, thus increasing the proportion of their related phenotype. Most phenotypes go extinct. WAS 4.250 02:42, 9 March 2006 (UTC)
Basically the answer is, organisms aren't able to pass on altered traits to their offspring. So, in fact, that isn't part of Darwinian evolution. Like Alterprise wrote, what you described is part of the now-discredited Lamarckian evolution, not Darwinian evolution JianLi 03:14, 9 March 2006 (UTC)
God created the universe! —Preceding unsigned comment added by Im in ur house (talk • contribs) 05:25, March 9, 2006 (UTC)
- If G-d lived on Earth, people would break all His windows. (according to Yiddish proverb) GangofOne 08:27, 9 March 2006 (UTC)
The way the enviroment affects a phenotype is to either kill it ( or otherwise prevent it from reproducing) or not. Those that reproduce dominate the next generation. That's evolution. GangofOne 08:27, 9 March 2006 (UTC)
- Yeah. As far as classical evolution is concerned, environment cannot have such a direct effect. On the other hand, there are exceptions to such a rule. For example, exposure of some organisms to stress can cause a faster rate of mutation - not exactly the same, but close. Some research may also be concerned with feedback effects between organisms and their habitats - as an organism's phenotype changes, it may cause changes to its environment that continuate its own phenotype. (If we use rather broad definitions, we can speak of human society and education in particular as an example of this) - User:Fangz (not logged in)
- There's still an ongoing debate on directed mutation (which might need an article), as Fangz noted. I seem to recall that there was a study involving exposing fruitflies to high heat. This may be it, the various references on this are also interesting.
- Supporters indicate that there is experimental evidence of certain cellular mechanisms for repairing 'damage' mutations which may be suppressed under environmental stress; this is posited to be a fitness trait of another order which allows a species to 'speed up' selection by increasing mutations when there is high environmental stress. Detractors usually mis-identify directed mutation with Lysenkoism by defining it as "the hypothesis that mutations that are useful under particular circumstances are more likely to happen if the organism is actually in those circumstances."[25] However, there is some very interesting experimental evidence that the cell may be able to "choose" the mutation.[26]
- I also browsed a science fiction book called Darwin's Radio by Greg Bear which seemed to have a plotline along this angle. KWH 17:42, 9 March 2006 (UTC)
- The gender ratio of offspring can be affected by the environment, such as the average temperature of the father's testicles. StuRat 19:53, 9 March 2006 (UTC)
There was an account of an interesting evolution-environment link in Orson Card's Ender's Game series, I think he mentions a Chinese philosopher, but I'm not sure if it was a reference to a real person or not.
The basic gist of it was that there's a planet completely covered with only one species, a sturdy little flower; let's call it a daisy, and assume it's yellow. When the environment of the planet begins to heat (hot cycle) the portion of the planet's plants that had evolved (by random traits) to have slightly darker petals would die because of the absorbtion of too much heat, leaving the lighter colored daisies to multiply and thus lightening the surface of the planet, reflecting greater amounts of light and cooling the atmosphere. If the planet were to suddenly cool down, it would have the opposite effect and the surface would darken causing the atmosphere to warm up again.
I realize this has little to do with the question but it's such a pretty story about cute little yellow flowers, and I wanted to share it with everyone! freshgavinΓΛĿЌ 05:40, 10 March 2006 (UTC)
- The oscillation is interesting, but there are cases where life has changed the Earth in a negative way for it's own survival. Early plants gave off oxygen and thus filled the air with it. However, free oxygen was toxic to these early plants, so later plants, and then animals, needed to develop a defense against free oxygen. Our layer of dead skin on the surface protects us nicely. A current example might be global warming, which may be disastrous for human life. StuRat 10:29, 10 March 2006 (UTC)
- I haven't read Ender's Game, but that sounds a lot like the Daisyworld simulation of James Lovelock's Gaia hypothesis. KWH 14:59, 10 March 2006 (UTC)
- Yes, yes that's it! So they were daisies after all. I highly recommend the books, anyway. Mostly written in the 1980s, they're not perfect sci-fi books (Card is not a scientist, but a religious man), but if you can imagine it as an adventure series they can be quite inspiring. freshgavinΓΛĿЌ 05:51, 13 March 2006 (UTC)
Effect of bodies of water on surrounding areas
[edit]I was wondering what the name of the phenomenon might be where proximity to a large body of water regulates the climate and weather of the land directly adjacent to said body of water. For example, the way in which the climate of the coast of British Columbia remains so temperate due to its proximity to the Pacific Ocean. If I could just get the name of the phenomenon or concept, I could go from there. Thanks.
- See continentality, which is the opposite of oceanity or oceanicity, which is something like what you're talking about. If you do a google search for "climate", "body of water", and "specific heat" you'll find more. —Steve Summit (talk) 06:20, 9 March 2006 (UTC)
- Moderation. The water moderates the climate. --Anonymous, 08:48 UTC, March 9.
- I looked around. There's a little bit on microclimate. Googlize and you find a bit more. --Zeizmic 12:36, 9 March 2006 (UTC)
- Lake effect snow, Orographic lift, http://www.noaa.gov/questions/question_011602.html ,http://meted.ucar.edu/norlat/snow/lake_effect/1.2_instability.htm. WAS 4.250 14:55, 9 March 2006 (UTC)
Parabolic hotdog cooker
[edit]Hello friends! My friends and I building a solar hot dog cooker. We are looking for a way to make the parabolic dish shape and for a good reflective material (that doesn't diffuse). Any searching I do lands pizza-box or traugh level work, but we're looking for something a little more kick-but. Any help appreciated. --orphan frequently 05:31, 9 March 2006 (UTC)
- May I suggest the Solar death ray? :) --Obli (Talk)? 06:40, 9 March 2006 (UTC)
- Did you see solar cooker? And there are some interesting links from there. --Shantavira 08:25, 9 March 2006 (UTC)
- I remember when I did that in 8th grade, I shared my two hotdogs with my friends. *tear* A Frensel lens off Ebay is what you want to do. You can find directions for building the box and doing the right math (if you are doing this for school). Have fun! Also try cooking other things, and things that aren't food. (Shotgun shells don't work well). Ow. --
 Mac Davis] ☢ ญƛ. 10:05, 9 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 10:05, 9 March 2006 (UTC)
- I believe you meant to say a Fresnel lens. StuRat 19:45, 9 March 2006 (UTC)
How long does it take for the effects of this to wear off? I took a 40mg dose ~36 hrs ago and while it's had the desired effect on my symptoms, I'm getting a bit concerned that it's been too effective. (Apologies for TMI.) --Bth 08:21, 9 March 2006 (UTC)
- What did the packaging say about dosage? - Mgm|(talk) 10:19, 9 March 2006 (UTC)
- To intially take two 20mg pills and then another one every time I had a loose bowel movement (which I haven't had so the initial dose was the only one). Looking at the infobox in the article, the elimination halflife is ~10 hours so I should still have about 5mg floating around my system, which makes me a bit less worried. (And my stomach just rumbled, which makes me thinks might be coming back to life.) --Bth 10:29, 9 March 2006 (UTC)
- By the way, don't take anti-diarrheals if you think it's being caused by an infection. Diarrhea is a good thing since it flushes out the organisms. Just be sure to stay well-hydrated with an oral rehydration solution preferably or a sports drink like Gatorade. --Uthbrian (talk) 19:21, 9 March 2006 (UTC)
PC catastrophe
[edit]I bought a graphics card from Dabs.com, marked Used. "dabs.com used stock has been price discounted because there’s a minor problem with it. For example, the packaging could be damaged, or there might be something missing such as the software or its user manual. Alternatively, it may have been opened, used and simply returned to us."
I installed it, with no problems that I could notice during my installation. Then my problems began. Windows booted, I tried installing drivers for my card, but the screen went black and my PC hanged in the middle. When I tried to restart, Windows rebooted before the login screen. Safe mode got stuck at amdagp.sys. I took the new graphics card out and put my old one back in, but the problems remained. I tracked the reboot problem down to one of the nVidia drivers, so I deleted them in Ubuntu Linux, but alas I got another error. I finally got Windows to boot by using "Last Known Good Configuration", but then I got a blue screen a few minutes later. This morning the PC seemed to be up and running again, so cautiously I rebooted and scheduled a CHKDSK. I came back to find the error "Windows cannot start because the HAL.sys file is missing" (or something similar) and a very worrying smell of burning. Opening the case, I found the smell was coming from my processor.
So, two queries: 1) Has anyone any ideas at all what the problem might be? I think it might be something to do with the PSU... but would that explain my hard drive/Windows problems as well?
2) Is there any way I can get compensation from Dabs for selling me a product that could end up costing me my whole PC?
Thanks. I think I have burning plastic fumes in my lungs. Sum0 12:20, 9 March 2006 (UTC)
- Wow! I just got a mirror delivered by UPS. It was dropped on my porch (I think they waited for me to go away a few minutes). It looked liked it had bullet holes through it. The box was falling apart, so it looked like the guy tipped it to stop glass shards from falling out. Does this answer the question? No -- shit happens. --Zeizmic 12:38, 9 March 2006 (UTC)
- I had a server delivered by FedEx about 10 years ago. It was in fine condition if you ignored the steel rod going through the package (and server). I brought this to the delivery guy's attention and he replied, "So, you're saying the rod isn't yours?" Does that answer the question? No. But, an answer could be: The card is apparently an AGP card. Windows failed on an AGP library. A hard reboot was apparently done, which messes up the Windows files. A reinstall is required. See if you can disable AGP in the BIOS and use the card like it is a PCI card. If you have an LCD display, ensure you are using a resolution it can display - otherwise you will get a blank screen even when the card is working perfectly. Expect 90% of what you purchase through cheap discount dealers to be garbage. If it was actually a great deal, someone would have bought it before you saw it. --Kainaw (talk) 13:22, 9 March 2006 (UTC)
- Is it possible that you might have inadvertently loosened the heat sink on your CPU when you swapped graphics cards? Poor contact between the CPU and heat sink will result in inefficient cooling; a processor that's running hot will cause the PC to behave erratically and hang periodically (whenever the CPU warms up). Running for extended periods with a poorly-attached heat sink will eventually cook your CPU (possibly leading to the smell of burning and the release of the magic smoke.) TenOfAllTrades(talk) 13:55, 9 March 2006 (UTC)
- What is the material that actually burns to produce this smoke? --Username132 22:28, 9 March 2006 (UTC)
- The smoke is installed when the chip is produced. We can only speculate as to what it's actually made of. freshgavinΓΛĿЌ 03:50, 10 March 2006 (UTC)
- :D I assume it's actually the plastic casing of the chip melting. Sum0 09:21, 10 March 2006 (UTC)
- The smoke is installed when the chip is produced. We can only speculate as to what it's actually made of. freshgavinΓΛĿЌ 03:50, 10 March 2006 (UTC)
- Good thinking, TenOfAllTrades. The heat sink appears to be fine, but the CPU fan isn't spinning. I think what has happened is that when I was putting the new card in I invadventantly damaged the connection between the CPU fan speed controller (a knob on the back) and the fan. So... sorry Dabs, seems like it was my fault all along. Just need to get a new fan. Either way, thanks to everyone for the replies. Sum0 16:07, 10 March 2006 (UTC)
- Hopefully you just need a new fan! You may need a new motherboard and processor too! I mean you smelled the thing burning... Good luck anyway. --Username132 17:15, 12 March 2006 (UTC)
- Thanks for your concern! I installed a new fan yesterday and everything is now working swimmingly. I'll be sending the card back, incidentally. Sum0 23:03, 12 March 2006 (UTC)
- I'd imagine the burning smell was dust or something. You get it all the time when something gets hotter than normal but not hot enough to destroy itself or when stuff that normally runs hot has been in storage for a while. I can't imagine a fan failure alone killing any modern chip (most have protection systems and whilst they are sometimes a bit slow to deal with complete heatsink removal fan failure is a much more gradual overheat).
- Thanks for your concern! I installed a new fan yesterday and everything is now working swimmingly. I'll be sending the card back, incidentally. Sum0 23:03, 12 March 2006 (UTC)
- Hopefully you just need a new fan! You may need a new motherboard and processor too! I mean you smelled the thing burning... Good luck anyway. --Username132 17:15, 12 March 2006 (UTC)
- What is the material that actually burns to produce this smoke? --Username132 22:28, 9 March 2006 (UTC)
Horsepower
[edit]Can anyone please explain the following to me: Engine Horsepower (EHP), Thrust Horsepower (THP) and how do I explain it to a student?
Confused pilot?
- EHP is the horsepower that the engine produces, equal to torque times angular speed. THP is the horsepower required to push the aircraft along, and is equal to drag times speed (assuming constant speed). The difference between the two is due to losses such as heating of the air [27]. --Heron 21:33, 9 March 2006 (UTC)
- Wow, that should be in an article (if it isn't already). - Cybergoth 21:52, 16 March 2006 (UTC)
should the distance between eyes and monitor vary with the size of the monitor?
[edit]How close is too close to watch a computer monitor? It is a well known fact that while viewing a 15 inch monitor, we should keep a distance of 25 to 27 inches between the monitor and the eyes. Should the same distance be maintained while viewing a 10 inch monitor? (Please note here that the area of a 10 inch monitor is approximately half that of a 15 inch monitor). Can I use small fonts in the 10 inch monitor and sit closer than what distance I keep with a 15 inch monitor? Generally, should the distance between eyes and monitor vary with the size of the monitor?
If 27 inches is okay to 15 inch monitor, whats ok for 10 inch?
- Eyes are unique to each person. So, distance to a monitor is unique to each person. If your monitor position makes your eyes hurt, movie it. If your neck hurts, the monitor is usually too far away or too low. There is no "well known fact" about specific monitor distance. There are only assumptions based on poor science. --Kainaw (talk) 15:46, 9 March 2006 (UTC)
- I can tell you that the top of your monitor should be roughly at eye level, so you never have to look up to read pages.
- If I do that, I get severe migranes. I keep the bottom of my monitor at eye level. As I stated before, monitor position is dependent on the person looking at the monitor. Any "rule" can, at most, be applied to most people, not all people. --Kainaw (talk) 17:26, 9 March 2006 (UTC)
- In my iMac's manual I think it said I should have my eye level a few inches above the bottom of the monitor. --
 Mac Davis] ☢ ญƛ. 07:29, 10 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 07:29, 10 March 2006 (UTC)
- In my iMac's manual I think it said I should have my eye level a few inches above the bottom of the monitor. --
- Further on this point, the desirable height may be different for people who use bifocal/multifocal glasses, or have (say) neck problems affecting what position they can hold their head in. --Anonymous, 23:33 UTC, March 9.
- I think you need to invest in a larger monitor, 10 inches is pathetically small. I use a 19 inch monitor, so almost 4 times the area, and wish I had a larger one. Also, I would avoid traditional cathode ray tube monitors and go with a plasma screen. That technology can produce a bright, high contrast image with a minimum of electromagnetic radiation, unlike a CRT. The size, brightness, and contrast of the screen are more critical to preventing eyestrain than your distance from it. StuRat 19:15, 9 March 2006 (UTC)
- I don't think radiation is an issue in modern monitors. I'd go for a 17-19 inch CRT off ebay or something. If you're using a 10" monitor, you probably havn't got money to spend on a plasma anything. I certainly don't. Vive le CRT! --Username132 22:20, 9 March 2006 (UTC)
- Well, that's the reason it's called a "plasma" screen, you need to sell blood plasma until you can afford it ! :-) StuRat 10:20, 10 March 2006 (UTC)
- Only in America... The UK (at least) blood service don't pay for blood products. --Username132 13:36, 10 March 2006 (UTC)
Possible allergy?
[edit]I'd never had Tasty Food Product (hereafter referred to as TFP) until about a year ago. When I eat it after abstaining for a while, I get mild stomach pains and moderate diarreah. After that, I can eat it daily with no troubles -- but if I go a few days or a week or two without it, then try it again, I get the diarreah. Is this an allergy or what?
- First, a disclaimer: Wikipedia is not a medical doctor. All responses should be treated as random hearsay. Anyway, I've had similar stuff (in my case, red meat, which I didn't eat as a poor college student) where my tolerance could fluxuate. However, in my case, it was simply said tolerance and the need to develop one. — Lomn Talk 16:27, 9 March 2006 (UTC)
- I can think of a possible mechanism for this observation: perhaps a helpful bacteria exists in the digestive system initially in insufficient quantities to digest large quantities of that food. However, the bacteria quickly multiplies by eating that food, and thus the numbers become sufficient to properly digest the food after a few days. Then, when you abstain from eating the food for a long period they are flushed from the system. The appendix seems to serve the function of allowing at least a few of the bacteria to stay in that pouch and not be flushed out. I would be interested to know if you each still have your appendices. Four possible solutions are:
- StuRat 19:00, 9 March 2006 (UTC)
- Did you just invent a function of the appendix? Or did I miss a new discovery? And what is TFP? Is it some food brand or fatfood chain? Suitly emphazi (there, now I'm talking in riddles too, for those not native to this ref desk :) ). DirkvdM 19:16, 9 March 2006 (UTC)
- No, I've heard that theory before, especially with respect to storing bacteria necessary for the digestion of raw meat by cave men, who would likely go for long periods betweens "kills" and thus need a way to preserve the helpful bacteria. If our appendix article omits this info, that's just one of Wikipedia's shortcomings, I guess. Also, they were using TFP to refer to any food which causes the problem in question, not any specific food. StuRat 19:32, 9 March 2006 (UTC)
- Appendix intact and in place here, and "TFP" is just a catch-all for "whatever food is responsible here". — Lomn Talk 19:33, 9 March 2006 (UTC)
- Thanks for the advice. I still have my appendix, and the food in question is soymilk. --Original Poster
acute renal failure
[edit]Can acute renal failure be caused by Ptomaine poisioning? Any information would be helpful. Thank you in advance.
- Where did you pull Ptomaine from? "Ptomaine poisoning" is a rather old term for "food-born bacteria poisoning". In the area of food-born bacteria, you will find E.Coli, which is known to cause renal failure. --Kainaw (talk) 17:56, 9 March 2006 (UTC)
operational amplifiers
[edit]why level shifting is necessary in operational amplifiers?---61.1.127.74 18:31, 9 March 2006 (UTC)
- This looks like a good reason to do your own homework, but you might also check out operational amplifier and search for "level shift". — Lomn Talk 19:39, 9 March 2006 (UTC)
RENAL FAILURE
[edit]My son was working in a crawl space under a residence, he saw mold, mouse droppings and smelled sewage, the man working above him was drilling holes in the floor boards for elec. wires. The debris rained on him. His employer provided no protective gear. My son soon therafter came down with acute renal failure. The workmen's comp doctor says my son must have had Ptomaine poisioning. My doctor feels he came in contact with a number of toxic items, and even feels he may have contacted a virus such as hunta virus. I want to know can Ptomaine poisoning cause renal failure. We all had the same lunch my son ate. Dry Salami and no one got sick? My son almost died, he has now recovered and has never had any renal problem before.
- I think your analysis is correct, and they are just trying to protect themselves from a costly lawsuit. If you have samples of the materials he was exposed to, say from those could be analyzed to find the toxic agent. StuRat 18:50, 9 March 2006 (UTC)
- Wow! Two people with renal failure caused by ptomaine poisoning in the same day! --Kainaw (talk) 19:10, 9 March 2006 (UTC)
- I bet they both had salami too, without getting sick with a question mark. DirkvdM 19:21, 9 March 2006 (UTC)
- I don't understand what either of you are saying? Only one person got poisoned - his son? And why did Dirkvdm verbalise his question mark? --Username132 22:12, 9 March 2006 (UTC)
- The question got asked twice, both times unsigned. Of course it's obvious that it was the same person who asked it but we were just having a bit of fun. And this version of the question had an oddly placed question mark; "Dry Salami and no one got sick?" so I made a little 'joke' about that? Aren't we having a lot of fun here! (in case you wonder about the misplaced exclamation mark - it's intentional?). DirkvdM 12:42, 10 March 2006 (UTC)
- StuRat is right, you should probably consult a lawyer; if your son is in a union he should probably speak to the union people as well. --Robert Merkel 01:48, 10 March 2006 (UTC)
- That would be hantavirus, btw, not hunta virus. - Nunh-huh 09:36, 10 March 2006 (UTC)
- Well, if you don't know where a virus is, you need to hunta virus, right ? :-) StuRat 10:17, 10 March 2006 (UTC)
Well, yes I am hunting the cause and I am his mother, since he has no medical insurance.(Of course the medical bill is enormous and I am on SS) And we can't find a lawyer to take the case because it is a workmen's comp. case. and my son was out of work for 3 weeks, so there is no money to speak of for an attorney to get motived to take the case. Since we are not allowed to hire an attorney and pay him out of our own pocket. We are very "nicely" aced out of legal representation. Thanks anyway. Francesca
- The solution to your problem is publicity. This is a human interest story. Newspapers make money from circulation, and get circulation from interesting and alarming stories. Trying to help your son all by your self against the only-profit-matters corporations makes a great story. Further people who read it will offer to help. Some will send a dollar. A law student might decide your publicity could be his publicity and offer free legal help. But to get the ball rolling, you have to talk to reporters, church groups, neighbors, etc. Set up a web site with photos and a way to donate. WAS 4.250 18:56, 10 March 2006 (UTC)
You need to find lawyers who are willing to do the work for free (pro bono). See our Legal Aid article, and follow the links at the bottom for specific contacts. StuRat 03:04, 14 March 2006 (UTC)
analogy of rotational equilibrium
[edit]I want to know that Is "rotational equilibrium with constant angular velocity" a dynamic rotational equilibrium?, since "Translational equilibrium with constant linear velocity" is Dynamic Equilibrium
It is, I'd say. deeptrivia (talk) 20:13, 9 March 2006 (UTC)
- I'd say you're confusing dynamic equilibrium with mechanical equilibrium. —Keenan Pepper 22:01, 9 March 2006 (UTC)
Fiber in Tropicana "Lots of pulp" juice
[edit]How come there is 0 grams of fiber in Tropicana "Lots of pulp" juice? Isn't the pulp fiber? What's the benefit of pulp in juice if it's not fiber? deeptrivia (talk) 20:13, 9 March 2006 (UTC)
- The pulp does contain fiber, and other carbohydrates, but probably the pulp makes up a small enough proportion of the total juice that one serving contains less than a gram of fiber. (0 grams, really means less than one gram per serving). What's the benefit of pulp? Maybe some people just like the pulp because it makes the juice seem more like fresh squeezed, rather than processed, juice. Edgar181 21:59, 9 March 2006 (UTC)
- Have you ever poured a jug of Tropicana into a sieve just to see how much pulp there actually was? There's lots of it, a lot more than 1 gram per serving. By your logic, it should at least get a "traces" rating for fiber. I'd say the answer to this Tropicana mystery is that the company figures that people don't really care about the fiber content, and rather just like getting their faces sloppy, and it puts the same specs on the box for "Homestyle" and "Original". I personally prefer the pulp. freshgavinΓΛĿЌ 07:07, 10 March 2006 (UTC)
- I'm confident that if you filtered a serving of juice, collecting the pulp, and then completely removed all the water, the remaining dried solid would weigh less than a gram. Edgar181 13:24, 10 March 2006 (UTC)
- Have you ever poured a jug of Tropicana into a sieve just to see how much pulp there actually was? There's lots of it, a lot more than 1 gram per serving. By your logic, it should at least get a "traces" rating for fiber. I'd say the answer to this Tropicana mystery is that the company figures that people don't really care about the fiber content, and rather just like getting their faces sloppy, and it puts the same specs on the box for "Homestyle" and "Original". I personally prefer the pulp. freshgavinΓΛĿЌ 07:07, 10 March 2006 (UTC)
- I hate those chunks of pulp that stick between my teeth. I once bought "homestyle", not knowing that apparently means "nothing but pulp" ! StuRat 08:57, 10 March 2006 (UTC)
The pulp is low in fiber, as most of it is digestible. Only a small portion of the pulp goes through the system undigested (and thus can be called fiber). StuRat 09:02, 10 March 2006 (UTC)
- Well there you go. Gotta give it to fruits. They really know how to be eaten. freshgavinΓΛĿЌ 05:47, 13 March 2006 (UTC)
Hard disk
[edit]If you save data on hard disk and remove the hard disk from CPU ? you will lose the data
if you removed hardisk too much this remove will effect on hard disk?
- Generally, hard disks can be unplugged, removed, placed in another computer, etc. without damaging data. Doing it too much shouldn't be dangerous, but it increases the chance that something might go wrong. Hope this helps! Sum0 20:51, 9 March 2006 (UTC)
- As long as you treat your HD with care as you do this, there shouldn't be any problems. — TheKMantalk 21:15, 9 March 2006 (UTC)
- It may go without saying, but I'm going to say it anyway—don't remove the hard disk while the computer is running. This will often result in data loss, and may result in physical damage to the drive.
- On a practical note, IDE connectors have a lot of fiddly little pins that don't like being repeatedly plugged and unplugged. Moving a hard drive between computers too often may result in premature failure of these pins. TenOfAllTrades(talk) 21:20, 9 March 2006 (UTC)
- External USB harddisks maybe a better solution. Theresa Knott | Taste the Korn 21:43, 9 March 2006 (UTC)
- Treating with care includes full anti-static precautions. The vulnerable part of a hard disk (unless you drop it) is that circuitry on the outside. Consider an external hard disk, though, may be much easier, or a memory key. Notinasnaid 09:14, 10 March 2006 (UTC)
- Oh yes, and make sure the computer was shut down normally before you take the disk out, NOT just crashed or switched off. That can reduce the chances of damage. Notinasnaid 09:15, 10 March 2006 (UTC)
- That's a tricky one - in my experience, shutting down 'normally' is not normal for msWindows. DirkvdM 12:45, 10 March 2006 (UTC)
- And sing to it - and care for it - and love it. Celcius 11:39, 10 March 2006 (UTC)
- At the risk of being pedantic, SATA hard drives (as well as external USB or FireWire ones) are hot-swappable. However, the operating system needs to be aware of this capability, so for instance in linux, be sure to umount the hard drive first. In Windows, SATA drives don't seem to be considered removable storage, so you don't have the handy safely remove hardware icon for it, so you must shut down windows before removing or reconnecting the drive. --WhiteDragon 17:33, 10 March 2006 (UTC)
I like my apples waxy... or not
[edit]I took an apple out of the pack and it felt really waxy, and then so did the others. What's this weird waxy coating... it's not conveinient to wash them before eating... --Username132 13:34, 10 March 2006 (UTC)
- Take a look here. Hope this helps — TheKMantalk 21:16, 9 March 2006 (UTC)
- Oh thanks. Perfect. :) --Username132 22:21, 9 March 2006 (UTC)
- In future, if you ever come across a mystery waxy or oily substance that you're not sure if you're supposed to eat or not, I recommend you eat it. Not because I wish any harm on you, but because waxy and oily substances are hydrophobic, and will (generally) cluster (more or less) in your stomache, and won't cause any damage (probably, if you're lucky). On second thought... freshgavinΓΛĿЌ 03:06, 10 March 2006 (UTC)
- Oh thanks. Perfect. :) --Username132 22:21, 9 March 2006 (UTC)
- lol, that's the best advice I've ever heard. =P —Keenan Pepper 04:52, 10 March 2006 (UTC)
- Bile salts may emulsify the wax... Plus being hydrophobic/lipid soluble, they should be able to freely cross cell membranes into the blood. --Username132 13:34, 10 March 2006 (UTC)
DARPA??
[edit]What did this have to do with the formation of the internet? Just wondering??Do any of you know?--205.188.116.11 06:32, 24 February 2006 (UTC)
- The preamble at the beginning of this bit has something on "It's quicker just to do your own search rather than bother people". You can type 'darpa' into the magic box, and find something wonderful. --Zeizmic 22:28, 9 March 2006 (UTC)
March 10
[edit]Evil Gene Patenting
[edit]I was reading about gene patenting. What was it the Netherlands was trying to do? Did they want the patenting stopped? --Username132 03:18, 10 March 2006 (UTC)
- The evil gene cannot be patented; for national security reasons it is subject to a government monopoly. --Trovatore 03:21, 10 March 2006 (UTC)
- Patenting genes is patent nonsense - so a lot of countries want it stopped. Celcius 03:35, 10 March 2006 (UTC)
- Then why did the legislation get passed in the first place? --Username132 04:08, 10 March 2006 (UTC)
Wait so what happens if someone patents a gene and my child is born with it? :\ — Ilyanep (Talk) 04:09, 10 March 2006 (UTC)
- You get payed a penny every time your kid repeats the company slogan in front of strangers. Or, if your kid is ugly, you have to pay a penny. freshgavinΓΛĿЌ 05:12, 10 March 2006 (UTC)
- Or you can't actually claim anything over natural expression of the gene. But that's not the point. The point is, some corporation discovers the gene involved in, say, I don't know, some kind of cancer. The fat business executives who couldn't care less about people's welfare (they studied business and finance, not biomedical science) can then charge anyone who tries to distribute a treatment for that disease. --Username132 13:32, 10 March 2006 (UTC)
- The businessman would say, "Listen, this isn't about people's welfare. I have a big company and spend a huge amount of private money in searching for genes related to disease. Of course I try to make a profit off of this once I've discovered something useful -- if I didn't, how would I ever get the money to look for genes in the first place? Sure, it'd be great if governments ponied up the dough for this, but we all know that it doesn't always work out. We'll try to set things at a fair price, to guarantee our future research abilities. And hey -- we all know that most of the money from these treatments is paid by insurance anyway, so what's the big deal?" I'm not saying that's the last word on the subject, but viewing it as just about "fat business executives" is a very one-sided and unfair approach. --Fastfission 01:52, 11 March 2006 (UTC)
- Sounds like something a fat business executive would say... I'm in this gig for the people and what do I get? Exploited. It's fine to make money to cover future expense etc. but they're in it for their flash cars, homes and swimming pools. When my mum was younger she had to boil her asthma cartridges (to pressurise any remaining medicine) SO SHE COULD BREATHE. --Username132 16:57, 12 March 2006 (UTC)
- Again, I think that's an overly narrow view of things. While I agree that there is much to be wary about in the big pharma industry, it is important not to mistakenly collapse things down into simple categories of "good guys" and "bad guys" because in most cases that's simply not how the world works. --Fastfission 03:33, 17 March 2006 (UTC)
Computer program equation input
[edit]If I want users to enter an equation into a program I write, and then want the program to use the equation, I only seem to have two options, neither of which seem very good:
1) Parse the equation in the program, allowing for every possible combination of functions, like trig functions, absolute value, hyperbolic functions, log functions, etc. This seems like a lot of work to redo what the program could already do had the equation just been hardcoded in.
2) Write the entire program out to a file, including the equation supplied by the user, then recompile and relink the program, and run it. This requires that anyone running the program also have a compiler.
The language I am using is FORTRAN and the application is a graphing program, but this seems like a more general issue to me. An example would be, if the user enters Y = X^(pi^e) + abs(sin(X^3))/log(1/sqrt(X)), I would then graph that equation. Are there any languages where a user supplied equation can just be used directly by the program ? Does anyone have any other ideas for how to do this in FORTRAN ? StuRat 05:04, 10 March 2006 (UTC)
- Yay procedural programming! Not specific to FORTRAN but I learned a little about this in compiler theory (which I didn't enjoy too much), so you might want to check out a book on that. It's possible that someone has made a library that can recieve code as a parameter and parses it into instructions, but they probably did the exact same thing that you said in (1), though likely with a low-level language. High level langauges aren't very good at analysing their own code, which is why many of them have their own scripting languages (I assume FORTRAN doesn't). Does FORTRAN have Assembly commands? freshgavinΓΛĿЌ 05:25, 10 March 2006 (UTC)
- Not that I'm aware of, but it can make system calls, so if assembly language commands are available at the O/S level they could be accessed via a system call. StuRat 08:43, 10 March 2006 (UTC)
- This would be relatively trivial in Matlab, but whether that's a real language or not is open to debate.
- It wouldn't just be trivial, it wouldn't require any programming at all! freshgavinΓΛĿЌ 07:01, 10 March 2006 (UTC)
- Just look for almost any interpreted language. These tend to show off by having a function like "eval" which takes a string in the same language and execute it. They may be slower than compiled code, but this sort of thing is cute. Believe me, writing an expression parser is not simple, especially if you've never studied formal grammars and parsing techniques. Actually executing the functions is the trivial part. Notinasnaid 09:12, 10 March 2006 (UTC)
- Yea, it looks like a lot of work to me. I was actually leaning towards option 2, which requires that the code be recompiled and linked after the user enters the equation to graph. StuRat 10:12, 10 March 2006 (UTC)
I can attest that it is a lot of work because I've written a parser and interpreter that does that; it's not that difficult (recursive descent parser), it just takes forever. You might consider C# which can compile code on the fly without the need for the user to have the SDK on the computer. enochlau (talk) 14:43, 10 March 2006 (UTC)
- Expanding on what enochlau said: yes, option 1 is straightforward, though a certain amount of work. But you don't have to do all the work yourself; there are libraries which can do it for you. The C FAQ list mentions a few, although they're pretty dated. And if you know what you're doing, an expression evaluatior really isn't that much work, after all; you can write a simple one in an hour or two. Here's a condensed, mildly obfuscated one I coughed out once: eval.c.
- Back in about 1987 I was thinking about precisely the problem you describe -- I wanted the user of a graphing program to be able to enter expressions using FORTRAN-like syntax. I wrote an expression parser and evaluator which I'm still using for various things. Like so many personal projects, it's not quite packaged and documented well enough for others to use easily, but you might take a look at http://www.eskimo.com/~scs/src/#med . That package includes the expression evaluation library and a command-line tool to exercise it. I just entered the expression
c1**(pi**e) + abs(sin(X**3))/log(1/sqrt(c1))
- into that tool (where I substituted the FORTRAN
**for your^, andc1-- meaning "column 1" -- for X) and it worked fine. Your function blows up pretty fast:
X Y 0 divide by 0 1 divide by 0 2 5.76607e+06 3 5.19687e+10 4 3.32476e+13 5 4.99203e+15
- Finally, a few more words about your option 2. You don't need to write the entire program out to a file and recompile it; typically you just need to write and compile a stub function containing the user-entered expression, then use dynamic linking to call the just-compiled code from the main program. The C FAQ list talks about this, too. (You didn't say you're using C, but both of the links I mentioned contain information that might be useful no matter what language you're using. Oh: you did say you're using FORTRAN. Hmm. I don't know how much of this stuff even a modern FORTRAN compiler would let you do.)
- Wow, what a fortunate coincidence ! That math editor function looks quite useful. A few questions:
- The documentation says "in some versions" repeatedly. Do you have any documentation on what the version you are offering specifically does ?
- Do you have a FORTRAN stub that calls the function ? This would be useful to overcome all those silly FORTRAN-C function compatibility issues.
- Is there any support for +/- ? For example, if I give it Y = +/-sqrt(4), I want to get both +2 and -2 as output.
- Thanks a lot for your help ! StuRat 20:30, 10 March 2006 (UTC)
- Answering these on your talk page. —Steve Summit (talk) 16:45, 11 March 2006 (UTC)
- I would like to note that most of these suggestions are good if you're only using the program yourself, maybe if only for a department of a few dozen users, but the concept of using eval statements or open ended compiling is a Really Bad Idea from an information security perspective, if the program is destined to become a software product or mission critical system in any sense. Best case is that an errant user could enter an equation that could crash or drag the processor to a halt, worst case is that you give a malicious hacker the keys to the kingdom (by letting them compile and run any code they want on your system). This of course depends highly on the systems environment you are in, but it is a word to the wise.
- If you want to do this in any serious environment (where money or lives are on the line), you will want to know the appropriate compiler theory to parse the equation and build the code yourself, with appropriate boundary checks. KWH 19:11, 10 March 2006 (UTC)
- It's just for my own use. I saw many glaring deficiencies in most graphing programs that I wanted to fix:
- Can only graph an equation in Y = f(X) form. Mine handles Y = f(X), X = f(Y) and equations in neither form.
- Can only use constant X increments for point sampling. Mine allows constant X increments, constant Y increments, or both.
- Can't handle inequalities. Mine does.
- Can't handle Boolean operations on multiple equations. Mine can handle unions, intersections, subtractions, etc.
- Limited size output. Mine can create a graph any size up to the memory limit of the computer.
- Can't handle polar equations. Mine can, in R = f(theta), theta = f(R), and general forms.
- Limited grid lines. Mine can create up to 5 levels of rectangular or polar grid lines.
- Can't do 3D graphs. My program can.
- Can't do derivatives and integrals. Mine will do these using numerical methods, although I haven't written this code yet.
- I've already created several graphs with this program that are used in Wikipedia [28], but must edit and recompile the program each time I change the formula. This is annoying. StuRat 20:20, 10 March 2006 (UTC)
- Look at free software that already does equation parsing. No need to write your own (although you may be the type who likes to DIY) PARI-GP computer algebra system, Euler, YACAS (Yet another computer algebra system) come to mind. Probably others. List of computer algebra systems , Category:Free mathematics software --GangofOne 21:33, 10 March 2006 (UTC)
- I've already created several graphs with this program that are used in Wikipedia [28], but must edit and recompile the program each time I change the formula. This is annoying. StuRat 20:20, 10 March 2006 (UTC)
BIN and CUE files
[edit]I have a .bin and a .cue file which I have downloaded (possibly illegally ;) and contain a computer game. How do I get it to open? --AMorris (talk)●(contribs) 07:34, 10 March 2006 (UTC)
- Let's hope it's not illegal then. BIN and CUE files can be opened using a "virtual drive". This is a program which emulates a CD drive on your computer. Of course you could just burn the files onto a CD for same effect but it is easier to use the CD drive emulator method. I usually use Daemon Tools - which you can download here - but that's a religious choice - there are many simulators out there. Celcius 11:14, 10 March 2006 (UTC)
- An alternative to Daemon Tools is the equally popular Alcohol 120%.
- I hope it is one of the many .bin files I've put my remote desktop server hack in. I can always use another bot! --Kainaw (talk) 13:52, 10 March 2006 (UTC)
- I highly recommend Alcohol 120% over Daemon Tools, it's much more user friendly I think. Once you got Daemon Tools or Alcohol 120% just mount and unmount the image file(.ISO,.BIN). It's not a very good ideo to have a virtual drive on at all time because it will really slow down your computer, use it only when neccesary. If you want to use the image file constantly just burn the image file to CD using Alcohol 120% but if it's illegal, which i'm guessing it is, it may detect emulation and that you've stolen software, just hope that your OS doesn't send a report.--154.20.89.16 21:16, 12 March 2006 (UTC)
Telescopes
[edit]I've heard that some little telescopes can follow the stars so I don't have to turn it all the time. How is that possible? Thank you!! :) Blueiris 11:21, 10 March 2006 (UTC)
- I don't know anything about telescopes - but I do know something about computers. It seems like a fairly simple job to hook up a computer and a small motor to a telescope and let it follow the stars automatically. Technically it wouldn't be very difficult. Celcius 11:36, 10 March 2006 (UTC)
- The basic principle is that the sky appears to rotate around the pole (north or south). You have a wheel axis pointing to the pole, and have the telescope turn very slowly on it. These days, this can be coupled with a starfinder computer, which knows the location of celestial objects and will automatically use the motor to track to find them, before settling down to following the stars around. It is less expensive than I would have feared. I've been tempted to upgrade my old telescope with one of these. Notinasnaid 11:40, 10 March 2006 (UTC)
- Okay. Thanks for both answers! Blueiris 11:46, 10 March 2006 (UTC)
- This is very common for all except the smallest and cheapest telescopes, and is called an equatorial mount - for a simple version, all you need to do is set one of these up with the right ascension (also called polar) axis pointing at the celestial north pole, lock the declination axis, and rotate the polar axis so that it makes one revolution every 24 hours. Pretty much every equatorial mount comes with a little motor that does this job for you.
- Fancier telescopes with star-tracking software often use a simpler kind of mounting (an altazimuth mount, you've seen them on most binocular tripods), but with motors linked to a computer controlling both axes. This kind of mount is harder to follow stars with manually (as you need to constantly adjust both axes), but the computer does that job for you, while also automatically pointing itself at any object you particularly want to see. — QuantumEleven | (talk) 12:34, 10 March 2006 (UTC)
- I think two things get mixed up here. The question was about a little telescope and that may refer to the little one that is often attached to a big one to get the bigger picture. First you roughly aim the telescope, then you look through the small telescope for more precision and only the do you start looking through the big one (sort of like looking up from binoculars to get the bigger picture). Afaik automated star-tracking doesn't use such small telescopes (don't know the ins and out, though) and it will only be used for the more expensive (bigger) telescopes because the mount isn't really cheap (I guess). DirkvdM 12:57, 10 March 2006 (UTC)
- (not directly related to the original question, but still) No, you're right, Dirk, automatic star tracking doesn't use the small telescope (often called a finder scope) - how it works is that the computer knows where every star is at any point in time (a very easy thing to store, as stars don't move relatively to each other, and the Earth's rotation is very well known). You set up the telescope by pointing it at a particular star (the computer can't really do this... yet... :)), and from that and the current time and latitude, the computer knows where every star is and will be at any point in time. Therefore, it can control the two motors controlling the axes, and point the scope at anything you want, as well as keep it in the field of view as the Earth rotates. — QuantumEleven | (talk) 15:52, 10 March 2006 (UTC)
- By "little" I actually meant a hobbyist's telescope. Equatorial mount was maybe the thing I was looking for, because sometimes telescopes are said to be star-tracking and there's no computer nearby. But I thought it had something to do with gyroscopes because they keep pointing at the same star, too. Blueiris 13:15, 10 March 2006 (UTC)
- Yup, I think you probably got that right - a "star tracking" telescope without a computer is most likely one on an equatorial mount with a small drive motor. It takes advantage of the fact that, because the Earth rotates, from the Earth it seems that the stars are spinning around a point in the sky directly above the north pole called the "celestial north pole" (you can see a nice photo of this at Astronomy Picture of the Day - the camera shutter was left open for a few hours, and the streaks you see are the stars apparently moving). If you have a telescope with one of its mount axes pointed directly at this north celestial pole, and you lock the other axis, by spinning the first at the correct rate your telescope will follow a star as it seems to spin around the celestial north pole. Have fun observing! — QuantumEleven | (talk) 15:52, 10 March 2006 (UTC)
I would also think a computer could take video input from a static telescope and compensate for the Earth's rotation. However, this system would only work for small time periods, after which the celestial object being tracked would move out of the field of view. Such a system would have the advantage of eliminating any vibrations that might be introduced by a motor, especially a cheap one. StuRat 19:09, 10 March 2006 (UTC)
- Wouldn't a cheap fast spinning motor with a succession of gear-wheels eliminate the vibrations? DirkvdM 07:37, 12 March 2006 (UTC)
- I would expect that any moving parts during operation would have to introduce some vibrations. Those vibrations may, or may not, be significant depending on their magnitude and the magnification factor used. StuRat 03:21, 14 March 2006 (UTC)
- My thought was that actively moving parts vibrate, so passively moving parts should eliminate that. Not quite sure, though. Another thought would be to use several little motors which cancel out each other's irregularities. I believe that's how record players eliminate motor vibration. Hey, that would be a good one. Use an old record player drive. Oh, of course, a belt drive. There, problem solved. :) DirkvdM 07:31, 14 March 2006 (UTC)
- Those things might well reduce the vibrations caused by movement, but none would eliminate them entirely. StuRat 01:29, 15 March 2006 (UTC)
- Equatorial mounts with a clockwork drive were around many years before computers came along. --Shantavira 19:50, 13 March 2006 (UTC)
Physics
[edit]We know that like charges repel(coulombs law) and two wires carrying current in the same direction attract. Suppose there were two independent like charges moving with same velocity parallel to each other in the same direction then what would the force acting on them???61.1.131.142 12:55, 10 March 2006 (UTC)
- You appear to be asking: If you take two negative (or positive) ions, put them side-by-side, and send them flying down the road, what will happen? They repel. It doesn't matter how fast they travel. --Kainaw (talk) 13:50, 10 March 2006 (UTC)
- To explain this you really need relativistic electromagnetism (electromagnetism in the context of special relativity). The electric repulsion dominates the magnetic attraction until the charges are moving at a substantial fraction of the speed of light, and then relativistic effects like length contraction and time dilation kick in, so that the force in a comoving reference frame is always that due to the electric repulsion alone (because the charges are at rest in that frame). —Keenan Pepper 13:53, 10 March 2006 (UTC)
- "two independent like charges moving with same velocity parallel to each other in the same direction" if so, then there's no movement relative to eachother, and no magnetic force. ☢ Ҡi∊ff⌇↯ 18:11, 10 March 2006 (UTC)
- Well, yes, but if you're in a second frame of reference that observes the particles zooming off somewhere, you would see the force between them divided into magnetic and electrostatic, but the combined force would be equivalent to the electrostatic force between them in their frame of reference. In other words, it's an "everybody's right" kind of answer :) Confusing Manifestation 13:01, 11 March 2006 (UTC)
Well was wondering if i included relativity,may i now ask that the two electrons would feel repulsion if viewed on the particles,but attracton if viewed ouside the particles??
Earth Orbiter
[edit]What is the minimum speed and altitude a large sattelite would have to be to be in solarsynchronous orbit around the Earth without being torn apart.
- Before our resident astronomer comes along, Roche limit might help. Sum0 20:20, 10 March 2006 (UTC)
- In particular, note that the "without being torn apart" requirement depends on what the satellite is made of, as described in that article. The Roche limit is usually considered in relation to natural bodies that are held together by their own gravity. Sun-synchronous orbits, on the other hand, are a very specific category of orbits, and are usually considered only in relation to artificial satellites that might be deliberately launched into those orbits. (See Polar Sun Synchronous Orbit and heliosynchronous orbit.) So the question is somewhat odd. --Anonymous, 21:15 UTC, March 10.
Processor spead
[edit]I want to buy computer processor , what is the hieghest spead of processor until now ? have more than 3000 Mhz.
- I am ill-aware of the highest processor speed, but the higher the speed does not necessary mean a quicker computer. Kilo-Lima Vous pouvez parler 20:26, 10 March 2006 (UTC)
- From your question I take it you are not a computer person? It is not trivial to replace an old processor by a new one, unless you are fairly experienced. In most cases if you simply bought a different processor, it wouldn't fit in the slot, or wouldn't work with other components in your computer. It's also really easy to destroy the processor or other parts (like motherboard) during installation. So if you really want to buy a processor, you need to tell us what kind you have now, what's you motherboard model, memory, etc. --Ornil 20:31, 10 March 2006 (UTC)
Electrical Cost Calculations
[edit]If my utility charges $.1098 KWH how can I figure out what a 5 amp device costs to use?
This might be good to discuss generally. I beleieve people are curious.
Thanks, John
- Well, the first step is to figure out the wattage, do you know how to do that ? StuRat 21:02, 10 March 2006 (UTC)
- Not to mention how long the device in question will be working for. Here7ic 22:41, 10 March 2006 (UTC)
- Like StuRat mentioned, the power consumption is best to go by. Power (Watts) = voltage x current, but the problem is many devices give their current reading as the maximum instead of average, so the 5A could well be the peak current load on the device. - Akamad 23:20, 10 March 2006 (UTC)
- Rule of thumb - electricity costs (approximately) $1/watt-year. A 5 AMP device running continiously for one year would consume: 5 amps * 120 volts -> $600 in one year. (5 amps is *a lot* of current). This is the reason, in my field, that supercomputers cost more in the long term due to energy usage than it costs to build them. Raul654 23:27, 10 March 2006 (UTC)
- Well, your rule of thumb isn't too far off, assuming a few things about the question. The voltage of the questioner isn't specified, which makes quite a bit of difference. Assuming he's in the US (probably a good guess, based on his use of a dollar sign), this device will draw about 600 watts. Given John's cost, that results in a cost of $0.06588 per hour. This assumes that the device draws a constant 5 amps. So, with all these assumptions, this device running all year would cost $577.1088. kmccoy (talk) 03:21, 11 March 2006 (UTC)
- You only report the total to the hundredth of a cent ? What kind of accuracy is that ? Spock would be appalled. StuRat 03:33, 14 March 2006 (UTC)
Space Based Radio Telescope
[edit]Would it be possible to built an enormous radio telescope in GEO above Earth? I'm talking bigger than Arecibo. If possible, what are the cheapest viable construction resources that could be used? Polymers? Here7ic 21:58, 10 March 2006 (UTC)
- Sure, but why would you want to? -- Finlay McWalter | Talk 22:01, 10 March 2006 (UTC)
- A) Self indulgance, B) A better, clearer, and cheaper grasp of our universe, C) Wouldn't it just look cool? Here7ic 22:36, 10 March 2006 (UTC)
- Edit: plus, it'd help to make the Earth a more peaceful place. Here7ic 22:39, 10 March 2006 (UTC)
- How's so? ☢ Ҡi∊ff⌇↯ 23:39, 10 March 2006 (UTC)
- I think such a project would result in one of two outcomes. 1: The Earth governments would control it as a whole, and it would be one more step towards peace. 2: The Earth governments quibble about spying, good ole' cold war fears are back, and our home world buries itself deeper into political unrest. Here7ic 04:35, 11 March 2006 (UTC)
- How's so? ☢ Ҡi∊ff⌇↯ 23:39, 10 March 2006 (UTC)
- Edit: plus, it'd help to make the Earth a more peaceful place. Here7ic 22:39, 10 March 2006 (UTC)
- Arecibo is a radio telescope, placing a single radio telescope that size in space would not yield better results than the Earth-bound arrays we already use. In fact it would probably not be better than Arecibo itself as radio waves aren't as distorted by the atmosphere as visible light. Rmhermen 23:57, 10 March 2006 (UTC)
- What if you set up an array of satellite receivers? i.e. VLA but with a diameter of the Earth itself? --Fastfission 02:20, 11 March 2006 (UTC)
- Why use the diameter of the Earth ? If you put them in geosynchronous orbit you would have a much larger diameter and would avoid much of the local radio interference on Earth. StuRat 02:51, 11 March 2006 (UTC)
- Indeed, there already is a VLA-type array on Earth that's thousands of miles across: the VLBA. The VLA looks cool because of all the dishes being close together, but the VLBA does way better on resolution. According to the NRAO web site, it "has an ability to see fine detail equivalent to being able to stand in New York and read a newspaper in Los Angeles." Of course, as StuRat says, a high orbit in space would do even better. On the other hand, there is the downside that you'd need a radiotelescope to receive the signals from your radiotelescope... --Anonymous, 03:57 UTC, March 11.
- What if the Voyagers would have been used for this? That would have given a rather long baseline. DirkvdM 09:08, 11 March 2006 (UTC)
- One problem with that is the speed with which technology advances. In the amount of time it took Voyager to get there, its capability is outstripped by new local deployable technologies. Who would invest in a long term scheme when new technology might surpass it before it started it work? Already adaptive optic technology rivals the Hubble Space Telescope. Rmhermen 15:37, 11 March 2006 (UTC)
- What if the Voyagers would have been used for this? That would have given a rather long baseline. DirkvdM 09:08, 11 March 2006 (UTC)
- A radio telescope isn't all that high tech. Actually, it's pretty low tech I believe. So that isn't a problem and they would have known then that that technology wouldn't improve much. But, not knowing that space exploration budgets would collapse, they might indeed have thought that later faster satelites would overtake them.
- The precision of Hubble can't compensate for a lack of diameter (which is what this is about). And anyway, Hubble isn't a radio telescope. It wouldn't make sense to put a radio telescope in space because that radiation reaches Earth's surface almost unperturbed. And the corrections were needed to compensate for errors, not to improve on an already good system. DirkvdM 07:47, 12 March 2006 (UTC)
March 11
[edit]Combustion chamber
[edit]1) Is the flame ignited in a car engine's motor cylinder a premixed flame- being more efficient, and kinda bluish- thanks to the premixture of fuel and oxidizer?
- Since question 2 is not really related, I split it off into a separate question just below. --Anonymous, 04:10 UTC, March 11, 2006.
Color of overcast sky
[edit]2) Why does a cloudy overcast day have a hot color temperature as indicated by its bluish tint when it is usually cooler (what amkes it color hot)?
- First, color temperature is related to actual temperature only when you're talking about objects that are actually incandescent, like the Sun or a light bulb. That doesn't apply to sky light.
- I think the answer is that sky light on a cloudy day is only bluish in comparison to direct sunlight. On a clear day, scattering in the atmosphere affects high-frequency light more than low, so the sky is bluish while the direct light from the Sun is depleted of blue and therefore is slightly yellow. On a cloudy day, all the light is further scattered in the clouds and as much of it as gets through also gets mixed back together. So it's bluer than direct sunlight, but less blue than the sky light you get in a shadow on a sunny day. --Anonymous, 04:10 UTC, March 11, 2006.
- I would suggest taking a look at the article Rayleigh scattering 80.0.184.11 01:15, 18 March 2006 (UTC)
Earths Atmosphere
[edit]What are two different theories on the origins of life and what is the evidence that supports these two theories?
Try origins of life
Only two? Organic molecules may have formed in several differrent ways and that may have happened in different locations, such as in water, in rocks and in space. And then there are the oldest 'theories', from before the emergence of proper science, that some 'superbeings' created it, but that is of course begging the question because where did those 'superbeings' come from then? DirkvdM 09:17, 11 March 2006 (UTC)
I agree that there are many theories on the origins of life but synthetic theory of evolution is followed.Suraj vas 11:39, 11 March 2006 (UTC)
Search for life on other worlds
[edit]Does anybody out there have any worries on how NASA is actively trying to search for life in other worlds? I am all for exploration, but I am seriously afraid of a scenario in which we make contact with an alien civilization. Stephen Hawkins himself made a statement that basically stated that if we contacted an alien civilization that was far more advanced then we, then there was a chance that they would not come in peace. Does anyone know if the U.S. government or the military has any plans of dealing with such a situation?
- I can understand on why some people may be afraid of a situation in which we make contact with an alien civilization. History on this planet shows us that when a more advanced civilization interacts with a lesser civilization, death and destruction follows. This was the case when Europeans first came to the Americas with their superior guns and wiped out much of the native Indian population. I do not know if the government or military has any plans for this. One would assume that if aliens ever came here they would be possesing some serious technology since they would have traveled many ligh years. I don't think aliens that may be a million years more advanced than we, would have any trouble dealing with "primative" human defense capabalities.
- Actually, guns didn't do a great deal of damage to Native American populations. Smallpox and measles did. - MPF 21:53, 11 March 2006 (UTC)
- Although we have sent some signals, most searches for extraterrestrial intelligence are passive. If we do find a radio signal, the aliens won't know that we know. As for dealing with them, there are other things that we might actually need to defend Earth against; see our stub on Planetary defense. It'd be sad if we spent our anxiety on aliens and then got creamed by a rock. Melchoir 05:27, 11 March 2006 (UTC)
- The Earth has been blaring out radio transmissions to this sector of the galaxy for a century now - anyone looking for signs of civilisation that's within 100 light years could have picked us up. So if there are any alien civilisations out there, they may well know we're here. To be actively looking for alien life means meeting it on our terms, rather than have it suddenly arrive on our doorstep, enabling us to be just that little bit more prepared. If there are intelligent belligerent aliens out there, then whether we're seeking it or not is irrelevant, since it could easily find us (we're a very loud species) - but seeking it out at least gives us a chance of finding it and maybe parlaying with it rather than just have it move in. Mind you, the vast majority of the search for extraterrestrial life is simply looking for any form of life, intelligent or not. Just discovering primitive extraterrestrial life - even monocellular - would be one of the biggest discoveries in scientific history. Grutness...wha? 07:29, 11 March 2006 (UTC)
- Then again, a 100 lightyears radius is nothing compared to the size of the universe, the edge of which is said to be at 78 billion lightyears away. And our won galaxy is about 100 thousand lightyears wide. A main reason it is assumed there is life somewhere else is that there are so incredibly many stars that it would be statistically unlikely that something that happened here will not have happened somewhere else. The nearest star is about 5 lightyears away. Does anyone know how many stars there are in a 100 lightyear radius around us?
- Why would the aliens be interrested in the US alone? Or rather, why should the US alone prepare for this?
- Our intellectual and technological levels would likely be so far apart that they wouldn't be interrested in us. Look at animals in nature. They either compete, eat each other or ignore each other, with the last option being by far the most popular. Animals generally only eat what they know. Something from a different ecosystem (in this case Earth) might not go down well and would be avoided. Humans compete (such as in America) because they are so similar and use up each other's sources (land, food). But we pretty much ignore ants (as long as they don't come in our houses) and the aliens would likely be so totally different from us that they'd probably treat us in the same way. They might not even notice our presence and we'd be able to live here side by side. And we might not even notice them. Maybe they're too minute (hey, maybe ants are aliens) or maybe they take a from we don't recognise as intelligent. Maybe they're made of some gas. Hey, maybe they're already here and that's where ghost stories come from. :) DirkvdM 09:35, 11 March 2006 (UTC)
- There have been any number of theories advanced by science fiction writers and SETI researchers (who sometimes are one and the same) about what contact with an alien species might mean. here's an interesting discussion of the various possibilities, though the signal-to-noise ratio in the discussion occasionally gets a bit low. You might also have a look at SETI, Fermi Paradox, Drake equation, CETI, and First contact. The short version is that a) unless they're travelling faster than light it's not going to be our problem, b) if their technology is sufficiently advanced to permit interstellar travel, they're probably going to have kick-arse military abilities as well, c) we're already transmitting unintentionally, d) even if their intentions are benign, real contact with aliens could possibly cause all manner of societal ructions, as it has when more technologically advanced cultures have run into less technologically advanced ones, and e) as an actual threat goes, there are plenty of other things higher up the priority list than aliens, for instance near-Earth objects and random long-period comets heading our way. --Robert Merkel 12:00, 11 March 2006 (UTC)
- Don't know how many stars are within 100 ly, I too would be interested in knowing. Our List of nearest stars only goes out to 16 ly. Rmhermen 15:30, 11 March 2006 (UTC)
- Hold the horses people - why would aliens want to wipe us out? Earth dosn't contain anything of particular value to a space faring civilzation. All of our ressources could be mined more easily from the Kuiper Belt rather than the gravity well that is planet earth. Our planet will most likely not suit their living needs as they evolved in an entirely different enviroment - so what would they want with earth? Celcius 22:58, 11 March 2006 (UTC)
- I think usually (in popular science fiction) the aliens want land. Let's face it: Earth is a sweet ride. Melchoir 00:50, 12 March 2006 (UTC)
- Hold the horses people - why would aliens want to wipe us out? Earth dosn't contain anything of particular value to a space faring civilzation. All of our ressources could be mined more easily from the Kuiper Belt rather than the gravity well that is planet earth. Our planet will most likely not suit their living needs as they evolved in an entirely different enviroment - so what would they want with earth? Celcius 22:58, 11 March 2006 (UTC)
- They might want to eradicate the cancer called 'mankind' before it spreads into space (as it's starting to do now). This isn't eco-negativism but just a conjecture of what alien minds might work. When it comes to alien thinking I consider myself an expert. :) DirkvdM 07:54, 12 March 2006 (UTC)
- The reasons for wars just between different human societies are not always about rational reasons like resources, and for an alien civilization, who knows? Maybe they are like Nazis and want to exterminate 'inferior' life? Maybe they are mutant ant men and want to eliminate anyone existing contrary to their regimented existance? Maybe they are an ambulatory plant species, which has an imprinted hostility to animals (who preyed upon them until the plants learned to fight back)?
- Even the Nazis only bothered to kill people who got in their way. They weren't about to send out an army to murder people on Easter Island, for example. StuRat 01:04, 15 March 2006 (UTC)
- Ummm, no, they went out of their way. HighInBC 21:27, 18 March 2006 (UTC)
- Even the Nazis only bothered to kill people who got in their way. They weren't about to send out an army to murder people on Easter Island, for example. StuRat 01:04, 15 March 2006 (UTC)
- Here on Earth some plants (and fungi) have started to fight back by producing poisons. And then we fought back by starting to like some of those poisons (I recommend the fungi - no not Danish Blue, although that's good too). DirkvdM 07:37, 14 March 2006 (UTC)
- What are the chances of intelligent life? I used to believe that there were "aliens" out there that are at least as intelligent as us, but a certain documentary[29] changed my mind. :P Even if you ignore the mention of "God" near the end of the video, the scientific and statisical evidence against extraterrestrial intelligent life is staggering. —OneofThem 20:09, 15 March 2006 (UTC)
- We don't know. Melchoir 20:11, 15 March 2006 (UTC)
transistor
[edit]The area of the collector region in a transistor is greater than that of the emitter region. Why it is so?(Detail answer please).
Thank you
Mani reformatted from original all caps
Drawing too much attention to yourself makes me ignore you. For that reason I haven't read your question. DirkvdM 09:37, 11 March 2006 (UTC)
- I don't mean to be rude, but if you can't say anything useful... Sorry, it's nothing personal. Sum0 09:52, 11 March 2006 (UTC)
- Well, my remark looks a bit silly now that someone has cleaned up the question. Fyi, it was all capitals and indented to draw a box around it. So my point was that it was the questioneer who was being rude. DirkvdM 07:56, 12 March 2006 (UTC)
- You are forgiven, my child. Sum0 15:11, 12 March 2006 (UTC)
Nah, nah, Dirk got the 'rude' slam! ...But really, this is a true 'suitly emphazi' homework question. --Zeizmic 12:21, 11 March 2006 (UTC)
- The answer is in bipolar junction transistor, with a nice picture too. --Heron 13:56, 11 March 2006 (UTC)
Can anyone tell me the purpose of this page?
[edit]Adambrowne666 11:13, 11 March 2006 (UTC)
- My eyeballs are behaving like the tumblers of a one-armed bandit after reading some of the pages on this site. Either it's the work of manic surrealists or someone is trying to reproduce Finnegan's Wake. I've no idea what it is, but I've bookmarked it. Actually, it does bear some resemblance to the sort of weird things you get in some spam mail, so perhaps there's a connection there. Many of the links are like that, too, as are the upper level pages (e.g., http://www.pcnotdienst-muenchen.com/misc/intimating/). On the offchance that it is spam related, I'd be wary of doing too many clicks on it, though... Grutness...wha? 11:30, 11 March 2006 (UTC)
- Looks like an example of Markovian Parallax Denigrate to me. GeeJo (t) (c) • 11:53, 11 March 2006 (UTC)
- It is a randomly created website used to harass search engines and programs that search websites for email addresses. There is no specific name for it (yet). Some call it a quicksand pit. Some shorten that to sandpit. Some call it a stinger site. Others call it a honeypot. Regardless of the name, it is simply designed to appear like a real website to a program that automatically goes from link to link in search of content. Because it cross-links to itself with random links, the program gets stuck and never leaves. --Kainaw (talk) 15:15, 11 March 2006 (UTC)
- In fact cf. Honeypot (computing).--Dell Adams 06:54, 12 March 2006 (UTC)
- Is that to battle the likes of Google or spammers? Also, wikipedia links to itself, right? --Username132 21:50, 11 March 2006 (UTC)
- Google is smart enough to leave one of those sites. Spammers write clumsy programs to harvest email addresses from web pages. Those could spend weeks on a randomized site going round and round and round... --Kainaw (talk) 22:43, 11 March 2006 (UTC)
- I see. And how is wikipedia different from one of those sites? --Username132 07:40, 12 March 2006 (UTC)
- In that it doesn't go around in circles linking to itself, I suppose.
- Are all those English words? DirkvdM 08:01, 12 March 2006 (UTC)
- What are you talking about? Of course it links to itself. Cheese > Cheddar > Cheese > Cheddar... --Username132 16:58, 12 March 2006 (UTC)
- Trawlers are usually smart enough not to grab files that they have already grabbed.--Fangz 18:51, 12 March 2006 (UTC)
- So you're saying the page in question doesn't actually hold on to the programs spammers use? --Username132 00:29, 13 March 2006 (UTC)
- You're feeding the trolls again aren't you - now git you trolls and you kids come home right now! hydnjo talk 00:35, 13 March 2006 (UTC)
- Well, it is apparent that the word "random" is not in the troll dictionary. Otherwise, it would be rather obvious what the difference is between Wikipedia and a site that randomly creates web pages with random links to more randomly created pages. --Kainaw (talk) 13:49, 13 March 2006 (UTC)
- Who's trolling? It doesn't matter whether these links are "random" or not. The facts are
a) these website link to themselves "randomly" b) wikipedia links to itself, albeit usually according to some form of logic - how does a trawler differentiate between "random" links and links which are appropriate in their context? My guess is it doesn't. So again, I propose that either a) these websites don't confuse trawlers OR b) wikipedia confuses trawlers in exactly the same way
If your answer is a), please explain why this is so, beyond the fact that the links are much more "random". --Username132 19:33, 14 March 2006 (UTC)
- It is apparent that the point is not getting across. Wikipedia is editable, but it is fairly static. There are a certain number of pages. A handful are added each day. Less are removed. A handful of pages have major changes, but most pages just have minor changes or vandalism reverts. The website mentioned in the question here is as close to infinite as a URL will allow. Try
- The page is randomly created. It does not actually exist. So, no matter how long a spider spends trying to index that site, it will never ever finish (well, if it outlived the Sun and increased indexing speed by 200% every year, it may have a shot at it). Wikipedia, on the other hand, is finite. It can be indexed in a single day. Wow! That fast! Yes - it is mirrored constantly. Just copy the site (an hour at most) and then run a keyword indexer (less than a day will suffice). But, going back to the random page... you will never ever be able to mirror it. You will never ever be able to index it. Why? It is random. So, to repeat once again... Wikipedia is a set of real finite files on a server. The site mentioned in this question is a infinite product of a random page generator. --Kainaw (talk) 00:19, 15 March 2006 (UTC)
Ohhhh, I get it now. That's clever. Thanks! --Username132 (talk) 16:58, 15 March 2006 (UTC)
Raw rice
[edit]My friends told me that eating raw rice is harmful to our health. Is it true?If so how?Suraj vas 11:36, 11 March 2006 (UTC)
- Well, first off remember the conditions that rice is usually grown in. If it's left completely untreated and eaten shortly after harvesting, it's likely to harbour all sorts of nasties. I also seem to recall reading about a form of torture involving raw rice, in which the torturers would force a victim to eat as much of the stuff as they could, and then force them to drink a portion of water. The rice would take up the water and expand, stretching the stomach and causing extreme pain. I'm not sure on how apocryphal that story is though, and in any case, the stuff bought in supermarkets won't cause you any harm if eaten raw. GeeJo (t) (c) • 11:59, 11 March 2006 (UTC)
- Apocryphal. When rice soaks up water, it doesn't expand to more than the volume that the water and unsoaked rice occupied in the first place. - MPF 21:57, 11 March 2006 (UTC)
- Could be an urban legend but I heard its fairly common that you can't throw rice at some churches because birds eat it and subsequently "explode" - which is to say their stomachs crack open under the pressure of the expanding rice. Does anybody know if this is an urban legend? Does anyone have some birds and some rice and a keen mind to find out? Celcius 22:47, 11 March 2006 (UTC)
- Apocryphal. When rice soaks up water, it doesn't expand to more than the volume that the water and unsoaked rice occupied in the first place. - MPF 21:57, 11 March 2006 (UTC)
- snopes.com says this isn't true. User:Zoe|(talk) 23:21, 11 March 2006 (UTC)
- Birds probably know quite well how much raw grain they can eat because that's what they normally eat. Right? Not sure about raw rice, though, because it may be dried. Is it?
- Anyway, if you drink plenty of water it passes through your stomach. If that is filled with dry rice ,that will absorb it. And then you can keep on adding water with at least part of it being absorbed. So the riceball would expand to several times its original size. Then again, wouldn't that go down the digestive tract and come out before we get a Monty Python restaurant scene? DirkvdM 08:08, 12 March 2006 (UTC)
- The version of this story I heard is the birds do not get enough stones in their crop to grind up the rice and have problems because of it.
- Meanwhile rice farmers have too many stones in their crops and have problems because of it, how ironic. StuRat 00:57, 15 March 2006 (UTC)
Pluto
[edit]Recently NASA launched its mission to Pluto. So whats the use? They might discover more moons, water,life etc. So what? I mean they spend millions of dollars for this mission which could be used for the support of millions of poor people in the world. Isn't my point right? You might feel that I am not interested in astronomy at all but I really love this. This question was asked by my friend too me. I just couldnt answer him.Suraj vas 11:46, 11 March 2006 (UTC)
- They don't know what they'll find, so they won't know the point until they find it. In general terms, however, investigating the outer regions of the solar system may help us understand the process by which the solar system was formed, which is a good thing. That can help us with issues such as how life arose, and where else it might have arisen. In any case, the amount of money spent is trivial compared to the amount spent on buying bombs for the army, or the amount of money spent on chewing gum each year. You can bet that if the money wasn't being spent on this mission, it wouldn't be spent on relieving world poverty. Markyour words 12:02, 11 March 2006 (UTC)
- Yes, and what tells you that the money would have been spent to support poor people, or anything useful at all? It's at least as likely that it would have been used to drop a few more bombs on random countries with difficult to pronounce names, so the Pluto mission might actually have saved innocent lives :p dab (ᛏ) 12:11, 11 March 2006 (UTC)
- Tell your friend they are committing the logical fallacy of false choice which is a correlative based fallacy in which options are presented as being exclusive when they may not be. It is often used to obscure the likelihood of one option or to reframe an argument on the user's terms. There is nothing to prevent the government from doing both. This fallacy is also known as Morton's Fork. WAS 4.250 14:52, 11 March 2006 (UTC)
- So, your friend's argument is to fire everyone at NASA, make them poor, and give their money to poor people? How is that a more intelligent thing to do than sending a probe to Pluto? --Kainaw (talk) 22:41, 11 March 2006 (UTC)
- There's this silly idea that feeding the hunger is something terribly important for mankind, as if no money can be spend anywhere else until everyone is chubby. People just try too hard to "do a good thing" (and "feeding the hunger" is the stock "I care" idea) and forget there are other things that are also good for mankind, even on a higher level, such as scientific research. The issue with the poor people shouldn't be solved taking money from these other areas, but by correctly allocating resources and disappearing with corruption and white collar thieves. ☢ Ҡi∊ff⌇↯ 22:49, 11 March 2006 (UTC)
- In about 200 years, when the Earth is uninhabitable, these probes will have given NASA or whatever other agencies which succeed it (and those in other countries) enough information to help to transport humanity into space, where they can survive instead of dying out. User:Zoe|(talk) 23:18, 11 March 2006 (UTC)
- Now that's just rubbish. The Earth will still be habitable in 200 years. In any case it's the most likely probability. The "last perfect day" is a good several billions of years from now. At least that's the general consensus. Here7ic 05:06, 12 March 2006 (UTC)
- You guys seem to have crystal balls (no pun). One far-sighted, one short-sighted. DirkvdM 08:42, 12 March 2006 (UTC)
- I don't need a crystal ball. My contribution is based on the observed age of our sun and it's predicted life. I'd really like to see his sources... Here7ic 18:31, 12 March 2006 (UTC)
- I'm not talking about the sun burning out, I'm talking about the lack of energy sources and the soup of pollution. User:Zoe|(talk) 00:20, 13 March 2006 (UTC)
- Ever heard of expansion? Earth won't be our only home forever. Here7ic 04:12, 13 March 2006 (UTC)
- That's my whole point. If we don't explore the solar system/galaxy, how will we ever be able to expand? User:Zoe|(talk) 17:16, 13 March 2006 (UTC)
- It is expected that a large amount of money will be spent on space exploration within the next decades. Here7ic 01:32, 14 March 2006 (UTC)
- That's my whole point. If we don't explore the solar system/galaxy, how will we ever be able to expand? User:Zoe|(talk) 17:16, 13 March 2006 (UTC)
- Ever heard of expansion? Earth won't be our only home forever. Here7ic 04:12, 13 March 2006 (UTC)
- I'm not talking about the sun burning out, I'm talking about the lack of energy sources and the soup of pollution. User:Zoe|(talk) 00:20, 13 March 2006 (UTC)
- I don't need a crystal ball. My contribution is based on the observed age of our sun and it's predicted life. I'd really like to see his sources... Here7ic 18:31, 12 March 2006 (UTC)
I am one of those who supports the pursuit of knowledge as its own end (obviously, since I’m a knowledge seeker). Yet experience has repeatedly shown that the pursuit of science provides practical benefits. Obviously, without a space program, we would not have satellites for weather, commmunication, television, and so on. If not for this tendency to push our limits and to explore, humanity might have remained hunter-gatherers still confined to a small region of Africa. And while local sea transport certainly helped with coastal trade and so on, the deep-sea voyages of several centuries ago were extremely risky and expensive, with no clear knowledge of what lay beyond, to use an approximate analogy. — Knowledge Seeker দ 06:01, 12 March 2006 (UTC)
- Relatively speaking, these explorations are very cheap. Space exploration brings up the same argument and counter-argues that "space exploration itself receives a very small percentage of total government spending (nearly always under 0.5%)". And most countries don't even have any space program at all. A mistake often made is to just look at the cost and not at how often that cost is made. Space programs are expensive but rare. New Horizons (the project) says it costs 650 million US$ over 15 years. That's 40 million per year. A comparison with military cost is obvious, but let's take something closer to home. The US has 220 million cars. The total cost of driving a car is a few thousand $ per year. Say that comes to 400 billion per year. That would then be 10.000 times the cost of the program. So maybe if people would do more ride sharing that would save loads of money that could be spent elsewhere. You yourself can donate the money you save with this. Share a ride and save a life. That's something you can do yourself, so you don't have to bitch about the government not doing things right. DirkvdM 08:42, 12 March 2006 (UTC)
World hunger is not as simple as just not having enough food for poor people, the reasons it exists are complex. For instance, a country may have a dictator who uses collective farming techniques to force the farmers to raise cash crops like strawberries to generate revenue to buy weapons. Another problem is like the situation in Iraq where the UN was drawing off resources intended to provide food and medicine to the people. Also, just giving people food does not teach them how to feed themselves.
- Suraj, you might like to think about why your own country is doing things like a) building the BrahMos cruise missile, buying (and building, I believe) Su-30 fighter jets, and hosting the 2010 Commonwealth Games, all of which are considerably more expensive than the New Horizons mission? Are they really more important than the 300-million odd Indians who still live in absolute poverty? And would cancelling any of them help those people? --Robert Merkel 00:48, 14 March 2006 (UTC)
- How does collective farming promote poverty? Or did you just throw that one in for good measure? DirkvdM 07:41, 14 March 2006 (UTC)
- Well, I do not want to turn this into a collective farming rant, so just presume collective farming=command economy and do the math.
- Wait, communitarianism and collectivism is not necessarily by a command economy...there are gift economies and anarcho-syndicalist communes, for instance... Elle vécut heureuse à jamais (Be eudaimonic!) 22:58, 14 March 2006 (UTC)
- I did not want to get into an extended discussion of collective farming and command economies. For the example stated above, the dictator uses a collective farming techniques in it's command economy sense to starve the people.
Origin of life
[edit]In wikipedias article about this there is no mention about Synthetic theory of evolution. But my science book(CBSE, class 10) says its the currently accepted theory of evolution. So which is right?
friction
[edit]what is rotational friction.while a bicycle is running how is the frictions act on the tyres?--Mufleeh 12:27, 11 March 2006 (UTC)
- See rolling friction. Is "rotational friction" another name for it? --jpgordon∇∆∇∆ 16:59, 11 March 2006 (UTC)
- That's probably what the question was looking for, but in general, from a google search, "rotational friction" seems to mean any damping torque in general. Melchoir 19:29, 11 March 2006 (UTC)
Pre-heat/warm oven?
[edit]Why do we pre-heat/warm ovens?
Regards,
Reelstreets.
- Are you talking about an oven you would have in your kitchen? If so, to pre-heat the oven just means to turn the oven on a few minutes before you are going to need to use it so that the oven is up to the desired temperature before you put the food into it. Dismas|(talk) 15:22, 11 March 2006 (UTC)
- It's because foods cook differently at different temperatures. Let's say you were baking a cake. Cooking it for 10 minutes at 300 degrees(F) is not the same as cooking it for 20 minutes at 150 degrees(F) (twice as long at half the temp). All things are cooked at a specific temperature to achieve the best results. We preheat ovens so that from the moment we put the food in there, it is cooking at just the right temperature. A good example of how things cook can be found in pretzels. Hard pretzels are the result of high temperatures and short cook times. Soft pretzels are the result of low temperatures and long cook times. See how much of a differene it makes? --Chris 15:59, 11 March 2006 (UTC)
Oh! Right. Thank you so much for the information. Reelstreets.
I don't always preheat the oven, let me explain why I sometimes do and sometimes don't:
- Preheating is useful if you are cooking something based strictly on time, for example, at 300 degrees for an hour. That is, without it you don't exactly know how long it takes for the oven to get up to temp and how much cooking was done while at the lower temps.
- Cooking based strictly on time and temp is akin to "dead reckoning", however, which is finding a location by moving a certain amount in a certain direction with no regard for landmarks. If you use landmarks, you can navigate much more closely to your target. Imagine the difference between driving home with your eyes closed and with them open ! In cooking, you can look for browning, use a thermometer, stick a fork in the food, use smell, etc., to gauge when the food is ready. This is much more reliable than going strictly by time and temp, which may vary by altitude, oven design, humidity, slight differences in proportions of ingredients, age of ingredients, how much of your yeast is alive, etc.
- I prefer to put food into the oven and take it out, when the oven is cool, to prevent accidental burns. Yes, you can use pot holders, but one slip and it's easy to severely burn yourself. I also like to open the oven door and let it cool down before removing the food. Most food needs a cool-down period before it's served anyway, so this is not a problem.
StuRat 20:47, 11 March 2006 (UTC)
- You're odd. Everybody knows that the best way to prevent accidental burns is to first burn yourself on purpose so that you develop large calluses on your hands and all the nerves die. You can't grow up to be a respectable old lady with cared-for dove-soft hands. freshgavinΓΛĿЌ 05:44, 13 March 2006 (UTC)
- I don't want to be a respectable old lady. I was rather hoping to be a dirty old man. StuRat 04:01, 14 March 2006 (UTC)
multilingual webpage
[edit]What's the right way to make a page multilingual? Like, so that the right language gets selected automatically according to the clients language preference? -lethe talk + 17:59, 11 March 2006 (UTC)
- You have to set up your web server to serve them a different page based on the "Accept-Language" field sent by the browser. Exactly how to do it depends on which web server you're using. —Keenan Pepper 18:33, 11 March 2006 (UTC)
- Well, what if I don't have access to the server configuration? I was hoping for something I could include in the page. Basically I'm looking to replace a front page that says "choose your language". You know that wikipedia error page that you get when the site goes down? It's a single page, but it includes 5 or so different languages, which you can switch between (without loading a different page). Like, maybe it's Javascript? How do I see that page when wikipedia is not offline? -lethe talk + 18:40, 11 March 2006 (UTC)
- So does the webserver have to know what translations to use for every single page it's going to serve? Seems a bit inefficient, doesn't it? Every time you add a page, you have to update the server config? -lethe talk + 18:50, 11 March 2006 (UTC)
- The multilingual portal for wikipedia should appear here, and this page seems to address your 'automatic language selection' needs. Tzarius 01:25, 12 March 2006 (UTC)
Tracking Addresses
[edit]Hi is it possible for people to find out my location from posting on Wikipedia, cos people get your I.P address? Also can people do the same from emails, i have a hotmail account can they find my address? thanks Kingstonjr 18:07, 11 March 2006 (UTC)
- Short answer: in principle, yes, your IP address tells your physical location, but in practice, no, no one can find out your address. Yes in principle because your IP address is just that, an address. Packets on the internet use it to get to and from your computer, and if internet packets can do it, then so can people. Only, most people don't have access to all the routing information of the internet, so in practice, it's very hard.
- On Wikipedia, if you have a user account, no one can get your IP address without developer or m:CheckUser access. Thus having a user account actually makes you more anonymous. If you edit wikipedia "anonymously", then anyone who cares to can see your IP address.
- With email, email headers generally contain the fully qualified domain name (FQDN) of the machine they originated from, which is equivalent to the IP address. Emails from webmail services do not actually originate on your machine, they originate on the webserver, although the webserver can choose to add the client's IP address to the headers. Hotmail does this, gmail does not.
- So what can people find out from your IP address? It depends on the address. When I'm in my office at university, my FQDN contains my last name, the department I work for, and the name of my uni. That's a lot of information; with that information, someone can look up my full name, see my picture, get my phone number, and my mailing address from the university's online directory. When I'm at home, my IP address contains the name of my internet service provider (ISP) and an abbreviation for the city, province and country that I live in. If I were using AOL instead, the IP address would contain less information; just the name of the ISP (AOL). It might also say whether I'm a broadband or a dialup user. In no case does your IP address contain information about your actual mailing address, though in some cases it can be almost as good.
- Your ISP does know your mailing address, that's how they send you bills and do maintainence on the connection, etc. So people who work for your ISP can figure out your address if they know your IP address. This also applies to people who can break into your ISP's database or people who can get a judge to issue a court order to the ISP to release that information. -lethe talk + 18:27, 11 March 2006 (UTC)
- In films people sometimes hide their location by being connected through several times (or something like that - I don't understand how that works either). But I suppose that is just to make it difficult to locate them, even for intelligence agencies. By the time the location is found they're gone. I suppose. DirkvdM 08:50, 12 March 2006 (UTC)
- Lethe makes a good point, though I would like to point out that to track somebody you need both their IP and the time they were using it. This is because many ISP's give the same IP to many people over the course of time. As for DirkvdM's comment about connecting through other computers, perhaps the article on proxy servers should be consulted for one method of doing this. HighInBC 21:40, 18 March 2006 (UTC)
- A lot depends on the internet connection in question. Here in the uk with dialup or DSL connections all you could find out without the ISPs cooperation is that someone is in the uk. With cable you can generally figure out what area of the uk they are in from the reverse dns (e.g. something.basi.blueyonder.co.uk would mean basingstoke. If your on a corprate or academic network it may be much easier to get a physical location though (e.g. A16lab.ee.umist.ac.uk is a dead giveaway as to someones location right down to room number!). Sometimes its more cryptic but easilly figured out at least down to site level with some local knowlage (e.g. hh052a.halls.macnhester.ac.uk) Plugwash 22:18, 18 March 2006 (UTC)
Antibiotic Sensitivity Testing (Antibiotic Discs)
[edit]Antibiotic sensitivity is tested with impregnated discs. The discs are available at a different antibiotic concentrations from the same company (e.g. Nofloxacin (10 mcg)-NF, Cefuroxime (30 mcg)-CR, Ciprofloxacin (5 mcg)-CI). Now is it fair to normalise the size of the clearance to the concentration of antibiotic on the disc - I mean is a clearance of 30 mm for 10 mcg of antibiotic A supposed to make it an equaly good choice as 30 mm 10 mcg antibiotic B? I mean antibiotic B could be lethal at a concentration of 10 mcg...
...so do they impregnate the discs at physiologically meaningful concentrations? Different companies even do different concentrations for the same antibiotics... how confusing! How does one decide what to give a patient? --Username132 20:05, 11 March 2006 (UTC)
- Different disks are used for different purposes, or are used with appropriate variations of method. Obviously, the concentrations need to be clinically relevant. Patterns of infection and resistance in a hospital are closely watched and if the sensitivity testing results were not correlating with clinical responses to infections, it would be noticed and investigated. The sensitivity report is only one of the pieces of information considered in choosing an antibiotic. I suspect you could pay a visit to the microbiology lab of your local hospital and one of the lab techs would be happy to show you how it is done and answer your specfic questions. alteripse 03:05, 12 March 2006 (UTC)
- In clinical use, you have to consider a number of things aside from antibiotic sensitivity, although it is definitely a good starting point. The antibiotic has to be able to reach the site of infection in an active form at adequate concentrations, and you have to consider possible side effects, contraindications, and drug interactions. For instance, tetracycline antibiotics may bind to a number of different metals such as magnesium, aluminium, iron, and calcium-- this will inactivate the tetracycline. Hence, they should not be taken with antacids or milk. Also, since they bind to calcium, they can also cause permanent stains in children's growing teeth, and the tetracyclines may also affect their bones.
- Some bacteria may not show resistance at low concentrations; however, when you test at higher concentrations, genes for antibiotic resistance may be activated, and the bacteria is then resistant. This is known as "inducible resistance", and is associated with the SPICE organisms (Serratia marcescens, Pseudomonas, Indole-positive Proteus, Citrobacter, Enterobacter). In these cases, the disc method might not pick up on this form of antibiotic resistance, and you may have to resort to other techniques [30].
- Additionally, some bacteria may form biofilms, which are aggregates of micro-organisms that can resist antibiotics-- this makes treatment of replacement joint infections difficult. Also, when you take broad-spectrum antibiotics, you usually end up killing a multitude of microbial species in your gut (i.e. gut flora). This may allow Clostridium difficile to take over, and you can get Pseudomembranous colitis as a result.
- Hopefully, I haven't overwhelmed you! But as you can see, there are a number of factors that go into choosing the most appropriate antibiotic aside from antibiotic disc sensitivity. --Uthbrian (talk) 06:01, 13 March 2006 (UTC)
Thank you! That's really helpul! Incidentally, that tetracycline thing is quite relavent. My dissertation involves its use as a controller of gene expression but I guess you wouldn't want to do this with children. Is the stain permenant? I mean bones are dynamic and constantly being rebuilt - surely this would also make adults susceptible? --Username132 19:38, 14 March 2006 (UTC)
visual acuity testing using the stars
[edit]I am preparing a talk to ophthalmalogy residents. It has been said that a test for keen visual acuity in ancient times was to be able to see the "double" star of the second star from the end of the Big Dipper- Mizor and Alcor. This separation is 12 minutes of arc. 12 minutes of arc is equivalent to what in Snellen Vision Acuity about 20/50 or 20/200? Thank you, George Bohigian MD Washington University School of Medicine St. Louis, Mo
- Hmm... Visual acuity and Zeta Ursae Majoris don't provide an immediate answer. It doesn't seem as if groups of dots are standard optotypes... Melchoir 21:02, 11 March 2006 (UTC)
- I know that answering this question is futile, but I can't help it. :-\ The letters on the Snellen chart are based on a 5x5 grid. At 20/20, each letter subtends 5 minutes of arc, so each grid cell subtends 1 minute. Recklessly assume that resolving two stars is the same as perceiving a single black cell between two single white cells (which of course is not what happens in a real Snellen test). At 20/20, the centres of the two white cells are separated by 2 minutes of arc. 12 minutes of arc is six times bigger than this, so the Snellen fraction would be 20/120. However, with star-spotting, you are violating the standard illumination conditions of the Snellen test just a teensy bit, so I doubt that this result is meaningful. The only true way to equate the Snellen scale with the star-seeing test would be by experiment, which would be an interesting exercise. I wonder if anyone has done it. --Heron 18:32, 12 March 2006 (UTC)
- Good response Heron. I think it would also the same as discerning 3 black cells in a row from two black cells separated by one white cell. I would guess that 20/120 would correlate quite well (at the very least it would be a rough estimate) with the star-example although it is true that experiment is the only way to tell. George, I'm wondering where you read about that being an acient test? Just curious, thanks. -Snpoj 07:08, 13 March 2006 (UTC)
Languages
[edit]How do you add foreign language support in mozilla firefox? I tried tools/options/edit languages/add language, but this did not work -Anonymous
- Depends on what you mean by "foreign language support". If you want to read pages in other languages, it should just work automatically as long as you have the right fonts. If you want the browser interface to be in a different language, you have to download a regionalized build here: http://www.mozilla.com/firefox/all.html —Keenan Pepper 23:40, 11 March 2006 (UTC)
- I meant the former, so I guess I don't have the right fonts...But there is nothing that automatically asks me to download the required fonts, like in Internet Explorer. For example, I can't read [31] -Anonymous
- It doesn't have to be any particular font, just one that includes the required characters. For example, if you install Microsoft Office, it includes a version of Arial with characters for a wide variety of languages. If you don't have Office, you may be able to find a freeware font with the same characters, such as Code2000, but I haven't tried any of those.
- By the way, that "Add Language" dialog is for something completely different. The list you enter there is sent to web servers, which can then decide which language version of a page to send you in case they have more than one of the same page. I don't think it's used much any more. —David Wahler (talk) 15:16, 12 March 2006 (UTC)
- That's strange...I already have Microsoft Office. I even went to Microsoft Office Tools/Microsoft Language Settings and added the language (Chinese) but i still couldn't read [32] -Anonymous
- Oh, wait, it works now. Thanks! -Anonymous
Problem with my mouse
[edit]I have a problem with my mouse (computer): It seems to jump across the screen, move up and down at random intervals, completely stop responding but then responds a few seconds later, and sometimes go into a slow, sticky movement (if you know what I mean, it's a bit abstract to explain). The connection from computer tower is fine; and I am using a ball mouse. Can anyone help me out on how to resolve the problem? Thank you very, very much. Kilo-Lima Vous pouvez parler 23:01, 11 March 2006 (UTC)
- If you haven't already done so, cleaning your mouse is a good idea. Take out the ball and scrape the grey gunk off the rollers (most mouseseses have three). If it's an old mouse, then the problem may be with the cable - if it's worn internally, it may not send a good signal to your computer. And of course, check the connection!
Slumgum 23:10, 11 March 2006 (UTC)
- I'd suggest you to just go get an optical mouse instead. They're pretty cheap nowdays and they're just too much better than ball mouses. ☢ Ҡi∊ff⌇↯ 00:58, 12 March 2006 (UTC)
- Alas you specified that it is about a computer mouse. Without that the question would have been a lot funnier. Especially the 'ball mouse' bit. DirkvdM 08:53, 12 March 2006 (UTC)
- More seriously, it sounds like your graphical system is overloaded. In that case you should have other graphical problems as well, such as when switching between windows being slow. Having too many windows open (say, over 20) could be a cause. I also once had something like this with a mouse-pad, althogh I can't remember the details. I think I had both a normal mouse and the mouse pad connected and when I disconnected the latter all was fine. Does the problem persist when you use a normal mouse? DirkvdM 08:59, 12 March 2006 (UTC)
- What about mice with a tracking ball - are they nice to use? --Username132 17:02, 12 March 2006 (UTC)
- If you don't mind people making fun of you, and if you can get used to doing everything backwards. freshgavinΓΛĿЌ 05:39, 13 March 2006 (UTC)
- I use a digitizing tablet and pen. Never needs cleaning to get it to work and I don't have to decide if more than one computer mouse are "mice" or not, LOL. StuRat 04:11, 14 March 2006 (UTC)
Download freezing my PC
[edit]I tried to download an .exe to upgrade one of the downloaded programs I have on my PC, and the download froze the computer right at the very end. I had to literally turn the computer off and back on again to get out of it. Now the mostly-downloaded file is sitting on my desktop, and every time I even touch it, either by clicking on the icon on the desktop or clicking on the file name in Windows Explorer, the PC freezes again. Any ideas on how to delete this file? User:Zoe|(talk) 23:08, 11 March 2006 (UTC)
- I am assuming you use Microsoft Windows, and if so, then you could try their Malicious Software remover. I have not yet downloaded this, so, unfortunately, I am unable eto give you a mini rave-review. It's available here. Hope this helps. However, after reading a few details on the page, it appears it scans your ccomputer, so I doubt the file will come up becuase every .exe file will come up. Kilo-Lima Vous pouvez parler 23:20, 11 March 2006 (UTC)
- (added after edit conflict) First off, a few specifics. What program were you trying to update? What was the name of the exe file? From where did you download it? It might be some sort of spyware mucking up your OS, so it'd be helpful to know what something about it.
- Other than that, it sounds like there was a problem with your browser downloading it. Since it froze before it was even finished, it seems like it doesn't have very much to do with the exe itself, other than it got malformed during download. Have you tried simply downloading the file again?
- As for deleting it, you may wanna try deleting it using the command line, instead of using Windows Explorer. Does this work? That is, open Run, type "cmd" browse to the correct folder using "cd", and typing "del [filename]". Hope this helps Oskar 23:23, 11 March 2006 (UTC)
- It was an update to IrfanView. It isn't a .exe file, it's a .part file, because the download didn't finish. I can't delete it, that's the problem, the minute I single-click on the file name or the icon, the computer freezes. Maybe I'll try re-downloading it, see what happens there. User:Zoe|(talk) 23:44, 11 March 2006 (UTC)
- So, what you're saying is, probably not spyware? :P Try downloading again and/or deleting it using the command line, those should work. Oskar 23:48, 11 March 2006 (UTC)
- Having more than one OS installed can often help in such situations. Preferably two different ones, so if you use msWindows, install Linux (takes about half an hour with a modern distro and you'll have all sorts of programs coming with it - unlike with msWindows). Then, from there, it should be no problem to remove the file (linux can access just about any file system, although ntfs is problematic). And then you can use Linux to download the program. That's what Linux is best at - internet stuff. And if things go wrong it won't freeze (well, extremely unlikely). DirkvdM 09:04, 12 March 2006 (UTC)
- I find Ubuntu Linux useful because the Live CD enables complete access to Windows folders without having to go to the trouble of installing it all. Sum0 15:07, 12 March 2006 (UTC)
- Have fun trying to mount it though. The wiki will come in handy if you decide to go the Ubuntu route. --Optichan 18:47, 14 March 2006 (UTC)
- I find Ubuntu Linux useful because the Live CD enables complete access to Windows folders without having to go to the trouble of installing it all. Sum0 15:07, 12 March 2006 (UTC)
Attempting to download it did the same thing -- download froze at 1 second left, and I had to turn off the PC. And Start/Run/cmd doesn't work, it says it can't find the program (I've never tried to get into MS-DOS from this computer, this is the first time I've tried the cmd command). User:Zoe|(talk) 00:04, 13 March 2006 (UTC)
- Which version of Windows are you using? Try Start/Programs/MS-DOS Prompt or Start/Programs/Accessories/Command Prompt. --Optichan 18:49, 14 March 2006 (UTC)
- You say the half-downloaded file was a *.part file, so I assume you're using Firefox. Firefox has frozen for me before at the end of downloads for no reason. Also, have you configured Windows so that a double click opens a file or a single click? If you're configured in the default way so that single clicks just select, and your computer freezes on merely selecting the file, that's not good. When you select a file in Windows Explorer, it finds out a bit more information about the file such as date created, modified, accessed, and information on its place in the file system. The file's gotta be pretty corrupted if it freezes like that. Also, it would be advisable not to run an incomplete *.exe file. It tends to hang, do nothing, you have to end its process and even then, it doesn't let you delete it except in Safe Mode (hold F8 while booting). -- Daverocks (talk) 03:01, 15 March 2006 (UTC)
March 12
[edit]aromatic oils in citrus
[edit]What is the purpose of the aromatic oils in citrus zest? Are they an insect repellent, or what? —Charles P._(Mirv) 00:44, 12 March 2006 (UTC)
- Does the word purpose mean you support intelligent design, or are you really asking why the citrus tree evolved the genes to make those oils? —Keenan Pepper 01:23, 12 March 2006 (UTC)
- the latter. sorry if that wasn't clear. —Charles P._(Mirv) 02:21, 12 March 2006 (UTC)
- Many fruits are "designed" (the parentheses are there to keep that anti-Intelligent Design guy from chiming in again) to be eaten, then the well fertilized seeds grow into a new plant. Having a bright color and fragrance makes it easier for animals, including us, to find and eat the fruit. StuRat 06:51, 12 March 2006 (UTC)
- Also, some of them might be chemical signals in the flowers, to bees or whatever pollinates them, that happen to remain in the fruit. —Keenan Pepper 07:04, 12 March 2006 (UTC)
- Your comment on intelligent design was way out-of-order and I expect you to bow deeply and resign from your post. It doesn't matter if it was an intelligent purpose or not, it still functions in the same way and achieves the same results no matter what you think of evolution. freshgavinΓΛĿЌ 05:38, 13 March 2006 (UTC)
- Also, some of them might be chemical signals in the flowers, to bees or whatever pollinates them, that happen to remain in the fruit. —Keenan Pepper 07:04, 12 March 2006 (UTC)
- Um...I leave orange peels and lemon peels on the grass so that the racoons and cats don't visit my place... --HappyCamper 03:17, 14 March 2006 (UTC)
- Be honest. You just put them there because you don't want to have to explain to people that racoons and cats don't want to visit your place! freshgavinΓΛĿЌ 03:41, 14 March 2006 (UTC)
- Um...I leave orange peels and lemon peels on the grass so that the racoons and cats don't visit my place... --HappyCamper 03:17, 14 March 2006 (UTC)
- I disagree with bringing ID vs Evolution into this question, but one might argue that they'd give different reasons for why the citrus has those oils. (Presumably, the former would say they are mainly for the benefit of humankind, the latter, that they are mainly for the benefit of the tree. However, some forms of creation might say that God made the tree to benefit itself (to become fruitful - pardon the pun - and multiply itself), that the fact that it benefits other species is simply good design. Circle of life and whatnot.)
- Either way, a lot of fruit actually "wants" to be eaten and goes out of its way to attract animals. About the insect repellant idea - I don't know if it helps in the tree, but citrus juice is used to handle insects outside: http://www.thriftyfun.com/tf000835.tip.html Bryce 14:06, 19 March 2006 (UTC)
Mustard oil
[edit]Mustard oil is being used in India for cooking for thousands of years.IBut I think it is banned in most of the countries including USA and Europe.Is it really hazardous to health? If it is so why is it hazardous?
- Have you read the article on mustard oil? —Keenan Pepper 01:18, 12 March 2006 (UTC)
- Mustard oil is the best! deeptrivia (talk) 01:21, 12 March 2006 (UTC)
Moment in a bimetallic strip
[edit]When we heat a bimetallic strip, differential expansion causes an induced moment in the strip, from where the curvature of the beam can be calculated (see Eqn. 4 on this page.) This page says: "Derivation of equation (4) is not particularly complex, but need not concern us (see Clyne T W, Key Engineering Materials, vol.116/117 (1996) p.307-330). It is based on the balancing of the bending moment generated by the misfit strain against the opposing moment offered by the beam." I don't have this reference, but since it says this derivation is not complex, I was wondering if someone has ideas on how to go about it. Any help would be highly appreciated. deeptrivia (talk) 03:26, 12 March 2006 (UTC)
- I have posted an usatisfying answer on his talk page Vicarious 20:58, 13 March 2006 (UTC)
what's a good common mesh/model file format with enough features?
[edit]I've been looking up mesh/model file formats, but I can't find any open ones with easily-found documentation for a format whose specification allows for not just regular static meshes but also animation channels (weighted or not) and UV texture coordinates.
The closest one I could find have been the .MD3 and .MD5mesh/.MD5anim file formats used in John Carmack's game engines, but I'm not sure if he wants royalties for using those formats. And even if the MD2/MD3/MD5 formats are free to use, exporters for Blender (the only 3D suite I can afford) for that format are only partially functional in that they don't export everything they ought to export.
The only mesh format I've become somewhat comfortable with has been the Wavefront .obj format -- being text based, it's easy to figure out a lot of it just by looking at it and I've written a flat-shaded model viewer for .obj files, but the specifications I've found so far do not indicate that it supports animation information or multiple texture channels.
Do I have to come up with my very own model format and write my own Blender exporter for it, all from scratch, if I want to write anything resembling a graphics engine? That seems to be what many videogame companies do anyway, using an in-house format that they no doubt wrote custom exporters for, but I really don't have time for that. Or is there some open-specification, well-documented, royalty-free, and pretty-much-fully-featured model/mesh file format out there supported by the majority of 3D modeling+animation programs that I haven't noticed yet? It's just frustrating that the only thing holding me back from writing an engine that does more than render flat-shaded, static models is the lack of information and availability of utility. --69.237.157.237 08:06, 12 March 2006 (UTC)
- Wow! Do you want to get a real name and work for us? The pay is lousy, but you get to make fun of people. As for your question, you'll have to go to newsgroups, or advanced graphic forums. Since programmers are very mobile, I am sure that somebody has solved the problem, and is no longer tied in-house. --Zeizmic 17:42, 12 March 2006 (UTC)
.m4a
[edit]are there any specific programs that can turn .m4a songs to the format of .wma of .mp3 format songs? urgent!
- iTunes can. Melchoir 10:30, 12 March 2006 (UTC)
- To turn m4a into mp3, use iTunes. To do this go to prefrences, and make sure you are importing to mp3, with the bps of your choice (128 is CD quality), and right click (control-click on a mac) on the song(s) you want to change and select "convert to mp3." I am pretty sure you can't convert from m4a to wma, because of codec issues. If you want to convert from wma to mp3 or m4a, then you would want to use Easy WMA. Love, --
 Mac Davis] ☢ ญƛ. 11:20, 12 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 11:20, 12 March 2006 (UTC)
- It's certainly possible to convert from m4a to wma. If nothing else, you could convert the m4a file to a wav file, then convert the wav file to wma. However any transcoding between lossy formats is going to do damage to the quality of the file, so should be avoided if at all possible. Markyour words 11:39, 12 March 2006 (UTC)
I presume that you are using a PC, since you want .wma, but what are you trying to do? A clearer idea of your purpose would help. For great justice. 02:51, 13 March 2006 (UTC)
Audio Conversion
[edit]How can I convert audio files from a CD into .mp3 files, for free?
- Use any CD ripping program. ITunes is popular but very bad; EAC is harder to use, but gives the best quality. Markyour words 12:28, 12 March 2006 (UTC)
- Can't Windows Media Player simply do it? Kilo-Lima Vous pouvez parler 12:42, 12 March 2006 (UTC)
- Newer versions can, but they're no better than iTunes. Markyour words 13:02, 12 March 2006 (UTC)
- Winamp Pro can. This costs $20. You can try this piece of freeware. Though, it doesn't look perfect. Computerjoe's talk</span> 15:34, 12 March 2006 (UTC)
- I use FreeRIP, my sisters use EasyCD-DA Extractor. In my opinion, they're both better than all these suggested so far. FreeRIP is the faster I know, too. ☢ Ҡi∊ff⌇↯ 01:12, 13 March 2006 (UTC)
- Can't Windows Media Player simply do it? Kilo-Lima Vous pouvez parler 12:42, 12 March 2006 (UTC)
- There is a LAME plugin for iTunes that replaces iTunes crappy codex with a kick ass mp3 encoder. For great justice. 02:52, 13 March 2006 (UTC)
- I've always used CDEX for ripping. Just another reliable option. It's small and freeware, allows plugins. freshgavinΓΛĿЌ 05:33, 13 March 2006 (UTC)
- I like to use DeepRipper. Its completely free and very easy to use. Try it out. You can download it from download.com or from the official website. If you use the official website, make sure you get DeepRipper and not DeepBurner. I suggest download.com though. Latest version is 1.1 for both sites. --Chris 16:14, 13 March 2006 (UTC)
Blood circulatory system
[edit]How long does it take for sugar in a can of cola to diffuse throughout the body? I need this info for a project im doing involving the "healthiness" of different canned drinks
Wargsmon
- Well, even although I don't have a specific andwer for this question, bu if coca-cola contains roughly the smae amount of sugar in honey, then it iwll take about 20 seconds for the suagr to get into the bloodstream. (Source: The Book of Useless Information.) Kilo-Lima Vous pouvez parler 14:52, 12 March 2006 (UTC)
- Note this is probably the first appearance of sugar from honey in the portal blood, not full absorption or "diffusion" into the systemic circulation. alteripse 18:47, 12 March 2006 (UTC)
Ingested sugar does not normally "diffuse throughout the body" as sugar in normal healthy, non-fasting people. Most is absorbed from the duodenum in less than 15 minutes, triggers insulin and incretins, is carried by portal vein blood directly to the liver, where it is further metabolized. Most is stored in the liver as glycogen, and some is used to make triglycerides or other molecules. A significant amount of sugar is passed to the systemic circulation to "diffuse throughout the body" only when insulin effect is low, which occurs mainly in the following circumstances:
- a person has gone a prolonged period without eating carbohydrates (usually more than 16 hours), or
- a person has a low blood glucose not due to excess insulin, or
- a person has untreated or grossly undertreated insulin deficiency due to any form of diabetes mellitus, or
- a person has severe insulin resistance due to type 2 diabetes mellitus.
Does that answer your question or is that more than you wanted to know? The unhealthiness of most sugar drinks has several dimensions:
- the amount of sugar
- the amount of phosphoric acid (bad for bones and teeth)
- the amount by which healthier food intake is reduced.
Good luck with your project. alteripse 14:59, 12 March 2006 (UTC)
Unknown plant
[edit]Not a homework question, but what plant is best known for its healing of burns? Is it chamomile, becuase I couldn't find it on any of the sub-articles it recommends. Thanks! Kilo-Lima Vous pouvez parler 18:15, 12 March 2006 (UTC)
- are you thinking of aloe? —Charles P._(Mirv) 19:18, 12 March 2006 (UTC)
Windows Media Player 10
[edit]Is it possible to export the information stored by Media Player (Play Count, Song Length, Rating etc) in the form of a spreadsheet/database? smurrayinchester(User), (Talk) 15:11, 12 March 2006 (UTC)
- Hi, I have tried to get it on a spreadshett and a databse, but can't seem to go it; so I don't really think you can, sorry. Kilo-Lima|(talk) 17:10, 18 March 2006 (UTC)
March 13
[edit]noninteractive XML editor?
[edit]Anybody know of a good, noninteractive XML editor? I'm thinking of something analogous to sed. It could be based on XSLT, or something simpler. —Steve Summit (talk) 01:54, 13 March 2006 (UTC)
- I use Kate. No annoying popups. No annoying auto-completes. Plus, you can open/edit/save on remote servers without doing any import/export garbage. --Kainaw (talk) 13:44, 13 March 2006 (UTC)
- Thanks, but that looks pretty interactive to me! Anybody know of any noninteractive ones? —Steve Summit (talk) 16:16, 13 March 2006 (UTC)
- I'm tempted to suggest rolling your own Perl program for whatever it is you want to do -- if you know sed then it's not much of a leap, and there's loads of XML parsing modules available. But then I love Perl and have a tendency to see everything as a nail that can be hit by its hammer. --Bth 16:31, 13 March 2006 (UTC)
- Oh, believe me, I'm an incorrigible wheel reinventor, and I'm always sorely tempted to roll my own! But for once in my sorry, narcissistic life, I thought I'd see if there wasn't something prewritten which I might deign to use. And, in fact, there might be: I've since discovered that Xalan comes with a command-line tool which will do just what I want, as long as I'm content to express my transformations using XSLT. I've downloaded the Xalan and Xerces sources and built them; now all I have to do is get my head wrapped around XSLT, which at first glance seems several times more complicated than it ought to be, but that might just be me, or the unfortunate result of the fact that all the on-line turorials I've found so far are, alas, somewhat less than scintillating. —Steve Summit (talk) 05:06, 14 March 2006 (UTC)
Graphics of the PS3
[edit]Does anyone know if the PS3 will be able to achieve superior graphics than the XBOX 360? I have seen the technical specifications of both of the systems GPU's but I am not sure of the meaning of all those numbers. It seems that the PS3's GPU has higher spec numbers. —The preceding unsigned comment was added by 4.234.222.77 (talk • contribs) 02:35, 13 March 2006 (UTC)
- I believe the XBOX 360 can pump out better graphics than the PS3. The 360's GPU is truly custom built. It was built from scratch by ATI while the PS3 uses a clone of the Nvidia GeForce 7800.
- —The preceding unsigned comment was added by 4.234.222.77 (talk • contribs) 02:54, 13 March 2006 (UTC)
- A few comments on this. If you're going to judge "superior graphics" as a direct relation to specs on benchmark testing (like 3D Mark), then don't expect a more concrete answer than "PS3 seems to be better than XBOX 360". Benchmarking works with hundreds of critera and there is often overlap between winners: XBOX 360 is likely the winner in some benchmark categories and PS3 is likely the winner in others. Tech specs are largely just part of the "numbers game" as well, because a lot of what is important is much more difficult to put into numbers that the public will understand. Sure 1 billion gigahertz is impressive, but it means absolutely nothing if there isn't efficient use of the cache system, or if the machine-code procedures are badly implemented.
- The fact that the 360's GPU is custom built means very little. NVidia uses tried-and-tested, industry standard equipment, and they've been building quality products for decades. Unsigned's comments are rather a statement of his trust in the manufacturers employed by Microsoft, though POV is not un-welcome at the reference desk.
- Besides, we all know that the games (and how they're built, who built them) matter a lot more than the system does (and I don't even play games anymore). This is especially evident when talking about the "ease of programming" that each system allows for it's programmers, something that PS2 had a huge leg-up on over Microsoft when XBOX was first released. freshgavinΓΛĿЌ 05:04, 13 March 2006 (UTC)
- Precisely. The PS3 is coming out a year after the Xbox 360, so presumably it'll have a year's worth of graphics advances. So I'd put my money on the PS3 having better graphics. But that's not certain, and it depends on the gamer's opinion and on the game itself. In addition, console graphics improve gradually as the console gets older (as programmers learn how to get the most out of the console), so the Xbox 360 may well catch up with the PS3 (especially if programmers find it easier to program for the Xbox 360 - I don't know if that's the case.) Sum0 20:09, 13 March 2006 (UTC)
- Don't forget deadlines, budgeting, sponsorship. I'd be willing to bet that games created on the XBOX 360 at the same time as the release of the PS3 will be of much better quality all-round, but it really depends on what you're looking for. freshgavinΓΛĿЌ 03:39, 14 March 2006 (UTC)
- Precisely. The PS3 is coming out a year after the Xbox 360, so presumably it'll have a year's worth of graphics advances. So I'd put my money on the PS3 having better graphics. But that's not certain, and it depends on the gamer's opinion and on the game itself. In addition, console graphics improve gradually as the console gets older (as programmers learn how to get the most out of the console), so the Xbox 360 may well catch up with the PS3 (especially if programmers find it easier to program for the Xbox 360 - I don't know if that's the case.) Sum0 20:09, 13 March 2006 (UTC)
Epistasis?
[edit]If you were to have a population of people that had one of two kinds of mouths, thin and oval, with 8 people having a thin mouth and 8 people having an oval mouth. Also, of these 16 people, 4 of the 8 oval mouths were black, with the other 12 mouths being white. Is this an example of epistasis, where the shape of the mouths is one gene, and the expression of color is the other? I've been working on this problem for at least a day, so your help would be greatly appreciated. Thank you.
66.191.1.142 02:37, 13 March 2006 (UTC)
- Sounds suspiciously like homework, but... Two genes, let's call them A and B. Both can be in one of two forms (say j and k), with a 50% chance of a gene being one or the other. So you can get Aj, Ak, Bj, Bk. A controls mouth shape. Aj gives thin, Ak gives oval - irrespective of what form of gene B is. BUT both genes have to be in the k form for the colour to be black. Anything else will give white (i.e., Black is a recessive gene). An average population of 16 would give four each of AjBj, AjBk, AkBj and AkBk. The first eight would have thin mouths, the last eight would have oval mouths, but only the four with AkBk would have black mouths - the others would have white mouths. Whether that would count as epistasis, though, I'll leave to others who know more about genetics... Grutness...wha? 06:01, 13 March 2006 (UTC)
- Yes, what you have described COULD be an example of epistasis. If you were able to find someone with a think black mouth, then you would disprove that the black-mouth-gene forced an oval mouth. In reality though epistasis would be much more complicated than this with genes encoding hundreds of different proteins all adding their two cents. That's why you don't see people walking round with only 'two types' of mouth!!
- Anyway, to reiterate, epistasis is when one gene, via the actions of the protein it encodes, is able to influence the actions (or even stop all together) of another gene (or the protein it encodes). --Username132 (talk) 16:07, 20 March 2006 (UTC)
Lethal Dominant Genes
[edit]What could possibly allow a lethal dominant gene to exist, if it exerted its influence at birth? I'm curious because the article for Hairless Dogs states that the gene for hairlessnes is lethal, and can be either dominant or recessive. Ironically, in nature, only the type of dog that has the dominant lethal gene exists. JianLi 05:39, 13 March 2006 (UTC)
- As I understand that article, the hairlessness is dominant, but the lethality is recessive. That is, dogs with two wild-type (normal) genes will be normal, with hair. With one affected gene, they will be hairless but alive. With two affected genes, they will not survive. Does this make sense? — Knowledge Seeker দ 06:02, 13 March 2006 (UTC)
- Ah, I didn't catch that, but upon further inspection you are certainly right! So "homozygous lethal for the dominant gene" is just a confusing way of saying, "the hairlessness is dominant, but the lethality is recessive." So what is the mechanism that relates (double-allele) hairlessness with lethality? Can it merely be two genes linked so close together that they appear as one?JianLi 01:18, 14 March 2006 (UTC)
Check out thanatophoric dwarfism for an example of a human lethal single gene disease: [33]. Obviously all cases represent new mutations as no one survives to reproduce. alteripse 06:11, 13 March 2006 (UTC) PS, as it is a red link, we obviously need an article on it.
Electricity
[edit]In the middle of the winter, Finland almost run out of electricity because of all the heating. What would happen if a country ran out of electricity and what does it mean? Would some lamps just not light any more, or would they be darker? Blueiris 06:47, 13 March 2006 (UTC)
Humans survived many thousands of years without electricity in bitter cold. Modern technology allows us humans to survive even better. No worries mate! WAS 4.250 06:59, 13 March 2006 (UTC)
- To answer Blueiris, there would be power cuts or power rationing. Water heating might be turned off, or power might be turned off in general between certain hours of the day. To see what happens when power gets close to completely running ut in a Westernised urban area, have a look at 1998 Auckland power crisis. Auckland was lucky - it happened there in mid-summer. In winter in slightly-less-than-tropical Finland, though...? Grutness...wha? 07:05, 13 March 2006 (UTC)
- Okay, thanks. :) Blueiris 07:26, 13 March 2006 (UTC)
- If there was no power rationing and everyone was still connected to and drawing from the grid, then I would say that lightbulbs would go dimmer because there is simply less power. I would also guess that voltage would remain constant and that the amps coming into your lightbulb would drop, making it dimmer. Whether a lightbulb would even go on or not is a more difficult question. That would require looking at exactly how much power is going into it and how much power is required for the filament to begin emitting black-body radiation (i.e. how much power it takes to get the filament to a certain temperature). -Snpoj 07:39, 13 March 2006 (UTC)
- Harrumph. If the voltage remains constant, the current (amps) through a particular load (resistance) must also remain constant. Ohm's Law, you know?
- In fact, the delivery of electric power requires a careful balancing of load and generating capacity. If the load increases beyond what the generators can supply, the voltage starts to drop, creating a "brownout". With lower voltage, the current delivered is also reduced. Light bulbs shine less strongly, motors don't deliver as much power. But the voltage isn't allowed to drop more than a few percent, because if it did, appliances might start to malfunction. If the generators can't maintain even the slightly reduced voltage, they'll automatically "trip offline" and shut down. It's also possible that an excess of "reactive load" (basically motors as opposed to lights) will cause the frequency to drop rather than the voltage, and again the genertor trips offline. (The same thing also happens if the load is reduced suddenly due to some problem and the generator is delivering too much power.)
- Power utilities will try to avoid this sort of thing if they possibly can; for one thing, an unplanned shutdown can take longer to recover from, and if compounded by other problems it can propagate to a much larger area than the area of the actual shortage, as in the Second Great Northeast Blackout (where the actual power shortage was confined to northern Ohio). To avoid it, as noted, they will ration power: for example, they may use "rotating blackouts" where different districts deliberately have their power turned off in turn for predictable periods of time.
- --Anonymous, 10:01 UTC, March 13, 2006.
- I guess I was assuming a transformer that took in whatever it needed to and spat out a constant voltage..but that transformer breaks Ohm's law eventually if you follow the reasoning through. If the load is more than the turbines can make then it's got to give.. -Snpoj
- Didn't exactly that happen in Russia a few weeks ago? People died. Well, that was a general lack of energy, not specifically electricity. I suppose that in Finland the decision was made that heating was so important in winter that energy sources weren't used for electricity. That way at least people won't freze to death like they did in Russia. We may have lived without electricity until just one or two centuries ago, but we've become so reliant on it that we wouldn't know how to deal with lack of it. People don't generally have a bunch of oil lamps to light the house. But domestically that's mostly a nuisance (in winter a fridge isn't necessary).
- The biggest problem, I suppose, wuld be with certain computerised systems not running anymore, but the most essential systems will probably have their own backup with a generator. For which they'd still need fuel, though, so that's rather begging the question and those generators would be much less efficient, thus aggravating the problem. DirkvdM 09:50, 13 March 2006 (UTC)
- Unfortunately, though, you can't always separate "electricity" from "heating". I don't know what the common types of heating systems in Finland are, but many of the ones I'm familiar with here require electricity also. For example, my house has a gas-fired boiler, but without electricity to run the pump to force the hot water through the radiators, I wouldn't be able to heat the house. (And of course this is one more reason for having rolling blackouts, as opposed to, say, shutting the electricity off for good.) —Steve Summit (talk) 16:13, 13 March 2006 (UTC)
- Thanks all... these were exactly the things I wanted to know. :) Blueiris 11:05, 13 March 2006 (UTC)
- Yes, I would agree with most of what has been said here. We know some people living in Zimbabwe at the moment, and there is not enough power in the country for everyone, due to the general collapse of government and lack of fuel in general. They said that they used to have a "rota" where they would be told, "Tuesday, Thursday and Friday from 2pm-7pm you will have no power". And this system was rotated around, so different areas of the country were given different times and days. They said that there was nothing wrong with this and that you just learn to work round it ie. hot meal in the middle of the day, cold meal in evening instead of vice-versa. However, the country is now in such anarchy, that for at least the past several months, the power goes on and off entirely randomly, sometimes for a day or so. —Daniel May-Miller 20 March 2006 (GMT)
Last column of elements
[edit]What is the name for the elements in the last column, where did this name come from
- See the "Etymology" section of Noble gas. Melchoir 08:57, 13 March 2006 (UTC)
- Blue blooded fart? DirkvdM 09:52, 13 March 2006 (UTC)
- Don't worry - just Dirk thinking like an alien again :) Grutness...wha? 13:11, 13 March 2006 (UTC)
- Noble gas. You figure it out. DirkvdM 07:44, 14 March 2006 (UTC)
- Ah. Five minutes earlier and one indent fewer, next time, perhaps. Melchoir 07:58, 14 March 2006 (UTC)
- Noble gas. You figure it out. DirkvdM 07:44, 14 March 2006 (UTC)
distributed compilers
[edit]i need information about the distributed compilers not compilers for distributed languages....any one who can guide me .... i will be thankful....
- Is this the sort of thing you're looking for? --Bth 11:26, 13 March 2006 (UTC)
thankss...well...the right one....if u got any more articles ..do tell me...and any information about the distributed compiler for C# language....what is their scope and where they will stand in the future(C# distributed compiler).....
Smoke inhalation
[edit]Yet another question from the Newcomer's help page:
There are some types of smoke , which cause injury to the system. Like cigarrette smoke, CO2 smoke etc. Similarly there must be some qualities of smoke which may be good. Ayurveda, an Ancient medical science from India has described burning of some herbs for creating the smoke. The benefits are mentioned having short-term and long-term effects. How can I find scientific information about such herbs.
Thank you,
Dr. Santosh
— QuantumEleven | (talk) 12:18, 13 March 2006 (UTC)
- "There must be some good quality of smokes which may be good." — That's not a logical conclusion. The best thing to breathe is pure smoke-free air. All kinds of smoke contain polycyclic aromatic hydrocarbons like benzopyrene and other nasties. —Keenan Pepper 13:18, 13 March 2006 (UTC)
- Don't be a buzz killer man. Just tell him that smoking pot is good for him and everyone will be so much happier. --Kainaw (talk) 13:41, 13 March 2006 (UTC)
You ask "How can I find scientific information about such herbs." meaning herbs with "some qualities of smoke which may be good". Read the articles I link here and some of what they link to and then googgle relevent terms. You want to know about pharmacologic agents, especially medicinal plants like Medical cannabis that can be turned into smoke so the route of administration is inhalation. While the sum of the effects on a healthy person may be bad, the sum on an unhealthy person may be good - which is why medical treatments for unhealthy people are generally not good for healthy people - or more exactly, most medical treatments are good for specific conditions not as general health aids. WAS 4.250 20:17, 13 March 2006 (UTC)
- Note that essences of some plants are inhaled regularly in westrn medicine, too - notably eucalyptus. Grutness...wha? 22:39, 13 March 2006 (UTC)
- Yes, because those vapors are not smoke. Smoke is the result of combustion and contains soot and tar, which are carcinogenic. Some vapors are also harmful, like chlorine bleach vapors, but others are not, like water vapor. StuRat 04:29, 14 March 2006 (UTC)
- And CO2 isn't smoke either. And whether something is good or bad has a lot to do with quantity and concentration. Many 'poisons' are healthy provided the dosage is right. Other than that, WAS said it. DirkvdM 07:51, 14 March 2006 (UTC)
Electolysis
[edit]Hey. In the past, I have performed electrolysis experiments by passing electricity through salt water (NaCl). Now, theoretically, the water is supposed to be split into hydrogen and oxygen, but I know this doesn't really happen. The results appear to be hydrogen and chlorine. Oxygen gas does not form because it is more readily attracted to the Na ion and forms NaOH in solution, while hydrogen bubbles out, and the chlorine remains a liquid in the cold water. This is as far as I understand the mechanism.
Now its time for my question. What is the true end result of electrolysis? I have read in the sodium hypochlorite article that NaOH and Cl2 react to form NaClO, NaCl, and H20. I also read that NaClO, which is bleach, partially breaks down into Na and ClO ions, which can become NaOH and HClO (hypochlorus acid). Also, the Cl2 can react with the water to form HClO and HCl. So...what is really in the water? It seems that it could eight different chemicals at once. Thanks. --Chris 16:27, 13 March 2006 (UTC)
Unless you have really overdone it with the salt solution (lots of salt as opposed to just enough to make a conductive electrolyte) you will get oxygen at the anode and a small amount of chlorine. Hydrogen will collect at the cathode plus a small amount of sodium (immediately reacting with water to liberate more hydrogen). NaOH and HClO will not coexist very long in solution as one is acid and the other is base, HCl will not last long either in the presence of a strong base. NaClO is relatively stable, although it slowly liberates Chlorine. So, yes, you will have 8 different chemicals together, but the acid and base will only be there for a brief time. If you keep the electrolysis running, you will continue to generate these chemicals, until the water is totally decomposed into hydrogen and oxygen and no more liquid is left. Since the Sodium is a solid and does not leave the solution, whereas the chlorine is a gas and some will escape, you will probably end up with a salt deposit contaminated with NaOH.
- Well, I have done this with filtered water and a lot of NaCl. I have also done it with mere tap water. With both, I never seemed to get oxygen. I know that chlorine more readily escapes from the solution that oxygen, but there are some salts that do not take part in the reaction, and are only there to conduct the electricity. I do not know which salts these are though. But I assume that what you are saying is that there isn't going to be too many secondary reactions. Just hydrogen, chlorine, NaOH, and remaining salt will remain. Thanks for your help. --Chris 21:58, 13 March 2006 (UTC)
- I tried to do this once and Wiki boogered it up. Try again. Tap water should have sufficient ions to make the water conductive, but minimize unwelcome contaminants in the Oxygen. If you are using metal electrodes, the oxygen and chlorine, etc. might be reacting with it. Try this, use distilled water with a small amount of NaOH dissolved (wear gloves) this will guarantee no Oxygen contaminants. Then get use graphite electrodes. Get some of those replacement leads for mechanical pencils, or pressure cook a few carpenters pencils and carfully peel the wood off. Use DC current. You might get some unwelcome CO2 at the anode, but leaving it idle with the current off will allow the CO2 to react with the NaOH and remove it.
- Interesting. Well, I'm at college now and I can't really do this until I get home. I am a chemistry major, but I'm only a freshman. I will definately try using graphite though. It sounds promising. Thanks a bunch. --Chris 05:02, 15 March 2006 (UTC)
- Chemistry major? That means you should have access to a lab... Why wait until you get home?
Chimpanzee IQ
[edit]What is the approximate IQ of a chimpanzee? Is it true that the smartest apes are smarter than the least intelligent humans? The Mad Echidna 16:53, 13 March 2006 (UTC)
- Given my experience with a lot of the vandals here, I'd say, yeah, easily. --jpgordon∇∆∇∆ 17:35, 13 March 2006 (UTC)
- As opposed to people who make smart ass remarks, you mean? (No, this remark is not meant to be self-referencing.) DirkvdM 08:07, 14 March 2006 (UTC)
- The smartest ant is smarter than the least intelligent human. Therefore, the smartest apes are smarter than the least intelligent humans. Note - the least intelligent human is a human with no mental capacity. This may be caused by birth defect or brain damage. Some humans have no brain activity at all and are kept alive purely by machines. So, they are the least intelligent humans. Anything with a heartbeat is more intelligent. --Kainaw (talk) 19:56, 13 March 2006 (UTC)
- I think it's a testament to all wikipedian's maturity, that no one has mentioned george bush yet
- That doesn't mean we weren't thinking it. :-) Dismas|(talk) 22:46, 13 March 2006 (UTC)
- I think it's a testament to all wikipedian's maturity, that no one has mentioned george bush yet
- The chimp is very good...at being a chimp, just as a human is good at being human. IQ is a general measure of human abilities, and by applying it to a chimp, you might suggest that a chimp with a very high IQ isn't actually a very good chimp. It's off putting a square plug into a square hole and solving equations instead of eating bananas. Just something to think about :)--inksT 01:45, 14 March 2006 (UTC)
- Somehow or other, someone deleted my response, so I'll type it again :/ . The question as posed is really a null question. The average chimpanzee IQ is 100 - because it will be tested using chimpanzee-based IQ tests. Since all species have different types of intelligence, it's really difficult to find any way of truly comparing intelligence, even with species as close as chimpanzees and humans. As to the smartest apes being smarter than the least intelligent humans, yes, that is true - buyt then again, humans are apes so it's bound to be true. If (as is likely) you mean non-human apes, then the chances are that yes, they are also more intelligent. then again, the least intelligent humans are - as Kainaw pointed out - so mentally incapacitated that the question is again meaningless. A better question would probably be what level of intelligence to chimpanzees reach compared to humans. Again, this is pretty difficult to judge, but it would probably equate fairly closely with a child of about 5-7, IIRC. Grutness...wha? 05:35, 14 March 2006 (UTC)
- That sounds about right. Obviously an IQ test is a bad example, but if you're talking about a problem solving, non-language-based test, I'd take a chimpanzee against the average human child any day. EWS23 | (Leave me a message!) 06:15, 14 March 2006 (UTC)
- Somehow or other, someone deleted my response, so I'll type it again :/ . The question as posed is really a null question. The average chimpanzee IQ is 100 - because it will be tested using chimpanzee-based IQ tests. Since all species have different types of intelligence, it's really difficult to find any way of truly comparing intelligence, even with species as close as chimpanzees and humans. As to the smartest apes being smarter than the least intelligent humans, yes, that is true - buyt then again, humans are apes so it's bound to be true. If (as is likely) you mean non-human apes, then the chances are that yes, they are also more intelligent. then again, the least intelligent humans are - as Kainaw pointed out - so mentally incapacitated that the question is again meaningless. A better question would probably be what level of intelligence to chimpanzees reach compared to humans. Again, this is pretty difficult to judge, but it would probably equate fairly closely with a child of about 5-7, IIRC. Grutness...wha? 05:35, 14 March 2006 (UTC)
- There is no one IQ test. There are different test for different levels of intelligence. So I don't see a problem with designing a test for chimps because they're not too far from humans. Apparently, some gorilla called Koko has "an IQ of between 70 and 95 on a human scale". An ant IQ test would be something different, though. DirkvdM 08:07, 14 March 2006 (UTC)
- There's no such thing as the approximate IQ of a chimp. Their IQ varies just as it does with people. - 131.211.210.13 10:02, 14 March 2006 (UTC)
- Hi! Not as relevant a comment as the others (I have only just found the Reference Desk and I think it is a brilliant idea). I'm sorry I haven't got a reference, but I heard that some studies suggest that chimps might be quicker at basic mental arithmetic (adding and subtracting small numbers) than humans. —Joe Llywelyn Griffith Blakesley talk contrib 10:56, 14 March 2006 (UTC)
- Can't resist this... If you think Dogs can't count, try putting three dog treats in your pocket and only giving the dog two.
- I did not know that the IQ test could be performed without language, can chimps communicate now?
AIDS transmission
[edit]How is the HIV transmitted by sharing infected needles ? Does the virus lie dormant in a dry needle ? How is it then that HIV is not spread by mosquito bites ? Jay 17:26, 13 March 2006 (UTC)
It is spread just the way you think it is, large numbers of HIV moving from one human's blood to another human's blood, lying "dormant" between human cell infection. Mosquitos don't suck blood from one person and then inject that blood into someone else. They digest the blood (and the viruses in it) as its food and don't catch HIV themselves. They aren't sloppy eaters so HIV doesn't tag along on their mouth parts. WAS 4.250 19:41, 13 March 2006 (UTC)
- But the mosquito saliva mixes with the blood and sometimes the virus infects the next person the mosquito feeds on as it's body fluids will mix with the victims. This is how malaria is spread, AIDS is a fragile virus and does not last long outside the body, so it it is hard to infect someone that way.
- I always thought that the HIV virus died within a few minutes of leaving the body and being exposed to the outside world. Sum0 20:00, 13 March 2006 (UTC)
- It dies shortly after leaving the blood. When a needle is used, blood is left inside the vial/needle. That infected blood is passed to the next user. So, the virus never has to leave the blood to get from one person to another with needle sharing. --Kainaw (talk) 20:08, 13 March 2006 (UTC)
- The only problem is AIDS is temperature dependent also, so if the needle is left idle for too long before reuse, the virus can't remain viable.
- Yes. But be aware there is nothing magical about blood in terms of HIV surviving. It needs moisture and warmth (ie dryness and cold and heat "kill" or denature it). It can survive in a warm moist vagina quite long enough. WAS 4.250 20:22, 13 March 2006 (UTC)
- You could have just said "warm moist environment" and saved all of us the distraction. freshgavinΓΛĿЌ 03:32, 14 March 2006 (UTC)
- Right. Because we all know AIDS transmission is all about random "warm moist environments" and not vaginas or rectums.... NOT. WAS 4.250 04:56, 14 March 2006 (UTC)
- Some kinds of distraction I don't mind at all. DirkvdM 08:09, 14 March 2006 (UTC)
- Doesn't the blood/fluid in a needle dry up, maybe in 24 hours ? Does the dry needle kill the virus (that was my original question)? So wouldn't there be a time limit for when a used needle becomes sterile for AIDS ? By the way the idea of blood remaining in the needle was totally new to me. Jay 08:03, 14 March 2006 (UTC)
- I can't imagine that the fluid in a syringe can dry up through the tiny needle hole. It would probably stay moist for years. But the temperature drop in most rooms would kill HIV in a short time. However, needle sharing is a common model, especially where free supplies of needles or needle exchanges are banned/scarce; people may be effectively waiting in line for the next use. Notinasnaid 09:23, 14 March 2006 (UTC)
- If the needle is kept in the junkies' pockets or underwear, it could be kept nice and warm, and least until they die and cool off a bit. StuRat 00:35, 15 March 2006 (UTC)
- I found this [34] --Username132 19:50, 14 March 2006 (UTC)
- HIV can survive much longer if kept in blood than outside. The needle holds the HIV virus inside of blood and protects it from air. In Victoria,BC we have services that give out small containers of bleach water that can be used to clean needles. This kills HIV, but does not kill all types of hepatitis. A good rule of thumb, is DO NOT use a needle that someone else used. HighInBC 21:59, 18 March 2006 (UTC)
Mission to Mars
[edit]I am currently 21 years old and would love to see a human being walk on Mars in my lifetime. Does anyone know a realistic date in which NASA would send humans to Mars.
- Well, NASA's Vision for Space Exploration doesn't set a date for Mars, but says 2020 for the Moon, while ESA's Aurora Programme says 2030 for Mars. These are both highly speculative, of course. See also Exploration of Mars. Melchoir 21:33, 13 March 2006 (UTC)
- It's a programming, budgetary, and risk issue more than a technological one. If one were prepared to cancel the shuttle immediately and/or give NASA (or some other group) lots of money immediately, and were prepared to accept a greater risk of the astronauts not coming back (maybe 10-20% rather than the 2% the Shuttle astronauts face), the job could almost certainly be done within the decade. As a further example of a technology that could get us to Mars quickly (in both senses of the word) but which is likely to remain undeveloped for various reasons, have a look at Project Orion.
- FWIW, I too hope to see somebody walk on Mars in my lifetime, and I'm 29. I remain reasonably confident that it will happen. --Robert Merkel 00:34, 14 March 2006 (UTC)
- Because most white-collared first-worlders don't believe there's a valid reason for a human to actually have to GO to mars (as opposed to a robot) I think the only way it could possibly become a reality is if the world were to suddenly erupt in a massive war. In an instant all of the men of the world would be looking for a way to puff their chest and a place to pee on and call their own. The moon, being "done", wouldn't be good enough, and so huge amounts of money ("to be used for something of value that until now was not realized") would be pumped into the Mars program. freshgavinΓΛĿЌ 03:30, 14 March 2006 (UTC)
- If you're talking about the American space program, there doesn't really need to be a reason other than rallying the common man around a common goal. In that case, I'd think it an almost certainty that a man will step foot on Mars by the end of our lifetime (myself being a 21-year-old male). I'd be very surprised if a man hasn't been to Mars 2 or 3 times in the next 40 years. EWS23 | (Leave me a message!) 06:08, 14 March 2006 (UTC)
- The same man? Give others a chance too! And why a man? (Let me be 'politically correct' for once.) Anyway, Freshgavin is right. The only reason to send people into space is because it looks cool on tv. It reduces science to mass entertainment, which is a major pain for scientists. For the price of sending one (wo)man to Mars, they could probably send dozens of robots, which would be hugely more informative. Luckily they can say "yeah but we need to send robots first, to explore, so we know what we're going to land on" and get some useful info out of the budget. Sending people to the Moon was a huge waste of money, but with sending them to Mars it will be much worse because of the distance, for starters. With a robot it's basicaly just the same thing, except you 'shoot a bit further'. A robot doesn't care about being in space for years (well, not entirely true, but you get the point). DirkvdM 08:17, 14 March 2006 (UTC)
- I disagree with "sending people to the Moon was a huge waste of money". At the time, robotics wasn't yet up to the challenge, so people were still needed. The cost of sending people to the Moon was actually far less than sending robots with the same capabilities, using 1960's technology. StuRat 00:30, 15 March 2006 (UTC)
- I didn't mean walking robots or anything fancy like that, but the kinds of robots that were actually sent at the time. The one that took photographs of the back side of the Moon made the greatest achievement of the exploration of the Moon up until now. Later, humans were sent so they could have a look for themselves, but that was a totally useless effort. DirkvdM 14:15, 20 March 2006 (UTC)
- Well, I don't think they had the technology at the time for moon rovers which could go out and take samples to return to Earth, so humans were needed for that task. StuRat 20:39, 20 March 2006 (UTC)
- One could come up with a list of scientific questions which could be answered by going to Mars, things which could be learned, etc. But the question is whether these outbalance the risk and the cost. Without even bringing up whether the proposal is a political stunt. Which in this case it clearly was. --Fastfission 16:34, 14 March 2006 (UTC)
- Thanks for dissecting my lazy English, Dirk. :o) I completely agree with you that the benefits of sending a (wo)man to Mars probably wouldn't justify the cost, and that doing so would be much more of a political/entertainment venture than a scientific one. However, perhaps the more appropriate question might be, "If we eliminated the possibility of sending a (wo)man to Mars, would the people/government still be willing to fund robotic missions there at all?" I imagine a large percentage of the (non-scientific) public considers sending robots to other planets a relatively minor achievement, and only a stepping stone for us traveling there in person. EWS23 | (Leave me a message!) 18:07, 14 March 2006 (UTC)
- Look, it's probably true that if you're looking purely at cost-effectiveness for short-term and medium-term scientific goals, then robots beat humans. That's not the point. If you believe in long-term human expansion into space, then you've got to start somewhere. It's not about politics or entertainment, although that might be the way to sell it to dreamless bureaucrats. It's manifest destiny. --Trovatore 18:26, 14 March 2006 (UTC)
- Furthermore, you guys are probably overestimating the capabilities of robots, particularly when they are multiple light-minutes away from human decision makers back on Earth. The efforts of the Mars Exploration rovers, wonderful though they have been, take days to accomplish what a human can do in minutes. And human explorers are approximately one squillion times more flexible. --Robert Merkel 06:21, 15 March 2006 (UTC)
March 14
[edit]Pykecrete
[edit]I have heard about a substance called pykecrete, where wood pulp is slurried with ice and frozen to create a very strong substance. Does anyone know what the proportions should be? Even a rough estimate to begin experimenting would be nice.
- Check out Pykrete, which recommends "approximately 14 percent sawdust or some other form of wood pulp (such as paper) and 86 percent ice by weight". Melchoir 00:20, 14 March 2006 (UTC)
- Thank you. The spelling eluded me as well apparently!
- Well, it's good you asked! Pykecrete is now a proud redirect. Melchoir 00:33, 14 March 2006 (UTC)
- Er... it will be when a certain cache updates. Melchoir 00:35, 14 March 2006 (UTC)
- Thank you. The spelling eluded me as well apparently!
Cutting Mirrors
[edit]For my parabolic dish solar hot dog cooker I need to cut mirrors. I've figure out a way to get the measurments, but what equipment do you guys recommend to actually do the physical cutting of the mirrors?
- To be clear, you are talking about using a series of small flat mirrors to approximate a paraboloid, not actually constructing a curved mirror, right ? You can cut mirrors with a glass cutter: [35] Typically you score the glass in a straight line, then break it off with one half supported by a table and the other half hanging over the edge. You should wear goggles and thick leather gloves when doing this. StuRat 02:01, 14 March 2006 (UTC)
- KISS! Just smash the mirror and make it a mozaic. You just want to roughly focus the light in a certain area, not build a newton telescope, right? And when you're done could you write the parabolic dish solar hot dog cooker article. We're sorely in need of that. DirkvdM 08:39, 14 March 2006 (UTC)
- I did consider that approach, but think carefully cut pieces of glass will likely get a better grade than random shards of glass. StuRat 00:18, 15 March 2006 (UTC)
- I think a glass cutter is a better idea. And it is less dangerous. Find one at a hardware store, you probably don't want to wait for ebay. Good luck! --
 Mac Davis] ☢ ญƛ. 11:07, 14 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 11:07, 14 March 2006 (UTC)
- I think a glass cutter is a better idea. And it is less dangerous. Find one at a hardware store, you probably don't want to wait for ebay. Good luck! --
Another option is to buy mirrored tile: [36]. The largest square tiles are the best price per area, but the circular tile would be safer (no sharp corners) and might look better. StuRat 15:21, 15 March 2006 (UTC)
I am also curious about how you are going to build a parabolic fixture to which you plan to attach the mirrors. I was thinking that strips of papier mache hung from a hoop might very well form a close approximation of a parabolic shape, from which you could use grout (or even gum) to adjust each tile's position to aim at the hot dog. (I suggest covering the existing mirrored tiles so you can see if the current tile is properly aimed.) You could use a hula hoop supported by a wooden frame. The weight of the apparatus would likely require many support points for the hula hoop, however, so a stronger hoop would be better, but I can't think of a good place to get a cheap, large, strong hoop right now. StuRat 15:41, 15 March 2006 (UTC)
- I thought of another way, how about using a circular garbage can for the hoop and support structure ? One caution, it may be too flexible, allowing the structure to shift when moved. StuRat 16:45, 15 March 2006 (UTC)
- The hanging thing won't work. The shape formed by a rope (or chain, string, etc.) suspended at its ends is a catenary, not a parabola. It's a curve, of course, so it'll look rather like a parabola, but it won't focus light the same way.
- Besides, if you're looking to cook a long skinny thing like a hot dog, you'd really want to make a cooker that looks like an extruded parabola rather than a rotated one. The extruded parabola will focus light along a line, thus cooking the entire weiner. The rotated parabola focuses all the light at one point, leaving you with a snack that's burned at one point and raw everywhere else.
- From a construction standpoint, the extruded parabola design is nice in that you don't need a million little bits of glass. You can use long, narrow strips of mirror laid edge to edge along a parabola. And you can construct a cooker that cooks four or five hot dogs evenly all at the same time by just making the cooker longer.
- I think you are picturing this a bit more precisely than this kid is going to be able to create it. Getting an approximation where some of the light from each mirror hits the hot dog somewhere is about the best he can hope for. The grout or gum will allow him to compensate for error from the shape not being a true parabola. If it's heated unevenly, this will be OK so long as it is heated slowly enough for the heat to distribute itself evenly. But your idea of making it elongated might work. I've seen elongated "tubs" for bathing children/dogs that might work for a structure from which they could suspend papier mache. StuRat 03:10, 19 March 2006 (UTC)
- I think it might be better to just use tinfoil rather than actual glass mirrors, because it's easier to shape. It isn't so flat as glass mirrors but I don't think you need that anyway. – b_jonas 00:34, 20 March 2006 (UTC)
- I disagree. Aluminum foil, which I assume is what you meant, will reflect light all over the place, not just at the hot dog. Also, some way to contain the heat, say a glass box around the hot dog, would be a good idea. One last thing WEAR DARK SUNGLASSES when you use this, or you could suffer retinal damage ! StuRat 20:45, 20 March 2006 (UTC)
Walking On The Moon
[edit]I am sixteen. I am going to join the Marines. I would like to walk on the moon. Based on NASA's projections, what is the probablility that my dream to walk on the Moon will become a reality? Here7ic 01:39, 14 March 2006 (UTC)
- As I mentioned above, NASA's Vision for Space Exploration proposes manned Moon missions by 2020. Assuming that actually happens, I guess it depends on how old astronauts are supposed to be. Melchoir 01:47, 14 March 2006 (UTC)
- Very interesting. Perhaps that info is in the Wikipedia on Astronauts. I must check this out. Here7ic 01:59, 14 March 2006 (UTC)
- Well, good luck on your research! For such a visible occupation, Wikipedia has surprisingly little information about the astronaut career path, especially in the United States. Hopefully you can educate us all! Melchoir 02:49, 14 March 2006 (UTC)
- Actually, on second thought, I should stop thinking about Wikipedia for a second and encourage you to ask your Marine recruiter the same question. Melchoir 02:55, 14 March 2006 (UTC)
- Um, I wouldn't take the recruiter's answer at face value. He has a quota to fill. He probably won't literally promise you the Moon, but he might try to make it sound like joining the Marines is your best shot at it, even if it isn't. (Has there ever been an astronaut from the Marines? I think, at least in the early days, a lot of them came from the Air Force.) --Trovatore 03:05, 14 March 2006 (UTC)
- Good point. I don't have any idea about the breakdown by services, but I guess it couldn't hurt, Here7ic, to talk to your Air Force recruiter as well! As long as you maintain a healthy level of skepticism, more information can't hurt, right? Melchoir 03:09, 14 March 2006 (UTC)
- Um, I wouldn't take the recruiter's answer at face value. He has a quota to fill. He probably won't literally promise you the Moon, but he might try to make it sound like joining the Marines is your best shot at it, even if it isn't. (Has there ever been an astronaut from the Marines? I think, at least in the early days, a lot of them came from the Air Force.) --Trovatore 03:05, 14 March 2006 (UTC)
- Very interesting. Perhaps that info is in the Wikipedia on Astronauts. I must check this out. Here7ic 01:59, 14 March 2006 (UTC)
- If you're sixteen, and there are plans to land on the moon by 2020, that would make you 30 at the very earliest, and the way things work in the real world it's much more likely that you'd be 40 or 50, if everything goes approximately according to NASA's plan.
That's no reason to give up your dream though, working in a field related to space exploration would certainly be a fascinating job for you, and even if you don't get to walk on the moon yourself, you could very well have a hand in helping somebody else get there. freshgavinGLL.KJ 03:08, 14 March 2006 (UTC)- Historically speaking, 40 is about right; Neil Armstrong and Buzz Aldrin were 39 when they hit the Moon. But that was a long time ago, and the target age may have shifted radically since then. Anyone know if it has? Melchoir 03:16, 14 March 2006 (UTC)
- Not radically, seems to be around the early 30s now, and there have been many more over-age astronauts than under-age. You're right though, 40 could be just right, though I'd say 50 is pushing it. freshgavinGLL.KJ 04:19, 14 March 2006 (UTC)
On a related note, do most astronauts have a good physics background? In my opinion, it'd be a shame if the few people humanity sends into space don't even appreciate how they got there. Then again, I doubt most race car drivers have engineering backgrounds. JianLi 03:54, 14 March 2006 (UTC)
- I'd have to guess that yes, they do. Melchoir 03:57, 14 March 2006 (UTC)
- Every astronaut I've heard of has an engineering degree. Can anyone name an exception? Fredrik Johansson 03:59, 14 March 2006 (UTC)
- I looked at List_of_astronauts_by_name. So far, the majority of articles on the list give an engineering/science degree JianLi 04:03, 14 March 2006 (UTC)
- I can find a few exceptions, such as Ellen S. Baker, who has degrees in geology and medicine, but not engineering JianLi 04:06, 14 March 2006 (UTC)
- Has anyone ever heard of an engineering student who was anything more than a glorified business major? so what's it matter if they're engineers or burger flippers, they'll know about as much physics either way--64.12.116.74 04:09, 14 March 2006 (UTC)
- In the immortal words of Michael Jackson, that's just ignorant. Melchoir 04:15, 14 March 2006 (UTC)
- You're an engineering student aren't you? oh well, know your audience and all that jazz--64.12.116.74 04:41, 14 March 2006 (UTC)
- Far from it! Melchoir 05:49, 14 March 2006 (UTC)
- You're an engineering student aren't you? oh well, know your audience and all that jazz--64.12.116.74 04:41, 14 March 2006 (UTC)
- In the immortal words of Michael Jackson, that's just ignorant. Melchoir 04:15, 14 March 2006 (UTC)
- Has anyone ever heard of an engineering student who was anything more than a glorified business major? so what's it matter if they're engineers or burger flippers, they'll know about as much physics either way--64.12.116.74 04:09, 14 March 2006 (UTC)
Why do you want to join the marines? Why do you want to walk on the moon? People who want to do one rarely want to do the other. I suggest you get some real world experience and don't make lasting commitments until you are 21. WAS 4.250 05:02, 14 March 2006 (UTC)
- Marines: I want to join because I feel like it's the only way I'll get the training, and the action, I feel I need. Moon: I want to go to the moon because I feel that it's just the first step. The first step to a world that isn't confined to our current residency, Earth. Chances are poor that I will be alive for the terraforming, or colonization of farther planets, and even poorer that I will even see the first contact with an intelligent extraterrestrial species. It's my insurance, if you will, that I will have done something so profound that it will impact the lives of every human being on this Earth. In short, I'm in it for the thrill. Here7ic 05:31, 14 March 2006 (UTC)
- You are 16 years old and want thrills and profoundness in your life. "Know thyself" is ancient wisdom, and Clint Eastwood's charcter said "A man's gotta know his limitations." Understanding your motivations is important. I recommend you seek thrills and profoundness NOW while also seeking to stay alive and out of jail so you can continue seeking thrills and profoundness as many decades as possible. What do you think you can do today to find thrills and profoundness (that don't land you in jail or the hospital)? When I was your age many decades ago I sought those in finding the love of a beautiful woman (married at 18) and trying to understand the nature of the universe (religion led me to philosophy led me to logic led me to physics led me to the computer sciences). It has been an exciting profound journey. WAS 4.250 17:43, 14 March 2006 (UTC)
- I think it's fantastic to have a goal like that. It will take hard work, and as noted in other responses, perhaps another branch of the armed forces might provide a better path, but I wish you all luck. Now move zig! For great justice! --LarryMac 15:20, 14 March 2006 (UTC)
- You may be interested in the How to become an astronaut FAQ. A military background is a good thing to have on the resume, but you will need a PhD in a relevant scientific field; if you want the fast track to getting this that means getting in as an officer (and earning your university degree), not an enlisted grunt.
- This time around, I expect that the Moon-Mars program will be more science focussed, so if you want to go, become one of the world's foremost expert on something Moon or Mars-related; not having any health problems whatsoever wouldn't be a bad idea either (and is, unfortunately, largely out of your control).
- That said, becoming an astronaut is somewhere up there with winning the lottery in the scale of probabilities. But you could have a fascinating career in science or engineering even if you don't become an astronaut. --Robert Merkel 06:04, 14 March 2006 (UTC)
Astronauts don't really navigate the spaceships. Well, for a Moon expedition anyway. That's all controlled from Earth. The astronauts are just on board for publicity reasons. And while there are people on board they might as well be scientists so they can be of some use. Another thing is that they need to solve things when they go wrong and for that you need intelligence and preferably a good broad scientific education. If you're stupid enough to want to join the army (and the marines at that) then you probably don't have what it takes to become an astronaut (oops, sorry, my political correctness in the 'mission to mars' question (two up) probably made me want to compensate here). Oh, and the physical fitness thing is important too. You don't need to be exceptionally fit, just plain healthy. Of the first Dutch astronaut, Wubbo Ockels it was said the only thing wrong with him was a crooked tooth. DirkvdM 08:57, 14 March 2006 (UTC)
- The "spaceships" are not controlled from Earth--they're mostly controlled by the onboard computer. Radio waves take approximately 2 seconds to travel from the Moon to Earth and back, which is way too long. For example, the Apollo moon landers had exhaust nozzles that could be gimballed to compensate for the astronauts' moving around. If the nozzles took 2 seconds to respond to the astronauts' movements, the lander will crash.
- I would suspect (speculate) that, like Robert Merkel and DirkvdM said, being a scientist is going to be more important than being in the army if you want to walk on the Moon. I also strongly recommend against joining the Marines. Ultimately, the goal of all military-related stuff is to kill/capture people or steal information. Why do you want to be trained to do those things? Why do you feel that you need the training and action? If you want to exercise, join a fitness club, run around the streets for an hour every day, play basketball, or something like that! Just don't do something as stupid as joining the army. --Bowlhover 15:53, 14 March 2006 (UTC)
The guys who go into space the most frequently are the commanders and the pilots, like Kent Rominger and Curtis Brown who seem to almost exclusively have military backgrounds. Specifically, they tend to have been pilots in the armed forces, and good enough at it to become instructors and/or test pilots. I also see a lot of people around me, here at the Massachusetts Institute of Technology, getting their graduate degrees in engineering and the sciences paid for by the US military. So if you're willing to take the risk that you're going to be sent to war, and possibly killed, an officer position in the military is a pretty good route to NASA. It's only going to work if you're bright, and coordinated, and good at math, and not gay or at least willing to be in the closet indefinitely if you are.
- So from that point of view, though, the Marines are out. The Marines, as part of the Army, are not permitted to operate fixed-wing aircraft, and I doubt that helicopter pilots are the sort of pilots we are talking about. --Trovatore 22:28, 14 March 2006 (UTC)
- No, in the U.S., the Marines are part of the Navy and the certainly use both fixed wing and rotary wing craft. Rmhermen 22:53, 14 March 2006 (UTC)
- John Glenn was a Marine as was Story Musgrave, the only man to fly on every space shuttle. And Charles F. Bolden, Jr., Robert Cabana, Gerald Carr, James F. Buchli, etc. Rmhermen 23:00, 14 March 2006 (UTC)
- As of Jan 2005, there were seven active astronuts from the Marines, another seven from the Army, but 31 from the Navy, 24 from the Air Force and 1 from the Coast Guard. Rmhermen 23:08, 14 March 2006 (UTC)
- Thanks to Rmherman for the info. However the Marines are not part of the Navy. It does look like I was wrong about them being part of the Army, and it also appears that they do operate fixed-wing aircraft. --Trovatore 23:23, 14 March 2006 (UTC)
- Yeah, the United States Marine Corps article says it's its own branch, separate from both the Army and the Navy, and that they fly both fixed- and rotary-wing aircraft. Still, I'd go with the Navy.
- "The Department of the Navy consists of two uniformed Services: the United States Navy and the United States Marine Corps." from the Navy website. The Marines report to the Undersecretary of the Navy, their officer's are trained at the Naval Academy, their crimes tried at the JAG-Navy, etc. Rmhermen 21:11, 15 March 2006 (UTC)
- Yeah, the United States Marine Corps article says it's its own branch, separate from both the Army and the Navy, and that they fly both fixed- and rotary-wing aircraft. Still, I'd go with the Navy.
- Not at all. Rmhermen's talking about the Navy department, a political (executive branch) structure, not a military one. The Marines are a separate service from the Navy, even if they're under the same cabinet secretary. Heck, they even use Army ranks and grades, not Navy ones. That's probably a big part of why I thought they were part of the Army; the other reason is that they mostly fight on land rather than water. I get the sense that Marines and Navy men generally don't think much of each other, or at least affect not to. --Trovatore 01:53, 16 March 2006 (UTC)
- Read The Right Stuff by Tom Wolfe.
Skip the movie, it's unrealistic.(SOrry, I was thinking of the movie of Apollo13; THAT's unrealistic.) Learn as much science as you possibly can. --GangofOne 23:37, 14 March 2006 (UTC)
- Read The Right Stuff by Tom Wolfe.
Military recruiters are salesmen, and right now their main goal is to enlist as many people as they can to go to war in the Persian Gulf, not to go to the Moon. There's currently no war on the Moon that I've heard of, and, as everyone knows, the business of the U.S. Marine Corps is war. If your idea of a thrill is being constantly in danger of being shot, blown up, or otherwise killed and maybe having to kill another human being, then the Marine Corps may be for you. Being an enlisted soldier, which is what you'll be if you sign those papers at your recruiter's office, is not going to get you any closer to the Moon. As far as I know, astronauts are frequently military fighter pilots with advanced degrees in engineering. Try the ROTC programs of the Navy or the Air Force instead, and get a degree in aeronautical or electrical engineering. Then apply for pilot training. See how it goes after that. Even if you qualify as a fighter pilot, your chances of going to the Moon are slim to none. Brian G. Crawford 00:22, 15 March 2006 (UTC)
- For some reason, a lot of boys in my class are interested in military stuff. I think it's because they like destroying other people's property spectacularly (without getting into trouble) and killing other people. But Here7ic: why do you really want to join the Marines? What kind of training and action do you think you need? Do you really want to kill other people or destroy property? Do you really want to see other people dying in pain? If not, why join the Marines? --Bowlhover 04:18, 15 March 2006 (UTC)
- But Bowlhover, why don't you tell us what you really think of the military ? In a perfect world, we would not need a military, true, but do you really think this is a perfect world ? Total unilateral disarmament is a really bad idea. StuRat 16:29, 15 March 2006 (UTC)
I would like to assure you all that I've got my bases covered. I've already worked out how I will be entering the Marines. Next summer (while I'm 17) I will go to Basic Training, then complete my grade school career. I am currently 9th out of the 134 students in my class. After I graduate, I will spend four years in college, paid for by the United States Marine Corps, then for and additional four years I will begin and Officer Training Course (depending on how I do), and will hopefully make this dream a reality.
- Bowlhover, do you really think that a .50 calibur hollow-point through the cerebral cortex will hurt?
More so, I want to join the Marines because I do want frontline action. You may think it wrong to kill, but sometimes it's just your final option. That aside, my reasons are my reasons. Some are hard to explain, others just an urge. I hear the back of my mind telling me that I have to, I need to do this. As StuRat pointed out, this isn't a perfect world. If we didn't have a military, and followed world politics with a passive attitude, some dictator with a cruel and murderous regime would long have taken us over. It's just like being Jewish, and standing with your guard down in a room full of Neo-Nazis who know your Jewish. You're going to get yourself killed. 198.110.63.66 17:02, 15 March 2006 (UTC)
"But Bowlhover, why don't you tell us what you really think of the military? In a perfect world, we would not need a military, true, but do you really think this is a perfect world? Total unilateral disarmament is a really bad idea."
I'm a pacifist, but that doesn't matter right now. I feel that people should not join the U.S. military just because they get a sudden urge to do so, because injuring/killing others is probably not going to be as fun and exciting as they think it's going to be.
- I respect your pacifist beliefs, Bowlhover, but you shouldn't try to apply them to the actions of others, only to your own actions. StuRat 00:39, 16 March 2006 (UTC)
"I am currently 9th out of the 134 students in my class."
Congratulations! If you don't join the military (and maybe get killed), you can use that intelligence to your advantage in the workforce.
"Bowlhover, do you really think that a .50 calibur hollow-point through the cerebal cortex will hurt."
No, but do you really think that the same calibur through an arm won't hurt?
"More so, I want to join the Marines because I do want frontline action."
Then don't get disappointed. Only a small fraction of those who enlist will get to fight in the front line.
"You may think it wrong to kill, but sometimes it's just your final option."
But in this specific case, it isn't your final option. If a stranger is trying to strangle you, you will lose your life if you don't kill that person. However, if you don't join the Marines, you will not lose anything.
"That aside, my reasons are my reasons. Some are hard to explain, others just an urge. I hear the back of my head telling me that I have to, I need to do this."
If you can't even explain to yourself why you want to join the Marines, it's likely just a sudden urge that you will regret following. You should make this decision based on the advantages and disadvantages of enlisting vs. not enlisting, not based on an unexplainable urge. --Bowlhover 20:07, 15 March 2006 (UTC)
- It sounds to me like he does have a plan, and Basic is probably pretty good at disspelling any sudden urges. Melchoir 20:30, 15 March 2006 (UTC)
- "9th out of the 134 students" 134 students? small town, life boring, seems like there aren't a lot of options? had recruiters in your school since you were in 6th or 7th grade? I've known a number of people like you, just don't, you'll regret it later on, and it will be too late to back out. Also, the last war the US fought that actually involded fronts, was probably korea. One more also, only a small fraction of soldiers wind up dead, true, but a much larger fraction wind up wounded, disfigured, and paralyzed, in fact I'd bet most wind up wounded in some way or another, which probably kills the health requirements for the space shuttle right there--152.163.100.74 22:00, 15 March 2006 (UTC)
- 134 students in my class. I think there's something around 400-500 students in my high school, but that's not an issue I want to get into. I can safely say that the recruiters that come to my school (only since last year, they don't bother with the middle school) haven't effected my decisions or opinions in anything. The only time I even talked to one, besides saying "Hi", anyways, was when I got a copy of America's Army. You say I'm probably going to regret it forever because it's a "sudden urge", but it's not. Something that sticks with you for five years isn't what I'd call a "sudden urge". Here7ic 01:43, 16 March 2006 (UTC)
- I'm not going to try hard to convince you not to join the military. Just be aware that there is a significant risk of you dying or being wounded. However, I recommend you join the Air Force rather than the Marines. It will give you a greater likelihood of being chosen as an astronaut. Superm401 - Talk 03:15, 16 March 2006 (UTC)
- "You say I'm probably going to regreat it forever because it's a "sudden urge", but it's not. Something that sticks with you for five years isn't what I'd call a "sudden urge"."
- OK, then it's just an urge. But as I said before, if you can't explain to yourself why you want to join the military, don't join the military. Try to think of what would happen if you get what you want--that is, if you get sent to war. I hope you've seen real photographs of injured people dying during a war, because if you're ignorant of such things, you'll get a real shock when you witness it. Also, I'm almost certain that if and when you get injured, you'll regret your decision to join the military. --Bowlhover 06:02, 16 March 2006 (UTC)
- You don't understand the concept of instinct, do you? Here7ic 16:37, 16 March 2006 (UTC)
- OK, then it's just an urge. But as I said before, if you can't explain to yourself why you want to join the military, don't join the military. Try to think of what would happen if you get what you want--that is, if you get sent to war. I hope you've seen real photographs of injured people dying during a war, because if you're ignorant of such things, you'll get a real shock when you witness it. Also, I'm almost certain that if and when you get injured, you'll regret your decision to join the military. --Bowlhover 06:02, 16 March 2006 (UTC)
- I do understand it, which is why I know you're misusing the term. According to the article you linked to: "Instinct is the inherent disposition of a living organism toward a particular behavior." Having an urge to join the military is not an instinct, since you didn't know there's something called the Marines when you were born (that was learned). See the article for things that are instincts. --Bowlhover 04:02, 17 March 2006 (UTC)
- You didn't answer me yet. Will you consider joining the Air Force instead of the Marines? It will give you a better shot at becoming an astronaut, you're still helping your country, and it's less likely that you'll be injured or killed. Superm401 - Talk 04:24, 17 March 2006 (UTC)
- Fine then. A slight mistranslation, but nothing crippling. Perhaps "sub-conscious motivation" would be better, a 'prime directive' that must be attained even at high costs. It's what some would call a 'gut feeling'. I know I have to do it, and I know it has to be the Marines, Superm. If I weren't to, I'd be betraying the most human part of my mind, my sub-conscious. And in this world, I don't think it's worth it to betray my sub-conscious. I'm sure as a Marine I'll have at least a slight chance of making it into NASA's Space Flight program, and maybe that's all I need. I guess only time will tell. Here7ic 17:06, 17 March 2006 (UTC)
- This is ridiculous--"betraying the most human part of my mind, my sub-conscious". I won't try hard to convince you to not join the military, but you should really think about your own actions, instead of relying on a "sub-conscious motivation" to make decisions for you (and then blaming it if the decision was a bad one). Get a conscious motivation! If you can't, you should probably resist the sub-conscious motivation. (If you get a gut feeling that you have to rob a bank, you should probably resist it.) --Bowlhover 05:08, 19 March 2006 (UTC)
- No, dear Bowlhover, what's ridiculous is to think that I haven't consciously thought this over. I have, and I do every day. I know there are risks to joining the military, but it's something I have to do. It's a sense of fate that every thing human about me tells me is right. If you won't buy that, and want a reason I can provide with a more logical reference, well then the military is currently the most secure branch of employment available. You say I have a good chance of dying if I join because they'll send me over(an accuasation I've pointed out to be improbable) to Iraq. Well, you have a better chance at being struck by a car tomorrow, or being murdered on your way home from work. Do you think that just because I enter a politically debated war that I will die or that I won't have any better chace of staying alive? Do you honestly think that by staying out of the war you've insured your life? Many things are reliable. Life isn't. Here7ic 05:29, 19 March 2006 (UTC)
networks
[edit]how pervasive computing is can be used in goal oriented programming paradigm?
plz if u can help me then tell me any thing u know about it............
- Have you looked at pervasive computing? --
 Mac Davis] ☢ ญƛ. 11:09, 14 March 2006 (UTC)
Mac Davis] ☢ ญƛ. 11:09, 14 March 2006 (UTC)
Air pressure
[edit]What is the differnce between Static pressure and Atmospheric pressure?? --198.54.202.210 11:29, 14 March 2006 (UTC)
- If you take a look at static pressure you'll see that atmospheric pressure is an example of it. --Bth 11:42, 14 March 2006 (UTC)
- By contrast, you would get dynamic pressure if wind was blowing on an object. StuRat 00:09, 15 March 2006 (UTC)
- And by using a Pitot tube, we use the concepts of both dynamic pressure and static pressure to find the speed of airflow. EWS23 | (Leave me a message!) 00:53, 20 March 2006 (UTC)
Special Relativity & Orbit of the earth
[edit]Dear users can anyone explain to me why things gain mass when they were accelerated at the speed of light? Why does it contracts in length?
Can anyone tell me why the Earth spins on its own?
Thanks!
- 1) We have a splendid article entitled special relativity for beginners, but feel free to come back here if you need anything explained in more depth.
- 2) Conservation of angular momentum. The stuff from which the Earth formed was spinning. In turn, that was because the nebula from which it condensed was spinning. And that's because it got a random off-centre kick at some point. --Bth 12:56, 14 March 2006 (UTC)
Atomic Shells
[edit]Can anyone tell me why things need 8 electrons in the outer shell to be stable? why not 5 or 10? thanks!
- It's difficult to know how complicated an answer you want. The glib answer is: because of quantum mechanics. The full-on version is at electron configuration. I'll try and give an answer intermediate between those two.
- Electrons are found in fuzzy regions called orbitals, which act as subshells of the shells. Each orbital can only have 2 electrons (because electrons are fermions and they obey the Pauli Exclusion Principle -- each orbital can have one spin up and one spin down electron). Each time you increase n, the number of the shell, you get another 2n-1 subshells that are available to be filled by electrons.
- So for n=1 there's only one orbital (the s-orbital), which can have 2 electrons -- that's why Helium is a noble gas with only 2. For n=2 there's the s-orbital and three new p-orbitals, each of which can have 2 electrons, so six in total. Therefore you have eight electrons in the outer shell.
- That works for n=2. But why isn't it 18 for n=3, 32 for n=4, etc.? That's because of the order in which the subshells get filled. The s-orbital for the next shell up is at a lower energy than the new subshells (named d,f,g in each case) so the next electron after filling up the n-1 shell's p subshells go in there, but the p subshells for shell n are at a higher energy. So the filling order goes: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p (and gets even more complicated after that; even that's ignoring some subtleties). This is why we get the transition metals -- they're what happens when you fill up the d orbital with 10 electrons in between filling the 4s and 4p. But when you get along to the end you've still only got 8 electrons in the outer shell.
- You have three quantum numbers involved in identifying an electron in an orbital. (Why this is, is a longer story) It has Spin (s, ±1/2), Orbital angular momentum (l, an integer) and the Magnetic quantum number (m, which can take a value from -l to +l). Only one electron can have one combination of quantum numbers (because of the Pauli priniciple mentioned above)
- So, for l = 0, m must be 0, and all you have is spin up and down, so you can fit two electrons in there. (an 's' orbital) For l = 1, m can be (-1,0,+1) and with spin that means six electrons. (a 'p' orbital). The octet rule (stable with 8 electrons) comes from counting only the outmost p and s orbitals, so 2+6=8. There are other orbitals (d and f, corresponding to l = 2 and l = 3), but they get filled before the outmost p and s ones, so that's why the rule works. --BluePlatypus 14:40, 14 March 2006 (UTC)
Chemistry--Is it Acid or Chemical?
[edit]I am an Artist, living in india. While travelling I saw an old man copying pictres and letters of news paper to a blank white paper.I wonder how he can trace pictures without using a machine.He only uses a liquid (I don't know Acid or Chemical) he pastes that liquid on that paper and presses that blank paper on the picture he want to trace..how it happening sir? can u clear my doubt? which liquid he is using?Thanks (Sathiyamoorthi)
- Most newspapers use powdery ink that comes off and turns your hands black. In the U.S., there has been a lot of study on making cheap ink that doesn't rub off. But, most newspapers around the world still rub off. Just add a little water (damp sponge perhaps) and press a sheet of paper on it and the much of the ink will move easily from the newspaper to the paper. Also note, an acid is a chemical. Perhaps you meant "acid or base". --Kainaw (talk) 15:54, 14 March 2006 (UTC)
- Yes, most US newspapers don't use ink that rubs off easily any more. They were losing customers who didn't like ending up with ink covered clothes and hands after reading the paper, so made the change. StuRat 00:02, 15 March 2006 (UTC)
- There is one other possibility. Was the old man, by any chance, selling this chemical? Notinasnaid 16:37, 14 March 2006 (UTC)
- If he was - wouldn't it be easier to just get a tub of Silly Putty and sell that instead? --Kainaw (talk) 16:41, 14 March 2006 (UTC)
- Continuing the thought, which perhaps wasn't very clear: if someone is demonstrating a wonderful substance with miraculous properties, it often turns out to be snake oil. It's easy to imagine a set up based on invisible ink which purports to demonstrate a "photocopier in a can". Notinasnaid 09:05, 15 March 2006 (UTC)
Regarding higher quality ink that doesn't run when it gets wet: I believe there was a process for creating copies using a chemical akin to paint thinner, which then transferred some of the ink from the original to the copy. This method was used to copy the US Declaration of Independence, for example. However, the process has some negatives, such as degrading the original and making a limited number of blurry copies, as well as the toxic fumes. So, once photocopiers came out, this process mostly died. You can now buy a standalone 8.5" x 11" flatbed copier for under US$100, so there isn't much economic incentive to using the chemical process anymore, either. (The cost of the toxic chemicals will be more than the copier after a few hundred copies.) StuRat 15:01, 15 March 2006 (UTC)
thanks for your answer
I tried to play the 2004 two-disc edition of The Meaning of Life with xine, with the latest libdvdcss (1.2.9) installed, but descrambling failed. Here was someone with the same problem one year ago (but he never got a reply). What is the meaning of this? Have they come up with a new scrambling algorithm now? It really puts you off not pirating movies when you find you are unable to play the ones you didn't pirate. dab (ᛏ) 15:36, 14 March 2006 (UTC)
- I don't think CSS is implemented as widely as commonly thought to be. Other scrambling algorithms have started to be used as well, especially recently. Are you sure it's been scrambled using CSS? -- Daverocks (talk) 05:47, 15 March 2006 (UTC)
browser history drop-down
[edit]In both Mozilla Firefox and Internet Explorer, when I use the browser's history dropdown box in an attempt to find a shortcut to a web page I recently visted (having previously entered the URL into the browser), I usually don't find the URL I want to use. But the browser offers up a lot of addresses I visited a few days or weeks ago. What gives? Is the list in the dropdown box supposed to be in LIFO order, as I assume? If not, is there a way to sort the list? (I think not.) How is a person supposed to make practical use of this "feature" in the browsers? What am I missing? Thanks, --Halcatalyst 19:31, 14 March 2006 (UTC)
- I usually do my best to provide references when I answer ref desk questions, but I'm going to wing it on this one. The drop-down list on the address/location bar contains only those URLs that you have specifically typed in, and then hit enter (or clicked GO, or whatever). Early versions of Netscape Navigator limited the drop-down list to the last X entries (where X varied from one release to the next). I believe that the lifespan of entries on that list is now governed by your "days to keep" History setting. I think you are correct on the LIFO nature of the list, at least for Firefox. I think IE might use a MRU scheme instead.
- When you start typing a URL in the bar, the auto-complete feature found in many current browsers searches your complete browsing history, which will contain all pages that you have visited, perhaps by linking rather than explicit URL entry (subject to the same "days to keep" setting).
- As far as using the feature . . . at work I have a limited number of entries in my drop-down list, and en.wikipedia.org is one of the ones that always stays there, as well as my direct employer's webmail (I'm a contractor; Outlook connects to the client companies servers). I just use those entries to get to the few sites that I can visit while at work. I hardly ever use bookmarks/favorites. --LarryMac 21:09, 14 March 2006 (UTC)
- I have a tip for when you want to type in an address in the address bar and not have it appear in the drop-down box. This tip only works in Internet Explorer though. After typing the URL, grab the white space to the left (the area that normally shows an IE document-type image) and drag it into the page. The page *should* start loading. I find myself doing this quite a bit. It's one of the reasons I still use IE. --Optichan 21:51, 14 March 2006 (UTC)
I think I was wrong when I said the address drop down didn't contain URLs I had typed in. It probably does. I was confusing that with the browser's ability to call up suggested URLs when I start typing in the address box. Well, at least that's clearer to me now. Thanks to both for your help! --Halcatalyst 22:59, 14 March 2006 (UTC)
- I've noticed that when my browser (AOL) locks up, I kill and restart it, but the drop down never seems to have the URL of my last page. I suspect that it doesn't update the stored list until it is closed down properly, which is pathetic, guaranteeing it won't work to recover my web page whenever AOL locks up. StuRat 23:57, 14 March 2006 (UTC)
Metal Racks in Microwaves
[edit]I've always heard that putting any metal in a microwave and turning it on results in arcing, which can ruin the microwave. But a lot of the over-the-range models these days are coming with metal racks in them. What's the deal with that?
- From my very first microwave to the one I have now, the whole casing - inside and out - is metal. It is an urban legend that "any metal" in a microwave will "kill the coils/blow it up/cause rips in the fabric of space and time..." In fact, my first microwave said, in the instruction manual, that the best way to reheat chicken legs is to wrap tin foil around the bone end while reheating. --Kainaw (talk) 20:29, 14 March 2006 (UTC)
- The metal casing including the mesh in the glass window is there to reflect/absorb microwaves so they don't heat things outside the micrwave (like people). The casing defines the interior microwave space and is not in it. Metal and water absorb the microwaves and heat up. Micowave racks, to the best of my knowledge, are not made of metal, although some look metallic ("metallic finish" says one adv.). WAS 4.250 21:05, 14 March 2006 (UTC)
- I've wondered about 'microwave-safe foil dishes', so I looked it up. You can put metal in a microwave, so long as it is rounded, and doesn't touch other metal. You can put in those foil packets, but not on metal trays. [37] Standard foil has too many crinkles and sharp edges, so it will spark. --Zeizmic 22:31, 14 March 2006 (UTC)
- OK, the story as I know it is, you have to have enough microwave absorbant material in the microwave uncovered in metal to absorb the energy or there is too much feedback on the magnetrons and they arc. In the early days, even a thin gold rim on a plate was anough to cause trouble, but maybe the ovens are more durable now.
I had a rare case of arcing with no metal in the mic. I was melting cheese on saltines with jalapeno peppers on them, and got arcing between two ends of a semicircular pepper slice that scorched the cracker. I can't explain it, but my best guess is it was some weird electrochemical reaction with energy supplied by the microwaves. The acid in the pepper and the salt on the cracker probably played a part. StuRat 23:47, 14 March 2006 (UTC)
- Our microwave manual says that to warm a cup of water evenly you should put a metal spoon in the cup. It also says that the spoon shouldn't touch the metal casing. And I've read somewhere that metallic things were a problem in pre-90's microwaves, but not any more. – Blue ҉ Iris 05:39, 15 March 2006 (UTC)
- That's a different thing though, it doesn't have to be a metal spoon. The reason you want to do that is that, in rare cases and if the cup is smooth, the water can be heated so fast that it doesn't nucleate and start boiling. The water then becomes superheated, which is dangerous, becuase when you take the cup out and disturb it can start boiling instantly and blow hot water into your face. Adding a spoon to the water creates nucleation sites for the bubbles, and reduces the likelyhood of the water becoming superheated. --BluePlatypus 14:23, 15 March 2006 (UTC)
How have microwave ovens changed to prevent metal arcing ?
[edit]Per the above convo, I would be interested in knowing how microwave ovens have changed to make them handle metal better now. StuRat 14:50, 15 March 2006 (UTC)
MRSA
[edit]A non-surgical consultant physician has MRSA in the throat following abdominal surgery. Is he/she permitted to return to work and what risks to patients and the employing hospital's liability would be posed if such return was allowed? This is a UK question.
- I checked the our CDC Infection Outbreak list (contains American data only) to see if there have been outbreaks of MRSA. There has been in correctional facilities, athletic locations with common showers, in the gay-male communities, and in childcare centers. These are areas where outbreaks of just about everything occur normally. So, that makes me assume that the chance of an outbreak at a normal working facility is the same as any other common bacteria or virus. According to the CDC, they are monitoring MRSA infections in hospitals, but not in office workspaces. So, since this is a physician, they may be at increased risk of getting MRSA because of the hospital setting - that is assuming that hospitals in the UK are similarly full of infections like the ones in the US. But, I have to say, I got sick all the time when I worked for Sony. Since I've been working in a hospital, I haven't even caught a cold. --Kainaw (talk) 20:04, 14 March 2006 (UTC)
- Here in the UK we have a big flap on (largely inspired by the Daily Mail and its ilk) about patients being infected by their doctors(' ties). (In fairness, we do have above average infection rates in our hospitals so it's not completely tabloid hysteria). I suspect the question is more whether the guy would be allowed back to work until he's fully recovered, given that he might be (perceived as) putting his patients at enhanced risk. Unfortunately I can't find anything helpful via Google. --Bth 20:19, 14 March 2006 (UTC)
I think I need to suitly emphazi MRSA. StuRat 23:37, 14 March 2006 (UTC)
sound in pdf or similar?
[edit]I want to create a pdf that has an embedded sound file. Actually, I think I want pdf, maybe something else - I want a mostly text file that I can send to collegues and be confident that they will not have to install something to read it. It is mostly text, but I want them to be able to click on a sound icon and get a sound file played. How do I do this? I am using OS X, but want to distribute mostly for win 2k / xp.
- I doubt you can embed sound in PDF, but I'm not sure. Melchoir 23:38, 14 March 2006 (UTC)
- Actually, I guess you can. The question is, do you have access to software that will do it? Melchoir 23:40, 14 March 2006 (UTC)
- Adobe Professional, which you need to make an PDF file and integrate sound into it, costs 449 USD. There is a 30-day trial version, but it's only for Windows. --Bowlhover 01:03, 15 March 2006 (UTC)
Is there another way to do it? HTML?
- Yeah, it's trivial to do it with HTML. ☢ Ҡi∊ff⌇↯ 00:58, 15 March 2006 (UTC)
- If you want to use HTML, you'll have to have a sound file first, and than link to it using <a href="soundfile.extension">link name</a>. I'm pretty sure this is not what you want, since the user will have to download the sound file first, and then use another application (like Windows Media Player or Realplayer) to play it. It's the same as sending the sound file directly except that you'll waste time creating an HTML file. Bowlhover 01:04, 15 March 2006 (UTC)
- You can use the embed tag to embed a sound file into the webpage and start it playing without having the reader do any action (other than waiting for it to download). --Kainaw (talk) 01:24, 15 March 2006 (UTC)
- Don't forget the similar bgsound tag, used like: <bgsound src="http://mycoolsite.com/sounds/coolsound.mp3">. Mind you, neither embed nor bgsound will work in Firefox, as it blocks background sounds. -- Daverocks (talk) 05:54, 15 March 2006 (UTC)
- It's not very good practice though; forcing users to listen to your music without them having done anything to request it is rather rude -- if nothing else, think of the skiving office workers being caught out when their PC starts blaring the Red Flag (or whatever it is you want to embed). And frankly, the majority of sites that use such things tend to be bad in one way or another. Another reason to love Firefox. --Bth 08:58, 15 March 2006 (UTC)
Large intestine
[edit]Quick Biology question:
How long is food digested in the large intestine?
Thank you.
- Well, it spends a few hours in there, but it isn't really digested there, that happens in the stomach and small intestine. The large intestine mostly just removes water. StuRat 23:33, 14 March 2006 (UTC)
- If you type "large intestine" into the search box, you'll come up with Colon (anatomy), the first section of which is "Role in digestion". Melchoir 23:37, 14 March 2006 (UTC)
I need to find specific hours when water, etc... are removed in the large intestine. If anyone could please help out before tomorrow, I would appreciate it.
March 15
[edit]short-term memory
[edit]Could you tell me in what ways would life be different without short-term memory?
- What did you say?
In a way, short term memory allows you to create a continuous timeline in your mind and memory. Without it, you'd be living in discrete, unconnected intervals of, say, 5, 10 minutes. This is a terrible thing. ☢ Ҡi∊ff⌇↯ 00:35, 15 March 2006 (UTC)
- The article Anterograde amnesia has, um...
Slumgum 01:03, 15 March 2006 (UTC)
It may be worth looking at the Korsakoff's syndrome article too. This is a rare disease of chronic alcoholics. Brian G. Crawford 01:16, 15 March 2006 (UTC)
- Also note that it is the common current assumption that long-term memory is created by transferring select bits of information from short-term memory. So, without short-term memory, you have nothing to transfer into long-term memory. But, this is a theory about the brain. It will probably all be bunk in another 10 years. --Kainaw (talk) 01:26, 15 March 2006 (UTC)
- However, if the problem with short term memory is just an inability to consciously access it, memories might still make it into long term memory. StuRat 03:06, 15 March 2006 (UTC)
This is a philosophical or definitional problem in that "life", "different", "short-term", and "memory" are so ill defined in common useage that the question bogs down at the level of the definition of the terms involved. Just for an example, take a bacteria (life), and its recognition of a gradient (different and memory): what does "short-term" mean in this instance? WAS 4.250 01:34, 15 March 2006 (UTC)
- Um, bacteria don't "recognize" anything, they have roughly the cognitive ability of your average taste bud, absolutely none, their receptors simply turn in the direction of a chemical stimuli, then tumble until they've started to move in the other direction--152.163.100.74 21:43, 15 March 2006 (UTC)
- Wrong. Totally wrong. Read this. WAS 4.250 22:11, 15 March 2006 (UTC)
- Recognizing simple chemical signals doesn't make them intelligent, no more so than my taste bud, or a pH meter would be considered "intelligent"--152.163.100.74 23:02, 15 March 2006 (UTC)
- Recognizing simple chemical signals doesn't make humans intelligent either. Both humans and bacteria do MORE than that. WAS 4.250 01:03, 16 March 2006 (UTC)
- Um, what? Higher order mammals have these funny organs that usually handle that whole conscious thought, thing, bacteria have, um, practically nothing, just a primary genome, and in some cases a plasmid, so barring any nonsensical/theological explanations, or some sort of junk-science about bacterial souls, I have no idea what you're talking about--152.163.100.74 05:40, 16 March 2006 (UTC)
- Recognizing simple chemical signals doesn't make humans intelligent either. Both humans and bacteria do MORE than that. WAS 4.250 01:03, 16 March 2006 (UTC)
- Recognizing simple chemical signals doesn't make them intelligent, no more so than my taste bud, or a pH meter would be considered "intelligent"--152.163.100.74 23:02, 15 March 2006 (UTC)
- Wrong. Totally wrong. Read this. WAS 4.250 22:11, 15 March 2006 (UTC)
- Doctor: "Had any trouble with your memory lately ?"
- Patient: "Not that I can recall, no."
An interesting screenshot from netscape.com's news listings (this oughta be some sort of fair use, I'd think): File:NetscapeNewsOops.jpg. --Trovatore 22:21, 15 March 2006 (UTC)
- LOL, is the article about how hungry the author is, and what they would like to munch on, and how big their hands are, and how words lose their meaning when you repeat them a lot ? StuRat 01:50, 16 March 2006 (UTC)
Microsoft Word
[edit]I recently came across a 2003 article on the security risks of Microsoft Word [38].
- Is this truly something to worry about
- how and with what programs would one be able to access the encoded information, and
- has Microsoft done anything to solve this since then?
JianLi 01:43, 15 March 2006 (UTC)
- There is a document cleaner in MS Word now. Some people do not trust it and convert the Word documents to evil PDF documents before sending them out to people. I don't use MS Word and I will quit my job before even saving a death-spewing PDF to my hard drive. So, I can't say what menu item does the document cleaning or how to convert a word doc to a satan-spawn PDF. --Kainaw (talk) 01:48, 15 March 2006 (UTC)
- Why're .pdf files evil? Slumgum 02:14, 15 March 2006 (UTC)
- I'll let someone else answer that, but I'll opine that they're also seethingly annoying. If you use them for distributing documents which you print out on paper and then read, they're fine. But when used for distributing documents which are to be intended to be read on screen (e.g. on web pages) they're just *wrong*. The screen is not a piece of paper, and the lengths which PDF goes to in an attempt to reproduce exactly what you'd see on a piece of paper are often unnecessary if not downright detrimental for screen reading. The fonts are often too small, and even if your PDF reader lets you zoom in (some do; web browsers with PDF plugins may not) you've then got the problem of scrolling around. The scrolling you do within a "page" is often distractingly different from whatever you have to do to "turn the page". And don't even get me started on the up-and-down zigzag scrolling you have to do if the document carefully uses a newspaper-like multicolumn format. —Steve Summit (talk) 05:25, 15 March 2006 (UTC)
- Kainaw: If you don't use Microsoft Word or PDF, what do you use? —Keenan Pepper 03:14, 15 March 2006 (UTC)
- To distribute electronic documents? Me, I usually use plain text. It's guaranteed that the recipient can read it, and I'm more interested in impressing my reader with my content than my typography. —Steve Summit (talk) 05:25, 15 March 2006 (UTC)
- First, pdf files are evil because, as mentioned above, they are not meant to viewed on a computer. They are meant to be printed. I print, at most, 2 things a month. I'm not going to suddenly start printing PDF documents so I can read them. While it is humanly possible to read a pdf on the screen, it is more annoying than getting a jalapeno enema while trying to take the GRE. I have been well known to go down 12 floors, leave my office, walk six blocks, go up 8 floors, and yell at someone for sending me a PDF attachment in an email with the note: "Read This".
- [Wow. You got it even worse than I do. Condolences, 'n' that. :-) —Steve Summit (talk) 04:27, 16 March 2006 (UTC)]
- I know that I will never be able to solve world hunger, end all wars, or stop the constant onslaught of movie remakes of old television shows. However, I hope that I will someday be able to put an end to the plague of PDF documents that pollute computers around the world.
- As for what I do use... Text. When I send an email, I just type plain text. If I am taking notes, I use plain text. When it is absolutely necessary to have text formatting and pictures, I use HTML. If it is not absolutely necessary to have text formatting and pictures, I stick with text. When I do receive a Word document, I use Open Office to read it. Unlike PDF documents, you can open a word document and view it online without scrolling up-right-left-down-shift-jumped a page(damn!)-up-up-right-left-(wait for a page to load)-down-right-left-down-jumped a page again(double damn!)... --Kainaw (talk) 13:24, 15 March 2006 (UTC)
- First, pdf files are evil because, as mentioned above, they are not meant to viewed on a computer. They are meant to be printed. I print, at most, 2 things a month. I'm not going to suddenly start printing PDF documents so I can read them. While it is humanly possible to read a pdf on the screen, it is more annoying than getting a jalapeno enema while trying to take the GRE. I have been well known to go down 12 floors, leave my office, walk six blocks, go up 8 floors, and yell at someone for sending me a PDF attachment in an email with the note: "Read This".
- Maybe it's the software you're using to view the PDFs. Evince is a pleasure to use. —Keenan Pepper 15:34, 15 March 2006 (UTC)
Haha, btw, this all arose because I got one of these in the signature line of an email:
Please do not send me attachments encoded in a secret proprietary format such as MS Word or Power Point; I am unable to read them. Please use instead a publicly available format such as PDF (Portable Document Format) or plain text (ASCII). For more information, see [39]
-JianLi
- Most versions of Word now have an option in their settings so that they will 1. warn you if a document contains hidden data (like tracked changes) and 2. allows you to strip the document of any personal data upon saving. It is in the preferences somewhere. That signature is somewhat humorous -- if I'm sending someone a document which they need to edit, neither PDF or ASCII will be very useful. A less proprietary format which allows semi-complex formatting and editing would probably be RTF, if one is not interested in the open source Word alternatives (i.e. AbiWord or OO.org). --Fastfission 04:01, 15 March 2006 (UTC)
My answers:
- Yes, the danger of "leaking" unintended information via Word documents is quite real. All bashing aside, Word .doc format is a less-then-appropriate format for distributing documents, especially confidential ones. The .doc file is much more than just a representation of what the final form of the document "looks like" -- the file typically also contains all sorts of information about how the document got created and the revisions it went through. Back in the day, when you released the final version of a paper document for distribution, you released the final version; you obviously wouldn't release the manila folder containing your rough drafts, or the filing cabinet containing your confidential research.
- When someone sends me a Microsoft Word document that I need to read, these days I usually use the Unix "strings" utility. This shows me all of the text, none of the formatting, and incidentally sometimes also (a) metadata such as the names of the authors or the document filename on the author's hard drive, and (b) some of the deleted ("redlined") text. I have also used a utility called wvware.
- Microsoft may have worked on it, but if you're paranoid in this regard, it's wise not to trust them. If you have secrets which you can't reveal, and if you accidentally do via a Word document which you or Microsoft mistakenly thought was "clean", then even if you can get Microsoft to apologize, it's too late to save your secrets.
There was recently a memo put out by none other than the U.S. National Security Agency (NSA) which, despite spending 10 or so pages describing "How to Safely Publish Sanitized Reports Converted From Word", also admitted that "Using original source formats, such as MS Word, for [sanitized documents] can entail exceptional risks". —Steve Summit (talk) 05:25, 15 March 2006 (UTC)
- I agree with the earlier statement that you should send documents as either plain text or HTML, which everyone can read with no security risks. Not everyone can read the proprietary formats like WORD, and then there is the extraneous info such formats contain, which make the file unnecessarily long as well as providing security holes. I also agree that people spend way too much time on the formatting of their documents and too little on the actual content. (I wanted to add some dancing babies at this point, but couldn't figure out how.) StuRat 14:41, 15 March 2006 (UTC)
- To avoid leaving hints about a MSWord document history, just copy'n paste into a new one. To avoid using Word, I tried OpenOffice, which is not too bad, you have to get used to it, maybe there should be extensions for OO, just like for Firefox.
- To avoid pdfs, just avoid customers : the rule in plenty of offices is to give your customers pdf to eat, because they can't change the text. Here, in 2006, we're still waiting for reliable open source tools, non proprietary formats, and so on. --DLL 20:41, 15 March 2006 (UTC
What was invented this decade?
[edit]What was invented in 2000, 2001, 2002, 2003, 2004, 2005, and 2006? (I already know Digital Satellite Radio, Artificial Heart, and Podcasting were invented this decade.) -Anonymous
Sub-question: If the last decade was called the nineties, what is the name for this decade? --JianLi
- The name is problematic, perhaps the "naughts" ? The 2010-2019 decade will be tricky, too. You could call it the "teens", but that excludes 2010-2012. I believe artificial hearts go back decades, although none ever worked very well. Perhaps you mean the first self-contained artificial heart ? StuRat 04:36, 15 March 2006 (UTC)
- Probably JianLi got the list of three from Timeline_of_invention#2000s. Jay 16:50, 15 March 2006 (UTC)
- Actually, the original question wasn't mine. I just wrote the subquestion. -JianLi
- This decade often gets called "the naughties" (and I know what they mean ...) JackofOz 04:48, 15 March 2006 (UTC)
Recent decline in inventions
[edit]I've plotted the inventions per decade since 1800, from Timeline_of_invention (doubling the number for the 2000's, since that decade is only half over), and got the following:
1800's ********* 1810's ********** 1820's ******* 1830's ************************* 1840's ************ 1850's ********** 1860's ***************** 1870's ******************************* 1880's ******************************** 1890's *************************** 1900's **************************************** 1910's ********************************** 1920's **************************** 1930's ******************* 1940's ************* 1950's **************** 1960's ************ 1970's *********************** 1980's ************** 1990's ************* 2000's ******
It looks to me like the rate of inventions peaked in the late 1800's and early 1900's. This is counter to books like Future Shock, which predicted an ever increasing rate of inventions. So, my question is, what's going on here and what are the implications for the future ? I personally think that science and engineering have become unfashionable, at least in the US, and that might have something to do with it. Will the number continue to decrease until we reject science entirely and enter a new dark age ? StuRat 17:28, 15 March 2006 (UTC)
- Geez, that was a lot of work! In my alter-life I am caught in a giant techno-bureaucracy. With these things, there are hardly any 'inventions' but lots of mundane improvements. So the patent rate (of silliness) goes up. There's not a lot of scope these days to invent something significant in a garage. --Zeizmic 18:48, 15 March 2006 (UTC)
- Is that chart a count of inventions or patents? It seems like software patents are flooding the USPTO. I'd expect them to outnumber real inventions. --Kainaw (talk) 18:51, 15 March 2006 (UTC)
- It's a count of stuff in the wikipedia article, so it's by no means unbiased. You'd be insane to draw any real conclusions from that. If anything, it's a measure of the timespan required for an invention to be considered significant. Also, you could interpret it how the "inventor-invention" idea is more rooted in the late 19th century when you did have a lot of individual innovators, whereas research and innovation today tends to be done in larger groups and more incremental steps. Rest assured that by any reliable metric such as number of patents, number of researchers, number of scientific papers published, amount of money spent on R&D, etc, the pace of innovation is higher today than ever before. --BluePlatypus 19:43, 15 March 2006 (UTC)
- This is my point. It seems like more money is being spent, and more patents obtained, for truly insignificant things, like yet another pharmaceutical ever so slightly different from all the others in it's class, which is not significantly better than it's predecessors in any way. At the same time, the number of real, meaningful inventions is falling dramatically. StuRat 01:59, 16 March 2006 (UTC)
- Exactly. To be precise, Selection bias. Melchoir 19:44, 15 March 2006 (UTC)
- The fact is that there are less births and deaths in the years 500-1000 than in 1850-1900 in the Selected anniversaries (WP's main page).
- It takes time to really recognize the value of anything - people, inventions, events. The most recent ones may still be ignored. Plenty of immense inventions died twenty or thirty years later ; floppy disks, telex, LPs ... may be forgotten soon. We might well have the same plotted graphic in 2050. --DLL 20:29, 15 March 2006 (UTC)
- I thought of that, but it seems to me that inventions 50 years old are old enough to be recognized as valuable or not by now, yet we still see a decline even then. It seems like a reasonable bell-shaped curve, too. I would think any bias by Wikipedia editors to be a recency bias, so would expect even more inventions to be listed recently. StuRat 01:43, 16 March 2006 (UTC)
- Also, I think what you may be missing is that while not that as much "new stuff" is being invented, the stuff we already have is being radically improved. Jet airliners may be over 50 years old, but these days you can fly across the Atlantic for a very small fraction of the cost you could then. --Robert Merkel 04:09, 16 March 2006 (UTC)
- Yes, but that isn't new, either. Our use of wheels, fire, and agriculture have been radically improved over the ages, too. So, are we destined to stop inventing new things and only work on tweaking existing ideas ? StuRat 20:00, 16 March 2006 (UTC)
- I think he's trying to imply that the distance between "improvement" and "new thing" is pretty subjective. --Fastfission 21:37, 18 March 2006 (UTC)
- As for patenting trends, they are hard to generalize about without crunching a lot of data and taking into account changes in industry, patenting law, and society. In the US, for example, from about 1870-1970 you have a pretty steady increase in patenting with the exception of World War II. From about 1970 to the present you have some major changes following modifications to the patent system. In the last twenty years there have been some major changes to the way patents are filed and prosecuted, which have led some people to argue that the patent system itself is now functioning counterproductively (see Innovation and its Discontents for one such book). But anyway, I think generally speaking, if one removes from consideration the number of frivolous patents out there (which are probably not too many, in the end), the more inventions one has, the more improvement upon those inventions one gets, some of which end up being quite radical. It is very easy to lose sight of exactly how radical some of these changes have been: just about every aspect of an iPod would have been technologically impossible only a decade ago, and now we are happy to take it completely for granted. In any case, there's no simple answer to even assessing the trends, much less explaining them. I don't think your "bell curve" model is at all correct, though. --Fastfission 21:37, 18 March 2006 (UTC)
- Well, the bell curve happened to correspond with my personal observation that truly new inventions seem rare these days (although I would have put the peak a bit later, perhaps at WW2). To just take one field, let's look at disease. When is the last time a disease was actually eliminated ? The last that comes to mind to me is the polio vaccine in the 1950's. Let's look at the last new way to produce energy that actually works on a large scale. Wouldn't that be nuclear fission reactors, also invented in the 1950's ? Let's look at major innovations in transportation. That would be jet airplanes, from the 1950's again. Am I imagining all of this ? (I suppose you could count maglev trains, but they are expensive and rare and really just a tweak on traditional trains and were patented as far back as 1969, in any case.) StuRat 04:36, 19 March 2006 (UTC)
I think you ought to consider bias in the article as the likely explanation. Just looking briefly around my house I can see many significant inventions (significant to me, anyway) not in the list: the condensing boiler, the electronic piano, thermostatically controlled central heating, handheld game consoles, wireless LAN, laptop computers, inkjet printers, pin tumbler locks, print on demand books, silicone sealant, chip and PIN cards, handheld electronic dictionaries, ... I could go on. Gdr 00:35, 20 March 2006 (UTC)
- Well, if we look at that list, we have:
- condensing boiler: combines boilers (millennia old) with a heat exchanger/condenser, which is also quite old.
- electronic piano: first sold in the 1950's. Pianos, of course, go back centuries.
- thermostatically controlled central heating: central heating via hot water goes back at least to Roman baths, with the thermostat going back maybe a century.
- handheld game consoles: just a smaller, more sophisticated version of the same old game system, like Pong, from 1972.
- wireless LAN: combines wireless technology (a century old) with digital signal processing (a half century old).
- laptop computers: just a small, powerful, portable version of computers from the 1940's.
- inkjet printers: just a variation on printers, which go back to the teletype machine, from 1906.
- pin tumbler locks: also quite old, 2000 BC !
- print on demand books: This isn't really a new invention, just more practical now with faster download speeds.
- silicone sealant: quite old.
- chip and PIN: a variation on credit cards, which go back to the 1950's.
So, nothing much new in that list, is there ? Only a few tweaks to old stuff.
StuRat 21:06, 20 March 2006 (UTC)
- Every invention is based on previous inventions, but that doesn't mean that these inventions aren't significant. For example, you might think that a piano is just an improved clavichord. But with a piano you can fill a concert hall and compete with an orchestra: without the piano, no Beethoven piano concertos. And as for your idea that these inventions are just a "few tweaks", I think that's breathtaking arrogance. Gdr 00:00, 21 March 2006 (UTC)
- I see no justification for you to resort to personal insults here. There are inventions that weren't tweaks of previous inventions. For example, a car was definitely not a minor tweak of a horse and carriage, and an airplane was definitely not a minor teak on a hot air balloon, and nuclear weapons aren't just a tweak on conventional explosives. However, mostly the inventions I've seen recently don't fall into the category of "major new invention". StuRat 01:23, 21 March 2006 (UTC)
- Change is not necessarily the same as invention, however; it can be evolutionary. The cars of today are very different from those 100 years ago, but you can't really blame that on an invention, though tens of thousands of little inventions have gone into it. Similarly the computers of today are very different to those of 30 years ago, but the basic concepts haven't changed much.
There is a school of thought that the pace of change is now so rapid we are heading for a crisis point where everything after that point is incomprehensible before. Increasingly, innovation is happening in software, rather than physical inventions, and the argument goes that once computers are smart enough to start innovating the world will never be the same again. For a discussion of this idea, which I have probably misrepresented, see Technological singularity. Notinasnaid 21:58, 20 March 2006 (UTC)
- Yes, that's similar to the Future Shock book I opened this question with. I don't see it happening, though. Which required more of an adjustment, switching from horse and wagon to a car or switching from a car without a navigation system to one with it ? StuRat 01:23, 21 March 2006 (UTC)
How do I get Quake 3 to run in Linux?
[edit](no question body)
- You could try Wine, which works pretty well for most Windows applications, but it doesn't perform too well with DirectX 3D games. On that note, you might be interested in Cedega, a fork of Wine which concentrates specifically on running Windows-designed games on Linux, and the DirectX API. Unfortunately, unlike Wine, it isn't free. In general, it's been a difficult journey to get anything Windows-based running on Linux, but DirectX, being proprietary and used extensively by Quake 3, is difficult to implement. I think I heard somewhere that an earlier legal battle will soon force Microsoft to release some of its rights on DirectX, making it easier for programmers to implement it on other platforms. Good luck. -- Daverocks (talk) 06:41, 15 March 2006 (UTC)
- Quake 3 uses OpenGL, and the source code was released some time ago. I'm almost positive there was a port done to Linux, but you wouldn't know it from the Q3A article... Tzarius 07:47, 15 March 2006 (UTC)
- Actually, an official Quake 3 port was released by id Software when the game was first released. If you don't have that, as far as I'm aware, you can use the Linux Quake 3 demo executable or the latest point release update executable with the .pk3 files from the full Windows or Mac version. There's a short HOWTO here, and Google will give you plenty more guides if you search. Ahh...I remember the old days of the Quake 3 Test release, which was first released for Mac, then for Linux, and eventually for Windows, and after the release of the Linux version, all Quake 3 forums were filled with people asking for help with their Linux installations...good times. --Aramգուտանգ 09:44, 15 March 2006 (UTC)
- Wow. Never knew about any of this. I just assumed Quake 3 used DirectX, silly me. Oh well, <cliché> you learn something new every day. </cliché> -- Daverocks (talk) 09:02, 16 March 2006 (UTC)
uranium
[edit]good uses and bad uses
- Have you looked at our article on Uranium? You might find the Applications section particularly useful. -- Daverocks (talk) 06:43, 15 March 2006 (UTC)
- Well, you need uranium to build the Manhattan Project wonder, and nuclear weapons (obviously those are bad uses). It speeds construction of some spaceship parts, too, I think (which would be a good use). And if you have more than one mine you can trade it to others, but that probably counts as bad because of what they'd do with it. .... Oh, wait, I've been playing too much Civilization IV, haven't I? --Bth 09:07, 15 March 2006 (UTC)
- There are some benefits to nuclear weapons. They ended WW2 (the Japan part), preventing perhaps millions of additional deaths, and prevented an otherwise inevitable war between the Warsaw Pact nations and NATO. Thus far, they have saved far more lives than they took, although their potential to take lives probably does put them back in the evil category. Uranium also is useful in producing nuclear power, which does far less environmental damage than most of the alternatives. Depleted uranium can also be used in armor or shells, and deciding weather these are good or bad uses depends on if you support the armies that use them, like the US military. StuRat 14:25, 15 March 2006 (UTC)
- Uranium can also be used to dye glass and pottery nice colors of green and orange. This was all it was really known to do before the discovery of radioactivity, so it was considered pretty innocuous for a long time. In the end, it is powerful stuff -- power can be used in many ways, both good and bad. --Fastfission 23:34, 15 March 2006 (UTC)
- It can even be used for "fast fission". :-) StuRat 19:56, 16 March 2006 (UTC)
C# distributed compilers
[edit]well...i asked this question before but no response from anyone.... i m putting it again...coz i need information about it immediately.... my question is.... what is the scope and future of C# distributed compiler? i will be thankful....
- I'm confused as to what you want, and in what context -- are you looking for an actual compiler, or just information about a potential one? Google doesn't seem to be turning up any actually-existing distributed C# compilers, just noodling on mailing lists and IRC logs about how they'd work and who might do it (eg [40] [41]). Hunting around the various external links from our C Sharp article doesn't produce anything when you search for the phrase "distributed compiler". You could try asking on the article's talk page where you might get more knowledgeable people (not everyone reads the Ref Desk), or better yet sign on at somewhere like CSharpFriends, and ask in their forums. --Bth 09:34, 15 March 2006 (UTC)
improving my inyelligence
[edit]please tell me how do i improve my intelligence??? thanks for replying.
- Well, for one thing it's not spelled inyelligence--152.163.100.74 21:39, 15 March 2006 (UTC)
- You could train your brain (ooh, it rhymes!) by doing e.g. IQ tests or the like. Or by cracking ciphers (my favorite way). But to be honest I don't think IQ tests are a real measure of intelligence. – Blue ҉ Iris 09:37, 15 March 2006 (UTC)
- Honestly? Just question everything and be always ready to learn and discover new things. Ask, study, learn, observe and try to understand everything, at all times, as long as you live. Seek knowledge just the same way you breath air, and always try to challenge what you know whenever you're given the chance. This is a concept I've invented a few years ago (but that's surely not a new thing) which I called "scientific spirit" (from Latin scientia = knowledge, spiritus = breath, also in the sense of attitude.) I dare to say that lack of scientific spirit is the fundamental flaw on the people of the world nowdays. ☢ Ҡi∊ff⌇↯ 09:56, 15 March 2006 (UTC)
- Yeah, he's right. keep and open mind, and always be learning, that is the way. Be skeptical of things to, don't just accept everything, ask why. As for your "IQ," you won't be able to get a better one without practicing different IQ tests. --
 Mac Davis] ⌇☢ ญƛ. 11:26, 15 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 11:26, 15 March 2006 (UTC)
- Yeah, he's right. keep and open mind, and always be learning, that is the way. Be skeptical of things to, don't just accept everything, ask why. As for your "IQ," you won't be able to get a better one without practicing different IQ tests. --
- MAke sure you get enough of each type of vitamins, eat plenty of oily fish, and get enough sleep every night. Logic puzzles and suchlike will also help you home your mental agility. Proto||type 12:30, 15 March 2006 (UTC)
- And get your daily supply of Wikipedia, that can help you recover from the brain damage caused by watching regular TV (excluding PBS, the Discovery Channel, Biography, and perhaps some good Sci Fi shows). StuRat 14:10, 15 March 2006 (UTC)
- The Guardian (UK) just had an article about this; apparently there was a BBC TV show also. --LarryMac 16:53, 15 March 2006 (UTC)
- I heard that if you're put in a different culture, get sick, or are placed under similar stressful conditions, your IQ diminishes significantly. How true is it and why? Are IQ tests really that reliable as an indicator of intelligence? Igor the Lion(Roar!) 14:45, 15 March 2006 (UTC)
- IQ is a measure of your ability to perform a certain set of mental tasks. That's much narrower than what most would call "intelligence" in general. What it indicates is entirely dependent on what kind of test you use. A magazine-variety "IQ test" along the lines of "what figure comes next?" isn't going to tell you much compared to the tests used by psychiatrists. But I think it's safe to say no simple test can assess the whole spectrum of what people call "intelligence". As for stress, it seems to me that it's quite normal that your ability to perform just about any mental task will be diminished if you're distracted. --BluePlatypus 14:55, 15 March 2006 (UTC)
- Then are they really that accurate as people think they are? Or do they merely calculate a person's intelligence at the given time and not his brain's "potential" for intelligence (or how intelligent he would be in an ideal, totally stress-free environment)? And is intelligence only relative to the culture one is in? Like I don't know anything about, say, farming, so if I were transported to a farming nation (which for convenience wouldn't value things like English literature or skill in calculus) would my "intelligence" drop significantly? Is intelligence equal to common sense? (A bit vague, but I hope the points get across) Igor the Lion(Roar!) 15:09, 15 March 2006 (UTC)
- No, they're not terribly accurate. Marilyn vos Savant, who has the highest IQ recorded hasn't done anything terribly remarkable, unless you consider writing a magazine column the pinnacle of human achivement. Intelligence isn't really relative to culture, IMO. People usually distinguish between knowledge and intelligence. But learning ability is usually considered to be an important part of intelligence, so general-knowledge questions can be used to measure intelligence. But you've got to choose the questions carefully. If you've never been exposed to information about farming, the fact that you don't know anything about it means nothing. If you live in a farming community where everyone is always talking about farming, the fact that you don't know anything about it means a lot. Common sense is an almost even vaguer term. But in the sense of "reasoning ability" (as in analyzing things, drawing conclusions, making generalizations), then yes it is. And there's a big difference between having the ability to reason in the abstract and doing it in practice; there are plenty of highly intelligent people who mostly use emotional reasoning, and plenty of average folks who are capable of being cold-headed. How you use intelligence is a personality and maturity issue. --BluePlatypus 17:10, 15 March 2006 (UTC)
- Then are they really that accurate as people think they are? Or do they merely calculate a person's intelligence at the given time and not his brain's "potential" for intelligence (or how intelligent he would be in an ideal, totally stress-free environment)? And is intelligence only relative to the culture one is in? Like I don't know anything about, say, farming, so if I were transported to a farming nation (which for convenience wouldn't value things like English literature or skill in calculus) would my "intelligence" drop significantly? Is intelligence equal to common sense? (A bit vague, but I hope the points get across) Igor the Lion(Roar!) 15:09, 15 March 2006 (UTC)
- Hey! Where would the world be now if Marilyn vos Savant hadn't correctly solved the Monty Hall problem!? --Kainaw (talk) 20:00, 15 March 2006 (UTC)
- are they really as accurate as people think they are? No they are not.
- do they merely calculate a person's intelligence Not even that.
- not his brain's "potential" for intelligence (or how intelligent he would be in an ideal, totally stress-free environment)? That's right.
- is intelligence only relative to the culture one is in? What is measured in some intelligence tests is, what is measured in other intelligence tests is not.
- Is intelligence equal to common sense? No. WAS 4.250 16:09, 15 March 2006 (UTC)
As I understand it, "intelligence" is an absolute, being the theoretical maximum "brain capacity" a person has. However, measuring intelligence is basically impossible, as any test is inherently biased towards the language and culture in which it is administered, and the person taking the test isn't likely to be at their full potential. So, while actual intelligence would not drop by changing cultures, the measure of it would. Thus, if you moved to China and were asked to interpret Confucius, you would likely test worse than if asked to interpret Shakespeare. StuRat 16:02, 15 March 2006 (UTC)
- Scientifically speaking, "intelligence" is an absolute, being the theoretical maximum "brain capacity" a person has is nonsense. WAS 4.250 16:13, 15 March 2006 (UTC)
- I hope you're not confusing what I said with "brain pan capacity", or the total cubic inches/cubic cm which the brain occupies. I am referring to the maximum number of neuron connections in a brain, which is independent of it's size. StuRat 16:18, 15 March 2006 (UTC)
- "the maximum number of neuron connections in a brain" is a nonscientific reference (ie scientific nonsense). What is that for a single cell animal? for an animal with 100 neurons? or 101 neurons? More for 101? Humans lose neurons throughout life after infancy. Stop making things up off the top of your head, please. WAS 4.250 21:26, 15 March 2006 (UTC)
- I didn't say the number of nuerons, but the number of neuron connections, which is not necessarily directly related. Stop misquoting me, please. StuRat 03:16, 16 March 2006 (UTC)
Too many people are giving replies about the exact meaning of intelligence, which wasn't the question. You can play chess (and studey the game properly) which should help. Also, always review your knowledge, especially any fundamental material you have covered. This preserves a strong sense of the basics, which helps mental adaptability. The other thing would be to take up an instrument, especially the cello or violin (these require a strong sense of pitch) since this will help your mathematical ability. The Mad Echidna 17:32, 15 March 2006 (UTC)
- We are discussing the meaning of intelligence so that we can better answer the question "How do I improve my intelligence?" In any case, I don't think a person is that intelligent if he only has all these bits of information in his brain. Where I come from, there are many "gifted" children who memorize the flags of the world or the distances from here to whatever star they fancy. There are also those who play 10 different instruments. That alone, IMO, does not constitute intelligence. What does is the ability to process information, comprehend it, and adapt to a given situation. Also, a person with expertise in one field may be more intelligent than one who dabbles in several different fields. Re comprehension, Richard Feynman once commented on the educ system in Brazil; the students memorized everything by rote, but when asked about a simple application, were unable to answer and were in fact astonished when the underlying concept was revealed. Not very intelligent, though great calculators. Igor the Lion(Roar!) 17:50, 15 March 2006 (UTC)
- I totally agree. Some people think I'm "gifted" simply because I know more about math, science, and computers than most of my classmates. However, that's knowledge, not intelligence. Even a stupid person would know a lot about math and science if they've been using a computer to read about it for 5 years. (By the way, my IQ is probably below average, somewhere around 96). --Bowlhover 05:38, 16 March 2006 (UTC)
- Whoa there! Feynman commented on the educational system of Brazil? I must read that one! He's totally right, though. People here don't learn anything, they just memorize it. It's depressing and it goes against everything I believe on. I never felt I've studied a single day in school on my entire life! I'm pretty much an autodidact in everything I know. ☢ Ҡi∊ff⌇↯ 13:21, 16 March 2006 (UTC)
- It's in Surely You're Joking, Mr. Feynman. Great book, lots of fun, too. GangofOne 21:33, 22 March 2006 (UTC)
- Whoa there! Feynman commented on the educational system of Brazil? I must read that one! He's totally right, though. People here don't learn anything, they just memorize it. It's depressing and it goes against everything I believe on. I never felt I've studied a single day in school on my entire life! I'm pretty much an autodidact in everything I know. ☢ Ҡi∊ff⌇↯ 13:21, 16 March 2006 (UTC)
- Sufficient iron intake is extremely important. [42] --Zeizmic 14:28, 16 March 2006 (UTC)
- READ HighInBC 22:03, 18 March 2006 (UTC)
Listen to NPR. Drink a lot of coffee and be passionate about stuff. Don't get hung up on little things like misssspellllling words. Think about the Big stuff. Eat beans. BDSIII 04:15, 20 March 2006 (UTC)
HIV-1 Genome Insertion
[edit]Is there any particular place HIV-1 inserts its genome or does it occur at random? Do any provirus have specific loci at which the insert their genomes? --Username132 (talk) 12:55, 15 March 2006 (UTC)
- "The integration of HIV-1 cDNA shows a significant preference for genes actively transcribed by pol II."[43] Addition data can be found at [44], [45], [46], [47]. WAS 4.250 14:23, 15 March 2006 (UTC)
Gene + Promoters/Enhancers = ?
[edit]Is there a term that can be used to refer to a gene and its respective promoter and enhancer sequences? --Username132 (talk) 16:35, 15 March 2006 (UTC)
Burning papa johns sticker
[edit]Okay, here's a wierd question. I wanted to heat up some breadsticks that I got from papa john's so I placed them in my oven at 200 degrees (F) inside the cardboard box. Remembering Fahernheit 451 I thought that the box would be okay. After a while I started to smell burning paper so I hurridly got the box out. The box was fine but the sticker on the front had turned black. The sticker was originally white with black printing. The glue on the back is still sticky and not discolored. When I touch the front it doesn't feel any different and no carbon residue comes off on my hand. The sticker has just shaded completely to black and emitted a burning smell. I will obviously take the sticker off before trying it again but does anyone have any ideas about what occured? I'm just curious.Trngl999 16:38, 15 March 2006 (UTC)
- Some inks are designed for use in thermal printers; they turn black when exposed to heat. Was it a stick-on receipt or address label? TenOfAllTrades(talk) 16:41, 15 March 2006 (UTC)
- I don't think it's the ink, but special paper designed for the thermal printers still used in many cash registers. They really need to upgrade such printers, those receipts are crap, the ink also rubs off if you use a highlighter on it, fades with time, etc. This low level of quality is not acceptable to me. StuRat 16:50, 15 March 2006 (UTC)
- I for one, want my highlighted boxes of breadsticks to last well into the next decade. --Username132 (talk) 16:55, 15 March 2006 (UTC)
- I've been on an expense acount, which requires that I submit receipts to get paid, and those blackened receipts with faded lettering didn't cut it. StuRat 17:34, 15 March 2006 (UTC)
- I agree, StuRat, receipts have ridiculously low quality these days. Sometimes I think they'd last longer if they wrote me one by hand using pencil, much less a higher quality ink. At one of the grocery stores I go to, the combination of poor quality paper and ink causes the receipt to turn yellow and the ink to fade within 2-3 days of purchase (and yes, this does matter, because like StuRat, I sometimes need these receipts to get reimbursed for certain items). EWS23 | (Leave me a message!) 18:05, 15 March 2006 (UTC)
- Thermal printers have the advantage that the paper is the only consumable used, rather than the paper plus the ink cartridge/ribbon/toner of other technologies. I've actually had the receipt stuck to a pizza box turn black just from the heat of the pizza, no oven necessary. --Bob Mellish 17:30, 15 March 2006 (UTC)
- Sounds kind of like pyrolysis to me. And I don't think those stickers are paper..some type of plastic I thought. -Snpoj 03:47, 16 March 2006 (UTC)
Open Access "E-Prints" Respositories
[edit]If an institution maintains its own Eprints repository, and someone searches for the article using ISI Wed of Knowledge or PubMed, will the database know that it's out there, available, for free? --Username132 (talk) 18:08, 15 March 2006 (UTC)
- I doubt that ISI Web of Knowledge or PubMed use spiders, so no, they probably don't know about something unless someone adds it manually. Only databases with spiders (like Google) update automatically. --Fastfission 23:36, 15 March 2006 (UTC)
nitrogen
[edit]if there is a total of one liter of nitrogen gas dissolved in the body tissues at sea level how much would be dissolved in a diver breathing air at 99 feet below sea after the tissues have been equilibrated with the higher nitrogen pressure?
- That's an excellent homework question! The solution is closely related to the pressure under 99 feet of water, why not start there? — Lomn Talk 18:59, 15 March 2006 (UTC)
I UNDERSTAND THAT 3 ATM PRESSURE COMING FROM THE WATER AND 1 ATM FROM THE AIR. BUT CANT FIGURE OUT THE REST OF THE PROBLEM. THIS IS MY DAUGHTERS PROBLEM THAT I AM ASSISTING HER WITH. SHE IS 13 I DO NOT UNDERSTAND HOW THEY EXPECT HER TO KNOW THIS??? APPRECIATE ANY INPUT
thanks i got the answer --4 liters..
- I was about to say: They're probably expecting her to reason that if "the pressure" rises by a factor of 4, the dissolved volume also rises by a factor of 4. It's a naive question to begin with, since the body actively regulates its blood content, and there's no reason to think that the gas mixture in one's oxygen tank is the same as the mixture in the atmosphere. At the 13-year old level, though, the question probably isn't asking her to think that hard. Melchoir 20:08, 15 March 2006 (UTC)
- For most diving at 99 ft I think the gas inside your tanks is going to be compressed air. afaik, only very advanced divers actually use "oxygen tanks" and when they do they're going far beyond 99 ft. So I think it's a very good assumption that the gas mixture in your tanks is the same as the atmosphere. -Snpoj
- I don't think any SCUBA divers use pure oxygen, but some use trimix, to prevent nitrogen narcosis. StuRat 03:06, 16 March 2006 (UTC)
- It doesn't necessarily take very advanced diving to start using gas mixtures other than air. All recreational dive agencies now teach the use of nitrox, which is gas mixtures between 21-40% oxygen (with the remainder nitrogen), typically 32% or 36% oxygen (which are actually also NOAA standard gases called "Nitrox I" and "Nitrox II" respectively). It also doesn't take exceptional depth to start using oxygen on decompression -- I used it yesterday to decompression from a 70 minute dive at ~100 ft (where I was using a 30% Oxygen / 30% Helium / 40% Nitrogen Trimix mixture), switched to 100% O2 at 20 feet for 15 minutes. However, in the context of the problem I'm pretty certain they were assuming air... Although if the diver was using a 32% O2 nitrox mix at 100 ft (fairly common in some places where nitrox is available) then the answer would have been 3.44L instead of 4L. - lamont@scriptkiddie.org
Courier typeface
[edit]I would like to reference the article on the courier typeface and would much appreciate the name of the author to this article.
Regards
Ed
- Like most articles on Wikipedia, that article was written by several people. I think you'll find the citation info you need here. Melchoir 21:34, 15 March 2006 (UTC)
- See also Wikipedia:Citing Wikipedia for guidelines and hints for how best to cite Wikipedia articles. TenOfAllTrades(talk) 23:18, 15 March 2006 (UTC)
March 16
[edit]Why is iodine coloured?
[edit]hello , please could you tell me why iodine is coloured? I cnt seem to find any information on it. Does it have something to do with the electron configuration? Please msg me if you can. thanx
- I'm no fan of spectroscopy, so I'll just point out [48] and [49] and let someone else explain. Melchoir 23:40, 15 March 2006 (UTC)
- All the halogens are colored because their bonds are weak compared to those of other diatomic molecules (such as oxygen and nitrogen), which makes them absorb visible light rather than ultraviolet. Iodine's bond is the weakest of them all, so it vibrates slowly and absorbs low-frequency red and yellow light, leaving a mixture of light that looks purple. —Keenan Pepper 01:15, 16 March 2006 (UTC)
- Here's another good link: [50] —Keenan Pepper 01:23, 16 March 2006 (UTC)
Learning Computer Programming
[edit]I'm currently a high school senior who has never taken any computer programming courses. When I go to college next year (as a mathematics/physics major), I'm assuming that I'll be at a considerable disadvantage because of it, so I want to learn programming before college starts next year.
I'm considering going to a community college or doing distance learning, but, if those are not possible, what are some effective self-teaching materials/methods that perhaps you personally have found useful? And is it important to learn a specific language, provided that I'll be going to college next year?JianLi 00:08, 16 March 2006 (UTC)
PS, I tried teaching myself Pascal a few years ago. I think that I got up to arrays, but became discouraged shortly thereafter.
- First of all, your college should teach you from the basics, and that's a good thing. Many people show up to college with some bad programming habits, and it's harder to teach them than the beginners. If you want to learn on your own, go through a formal system like a recent book or a well-recommended online tutorial. You should also figure out what language you want to learn. Many schools these days are starting with Java though some with less trendy faculty still like C, and some more trendy schools like other things entirely, like Smalltalk or Python. See if you can find the academic calendars of the schools you're interested in, and see what language their "Introduction to computer programming" classes use.
- I wouldn't worry about knowing a specific language -- that's what they are there to teach you. If you want to get ahead though, learn the concepts of Object-oriented programming, which is usually all they care about teaching you at first (the language is often just a means to learn the concepts). -Quasipalm 00:36, 16 March 2006 (UTC)
- I would have to disagree with that, if you haven't programmed much he'll only be confused by it. I tried explaining polymorphism to a guy that had only programmed a while, and his head literally exploded (I really mean literally, I was cleaning gunk out of my eyes). I suggesst getting the very basics down first, so you know them well. Stuff like:
- If-then-else statements
- Functions and subroutines
- Loops (all the different kinds)
- Arrays
- Basic typing (structs for instance).
- These are the basic things that you should know through and through. Since you say that you are good at maths, some very basic algorithms shouldn't be to difficult to learn. I think that if you are smart and have aptitude for maths, you will probably learn faster this way than going through meaningless excercises. For instance, you could try one of the most basic problems first: Given an array, program a subroutine that sorts it into ascending order. Don't worry at first if it's fast or anything, just try to make something that works (if you wanna cheat, wikipedia has all sorts of different sorting algorithms in it). You could also look up simple algorithms and try to understand them. If you understand the Tower of Hanoi recursive algorithm, you've come a long way =)
- As for languages, I'd recommend QBasic. Basic gets a lot of hate from people, but it's a magnificent language to learn programming in. It's simple, intuative, and still a pretty decent language. Hope this helps at all. Oskar 01:01, 16 March 2006 (UTC)
- I would have to disagree with that, if you haven't programmed much he'll only be confused by it. I tried explaining polymorphism to a guy that had only programmed a while, and his head literally exploded (I really mean literally, I was cleaning gunk out of my eyes). I suggesst getting the very basics down first, so you know them well. Stuff like:
- I agree, learn BASIC first. But then again, I'm a FORTRAN programmer, which is similar to BASIC, so I may be biased. StuRat 03:00, 16 March 2006 (UTC)
- If you're going into science, do not take a course from a computer science department, and don't learn any of the fancy masturbatory languages. Linked lists and sorting algorithms are great, but they're not your job. In physics research, you'll want to know C; failing that, maybe Fortran. In your classes, you probably won't need to program at all, but it's still a good skill. Once you've learned the basics outlined by Oskar, make friends with Numerical Recipes in C. Melchoir 03:36, 16 March 2006 (UTC)
- eh, I wouldn't worry about being at a disadvantage. The physics/math classes you'll be taking freshman year almost definitely will not require you to do any programming. Just take a basic computer science course first semester (one that's meant for science/math people) and they'll teach you everything you need to know. I've found that many researchers/professors heavily use VisualBasic with Excel because of it's simplicity, availability and versatility. -Snpoj 03:41, 16 March 2006 (UTC)
- Visual Basic? Seriously? Huh. In my world it's all Matlab and C. And the occasional forward-looking prof likes Java, and the oldschool ones like Fortran.
colors of planets
[edit]where did the colors of planets came from/originated?
- The planets have colors for the same reason most other objects have colors: they are made of colored materials. For example, Mars is red because of all the iron(III) oxide on its surface. —Keenan Pepper 00:51, 16 March 2006 (UTC)
- Planets, gods, metals, colors, days ... were associated long time ago, half symbolically, half for other reasons such as plain appearance. --DLL 17:28, 17 March 2006 (UTC)
Odor of some black people
[edit]This is not a racist comment. It is a simple observation.
Some (not many) black people have a peculiar smell. The smell is only noticeable within (say) a meter, or less, of them. I don't think I have ever met any white people who smell like this.
What is the source of this smell?—Preceding unsigned comment added by 172.146.236.219 (talk • contribs)
- Perhaps you are noticing the lingering odour of food that is unusual to you. People often tend to eat foods traditional to their roots, and people from different backgrounds may notice different odours because they seem foreign to them. For example, I've been told that Japanese people can notice the faint smell of potatoes on westerners.
Slumgum 02:07, 16 March 2006 (UTC)
I'm partial to lots of garlic and onions! Perhaps there is some odour there.. --Zeizmic 02:28, 16 March 2006 (UTC)
- Or it's entirely possible that they have a gene for a kind of sweat gland that white people don't have. —Keenan Pepper 02:36, 16 March 2006 (UTC)
- I don't know how to describe the smell, except that it is (to me, at least) unpleasant. One black stripper who was dancing for me seemed to have this smell rather strongly. And it was NOT her perfume! I don't think it was food. I really think I was smelling HER. What is it, some strange kind of pheromone or something?
I believe one culprit is "hair relaxer", which makes curly hair straight. It's a rather nasty chemical and smells like bug spray. Whites who have curly hair don't typically try to make it straight using such products, so don't give off that odor. StuRat 02:53, 16 March 2006 (UTC)
StuRat's right. It's probably the hair care products and other grooming products that are marketed to and consumed by black people exclusively, and many of them say right on the box or bottle that they contain cocoa butter. Cocoa butter lotion, which is heavily marketed towards black people, has a distinctive smell on skin. Maybe you could get some and put it on yourself, and see how you smell. Brian G. Crawford 13:58, 16 March 2006 (UTC)
Additionally, different people sometimes just smell different, and there are properties of this (smell of perperation, for example) which could easily be correlated amongst populations in a way colloquially identified as "race". But just remember this: it's not that they smell strange -- to them, you probably smell pretty strange too, but you're just used to your own smell and as such regard it as the baseline. --Fastfission 16:42, 16 March 2006 (UTC)
- If the people have dreadlocks perhaps you are smelling patchouly. This essentail oil is believed by many to drive insects out of hair. This is not something specific to black people. HighInBC 22:10, 18 March 2006 (UTC)
- You don't see many non-blacks wearing dreads, not even Judge Dredd. :-) StuRat 14:09, 21 March 2006 (UTC)
tracking a path of a helium-filled balloon
[edit]What information do scientists gather bt tracking the path of a helium-filled balloon?
- If you are talking about a weather balloon, you can track the balloon itself for wind data, and it also carries instruments to measure atmospheric pressure, temperature, and humidity. In short, they help scientists predict the weather. Oskar 02:18, 16 March 2006 (UTC)
- Could a balloon constructed by say, Russia not blow over to the UK? --Username132 (talk) 08:40, 16 March 2006 (UTC)
- Yes, of course. --HappyCamper 13:45, 16 March 2006 (UTC)
- Do we end up with concentrations of balloons in particular areas? How many balloons are there? And how much damage is caused by balloons bursting and dropping their equipment? --Username132 (talk) 18:02, 16 March 2006 (UTC)
Venus Rotation
[edit]Given the speed at which planet Venus rotates, is it probable that it's core has slowed considerably? This would explain the planet's negligible magnetosphere, correct? What would be some logical ways of returning the planet's rotation to a prograde standard (if ever it was a prograde orbit)? Here7ic 01:57, 16 March 2006 (UTC)
- I'm not sure what you mean by it's "core has slowed considerably". I believe terrestrial planets, like Venus, have their core, crust, and atmosphere all rotate at the same rate. As for us significantly changing the rotation rate of a planet, forget that. That would require far more energy than all of humanity has ever used. StuRat 02:48, 16 March 2006 (UTC)
- I guess I could've meant the planetary rotation, or the outer core (if Venus is that similar to Earth). As for accelerating the rotation, if this should become a question of the future, I suppose Dark Matter would have a place in the topic. Would you agree? Here7ic 03:57, 16 March 2006 (UTC)
- No, unless you include science fiction writers. Why would anyone use dark matter to change Venus's rotation, and how would it be used? --Bowlhover 06:09, 16 March 2006 (UTC)
- Are you suggesting that humans can not and never will attain the ability to harness any forms of energy which results from experimentation with Dark Matter? Namely Hot dark matter? Truth be told we haven't even beached onto any real knowledge of it's potential. We didn't know the potential of a Hydrogen atom until we split it. It is, in all probability, plausable that some day, albeit in our distant future, we, as humans, will harness great quantities of usable energy from such experimentation. However, as a logical person, I must volunteer that any real discovery of the potential of dark matter will most likely come at a point in time far beyond our colonization on Venus. The future is rather like an ocean; the further you look into it, the darker and stanger things get. Here7ic 17:34, 16 March 2006 (UTC)
- No, I'm not suggesting that "humans can not and never will attain the ability to harness any forms of energy which results from experimentation with Dark Matter". I just don't see how dark matter can be used to change Venus's rotation. --Bowlhover 22:22, 17 March 2006 (UTC)
- Venus is totally unsuitable for human colonization. It rains sulfuric acid (battery acid), for one thing. We are lucky to get a lander to last a few minutes before it is destroyed. Mars, the Moon, and space colonies would all come before we would even consider colonizing Venus. If you want a way to change it's rotation, how about a linear accelerator around the equator and extending into space which could launch iron mined from the planet at the speed of light into space ? A huge fusion reactor could power it. It would still take years, perhaps millennia, to significantly change the Venutian day. StuRat 19:45, 16 March 2006 (UTC)
- Are you suggesting that humans can not and never will attain the ability to harness any forms of energy which results from experimentation with Dark Matter? Namely Hot dark matter? Truth be told we haven't even beached onto any real knowledge of it's potential. We didn't know the potential of a Hydrogen atom until we split it. It is, in all probability, plausable that some day, albeit in our distant future, we, as humans, will harness great quantities of usable energy from such experimentation. However, as a logical person, I must volunteer that any real discovery of the potential of dark matter will most likely come at a point in time far beyond our colonization on Venus. The future is rather like an ocean; the further you look into it, the darker and stanger things get. Here7ic 17:34, 16 March 2006 (UTC)
- No, unless you include science fiction writers. Why would anyone use dark matter to change Venus's rotation, and how would it be used? --Bowlhover 06:09, 16 March 2006 (UTC)
- I would like to correct a statement in your last post. Venus is currently totally unsuitable for human colonization. It is entirly possible for human intervention to alter or reconstruct the Venutian atmosphere. Perhaps in this era we are still only in our technological infancy, but to terraform a planet is still a good-looking prospect for our future development. As you may have gathered from my recent flare in activity, I have a passion for thinking about the future, a characteristic for which I hope you will hold me to no less a standard. Here7ic 20:29, 16 March 2006 (UTC)
- The point is that it is far less suitable to terraforming than Mars, the Moon, or even space. Less protection would be needed for a colony in space than one on Venus, as a vacuum is less corrosive than sulfuric acid rain. StuRat 04:57, 19 March 2006 (UTC)
bittorrent beta releases
[edit]Where can I find a beta release of MS Office 12 BETA 1 refresh (not through microsoft, of course) for free (no reg, preferably bittorrent)? --hello, i'm a member | talk to me! 02:01, 16 March 2006 (UTC)
- I'm not sure I should be publicly aiding this unlawful pursuit, but let's just say that The Pirate Bay can be very useful. Oskar 02:09, 16 March 2006 (UTC)
- Arr!! The Pirate Bay is my favorite, because it is most user friendly. You can also get Scientific American and Penthouse searching for Ebooks under browse. :) A bigger site, not that you would use it, because its evil is [[51]]. --
 Mac Davis] ⌇☢ ญƛ. 07:31, 16 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 07:31, 16 March 2006 (UTC)
flu pandemic
[edit]Is it possible that the flu pandemic that eveyone's worried about may never happen? KeeganB
- Sure, look on the bright side. A gamma ray burster might wipe us all out first. --Trovatore 06:29, 16 March 2006 (UTC)
- Yes, its very possible it may never happen. --
 Mac Davis] ⌇☢ ญƛ. 07:32, 16 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 07:32, 16 March 2006 (UTC)
- Sooner or later, there will be another flu pandemic just by the law of large numbers. It might be soon, arising from H5N1, or it might be in 50 years time arising from some completely other source. But barring biosphere-destroying cosmic accidents there's going to be one sooner or later. --Bth 08:19, 16 March 2006 (UTC)
- The world freaks out about "pandemics" too much for one to do considerable damage, in my humble opinion. Even someone as young as I has lived through periods when people lived in fear of Mad Cow Disease, West Nile virus, Anthrax, and SARS (and now bird flu). Also think of the ever-present "threat" of a meteor impact, nuclear war, and supposed "global warming". Do you not think that our world leaders, as well as nature itself, are doing whatever they can to prevent the destruction of mankind? Basically, don't worry about it. :P —OneofThem 18:40, 16 March 2006 (UTC)
- Actually, world leaders are quite pathetic at protecting their citizens from infrequent, but predictable disaster, such as the tsunami in the Indian ocean, hurricane Katrina, and the 1918 Spanish flu pandemic that killed millions. You might think we are better prepared today, but we are not. Politicians tend to only look at the chance a disaster will happen while they are in office. If that chance is small, they don't worry about it. Some factors, like airplane travel, and intentional spreading of disease by terrorists, could cause even more than the 50-100 million deaths due to the 1918 outbreak. StuRat 19:30, 16 March 2006 (UTC)
- Two of those are unpredictable natural disasters, you know. :P —OneofThem 19:42, 16 March 2006 (UTC)
- Natural disasters aren't all that unpredictable, in the long run. Hurricanes hit the US every year. At the very least, a hurricane would hit New Orleans every 300 years, on average, which was powerful enough to breach the levees. However, global warming probably made this a much more likely occurrence. Large tsunamis also happen with regularity, although infrequently enough for politicians to ignore them, apparently. StuRat 05:09, 19 March 2006 (UTC)
Yes it is possible because within the next hour a huge astroid will smashed into the earth and kill all living things except for cockroaches. Ohanian 11:49, 16 March 2006 (UTC)
- There's also the possibility of a huge solar pulse. I read somewhere about a hypothesis that one big enough to fry all the electronics on one side of the globe would occur every 300 years on average. --Halcatalyst 03:08, 17 March 2006 (UTC)
RE:Copying content of paper
[edit]Me, A student want to know that how we can trace a paper content to another one by pressing a blank paper by putting top of the source paper. For example: If I want a image of a magazine or currency to another blank paper, What chemical should I use in between source paper and blank paper before pressing these two?--Afsalh 08:24, 16 March 2006 (UTC)
- Sometimes it's not possible or necessary to have access to all mod cons. As to the original question, it really depends on your source image. Just saying it's "on paper" is not sufficient - is it painted, drawn with pencil or ink, part of a magazine, an inkjet print, a laser print, a photocopy ...? Actually, in the last two cases, you can do an image transfer using acetone or xylene. Googling for "image transfer acetone" got me several pages, including this one. No heat is required for this method, so there might still be something else. More clarification on the question would be helpful. --LarryMac 14:23, 16 March 2006 (UTC)thanks
- Did you post the very similar question earlier? If so, you never answered my question: was the old man selling this liquid? NO sir he is not selling this liquid but he sell a candle like meterial(light red colour)Notinasnaid 15:07, 16 March 2006 (UTC)thanks
- Last year of my high school abortion i did an experiment on the subject and came up with the following-
1) find the image you wish to reproduce 2) print it out using a laser printer (not needed if using magazine) 3) In a tray of sort place the image facing up 3) get some GooGone and pour a sufficient amount in the tray 4) place the blank page a top of the image 5) using a metal spoon begin rubbing over the blank page with enough force to transfer the image When all is said and done when you lift your blank page and it will have the mirror image imprinted on the previously blank page. Remeber that the the image will only transfer where you rubbed the spoon on the paper.
pervasive computing
[edit]what is the basic idea behind goal oriented programming paradigm and how it is linked with pervasive computing?
plz help me to answer this question,ill be grateful to u
see Ubiquitous computingSuraj vas 09:53, 16 March 2006 (UTC)
Also see programming paradigm Suraj vas 09:56, 16 March 2006 (UTC)
mentos and coke
[edit]why when we put the mentos inside the coke ,explosion happen? why this can be happen?
- One of the main ingredients of Mentos is gum arabic, which is extremely good at lowering the surface tension of water. When you drop a Mentos inside carbonated water (water with CO2), this reaction releases this gas from water in large amounts, causing it to release bubbles. Since the process is fast and the released gas occupies more volume, the pressure inside the bottle raises quickly, pushing the liquid upwards. ☢ Ҡi∊ff⌇↯ 13:10, 16 March 2006 (UTC)
- So, it isn't an explosion - just a gush of water (like when you drop your root beer bottle and then open it). I was hoping for an explosion big enough to make the TSA start confiscating mentos and cokes from people as they board the airplane. --Kainaw (talk) 13:54, 16 March 2006 (UTC)
- No, of course not, otherwise they'd be arresting flight stewards and stewardesses for "possession of dangerous items". Applies for everybody else who has mentos and a coke. Now, the big question is exactly how do we make a softdrink that really explodes when you drop a mentos in it? ;)
- The easiest way would be to just carry a bottle of nitroglycerine; if you throw the mentos into the bottle with sufficient force, a fun explosive is bound to happen. On the other hand, it might not be the most refreshing softdrink imaginable... -- Ferkelparade π 14:43, 16 March 2006 (UTC)
- Though it is used to drastically lower blood pressure. My mother carries a bottle around for her anginas. We haven't been arrested. Yet.
- Well, if the Mentos were on fire, then whiskey, which is drinkable (but not a soft drink) would probably at the very least go POP! smurrayinchester(User), (Talk) 17:33, 16 March 2006 (UTC)
- Or O'Pop, if it's Irish whiskey. JackofOz 19:28, 16 March 2006 (UTC)
- --groan-- for JackofOz. :-P Now I want to go out and buy some Mentos and Coke just to try this... Dismas|(talk) 22:35, 16 March 2006 (UTC)
- If you eat Mentos and drink Coke simultaneously, will your mouth explode? :)
- I doubt it, but you'd probably get an interesting facial expression. As for the original question, it sounds like someone saw the first half of an episode of Clever and missed the ending. The result was a pretty nice fountain of soft drink. Confusing Manifestation 23:20, 16 March 2006 (UTC)
- If you put a carbonated beverage in a paint shaking machine you can get a nice 20 ft fountain out of it. StuRat 02:04, 17 March 2006 (UTC)
Website listing and reviewing main competitors in Internet fields?
[edit]I am looking for a website which lists and gives information/reviews on the main competitors in various computer/internet fields.
For example, if the field is webmail, the website would list AOL Mail, Gmail, Yahoo! Mail and Hotmail. It would then give information regarding the features and storage, and give reviews, or allow users to post reviews.
If the field is operating systems, the website would list Windows, Mac OS and Linux (and tell me the main Linux distributions), and then procceed to provide information regarding the features and interface. Again, it will review each OS and allow users to give feedback.
If the field is web browsers, the website would list Internet Explorer, Mozilla Firefox, Opera, Netscape, Mozilla, Safari and some others I might miss. As I said, it will compare the security and features of each browser and offer reviews, and allow users to review them.
Such a website would be help Internet users make the right choices regarding the provider of a product. If someone wanted webmail, by reading the reviews, he would probably pick Gmail. If he was dissatisfied with Internet Explorer, he would go to the website to look for alternatives and probably pick Firefox. (These are guesses on my part.)
Such a website would also help web developers in designing compatitible products and services. For example, owners of social networking sites which allow you to import your webmail address book and send invites to friends would, after reading the webmail reviews on the site, offer options for importing contacts from AOL Mail, Gmail, Yahoo! Mail and Hotmail (Surprisingly, Friendster only allows imports from Yahoo! and Hotmail). Web designers must know the top browsers to optimize their websites for (besides Internet Explorer).
Does such a website exist? If so, may I have the URL of the website, and a link to a Wikipedia article about it, if such an article exists? --J.L.W.S. The Special One 15:08, 16 March 2006 (UTC)
- Why not try Wikipedia itself?
- These articles are really helpful! :D —OneofThem 18:56, 16 March 2006 (UTC)
Website listing number of accountss on various other websites?
[edit]Is there a website tracking the number of accounts on various websites? For example, if I go to the website and enter http://neopets.com in their search bar, they will tell me that NeoPets has 115 million accounts? If not, how many accounts do RuneScape and Blogger.com have? —This unsigned comment was added by Hildanknight (talk • contribs) 16:08, March 16, 2006 (UTC).
- Not that I know of. I'm not sure how it would be possible to get this kind of information unless you own the website and keep records for yourself. -- Daverocks (talk) 06:22, 17 March 2006 (UTC)
- Also, it can be difficult to define "accounts" in a meaningful way - for instance, do you count multiple accounts belonging to the same person as one or several? What about deleted accounts? Inactive accounts? The 115 million number for NeoPets in particular smells like exaggeration to me. — QuantumEleven | (talk) 10:22, 17 March 2006 (UTC)
- The Neopets article lists (or used to list) a reference that claims to know how many inactive/frozen/etc. accounts there are, but I have no clue how that website got the information. It might be okay for something that's not very important, but I wouldn't trust it if I would you. The count on Neopets is just the raw count, I think - how many have been created, including multis and inactive. (115 million is not suprising considering that Neopets is now on a very international scale, though a significant number of those are probably multis or automated.) --AySz88^-^ 02:31, 18 March 2006 (UTC)
slow scan TVs and Copthorne McDonald
[edit]Hello
Does anyone have any idea how I can find an old article (from the 50s) for my 83 year old blind dad, by Copthorne McDonald regarding slow scan TVs that appeared in Stuart Brand's Whole Earth Catalog?
Thanks for any insight.
Sincerely,
Carol Adams
- If he's really blind, you will need to read it to him or find it on audio, but I doubt if an article from the 50's would be in Books for the blind. StuRat 16:55, 16 March 2006 (UTC)
- Hi Carol; I don't quite understand your request - the Whole Earth Catalog was only published regularly from 1968 through 1972. If you click through to our article on the WEC, there is more info on subsequent editions after that. I found a history page that mentions articles written by McDonald in 1958 and 1961 in something called QST, but I haven't yet discovered what QST is (or was). I suppose it is possible that the WEC reprinted one or more of those articles; I'll see what else I can find. --LarryMac 20:57, 16 March 2006 (UTC)
- QST is the publication of American Radio Relay League. ARRL http://arrl.org . Search there for SSTV, etc. (The Whole Earth Catalog had issues in 1980 and 1995? "Whole Millenium Catalog", FYI.) GangofOne 23:02, 16 March 2006 (UTC)
flash bulbs
[edit]What process did the manufacturers use when they inserted the shredded thin metal filament inside the bulbs so it expanded and evenly filled the space?
- I think it does that automatically when you try to insert a stiff wire into a small volume. StuRat 19:22, 16 March 2006 (UTC)
enlarged breast in a pit bull dog
[edit]I own a pit bull she has had her first heat and shortly afterwards began getting large breast, like having milk in them but there is none. She has not been breed. Please help thank you vicki
- A couple possibilities come to mind:
- 1) She got away from you and got knocked up. It only takes a minute.
- 2) She has a medical problem, like an enlarged ovary pumping out too many female hormones.
- In either case, I suggest seeing a vet. StuRat 01:59, 17 March 2006 (UTC)
- ...and bring your dog to the vet, too, while you're at it. StuRat 21:44, 20 March 2006 (UTC)
Magnesium
[edit]Does magnesium have a shortened name like a nick name of some sort. I've looked every where and can't figure out if there's one or not.
- Mag? Magne? I dont think so! --Chachu207 ::: Talk to me 18:05, 16 March 2006 (UTC)
- Actually, I have heard "mag" used, as in mag wheels. StuRat 18:54, 16 March 2006 (UTC)
- In the hospital, it's quite common to hear magnesium citrate as "mag citrate", magnesium oxide as "mag oxide", and so on. — Knowledge Seeker দ 19:15, 16 March 2006 (UTC)
- "Mg" would work, I should think. Here7ic 20:13, 16 March 2006 (UTC)
- Except for the obvious confusion with milligrams. StuRat 21:14, 16 March 2006 (UTC)
- Oh, come on. First, milligrams are "mg" while magnesium is "Mg". (Megagrams are "Mg", but nobody calls them that; we all say tonnes or metric tons.) Second, in what context could they be confused anyway? --Anon, 00:08 UTC, March 17, 2006.
- Using capitalization to distinguish between completely different things only makes sense to C programmers (who also start counting at 0, not 1). In a list of vitamins and minerals you might very well see both. StuRat 01:55, 17 March 2006 (UTC)
- Meh, it happens all the time in science. We only have twenty-six letters to work with in the alphabet, we're lazy (efficient!) enough to want abbreviations for commonly-used terms, and we tend to impose a further requirement that the abbreviations should bear some relationship to the term that the represent.
- One of the many hats I wear from time to time is 'chemist', and in that capacity I would have no qualms about writing (or understanding) something like 'add 10 mg Mg to the reaction...'. When I'm wearing my 'biologist' hat, I'm not worried about confusing km with KM. If I'm pretending to be a physicist, I have to know that I can't just cancel out the ds in dy/dx.
- We have to accept that case and context are important in scientific shorthand. The price of brevity is a reduced redundancy and a need for greater care in writing. TenOfAllTrades(talk) 04:58, 17 March 2006 (UTC)
- The problem would be that a slight error could make it unreadable,
- Um, did you read what TenOfAllTrades wrote? He conceded that point. One of the prices of brevity is the possibility of error, unless you're careful. —Steve Summit (talk) 15:19, 18 March 2006 (UTC)
- ...like:
- The problem would be that a slight error could make it unreadable,
LIST OF REACTANTS = 10 mg Na, 5 mg, 15 mg Cl
- This could mean 5 milligrams of an unlisted reactant or 5 units (presumably milligrams) of magnesium. Having more difference between abbrevs would make it clearer what portion is missing from the instructions. StuRat 15:54, 17 March 2006 (UTC)
- Yes, but notice that capitalization is the key. It would solve your problem very easily if capitalization was involved. I would automatically interperit this as 5 milligrams of an unnamed substance; this is further supported by the fact that you've capitalized Potassium and Chlorine in the list, and didn't capitalize milligrams. Here7ic 17:15, 17 March 2006 (UTC)
It's Mg on the Periodic table. -LambaJan 04:28, 17 March 2006 (UTC)
- Yes it is, but I'm not sure if that should be used as a standard for non-scientists or we will end up using Au = gold, Ag = silver, Hg = mercury, W = tungsten, Fe =iron, K = potassium, Na = sodium, etc. (did I get those all right ?) Also note that "Mg" is only a written abbreviation. You would presumably still say the whole word. "Mag", on the other hand, is both a written and oral abbreviation, like gym or lab. StuRat 15:34, 17 March 2006 (UTC)
youngest fossils
[edit]We all know that fossils can be very old. But how young can they be? Is the age of any fossils measured in mere thousands of years? Generally what in the making of fossils requires time? Thanks, --Halcatalyst 18:39, 16 March 2006 (UTC)
- Are you talking about the type of fossil where bone is replaced by rock ? Other types, like an insect caught in amber, can form in just a few days. StuRat 18:52, 16 March 2006 (UTC)
- Yes, the type where minerals are infused into bone. --Halcatalyst 19:27, 16 March 2006 (UTC)
- I recall reading where bones in the vicinity of a mineral springs were mineralized in a few hundred years.
- The Fossil article reminds me that other body parts than bone can be fossilized. It also says, "Because fossils are by their nature old, the word has also entered the modern vernacular as a derogatory term for an elderly person." That would be me, ha. The article refers to fossiliferous rock and has a link to Paleontology. However, neither article suggests an age when fossils might be "new." --Halcatalyst 21:16, 16 March 2006 (UTC)
- I searched all over for this. There are various 'grades' of fossilization. Things are about to be fossilized go through a pre-mineralization stage, which may be only hundreds of years. Full fossilization may take 10,000 years or more. BTW, you can't search far before running into tons of 'biblical literalists' who insist that dinosaur fossils are the standard 4000 years old. --Zeizmic 21:42, 16 March 2006 (UTC)
- LOL, that would explain why the Bible is so full of stories about shepherds protecting their sheep from T-Rex attacks. StuRat 01:51, 17 March 2006 (UTC)
- I think it really depends on the chemical environment. Presumably the decomposition of the organic material would occur much faster than the mineralization, so it's basically just a question of how fast can you form the rock. So the mineral-springs idea above seems quite plausible. --BluePlatypus 01:17, 17 March 2006 (UTC)
Thanks, all. I'm querying "thousands of years" in a 5th grade science book I'm editing and was going to suggest "millions of years." Guess I'll just suggest they be vague about it. Very old fossils are what we're talking about. Obviously it's not a huge issue at that level, but we do want to be accurate. --Halcatalyst 02:22, 17 March 2006 (UTC)
- The range varies considerably, as the others mentioned. Generally a geologist can place a good guess without any carbon dating based on where the fossil is found. Conversely, a fossil of a known species can date a layer of rock to a particular period. -LambaJan 04:25, 17 March 2006 (UTC)
- A quick Google for "youngest fossils" turns up a rough minimum of ten thousand years for fossilization. An article from the journal Science also comes up in the first page or two of hits on that search, giving this article (PDF, subscription required) which mentions in passing fossils that are fifty thousand years old. (I count Science as a highly credible source, so fossils can definitely be as young as 50,000 years.) So certainly fossilization can happen in 'thousands' of years. TenOfAllTrades(talk) 04:40, 17 March 2006 (UTC)
robins playing baseball
[edit]Today, at the start of a gentle snowstorm, I noticed about 50-60 (American) robins congregated on a baseball field. They were mostly just standing around rather than hopping about feeding. I've never seen this many robins in a single small area at once. To me, they've never seemed particularly social birds. I wonder how this behavior could be explained? They must have been migrating. Do robins migrate in flocks of this size? --Halcatalyst 23:13, 16 March 2006 (UTC)
- Apparently they do: [57] [58]. I wonder if those are reliable enough to use in the American Robin article, which says nothing about flocking... Melchoir 23:41, 16 March 2006 (UTC)
I've heard of Cardinals playing baseball, or at least trying to. Never heard of robins trying it, though. StuRat 15:18, 17 March 2006 (UTC)
- Tony Robins could probably be a pretty good catcher, if he put his mind to it... Superm401 - Talk 22:11, 17 March 2006 (UTC)
March 17
[edit]The accumulator principle as it pertains to hydraulics .
[edit]Could someone please explain the accumulator principle as it pertains to hydraulics ?--70.251.211.166 01:50, 17 March 2006 (UTC)
Wow that was quick , thank you very much .69.153.194.86 15:26, 19 March 2006 (UTC)
natural warming trends vs global warming?
[edit]isn';t it silly to presume that there is any such thing when it's a well known fact that every few hundred tofew thousand years the earth goes through a perfectly natural trend of warming? If we were to stop this natural cycle on the grounds that it was some sort of "human influence" on the earth, wouldn;t we b dooming the earth to a new ice age?--How cme tkn4 03:19, 17 March 2006 (UTC)
- You might try reading our article on global warming; it will, at the very least, give you a better understanding of the terms of the debate and what the current scientific evidence is considered to be. --Fastfission 03:39, 17 March 2006 (UTC)
The temperature of the Earth is determined by many things that operate at different ranges of time. Day and night, summer and winter, Maunder minimums (maybe), Milankovitch cycles. And there are also indirect effects like the effect of the temperature on the amount of cloud cover. All these things (and more) combine to produce the resultant temperature. The idea is that human-caused global warming is an additional effect that will combine with the others as well. If we could turn it on and off then it might be useful if an ice age was expected anytime soon, but we can't: so we have to worry that a "heat age" is what's coming and will be intensified. For that matter, it seems as though global warmning operates on a faster timescale than the things that cause ice ages anyway, giving people less time to react to it. --Anonymous, 04:25 UTC, March 17. 2006.
- isn';t it silly to presume that there is any such thing when it's a well known fact that every few hundred tofew thousand years the earth goes through a perfectly natural trend of warming?
- It is not silly, because it is a well known fact that the Earth constantly goes through trends of warming and cooling. [59] [60] [61] [62] Notice the distinct trends and/or wildness in the graphs, especially 34. —This unsigned comment was added by Mac Davis (talk • contribs) 08:23, March 17, 2006 (UTC).
Let me use an analogy. Many natural things cause lung cancer, such as radon. Does this mean we should ignore the fact that the most serious factor causing lung cancer is smoking ? Perhaps we should put cig vending machines in elementary schools because "other things cause lung cancer, too". StuRat 15:10, 17 March 2006 (UTC)
- The radon analogy works on a number of levels, too. For example, exposure to elevated levels of radon only increases the risk of getting lung cancer from a non-smoker by a tiny percentage -- almost statistically invisible. However, if a smoker is exposed to elevated levels of radon the chance of getting lung cancer becomes a near certainty. The smoking takes something which would be naturally not a big problem (in comparison with other risks) and turns it into a big problem. --Fastfission 16:29, 17 March 2006 (UTC)
The Hockey Stick graph is pretty convincing evidence that something out of the ordinary is going on. Gandalf61 16:21, 17 March 2006 (UTC)
- Where does that name refer to? The article doesn't say. Superm401 - Talk 22:16, 17 March 2006 (UTC)
- I think the graph looks like a hockey stick with the handle on the ice:
*
*
*
**********************
- The Hockey Stick is highly controversial and questionable.
Help me, I'm an adict
[edit]To wikipedia, help me quick my wikiaddiction!--64.12.116.74 03:44, 17 March 2006 (UTC)
- Unfortunately you are using AOL, which means that simply blocking you wouldn't work very well. ;-) If you are interested in technical (rather than behavioral) means, one way is to block which websites you allow yourself to access by means of editing your HOSTS file to specifically block yourself from accessing Wikipedia, or by using content filters of one type or another. --Fastfission 03:48, 17 March 2006 (UTC)
- I bet your case is not that bad. For some bad cases see Wikipedia:Are_You_a_Wikipediholic_Test. Dang, I'm down to 17 place now! --
 Mac Davis] ⌇☢ ญƛ. 07:14, 17 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 07:14, 17 March 2006 (UTC)
calculating the goastrophic wind speed
[edit]Calculate the geostrophic wind speed (ms^-1) when tha distance between the 1000 and 1002 hPa isobars at 28° S is 1.2cm on a map with a scale 1:20*10^6. Assume ρ = 1.2 kgm^-3 and Ω = 7029*10^-5 rads^-1.
- Assuming a uniform stagnation temperature distribution, do a path integral around the Earth (making sure to take compressibility into account), then hop twice on your left leg while making clucking noises. Offer a small aubergine as sacrifice to the deity of the Bernoulli equation. Repaint the walls of your room bright orange. Then sit down at your desk like a good student and do your own homework. — QuantumEleven | (talk) 08:33, 17 March 2006 (UTC)
- One wonders how people who think "goastrophic" is a word get signed up to these sorts of courses. But I digress. Sum0 21:30, 19 March 2006 (UTC)
- It is geostrophic wind. Look at geostrophic wind article in wikipedia and you will be able to solve this problem without difficulty. Pflatau 17:23, 24 March 2006 (UTC)
Joules per mole to Kelvin?
[edit]Is it possible to convert kilojoules per mole to Kelvin, and if so, what is the conversion? If not, which unit is missing and how could it be used to complete the conversion if it were known? --James S. 09:41, 17 March 2006 (UTC)
In other words, is one an energy and the other a power, or are they compatible, and if so, what is the conversion formula? I only need a couple or three significant digits. Thanks. --James S. 09:58, 17 March 2006 (UTC)
- The question is somewhat garbled, maybe my answer will match it. KJ/mole is energy/mole. Power is energy/time. Kelvin is an intensive varible (temperature) , it is not power. However, for a given substance, if you add a certain amount of heat, you will get a certain temperature rise. This is expressed in the concept heat capacity, the change in internal energy per degree of temperature. THe heat capacity is different for each different substance, it is a property of the substance. To be more specific, there is heat capacity at constant volume,Cv, and heat capacity at constant pressure, Cp.
by definition, Cv = dQv/dT = (partial dU/ dT )v
so dU = Cv dT (infinitesimal changes). THe kicker is Cv also changes with the T.
so , integrating, delta U = Integral Cv dT |between T1 and T2
(I am pulling this out of a physical chem text.) So, it depends on the particular substance or system you're interested in. WHich is? Going back to your firest sentence, FOR a SPECIFIC SUBSTANCE, if you add a certain of heat energy, you will get a certain temperature change. Trying reasking the question for another iteration. GangofOne 10:21, 17 March 2006 (UTC)
- You can't convert one into the other - mol and K are both base units, and J is a derived unit equal to (due to the fact that work done = force x distance = mass x acceleration x distance). kg m and s are all base units, so the three units K, J and mol are all completely unrelated to each other. See SI units. — SteveRwanda 10:04, 17 March 2006 (UTC)
- Also see specific heat capacity, which is what you need to convert an added amount of energy per amount of substance to a change in temperature, and is different for different materials. --Bth 10:09, 17 March 2006 (UTC)
- Mol isn't a unit. It's a quantity. --BluePlatypus 16:23, 17 March 2006 (UTC)
- Yes, because it simply refers to a certain number of atoms or molecules. You know that and I know that, but the people who control the SI have decided that it is a unit of something called "quantity of substance", so we pretty much have to go along. Incidentally, responding to the original poster on another point, proper SI usage is "kelvins", not "(degrees) Kelvin". --Anonymous, 00:53 UTC, March 18, 2006.
- Crazy. I didn't know that. What next? An SI unit for a 'dozen'? :) --BluePlatypus 15:02, 18 March 2006 (UTC)
- "Proper" usage is "kelvins"?!? You seem to know what you're talking about, so I'll take your word for it, but to me, saying "kelvins" sounds (and will always sound) like some kind of uneducated yokel. —Steve Summit (talk) 04:09, 19 March 2006 (UTC)
- Yes, because it simply refers to a certain number of atoms or molecules. You know that and I know that, but the people who control the SI have decided that it is a unit of something called "quantity of substance", so we pretty much have to go along. Incidentally, responding to the original poster on another point, proper SI usage is "kelvins", not "(degrees) Kelvin". --Anonymous, 00:53 UTC, March 18, 2006.
- I have never, ever heard any educated scientist refer to "kelvins" apart from , perhaps, referring to their numerous children... Always "kelvin", please.G N Frykman 17:32, 20 March 2006 (UTC)
Laptop price?
[edit]Sorry if this is an inappropriate question for this forum, but it is kind of computer related.
I'm looking into selling a laptop with the following specifications:
PII. I think 400MHz 512Mb RAM Dell Inspiron. On board Ethernet (10MHz) Running W2K.
How much should such a machine sell for in today's market? Cheers. — SteveRwanda 09:53, 17 March 2006 (UTC)
- Computers are getting a lot cheaper nowadays, especially from the big companies like Dell. I can't really give you an amount it would sell for, but it probably won't be for as much as from retailers. (you probably already figured that out.) OK, so you probably should go to someone else for your answer, hehe. :) -- Daverocks (talk) 22:41, 17 March 2006 (UTC)
- Also, which market? I mean, going by your username it could be a couple of hundred thousand francs... Grutness...wha? 05:31, 18 March 2006 (UTC)
- Look on ebay and see what similiar machines are going for. GangofOne 04:41, 19 March 2006 (UTC)
- Also, which market? I mean, going by your username it could be a couple of hundred thousand francs... Grutness...wha? 05:31, 18 March 2006 (UTC)
- I was looking at a similar computer (a ThinkPad, as it happens) costing £180 second-hand from an online shop. People might not pay as much to get it directly from you as they would from a shop which tests and warranties the secondhand machines, checks the software license is valid, etc. Try looking here to find something closer to the spec of the computer you want to know the price for. Ojw 15:45, 19 March 2006 (UTC)
Transplantation Immunology
[edit]Respected Sir! I've not been able to find detailed mechanism of graft versus leukemia effect. I'll be obliged if supported with illustrations.
Thanx--58.65.136.146 10:41, 17 March 2006 (UTC)
- Are you asking about the effectiveness of bone marrow transplants in treating leukemia ? StuRat 19:18, 17 March 2006 (UTC)
frank hertz experiment
[edit]why we use argon atom in erank hertz experiment instead of hydrogen or carbon —This unsigned comment was added by 210.211.243.46 (talk • contribs) 12:48, March 17, 2006 (UTC).
- This description says it's done with mercury and helium. I think you can do it with any element. —Keenan Pepper 18:39, 17 March 2006 (UTC)
# of volts to create a spark
[edit]I'm wondering if someone can help me? My bosses are discussing how many volts of electricity it takes to create a spark. I figured I'd take the quick route and ask here. My boss is calling our office in Houston to get the answer and I'm hoping to get it first. Anyone??
Thanks
- That depends on the distance of the gap the spark will jump and the medium it will be jumping through. There is no distinct number of volts for every gap/medium combination. Did you know that your light switch sparks every time you turn it off? It is a very small spark, but it eventually destroys the connection in the switch and you have to replace it. --Kainaw (talk) 14:21, 17 March 2006 (UTC)
- I think the rule of thumb is that every inch of arc takes about 10,000 volts.Image:Arcing_pickup_sh That's assuming you're patient and only want a fairly calm spark to happen, and that youre in typically composed air with a medium humidity level. All of these are variables that can impact the outcome. As a reference, the sparks you get jolted by when its dry out and you generate static electricity can range from 600-1000 volts, but they are so quick that the actual energy exchanged is somewhat minimal. In short, there is no answer for 'what creates a spark'. I can get a 9v battery to make a tiny little arc, but if i wanted to properly run a spark plug in an engine it would take 50,000 volts. --Jmeden2000 14:49, 17 March 2006 (UTC)
- To go all mathematical on it, the electric field strength in a region of space is equal to the voltage difference across it divided by the size of the region (at least for a nice simple case like parallel plates). For air, the field strength at which dielectric breakdown (the phenomenon that causes the spark) occurs is ~3,000,000 V/m. So for a 1m gap you need 3 MV but for a 1mm gap you only need 3 kV. Or in Volts per inch, ~75,000 so Jmeden2000's rule of thumb is a little low. (Then again, maybe it works when you consider different topologies?) --Bth 15:01, 17 March 2006 (UTC)
- I don't know what scientific evidence that '10,000 rule' was based on, but I did peruse the article on Lightning and per the specs, a typical bolt has a potential of up to 100MV and will presumably travel around a thousand of feet from end to end. This highlights the variable aspect of arcing through air, it can have a lot to do with the base impedance of the medium and the amount of energy prepared to create plasma, effectively lowering the impedance of the pathway.--Jmeden2000 20:07, 17 March 2006 (UTC)
Take a 9v transistor radio battery and rub a metal object across both poles in the dark, you will see a spark. I have not tried it with lower voltage batteries.
- This is a really neat reference. [63] It basically states that you need a lot of voltage to jump an air gap of a few mm's. However, as we explored in an earlier question, it takes very little voltage to pull a plasma arc from a contact. In fact, the emergency switches in a utility switchyard have huge air blasters to kill the very long arc that can be pulled when a breaker opens. --Zeizmic 23:20, 17 March 2006 (UTC)

Here's a nice dramatic example of "pulling a plasma arc from a contact", one familiar to many people who use railways electrified with a third rail, such as most subway systems. The voltage here is only 630 V or so, but the current is very large, and look at the size of the arc you get when it's interrupted. --Anonymous, 01:20 UTC, March 18, 2006.
*nix shutdown options
[edit]I want to set up our Linux server so it will auto-update every Saturday night and then reboot. That is easy. The catch is that I do not want it to reboot if someone is logged in and working. I want it to wait for everyone to log off and then immediately reboot. I cannot find an option in the shutdown command to "wait for everyone to log off". Is there a hidden option or an alternative command that will perform this function? --Kainaw (talk) 14:24, 17 March 2006 (UTC)
- I think your best bet is to write a very short shellscript involving `who`. dab (ᛏ) 14:36, 17 March 2006 (UTC)
- Like this? "while [[ `users|wc -w` -gt 0 ]]; do sleep 10; done; shutdown -ry now" --Kainaw (talk) 14:45, 17 March 2006 (UTC)
- You can test whether people are logged on, but you can't test whether they're logged on and working. For that matter, they may be working while not logged on, because they may have left a program running. --Anonymous, 00:21 UTC, March 18, 2006.
MS Windows shortcut keys driving me crazy
[edit]I'm old enough to remember the typewriter and dial phones and DOS. In DOS it was handy to have shortcut keys to get around and do stuff. It's still convenient in Windows; I use CTRL-C, CTRL-V, CTRL-X, and CTRL-A all the time
HOWEVER... I'm a poor typist who sometimes brushes the Shift, Ctrl, Fn, and Alt keys, and even Caps Lock, with my left hand or fingers while entering some character with my right. It's because Shift is located right in the middle of this group of control keys. This often causes things completely unexpected; maybe even an e-mail will be sent before I'm ready.
No doubt I'm touching some of these keys simultaneously. What does Shift-Ctrl-Fn-Alt-CapsLock-Z serve up?
Is there a way I can keep the few shortcut keys I like and disable the rest?
I did find the Microsoft list of shortcuts. It looks like you could write an operating system using them alone. --Halcatalyst 16:52, 17 March 2006 (UTC)
- It would be possible to write a short script that binds all the shortcuts you don't want (Ctrl-S, Ctrl-T, etc) to a "nothing" action. So whenever you press them, the script captures it first and does nothing. I would write it for you, but I probably need more information about which shortcuts you want to keep. -- Daverocks (talk) 22:51, 17 March 2006 (UTC)
- Actually, just the ones above. That would be cool, and I'd be very grateful. However, I don't know the key combinations I don't want. They're the ones that cause me trouble when in my awkwardness I hit them unknowingly. --Halcatalyst 00:02, 18 March 2006 (UTC)
- The http://www.autohotkey.com/ tool allows you to write the scripts you want. helohe (talk) 11:52, 18 March 2006 (UTC)
- Actually, just the ones above. That would be cool, and I'd be very grateful. However, I don't know the key combinations I don't want. They're the ones that cause me trouble when in my awkwardness I hit them unknowingly. --Halcatalyst 00:02, 18 March 2006 (UTC)
What's required to make vodka at home?
[edit]What equipment and substances would be required to make small amounts of vodka at home?
- Rubbing alcohol and an empty Smirnoff bottle ? :-) StuRat 19:10, 17 March 2006 (UTC)
- NOTE WELL. Rubbing alcohol is NOT drinkable. It's a different alcohol. Just to make that clear. GangofOne 19:38, 17 March 2006 (UTC)
- ferment some starchy liquid (like potatoe juice) and distill the result. For proper vodka, you will want to filter it through a charcoal filter before distilling. --Kainaw (talk) 19:13, 17 March 2006 (UTC)
- Note that distillation is potentially a very hazardous process--ethanol vapour is very flammable--so home manufacture of spirits is not recommended. It's also illegal in most places. TenOfAllTrades(talk) 19:17, 17 March 2006 (UTC)
- Note, vodka is made from wheat. But, why not start easier, with beer and wine, to get experience with the fermentation process? Making your own beer and wine is legal in the US. It's easy, they do it in prisons. See Pruno. But if you distill it, you have to pay the taxes, by law. -- GangofOne 19:38, 17 March 2006 (UTC)
- Vodka can be made from wheat - but it is also made from a large number of other things. Rmhermen 03:57, 18 March 2006 (UTC)
- Why is it that "Inmates are obviously not permitted alcoholic beverages" (according to the article on pruno)? --Bowlhover 22:07, 17 March 2006 (UTC)
- I would assume several reasons:
- Alcohol decreases inhibitions and judgement. Given that convicted criminals tend to have low self-control already (generally speaking), this is highly unwanted.
- The ban is simply part of the punishment...err...rehabilitation.
- If the ban worked, it would help wean prisoners off addictions. Superm401 - Talk 23:59, 17 March 2006 (UTC)
- I would assume several reasons:
- Why is it that "Inmates are obviously not permitted alcoholic beverages" (according to the article on pruno)? --Bowlhover 22:07, 17 March 2006 (UTC)
I come from one of the few areas in the country where people still make their own moonshine. It's dangerous because of the flammability of the alcohol and the illegality of the enterprise itself. Also, improperly distilled spirits often contain toxic amounts of methanol, which can cause blindness or death. Leave it to the experts. In answer to other questions posed above, vodka is usually made from rye, and inmates are not allowed alcohol because it would make them harder to control, and alcohol is simply not a necessity.
- I have yet to see evidence or proof that "improperly distilled spirits" contains levels of methanol that would cause blindness or death. All I've seen is that during the 30's bootleggers will "water-down" alcohol with methanol. Also, note that if methanol is injested ethanol is actually an antidote (according to Methanol). Is there really any harm in distilling on your own unless the drink is intentionally "watered-down" with methanol? -Snpoj 05:53, 18 March 2006 (UTC)
- I'll concur with Snpoj on this one; it would take a pretty deliberate effort or some really bad luck to produce a distilled beverage with enough methanol to hurt you (before straight ethanol poisoning got you). If you're worried about methanol, just discard the first little bit of liquid that comes off the still—methanol tends to evaporate first. On the other hand, having methanol (and/or an excess of the heavier fusel alcohols) in your distilled beverage can result in unpleasant tastes and even more unpleasant hangovers. TenOfAllTrades(talk) 17:28, 18 March 2006 (UTC)
- All you need is to cut up some ripe pineapple, put it in a close container, and leave it in the fridge for a week or two, allowing it to ferment in its own juices. We did this once, inadvertantly. It wasn't too bad...kinda fizzy, but it works, lol. --Chris 03:06, 19 March 2006 (UTC)
converting to svg
[edit]I'm searching for a freeware that converts png and jpeg images to svg. What do you suggest? CG 20:12, 17 March 2006 (UTC)
- The Gimp can open jpg and png images and can save as svg. Depending on your OS, you may need to download the SVG plugin for Gimp. --Kainaw (talk) 20:18, 17 March 2006 (UTC)
- Erm, be aware that you are converting from a raster format to a vector one. Your results may be rather non-ideal, regardness of what you use. - Fangz (not logged in)
Yes, you need the equivalent of Corel Trace. But, I never got anything good out of that. You make it 'accurate' and you get a zillion tiny curves. You loosen it up, and it's really ugly. --Zeizmic 23:03, 17 March 2006 (UTC)
- If you don't mind a two-step conversion, one of the tools of choice for this sort of thing is netpbm. It
just gainedwill have an SVG converter in its next release, and it claims to be one that does curve tracing (based on Autotrace). But as Fangz was saying, make sure you understand this raster versus vector distinction and the limitations of the conversion process, because they're behind the surprising or disappointing results you're at least somewhat likely to see at first. —Steve Summit (talk) 23:19, 17 March 2006 (UTC) [fiddled 02:36, 18 March 2006 (UTC)]- Is is too much to ask anyone in Wikipedia that hast the right tool to convert an image (a PD one) to svg and upload it in Wikipedia? CG 08:23, 18 March 2006 (UTC)
- As the previous respondents have tried to point out, the "right tools" for the job just aren't very good. The only tool that does a really good job is a skilled human with a considerable amount of time. --Robert Merkel 11:16, 18 March 2006 (UTC)
- I'll be glad to give it a try when the netpbm-related tool I mentioned is ready. (The maintainer says it'll be in the next release.) But I can't promise results. What's wrong with the image you have? Why can't you upload it to Wikipedia as-is? —Steve Summit (talk) 14:45, 18 March 2006 (UTC)
- As the previous respondents have tried to point out, the "right tools" for the job just aren't very good. The only tool that does a really good job is a skilled human with a considerable amount of time. --Robert Merkel 11:16, 18 March 2006 (UTC)
- Is is too much to ask anyone in Wikipedia that hast the right tool to convert an image (a PD one) to svg and upload it in Wikipedia? CG 08:23, 18 March 2006 (UTC)
Inkscape is a nice free SVG editor, and includes a tracing tool based on potrace. —Ilmari Karonen (talk) 03:25, 19 March 2006 (UTC)
- I've never seen a tracing program that worked very well, even with very simple designs (i.e. black and white woodcuts). It is better to just trace it yourself, usually. But as noted that takes some knowledge of the program and some experience with it. --Fastfission 21:40, 19 March 2006 (UTC)
- I've found that tracing results can often be significantly improved by preprocessing the image in a bitmap editor to increase its resolution and contrast. This generally means scaling up the image 3- or 4-fold, followed by Gaussian blur (and possibly unsharp mask) to remove scaling artifacts, and finally conversion to a black-and-white bitmap using a suitable threshold. This is mainly to work around the fact that current tracers don't make proper use of the additional information found in antialiased edges, although it also serves as a useful noise-removal step for scanned images. Of course, this doesn't work for all images. —Ilmari Karonen (talk) 18:58, 21 March 2006 (UTC)
green snakes
[edit]Today's date got me to thinking: I wonder how many snakes are there in Ireland today? —Steve Summit (talk) 20:48, 17 March 2006 (UTC)
- Other than zoos and pets? None. --Kainaw (talk) 21:05, 17 March 2006 (UTC)
- Actually, I was thinking including zoos and pets. —Steve Summit (talk) 22:56, 17 March 2006 (UTC)
- If Ireland's like the other large snake-free islands in the world (e.g., New Zealand) they're not allowed into the country period. None in zoos, and definitely none as pets! Grutness...wha? 05:34, 18 March 2006 (UTC)
- They have both: [64]. That report gives a figure of 150,000 for all reptiles, though it's not clear from the context if that's the whole island or just the north. Markyour words 11:18, 18 March 2006 (UTC)
- Aha! That's exactly the kind of source I was looking for. Thanks, Mark. —Steve Summit (talk) 14:40, 18 March 2006 (UTC)
- Of course there are no snakes in Ireland! Saint Patrick scared them all off! Remember the story? :P Wikipedia says:
- Pious legend credits Patrick with banishing snakes from the island...
- —OneofThem 17:35, 18 March 2006 (UTC)
- I'm of the understanding that those 'snakes' were actually the Pagans. In Ohio there are a number of Pagans that celebrate 'All Snakes Day' on the same day as the Catholic St. Patricks's Day. -LambaJan 22:07, 18 March 2006 (UTC)
Who recognized potassium and magnesium?
[edit]Who discovered that potassium and magnesium are vital/essential? Was potassium known to alchemists from antiquity? Is this related to the history of banana cultavation? --James S. 21:34, 17 March 2006 (UTC)
- Article Potassium says "was discovered in 1807 by Sir Humphrey Davy". Bananas go back further. GangofOne 03:00, 18 March 2006 (UTC)
Genetics Experiment Went Hoorrribly Wrroonng!
[edit]At this website there's a DNA microarray with a green splodge towards the left - what went wrong? --Username132 (talk) 21:41, 17 March 2006 (UTC)
- And a dull red splodge above it and to the right. It's the end of the world as we know it.
mtDNA and genealogy
[edit]If I have an 84 percent mtDNA correlation with someone, could anyone tell me approximately how long ago our family lines seperated? --Thanks (unsigned from 206.8.175.238 , March 17, moved from near the top, to preserve chronologically)
- The mutation rate is not known very precisely, but suppose it was 0.02/site/Myr. Then with 84% correlation, your mtDNA lines would have diverged at most 8 million years ago. Gdr 00:12, 20 March 2006 (UTC)
This doesn't make sense to me... "Mitochondrial Eve," by whom every human on the planted is related, lived about 150,000 years ago, which means that my family line must have split sometime after that. What am I missing?
- Gdr's right. If 84% refers to sequence divergence, then either you or the someone you are referring to isn't human. If this value came from a geneology company then they may have excluded all or most sites that do not vary among humans. In this case, the crude molecular clocks based on mtDNA sequence divergence would not apply. --Aranae 01:38, 24 March 2006 (UTC)
Hurler's Syndrome
[edit]I read some articles on the site regarding Hurler's Syndrome, but I stll do not have a clear answer to a question because it's difficult to understand medical/biology terms. My husband's parents has conceived fetus with Hurler's Syndrome. In fact, one was born with all the symptoms and eventually died as a teenager, exactly described in the articles. So, it is possible that my husband has inherited the gene. If we try to conceive, will our fetus have the chance of being infected with the disease?
- from the article: "Because Hurler syndrome is an autosomal recessive disorder, affected persons have two bad copies of the IDUA gene. If someone is born with one normal and one defective copy of the gene he is called a carrier and will produce less alpha-L-iduronidase than an individual with two normal copies of the gene. The reduced production of the enzyme, however, isn't strong enough to cause the person to show any symptoms of the disease." Your husband has 50% chance of having the defective gene. If he has it, his child has 50% of getting the defective gene. However, since it's recessive, to get the disease, you need 2 copies of the defective gene, one from each parent. If you do not have the defective gene, then there is no way for the child to have two copies, and thus get the disease. See if they can test your husband for the presense of the defective gene, if you care. Ask a doctor these kinds of medical questions, not unqualified persons on the internet , such as myself. There may be other factors that the doctor can take into account. --GangofOne 00:25, 18 March 2006 (UTC)
- (I am not a doctor either). Hopefully, this won't spark a bitter probability dispute, but given the information we have, there is a a 2/3 chance he is a carrier. You ignored the fact that he does not have the syndome. There are two possibilities left: carrier or homozygous dominant. Carrier is twice as likely, so there has a 2/3 chance (1/2 / 3/4). That means the child has about a 1/3 chance of being a carrier (given what we now know). Superm401 - Talk 01:28, 18 March 2006 (UTC)
- You are right. I agree with your analysis. GangofOne 03:06, 18 March 2006 (UTC)
- (I am not a doctor either). Hopefully, this won't spark a bitter probability dispute, but given the information we have, there is a a 2/3 chance he is a carrier. You ignored the fact that he does not have the syndome. There are two possibilities left: carrier or homozygous dominant. Carrier is twice as likely, so there has a 2/3 chance (1/2 / 3/4). That means the child has about a 1/3 chance of being a carrier (given what we now know). Superm401 - Talk 01:28, 18 March 2006 (UTC)
Now you've done it-- you have evoked the Monty Hall problem. Using the ostensibly correct line of reasoning, the original poster's spouse had a mother and father who were each heterozygous for the trait (obviously, neither were homozygous, else they would not have reached reproductive age). This confers a risk of being a carrier (heterozygous) to the OP's husband of 2/4, or 50%, and a probability of 1/4 that he is either homozygous for the trait (not likely, since he is apparently disease-free) or completely lacking the trait. Since his probability of being a carrier is 1/2, his children each have a 1/2 chance of being carriers of the trait, assuming the original poster does not carry the trait. In the Monty Hall problem, the fact that the husband does not have the disease is insufficient reason to remove the homozygous condition from the number of potential gene combinations. Never understood it, but better minds than mine agree.--Mark Bornfeld DDS 15:11, 18 March 2006 (UTC)
- Er, I'm not sure how the Monty Hall problem would apply to this question. Assuming that the information in our article is correct – that is, that a single copy of the gene produces no symptoms, whereas a double dose of the mutant is invariably symptomatic and fatal – then Superm401's analysis is correct; the husband has a 2/3 chance of being a carrier, his children will each have a 1/2 probability of receiving a copy of the mutant (if he has it), for odds of 1/3 of them becoming carriers.
- Now, it appears to be possible to test for IDUA enzyme function and mutations in the IDUA gene; it is also possible to test a fetus for Hurler's in utero, to allow for early interventional treatment. Seeking the advice of a genetic counsellor on how best to proceed would be the best advice we could give here. TenOfAllTrades(talk) 17:23, 18 March 2006 (UTC)
- You say there is a "probability of 1/4 that he is [...] homozygous for the trait." That is not true. There was a 1/4 probability of a child (from these parents) being homozygous recessive (knowing only that). The husband, however, does not have the trait. That means there is a 0 probability of him being homozygous recessive. Superm401 - Talk 22:11, 19 March 2006 (UTC)
March 18
[edit]Turkey?
[edit]Is it true that turkies really have a special trippy amino acid that can put people to sleep? Do wild turkies use this as some sort of defense mechanism to parylize predatory animals?TurkayTurkey 00:09, 18 March 2006 (UTC)
- This is a well-spread myth. See Tryptophan#Tryptophan and turkey. Melchoir 00:14, 18 March 2006 (UTC)
- Yes. Tryptophan is an essential amino acid (i.e. if you go without it long enough you die) and it's in many different foods. Sometimes I think I can tell when I haven't had enough, and I feel better after eating a banana or some yogurt, but that's probably just a placebo effect. —Keenan Pepper 01:13, 18 March 2006 (UTC)
- The acid brought by turkeys creates a hot state of dependance and you freeze without it (it's called cold turkey). --DLL 22:30, 19 March 2006 (UTC)
Hitler's Syndrome?
[edit]What's the technical name of this disease? --LHbyot 02:28, 18 March 2006 (UTC)
- Is there even such a syndrome? I've never heard of it. ☢ Ҡi∊ff⌇↯ 10:34, 18 March 2006 (UTC)
- That's more political than scientific. However, the Napoleon complex may be somehow related. -LambaJan 22:02, 18 March 2006 (UTC)
- I think what the questioner was asking was not,"Describe the Hitler Syndrome," but rather, "What (mental) illness possessed Hitler?" Good question. He certainly was a strange man and did strange things, to say the least. He was, or thought he was, and in this thought he was joined by many, that he was a Wagnerian Hero; he won the loyalty of large part of an entire generation of "good Germans." He was a madman: so what, specifically, was he mad about? or to put it in Americanese, the guy was nuts, but what kind of nuts? Was it some really wierd (or maybe garden-variety) schizophrenia? If so, what kind? paranoid? Alas, Mr. Hitler never had the opportunity to be examined by a psychiatrist, or even a psychoanalyst. Too bad, since much of that discipline originated in Germany. Of course, it was regarded by Der Führer as Jewish trash. --Halcatalyst 04:26, 20 March 2006 (UTC)
- Was he mad, or just an ambitious asshole? We may never know. Superm401 - Talk 21:22, 21 March 2006 (UTC)
- You might also say the gentleman was a megalomaniac, for which the formal name is the rather common Narcissistic personality disorder. --Halcatalyst 04:30, 20 March 2006 (UTC)
The State of Bubbles
[edit]Is a bubble a solid, a liquid or a gas? It has a definite shape (but whether it has definite volume, I'm not so sure of), it has a "shell" of liquid, and it contains air! One science student in this world (me!) is miffed and mystified by this seemingly simple question... --70.237.200.25 01:26, 18 March 2006 (UTC)
Bubbles are gaseous. Brian G. Crawford 03:19, 18 March 2006 (UTC)
- The inside of a single bubble is a gas, but the really interesting case is when you get a bunch of bubbles together; the result is a foam that's somewhere between solid and liquid. Even in the case of a two-dimensional layer, you get interesting behavior:
- Understanding these structures is important in industry, and as you can see, research continues to this day! Melchoir 03:35, 18 March 2006 (UTC)
- Also see Antibubble, a drop of liquid separated from another liquid by a hollow sphere of gas. —Keenan Pepper 03:55, 18 March 2006 (UTC)
- Whoa, that's just crazy! Melchoir 04:33, 18 March 2006 (UTC)
- They're not that hard to make either. You just need a straw and some soapy water. —Keenan Pepper 05:13, 18 March 2006 (UTC)
- Or a light n'frothy German beer, where they were discovered. -- User:Mac_Davis
As for volume, I got this from sphere:
The surface area of a sphere of radius r is:
and its enclosed volume is:
So, there isn't a definite volume, but there is a definite relationship between your bubble's radius, surface area and volume, assuming it's spherical. If it's a semi-sphere you would need to halve the outcome of the formula. -LambaJan 21:54, 18 March 2006 (UTC)
- On the usual size scale for bubbles, you have a hierarchy of forces, air pressure > surface tension > wind shear. So a given bubble forms into a sphere with a definite volume, rigidly fixed by the number of gas molecules inside; even if its shape does vary, the volume doesn't. Of course, there are bubbles with different volumes, just as there are rocks of different sizes. Melchoir 22:07, 18 March 2006 (UTC)
Just going back to the original question. As Melchoir said, the inside is gaseous. But isn't it the case that the surface film is a liquid? Which leads me to ask, what do we mean when we say "bubble"? Is it the visible surface, or is it the invisible gaseous contents, or is it both? JackofOz 23:40, 18 March 2006 (UTC)
- I'd say, when most people say bubble, they mean either 'those things that you blow out of a straw thingy that float' or a prefix for 'bath'. I don't think it goes much deeper than that. Black Carrot 00:00, 19 March 2006 (UTC)
- When we call those soapy things "bubbles", we are referring to either the external liquid film, or the film plus the gaseous contents, but (I would suggest) never just the contents (except perhaps in particular scientific contexts). To say "bubbles are gaseous" does not tell the whole story. That would be true of bubbles of gas rising through a liquid, but the questioner referred to a "shell of liquid", so they have the soapy version in mind. I believe a soap bubble cannot be characterised as one thing or the other. Part of it is liquid, and part of it is gaseous. JackofOz 05:59, 19 March 2006 (UTC)
Wow, thanks everyone for trying to help me solve this! [I created an account;)] I have a bit to contribute to this:
- a solid is technically defined as matter with a definite shape and volume,
- a liquid only has definite volume . . .
- a gas has neither volume nor shape (since it would exoand indefinitely without boundaries to hold it in)
But, this still raises an interesting question: Would bubbles be sonsidered as having a definite shape? They could be thought of as an amorphous solid, or a slow-moving liquid, I suppose, but I'm not sure;) all in all, I think we're back where we started again! 70.230.149.224 22:06, 19 March 2006 (UTC)
- Oops, I wasn't logged in when I posted that ^ Joyskii 22:07, 19 March 2006 (UTC)
- And I seem to need typing classes, because I have more typos than the rest of you all combined;) Joyskii
- I fixed your formatting. How are we back where we started? Having conclusively determined that bubbles are made of up to three entirely seperate substances (even on the macroscopic level, except in the case of foam), the original question can be answered. As JackofOz said, one part of it is liquid, another part is gas. The bubble as a whole, being made of several entirely seperate pieces, would no more have a phase of its own than my entire glass of water has a phase of its own. The water has one, the glass has another, and the air above the water yet another. And no, bubbles don't have a definite shape, but that's not the only criterion. Black Carrot 02:03, 20 March 2006 (UTC)
Fuel Oil
[edit]Does improper burning of fuel oil in an oil furnace produce carbon monoxide or carbon monoxide? (Vern)
- Yes. Also CO2, NOx, H2O, and hydrocarbons of various lengths. Carbon monoxide is the result of incomplete combustion.
--Nagle 04:57, 18 March 2006 (UTC)
Magnesium & sodium hydrogensulphate
[edit]I am in the process of completing a SATs mock exam for my science homework.
Can you please help with the following question:
Magnesium is added to a solution of sodium hydrogensulphate. What would you expect to see forming on the magnesium? —This unsigned comment was added by LGardiner (talk • contribs) .
White stuff-- Magnesium sulfate. 1590 —This unsigned comment was added by Alteripse (talk • contribs) .
- Precipitation of an insoluble salt and the formation of a gas are both driving forces in this reaction. Hydrogen sulfate ion is a strong enough acid to oxidize magnesium. —Keenan Pepper 17:10, 18 March 2006 (UTC)
- No. Magnesium sulphate is reasonably soluble. The question is obviously leading to what is observed on the surface of the magnesium, and that is bubbles of hydrogen gas. The strip of metal would dissolve, leaving a colourless solution - technically a mixture of magnesium sulphate and sodium sulphate.G N Frykman 09:49, 19 March 2006 (UTC)
- Hmm, must have remembered wrong. The sulfates get less soluble going down the group, which is the opposite of the hydroxides. —Keenan Pepper 19:08, 19 March 2006 (UTC)
Microchips
[edit]Does a microchip have a magnet in it? Can a microchip have a magnet in it?
- Yes, the ferrite rod is an essential component. See microchip. --Shantavira 14:22, 18 March 2006 (UTC)
- For microchip implants, yes, the ferrite rod wrapped in copper wire acts as a radio antenna to send a signal to the scanner. Generally, ICs don't. — TheKMantalk 23:40, 18 March 2006 (UTC)
Dietary Advice
[edit]How healthy is a cup of minestrone soup from a packet? How would this compare with an equiavlent volume from a can? I like minestrone... --Username132 (talk) 18:29, 18 March 2006 (UTC)
- To give a good answer I would need to know specifically which products you're talking about. If high temperatures are used in dehydrating the food, this can cause the breakdown of some vitamines and proteins. However, the matter of ingredients is more important. What is the quality of the vegetables? probably not very high if they need to add natural/artificial flavors. Your best bet at a healthy product is to buy good organic vegetables, use filtered water, and make enough to store for your convenience. -LambaJan 21:43, 18 March 2006 (UTC)
- It depends on the comparative ingredients of the two soups (and nothing else, though the flavour might vary). If the products have nutritional labels, they will tell you more than we can. Notinasnaid 21:42, 18 March 2006 (UTC)
- Does it really matter if a protein has been denatured before I eat it? I mean, digestive processes are gonna do that anyway?
- Right. doesn't matter. When you fry an egg, you denature all the proteins. That is , the 3d structure of the proteins are mangled due to the heat. THat's why the egg whites turn cloudy. But the chain of amino acids is intact. The chain of a.a. s is taken apart in digestion, so you end up with a soup of amino acid molecules, that get's absorbed into the blood. When you cook meat, say, you denature meat proteins, but more importantly, you denature the proteins of bacterial and parasites, ie kill them. GangofOne 08:02, 19 March 2006 (UTC)
- Does it really matter if a protein has been denatured before I eat it? I mean, digestive processes are gonna do that anyway?
- Nothing is "healthy", or "unhealthy," its how much you eat of certain things that can be unhealthy or healthy for different people. --
 Mac Davis] ⌇☢ ญƛ. 01:30, 19 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 01:30, 19 March 2006 (UTC)
Intravenous Medicine Bottle Membranes
[edit]What are those membranes covering the tops of bottled of medicine destined for injection made of? --User:58.65.136.146 19:34, 18 March 2006 (UTC)
- Rubber. --BluePlatypus 20:49, 18 March 2006 (UTC)
Yeah, probably silicone rubber. Brian G. Crawford 21:37, 18 March 2006 (UTC)
- How does it seal itself back up? User:58.65.136.146 00:10, 19 March 2006 (UTC)
- Through the ordinary elastic properties of rubber. I don't think the bottles are meant to be stored for long after having been punctured though. I don't have any experience with IV bottles, but I've sealed GC vials which have the same kind of top (rubber disc and metal collar which you clamp on), and the rubber disc is a bit thicker than one might think, about 2-3 mm (1/8 inch). --BluePlatypus 01:41, 19 March 2006 (UTC)
Latin name for new species
[edit]I've also posed this question at the language reference desk: I'm writing a chapter that features microscopic machines, like the nano assemblers spoken about by Eric Drexler et al, and would like it if I could use a Latin species name to refer to them.
A strange question, I know, but if anyone out there thinks it might be fun to come up with a few suggestions, I'd be most appreciative.
Adambrowne666 23:26, 18 March 2006 (UTC)
- The short answer is no, it are not biological species. KimvdLinde 01:07, 19 March 2006 (UTC)
- I haven't made myself clear. I'm asking people to pretend they've discovered a microbial species which happens to be mechanical. What would it be called?Adambrowne666 06:27, 19 March 2006 (UTC)
- Microsoftium xbox3600000ium GangofOne 08:04, 19 March 2006 (UTC)
- I haven't made myself clear. I'm asking people to pretend they've discovered a microbial species which happens to be mechanical. What would it be called?Adambrowne666 06:27, 19 March 2006 (UTC)
- Machina automatos sapiens ☢ Ҡi∊ff⌇↯ 14:59, 19 March 2006 (UTC)
- Nice try but you mixed latin and greek and got all the endings disagreeing. alteripse 02:02, 20 March 2006 (UTC)
- So what, do you have any idea how many species names are simply "latinized" hell, people latinize their own names sometimes :)--152.163.100.74 03:04, 20 March 2006 (UTC)
- Nice try but you mixed latin and greek and got all the endings disagreeing. alteripse 02:02, 20 March 2006 (UTC)
- Be specific! back to see WP:RD/L. --DLL 22:25, 19 March 2006 (UTC)
To be more specific, the nanomachines are the traditional assembler type, but in a 17th century context (it's a long story) - in the novel, they're described as "Mechanical spiderlets so surpassing small that anything tinier could scarcely be imagined. Their enginery is wrought of microbes’ whiskers, and moves by wheels and spiral pulleys, as doth a clock." Hope this helps. Adambrowne666 07:04, 21 March 2006 (UTC)
Nanomycoplasma ubique WAS 4.250 11:23, 23 March 2006 (UTC)
How much power is used in the US/World
[edit]For a paper I'm writing I need to know how much energy is used (in watts or BTUs) in the world or US, thanks. --orphan frequently 23:28, 18 March 2006 (UTC)
- From the CIA factbook, the world produced 15.85 trillion kWh and consumed 14.28 trillion kWh in 2003. I have no idea whom the world is exporting electricity to, although the CIA factbook says we "exported" 536 billion kWh of it. :) --inksT 23:40, 18 March 2006 (UTC)
Thanks, and in case anybody's wondering, if everyone in the Us cooked their hot dogs with a parabolic reflector instead of a microwave, we would save approximately .005% of the world's power.
- That much? How many hot dogs are we cooking? Melchoir 04:44, 19 March 2006 (UTC)
- Show us your calculation. GangofOne 08:01, 20 March 2006 (UTC)
- That sounds dandy and all but what happens when I need my late night hot dog fix? It would be interesting to look at the fraction of foods requiring only minimal heat for preparation (like canned soups, or s'mores) and then figuring the timeslice of a day that they could reliably be prepared in, as a way to estimate potential savings from 'free-energy' like solar ovens. I predict it would be unparalleled by the amount of energy you would expend convincing everyone that it was a good idea to cook things in a big foil-covered contraption.
- When energy prices get so high noone can afford it, it won't take any to convince people to use free alternatives. (Ie, a free market argument.) However, I suggest looking at bigger picture. Your TOTAL household energy use, and ways it can be economized, and total energy use in society. It doesn't take much to see problems in the future. Shortfalls, rapid price rises, economic dislocations. According to what I read, rational creatures can use their rationality to plan ahead and avoid some unnecessary grief; but it's not clear that's true, based on what I see. GangofOne 20:29, 20 March 2006 (UTC)
- Here is the almost perfect graph for you Herzog, [65] I forgot where I got it, but it was either wikipedia or the US Department of Energy. --
 Mac Davis] ⌇☢ ญƛ. 05:11, 21 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 05:11, 21 March 2006 (UTC)
March 19
[edit]Uneven Bandwidth Sharing Network
[edit]I think my housemate has found a way to siphon off all the internet bandwidth for himself. Is this possible? --Username132 (talk) 00:12, 19 March 2006 (UTC)
- It depends on what kind of networking equipment you share. If it's something pretty fancy (such as a Cisco router, or a Linux box) it may feature traffic shaping, which lets the person who configured it do all kinds of stuff. If, however, its an off-the-shelf little ADSL gateway (linksys, belkin, netgear) then that feature isn't present (at least not in the UI). He's maybe just running lots of P2P apps flat out. -- Finlay McWalter | Talk 01:10, 19 March 2006 (UTC)
- Agreed. He might think he's just downloading one song, but he'll be uploading as well.--inksT 02:32, 19 March 2006 (UTC)
- P2P apps tend to use lots of connections and can therefore take bandwidth far more aggresively than the single TCP stream of a simple http or ftp download. IP doesn't really enforce fairness, its more a who pushes hardest wins type environment (ofc if you design protocols that push things too hard you'll just end up with loads of packet drops and noone will get good results, see ddos attack for an example of this situation). Plugwash 02:47, 19 March 2006 (UTC)
- With regards to traffic shaping, you can probably add that feature to your ADSL gateway if you are prepared to install OpenWRT. It's free, but installing and configuring it to your needs is a geek-only task. --Robert Merkel 03:18, 19 March 2006 (UTC)
Steel Wool
[edit]Could somebody please tell me the typical percentage compositions of the substances that make up standard steel wool? Thankyou Vollsa 06:14, 19 March 2006 (UTC)
- The article steel wool seems to suggest that it's 100% steel. Unless you're counting soap, I guess; is that your question? Or are you asking about the elemental composition of the specific variety of steel? Melchoir 06:21, 19 March 2006 (UTC)
- I very much doubt that steel wool material is tightly controlled; it would probably vary from batch to batch of raw material depending on what iron ore was used etc., but generally, steel wool is made of steel so one would expect certainly iron (probably 98-99%), carbon and oxygen, and possibly some trace sulfur and nitrogen. No way to know exact percentages without contacting a specific manufacturer --Fuhghettaboutit 07:31, 19 March 2006 (UTC)
- ...and asking them very nicely to run a sample of their product through a mass spectrometer. --inksT 09:22, 19 March 2006 (UTC)
- I very much doubt that steel wool material is tightly controlled; it would probably vary from batch to batch of raw material depending on what iron ore was used etc., but generally, steel wool is made of steel so one would expect certainly iron (probably 98-99%), carbon and oxygen, and possibly some trace sulfur and nitrogen. No way to know exact percentages without contacting a specific manufacturer --Fuhghettaboutit 07:31, 19 March 2006 (UTC)
Neutralization
[edit]Dear users,
Can anyone explain to me what is actually happening microscropically when a Antacid Tablet is inserted into Hydrochloric Acid? I need to write a hypothesis for this experiment (analysing and comparing the neutralizing capabilities of commercial antacids) and i need some very detailed explanation. Thanks!
p.s.: I am in the 9th grade so please don't include that much mathematics! Thanks!
- The basic replacement reaction is:
Acid + Base -> Salt + Water
- Note that "salt" doesn't mean just table salt, for example:
HCl + CaOH -> CaCl + H20
I'm afraid the above equation is wrong. It should read: 2HCl + Ca(OH)2 --> CaCl2 + 2H2O. But that is not the point. Commercial antacid tablets do not consist of bases as such, because they would be harmful to the stomach. They are much more likely to be made from sodium hydrogencarbonate, and the general equation is: acid + hydrogencarbonate --> salt + carbon dioxide + water. Thus: HCl + NaHCO3 --> NaCl + CO2 + H2O. The project thus boils down to an investigation of how much HCl (of known and equal concentration every time) reacts exactly with a known mass of antacid tablet. You could look up titration, but an easier way, if you had large numbers of each make of tablet, would be to take a fixed volume of acid each time, and see how many tablets you could add before the fizzing stops. The fewer the tablets, the more effective they are!G N Frykman 14:52, 19 March 2006 (UTC)
- Sodium bicarbonate isn't a "base as such"? What does that mean? This bottle of Maalox contains aluminum hydroxide and magnesium hydroxide, and the latter is definitely a "base as such". —Keenan Pepper 18:52, 19 March 2006 (UTC)
- He is refering to the strength of the base. All of the first and second group metal hydroxides are classified as strong bases, meaning that they completely dissociate in water. For example: Ca(OH)2 is a compound of Calcium, from group 2 of the periodic table, with Hydroxide. When put into water, all of the molecules of Ca(OH)2 will go into solution. When you are dealing with weak bases like sodium bicarbonate/baking soda, only some of compound actually dissolves into solution. (If you want to get technical, this amount is defined by the acid/base dissociation constant. I don't know the number off-hand). If Maalox has Mg(OH)2 in it, then that is a strong base, though it probably does not have a lot it. Remember that a strong base doesn't always have to be corrosive and deadly. Hope this clears some things up. --Chris 22:15, 19 March 2006 (UTC)
- There never were, or are, any "molecules" of calcium hydroxide, which is reasonably ionic in character, but not very soluble. I can't believe that people deliberately ingest aluminium hydroxide as an antacid without severe health risks. Of course aluminium hydroxide (very insoluble) and magnesium hydroxide (pretty insoluble) are both bases, as is sodium hydrogencarbonate (if we really want to get technical, of course, the hydrogencarbonate ion is amphoteric), because they are all proton acceptors. Strong bases are not necessarily corrosive, but strong alkalis definitely are, because they are very soluble. Sodium hydrogencarbonate solution has a pH of around 9 (depending on concentration, of course) and, when ingested into the stomach as an antacid, merely produxes salt water and a lot of burp-making carbon dioxide...G N Frykman 17:44, 20 March 2006 (UTC)
Airplane component question
[edit]On large passenger airliners, I have noticed that there are small rods, shaped a bit like small antennas, perhaps half a meter long, sticking out from the trailing edge of the flap tracks under the wings (like here). There seems to be one on each flap track, plus a few extra ones on the winglet (like in this picture). I know they're not antennas - what are they? — QuantumEleven | (talk) 13:06, 19 March 2006 (UTC)
- Your "like here" site can't be viewed by people other than you. I would guess those instruments measure air speed. StuRat 14:14, 19 March 2006 (UTC)
- I know the rods QuantumEleven is talking about, but I don't know what they are, either. If you can't view that picture [66] (though it worked for me), here are three more: [67] [68] [69]. The ones I've seen, and the ones in these pictures, are shorter, more like 20 cm than 50. I don't think they're pitot tubes (which are usually mounted forward on the fuselage, and which are the airspeed instruments I'm familiar with), but I suppose they could be related. Not all airplane wings have them; only some. —Steve Summit (talk) 16:42, 19 March 2006 (UTC)
- Ah, yes, I see the problem - the site blocks direct access to the picture in question, you need to click through their menu to get to it: [70] -> Walkarounds -> Airbus -> Airbus A330, then it's the middle picture, four rows up from the bottom.
- They definitely can't be pitot tubes, because pitot tubes (as Steve Summit said correctly) have to be forward-facing. As for airspeed measurement, that's taken care by the pitot and static ports at the front of the fuselage, where the air flow is least disturbed (it's pretty messed up behind the wing!). I had thought that they might be some kind of ion discharger - they act to stream off excess charge from the airplane so it doesn't build up a strong charge (which might damage instruments if it comes into contact with another object), but that's just speculation. Anyone have any better explanations? Thanks in advance! — QuantumEleven | (talk) 18:14, 19 March 2006 (UTC)
- Would a Boundary layer fence be installed on the trailing edge? EricR 18:55, 19 March 2006 (UTC)
- No, they wouldn't have any effect there. A boundary layer fence exists to prevent flow parallel to the wing (ie outward), so by nature it has to stretch for most of the wing chord, and it doesn't look anything like the little rods. A good illustration of one is here (the airflow is coming from the left).
- This is turning out to be quite a mystery! :) — QuantumEleven | (talk) 07:04, 20 March 2006 (UTC)
- No, they wouldn't have any effect there. A boundary layer fence exists to prevent flow parallel to the wing (ie outward), so by nature it has to stretch for most of the wing chord, and it doesn't look anything like the little rods. A good illustration of one is here (the airflow is coming from the left).
- Wouldn't it simply make the aircraft more aerodynamic? Kilo-Lima|(talk) 18:28, 19 March 2006 (UTC)
Is it possible that they are some sort of antenna? --Anonymous, 23:55 UTC, March 19, 2006.
- Not as far as I can tell (but, then, I could be wrong!). All the antennas are on the fuselage, in aerodynamically shaped fins. The end of a flap track seems a very daft place to put an antenna - why go to all the trouble to run wiring through the wing, and then through the flap track (which moves, making this a difficult task) if you can just stick it on the fuselage? But, then, again, I could be overlooking something... — QuantumEleven | (talk) 07:00, 20 March 2006 (UTC)
- The are called static discharge wicks and are for discharging any charges that accumulate on the surface they are attached to. Obviously charges on the surfaces are not a good idea because an aircraft wing holds thousands of gallons of fuel. I have no idea exactly how they do their job but I was responsible for calculating their drag when I worked in Aerodynamics at British Aerospace. They are found on an Airbus wing on the flap track fairings (that hide the flap mechanisms), the flaps, the ailerons and the winglets - Adrian Pingstone 09:19, 20 March 2006 (UTC)
- Presumably the wicks work by concentrating the static charge (incidentally, that article needs some work) at their tips, where the curvature is very large. Concentrated charge will be released into the air more quickly. --Tardis 18:55, 22 March 2006 (UTC)
Keratolysi exfoliativa
[edit]What is Keratolysi exfoliativa ? Is there any effective treatment for it ?
- That's Keratolysis exfoliativa, by the way. --jpgordon∇∆∇∆ 18:07, 19 March 2006 (UTC)
- Destruction of skin cellules by defoliation is what's meant. The most efective cure for skin diseases is ... general good health. That state may be obtained when you think that your life is good. IANAP, so do consult a true physician. --DLL 22:20, 19 March 2006 (UTC)
nuclear threats
[edit]what are nuclear threats?;how seriuos are they;what would be its global out come;what will happen with our energy consuption
- Are you talking about the use of nuclear weapons, dirty bombs, nuclear reactor accidents, intentional sabotage of nuclear reactors, nuclear waste disposal, depleted uranium pollution, side effects of nuclear medicine, radon gas, or what ? StuRat 17:21, 19 March 2006 (UTC)
- If you are talking about the threat of nuclear warfare, then it depends on exactly what sort of scenario you are proposing (i.e. a terrorist with one crude bomb or a massively nuclear state like Russia launching a full attack), and even then will be debateable. If you are talking about nuclear reactors, the global and local threats of major accidents is very low. --Fastfission 21:37, 19 March 2006 (UTC)
- Make that a low chance of things going wrong but a high risk in terms of potential results. Multiply a low chance with a high risk and both rather vague and the outcome is way too vague to conclude anything. But the waste takes a very long time to 'neutralise' (thousands of years, although that depends on what you call safe) and there's no telling what may happen to it or the place it's stored in in the meantime, and this uncertainty should be added. So the biggest problem is the impossibility to assess the risks involved. DirkvdM 15:15, 20 March 2006 (UTC)
Question; Shadow, reflection or ?.
[edit]This shadow? is visible on the left and right sides of a moving vehicle during times of no direct sunshine. ie; before sunrise, after sunset or during overcast conditions. The shadow is less distinct than that seen directly under the vehicle, and is not seen when the vehicle comes to a stop. The shadow appears to be in the shape of a truncated half-elipse. They are most easily observed when following a large truck but once a person knows what to look for, they can be seen beside any moving vehicle during times of indirect natural light. What are they? Thank you. -----
- (removed leading spaces to fix formatting) --LarryMac 15:43, 19 March 2006 (UTC)
- Essentially there are an infinite number of shadows under diffuse lighting, one from every particle in the sky which is reflecting sunlight down to the truck. The shadows reach the highest density under the truck, where it is darkest, and then the next most heavy density would be at the sides of the truck in the middle. You can see this for yourself if you shine multiple different colored lights at an object from different positions above the object. You will see many shadows, each a different color, but they will all come together underneath the object. StuRat 17:13, 19 March 2006 (UTC)
physical science
[edit]is it possible for south africans to uterlise free on-line turts for physics .please if the is may i get the web address for it
- Free online turtles ? StuRat 17:48, 19 March 2006 (UTC)
- Open Source turds on the Internet? DirkvdM 15:17, 20 March 2006 (UTC)
- Take a look at learn.co.za. Superm401 - Talk 22:00, 19 March 2006 (UTC)
http://www.lightandmatter.com/ free introductory physics and astronomy textbooks, available to everyone in South Africa or anywhere else in the universe that is internet-connected. For a list of books to search through with more books at all levels in English, visit the all-time great website http://onlinebooks.library.upenn.edu/ 25,000 books listed, all free, with new ones everyday. GangofOne 08:20, 20 March 2006 (UTC)
- Free High School Science Texts is written by South Africans for South African senior secondary students, and released under the GNU FDL. Anon 03:01, 24 March 2006 (UTC)
The feeling of being watched
[edit]Is the "feeling of being watched" alluded to so often in fiction only a myth, or is there any physical justification for it? Thanks.Loom91 15:20, 19 March 2006 (UTC)
- There are different ways you could take this. I'm assuming you mean something other than just being aware, perhaps subconsciously, of things around you which would give you clues as to whether you were being watched, but mean more specifically whether or not humans can tell, without other evidence, that people are looking at them. I'm pretty sure the answer is "no" on that last part, though I remember reading about some crackpot who had tried to prove that people had some sort of psychic intuition (the same guy also worked to prove that dogs were psychic, I believe). --Fastfission 21:34, 19 March 2006 (UTC)
- I find that when I'm looking down at something in a room full of a decent amount of people, and I look up, I always happen to look at the person who is looking at me. Maybe it's just because I notice it more often then not. I'm not sure if there is any explaination behind it. Just like how a room always tends to get silent at 20 after or 20 to. --Chris 21:53, 19 March 2006 (UTC)
- That kind of sixth sense must be real as it is experienced by various people and depicted by every fiction authors. Ancestral survival knowledge that, when in a place you do not know, intending to do certain things, you must be in alert. Then, when once you find that you are really watched, it may be mere coincidence but you were prepared to it. --DLL 22:14, 19 March 2006 (UTC)
- The person you're thinking of is Dr. Rupert Sheldrake. The competing ideas are that a)people notice things they don't realize they notice and b)people's eyes are drawn to someone's moving head as they turn around vs c)people are psychic. I'm replicating his experiment for Science Fair, and if nobody objects to me spreading a little original research, I'd be glad to share the results when I have them. BTW, do you know of any name for this, phrase that describes it, something in a book perhaps? Nobody seems able to come up with a good word for it. "Feeling of being watched" seems a bit long.
- Not really, DLL, it's quite possible for most people to believe something that has no basis in fact whatsoever. *cough*flat earth*cough* Black Carrot 22:23, 19 March 2006 (UTC)
- see Sheldrake's books, "Seven Experiments That Could Change the World" (1994) and "The Sense Of Being Stared At" (2003) GangofOne 00:25, 20 March 2006 (UTC)
Not quite being watched, but when you "feel a presence", I think it's because of low-frequency noises coming from the person. I paid attention to it and realized it's something in the hearing sense, but you can't quite put a finger at it. Eventually, after some thought on this, I did an experiment with low frequencies and found out the result is exactly the same. My guess is that it has something to do with these factos:
- You can't tell where bass sound is coming from
- If you hear such a sound but you can't associate it visually with anything, your brain assumes it comes from behind you.
- If you just stand somewhere you will produce such noises.
Now what else is involved, I dunno, and I never really bothered to do further experiments. But an interesting thing is that years later I saw a documentary on Discovery Channel where they used low frequency noises to stimulate "hauntings". People felt a presence and felt like being watched.
So my guess is, I was right. \☺/ ☢ Ҡi∊ff⌇↯ 00:53, 20 March 2006 (UTC)
- Awesome. But what if there's another sense (a genuine "someone's here" sense) and you just tend to mistake the one for the other without any training? Black Carrot 01:46, 20 March 2006 (UTC)
- I've read somewhere about an experiment similar to Kieff's. Several people said that a room was haunted, and it turned out that the reason was a table fan, which made a 15 Hz sound (too low to be audible by human ears). /130.238.41.144 13:54, 20 March 2006 (UTC)
explanation for the seasons of the earth
[edit]If a rocket is captured into orbit by a planet, it will always point the same face to the body it circles. Yet the earth does not do the same thing with the sun. Instead, it points its North-South axis in the same direction at all times (ignoring effects like the rotation of the galaxy). If it were like a rocket ship, and pointed the same face to the sun at all times, it would not have seasons. Why does the earth not follow the same principle as the rocket? The Mad Echidna 17:13, 19 March 2006 (UTC)
- The only reason a rocket always has one part facing a planet is that they choose not to spin it. In the case of planets and moons, they all initially have a spin that keeps them from having the same face toward the object they are orbiting. However, if the planet or moon is relatively close to the body it is orbiting, then tidal locking will eventually cause it's spin to change, typically so that one side always faces the body it is orbiting. This has happened to our Moon, for example. StuRat 17:41, 19 March 2006 (UTC)
- If one side of the earth always faced the sun there would be no day or night but we would still have seasons. They would be extra-crispy and super-crispy instead of summer and winter. EricR 22:04, 19 March 2006 (UTC)
- No they wouldn't. Seasons are caused mainly by days being of different lengths at different times of year. Were the same face of the earth to remain towards the sun at all times, we would have neither days nor seasons, other than a minor variation due to the earth's slightly elliptic orbit. Black Carrot 22:12, 19 March 2006 (UTC)
- See the seasons article. The irradiance of a give area of the earth's surface changes as the angle of incidence of the radiant flux from the sun changes throughout the year. EricR 23:55, 19 March 2006 (UTC)
The false claim "if a rocket is captured into orbit by a planet, it will always point the same face to the body it circles" is the source of the OP's confusion. Rockets are not special: they obey the law of conservation of angular momentum just like any other object. Artificial satellites that need to face the Earth at all times must be carefully spun so that their period of rotation matches their period of revolution, and this synchronization maintained by use of attitude controls. Gdr 23:58, 19 March 2006 (UTC)
- Right, I'd neglected the angle of incidence, but that doesn't change that there would be no seasons. Even factoring that in, all it means is that different parts of the globe would be different temperatures, not that any part of the globe would experience temperature change, since it would still face the same side towards the sun at all times. Black Carrot 01:41, 20 March 2006 (UTC)
- Since the earth's axis is tilted the latitude at which the solar radiation is normal to the surface changes as the earth revolves about the sun. During the equinox the sun's rays are normal to the surface at the equator, during the solstices at the Tropic of Capricorn or Tropic of Cancer. EricR 02:50, 20 March 2006 (UTC)
- "If one side of the earth always faced the sun," one side of earth would always face the sun. You're talking about the side of the earth facing the sun changing. Black Carrot 20:14, 20 March 2006 (UTC)
- I think i see the problem, take a quick look at the image on the bottom of the Axial tilt page. The direction in which the axis of rotation of the earth points remains constant (ignoring precession) during the year. The earth would still be rotating in order to keep one side always facing the sun. The direction the axis points would not change because of the conservation of angular momentum.
- Imagine you are standing on the Tropic of Cancer during the summer solstice, halfway around the "dayside" of the planet. The sun is directly overhead, 90° above the horizon. As the year progresses, your side of the planet always faces the sun--but the sun begins to drop towards the horizon. Half a year later during the winter solstice the earth has revovled 180° about the sun, and rotated 180° about its axis. But the direction the axis points has remained the same and you see the sun 23.45° above the horizon. EricR 00:47, 21 March 2006 (UTC)
- I see. I hadn't considered the axis of rotation not being able to be vertical in this hypothetical universe. I'd assumed that "the same face to the sun at all times" meant "the same face to the sun at all times", and reasoned from there. Were the axis to remain the same as it is now, yes, there would be seasons to some extent. Except in that broad circle on the back of the planet where there'd never be any light at all. Good call. Black Carrot 01:39, 22 March 2006 (UTC)
Electric current
[edit]Why is it that an electric current is generated when a coil is rotated between the magnetic flux that is what happens at the atomic level that leads to the flow of electrons in a mattel conductor? —This unsigned comment was added by Aggarwal kshitij (talk • contribs) .
- Have you read the article Electromagnetic induction? —Keenan Pepper 18:42, 19 March 2006 (UTC)
dopamine antagonist's
[edit]I am doing some simple research on parkinson's drugs to compile data so the question is I realize mirapex is a dopamine antagonist but is requip and comtan also dopamineanergict
- Umm... see Requip and Comtan? —Keenan Pepper 21:23, 19 March 2006 (UTC)
- By the way, you're probably thinking of dopamine agonists, not antagonists. --Uthbrian (talk) 02:17, 20 March 2006 (UTC)
Light travel from the early Universe to Earth
[edit]Earth is receiving light from the early Universe from over 13 billion years in the past.
- 1. Why didn't that light overtake the matter which is now earth in the first billion years?
- 2. Did the early inflationary period prevent this?
- 3. If so, how?
In my simplistic view, light from the early Universe must be vanishing as an ever expanding event horizon that passes Earth in all directions.
- 4. Will the curvature of spacetime prevent the early Universe from vanishing faster than the rest of the Universe for future observers?
--jwalling 19:38, 19 March 2006 (UTC)
- The furthest stars from us are moving away from earth at a rate approaching the speed of light, and proportional to the distance between us. See hubble constant. Light does not vanish, it diminishes in intensity by the inverse of the square of distance.--inksT 21:25, 19 March 2006 (UTC)
- In the early Universe, the average distance between all matter was a small fraction of the average distance today. For 13 billion years the average distance had to increase faster than the speed of light to prevent ALL the ancient light of the early Universe from reaching EVERY physical object, including Earth or its matter constituents. If that ain't so, I need another explanation that fits the conjecture the early Universe will be visible everywhere for billions of years henceforth at diminishing energies. --jwalling 00:22, 20 March 2006 (UTC)
- postscript: I was using the vernacular vanish to describe the effects of movement in relationship to an observation horizon. Objects far enough apart (Hubble distance) are receding at speeds greater than the speed of light. In theory you could physically travel away from a visible distant object to a place in space where light from that object would cease to reach you due to the expansion of the intervening space. That light source would vanish, so to speak. --jwalling 00:51, 20 March 2006 (UTC)
- p.p.s.: To close this circle, does the light from the early Universe have an observation horizon? I don't think any of the responses so far have answered that question. --jwalling 01:10, 20 March 2006 (UTC)
We don't see light from our part of the universe when it was young — that light is long gone from around here, as you point out. We see light from other parts of the universe when they were young, light that has travelled 13 billion light years to get here. The expansion of the universe has created those 13 billion light years of space for it to cross. See our Big Bang article.
Cosmic inflation is a red herring here. The inflationary epoch was long over by the time the Cosmic Microwave Background began to shine. Gdr 23:44, 19 March 2006 (UTC)
- Jwalling: Earth is receiving light from the early Universe from over 13 billion years in the past. Why didn't that light overtake the matter which is now earth in the first billion years?
- This is an excellent question, which is rarely answered properly.
- To rephrase, if the farthest quasars are about 13 billion light years away AND if the high redshifts indicate they're receding at nearly light velocity, how can both be true? If they're 13 billion light years away and receding at nearly light velocity, then 13 billion years ago they were much closer, therefore the light from them isn't 13 billion years old.
- The confusion comes from imprecise use of the term "distance", mainly by the popular media, and poor explanations of the expanding universe. In cosmology there are several "distances":
- Emission distance -- the distance the source was when the light we SEE NOW left the source (there and then).
- The co-moving radial distance (or reception distance) -- this is the tape measure distance if we could somehow at this cosmic instant measure the distance to the object when the light we see arrived here. This is what is used in the Hubble Law.
- When pondering distance, we tend to think of the above two. However there is another "distance" you see quoted in the popular print (because they think the above is too complicated to explain). It's the lookback time -- yes actually a time measuring how long the light we see NOW took to cross the EXPANDING universe and arrive on Earth. It is the light travel time in an expanding universe. This time is then multiplied by the speed of light and then quoted as the distance to the object (d = ct).
- Yet the distance based on lookback time can be non-intuitive because of popular misconceptions about the expanding universe. Remember, the universe is NOT expanding from objects "flying outward" through fixed space like particles from an explosion. If that were so, light from ancient nearby objects would have long reached earth's vicinity -- relativity says you can't out run a photon, nor does a rapidly receding object slow down emitted light. Rather objects are receding because the fabric of space itself is expanding, increasing the distances between objects.
- This "stretching" of space interposes additional distance during light's travel period. THIS explains why light emitted 13 billion years ago when a rapidly receding object was much closer takes 13 billion years to reach earth -- because the intervening spatial fabric expanded during the travel period, interposing additional travel time. Thus light from our relatively "local" early universe can still take many billions of years to reach us.
- At close distances (i.e. small redshifts), the emission distance, co-moving radial distance, and lookback distance will all be the same. At large distances (i.e. large redshifts), these distances will diverge. E.g, consider an object with a lookback distance of 13.2 billion light years. The emission distance would be about 4 billion light years, but the co-moving radial distance about 28 billion light years.
- This relatively simple concept is rarely explained clearly in most "Big Bang" articles. Joema 14:45, 20 March 2006 (UTC)
 Excellent response. Thank you for taking the time. --jwalling 02:04, 21 March 2006 (UTC)
Excellent response. Thank you for taking the time. --jwalling 02:04, 21 March 2006 (UTC)
- p.s. Another misleading example of the vernacular is asking how old light is that reaches Earth. Since photons travel at the speed of light, photons don't age. Photons from the early Universe arrive after 13 billions years as “young” as the instant they departed ;-)
quantum mechanics and the occupation of matter in space
[edit]Do particles really jump in and out of existance? If they do, is it possible for one put ones hand through a wall without ones hand disturbing the matter that makes up the wall and vice versa? -Danke (trancemammal)
- Particles cannot simply "jump in and out of existance", although they can exist without a definite location. If the wall is made of metal, for instance, then any particular electron in it is "smeared out" over a probability wave and has no definite location, but the total number of electrons is a conserved quantity and cannot change. You can't put your hand through the wall because the electrons repel the electrons on your hand, even if no single electron is definitely right there where you're touching it. —Keenan Pepper 21:08, 19 March 2006 (UTC)
- Well, you can argue for virtual particles "jumping in and out of existence", though on the other hand you can reasonably call that a bookkeeping trick. Shimgray | talk | 21:48, 19 March 2006 (UTC)
- Actually, they do jump in and out of existance, or the universe wouldn't work. Electric and inter-atomic and magnetic forces would all break... nothing would hold together. (We don't know yet on gravity, the fouth force. Stand by!) The book keeping refers to the probability theory some of the math used in the field of Quantum physics, but the specific field is Quantum electrodynamics. And according to current theorists, the particles are constantly coming into and out of existance in a complicated interchange governed by the Law of Conservation of energy. That's the 'bookkeeping' part– meaning debits and credits (of mass and energy) have to balance exactly, as in money matters (see: Double entry book keeping). Certain expermental results have indicated that in certain circumstances a particle or subatomic particle has to be two places at once, or the equivilent, they must each have a coresponding anti-particle, which may be expressed as a negative energy state. (bookkeeping again). The Electron's anti-particle is called the positron. Anti-matter refers to all the 'opposite' particles, as in Star Trek. Expermental data based on light wave behaviour gave strong evidence the quantum theory was indeed correct. Einstein never did accept quantum theory, saying that "God wouldn't play with dice" (or something similar). Another article on the topic would be unified field theory.
- These matters primarily involve subatomic particles, so you'd have difficulty putting even a single atom through your wall, much less your whole hand. Check back in 872 years, and we'll give you an update! FrankB
- Why 872? —Keenan Pepper 00:43, 20 March 2006 (UTC)
Video format change software
[edit]I want to change the video format 3GP File to anther kind of format of video? please I nead program to changes vidoe format.
- Have you seen the 3GP page? EricR 21:08, 19 March 2006 (UTC)
I cannot find anything about nuclear sap
[edit]Hello! I am doing an assignment on nuclear sap, however, it seems there is no information regarding it. Will you please tell me what its functions are? Thank you for your time. -Sandy
- Try searching for nucleoplasm. EricR 22:17, 19 March 2006 (UTC)
- I've created a direct for nuclear sap to nucleoplasm. Can experts please state the difference or equivalence in the article? Thanks. Samw 15:31, 25 March 2006 (UTC)
I've just reread Dune (still just as awesome as ever), and there's something I'm wondering. Frank Herbert supposedly took the time to understand most of the subjects that came up in the book. Are the Mentats based on sound psychological principles, or did he just make them up for the hell of it? Is it possible for someone to actually become one? Does anyone with those capacities currently exist? Black Carrot 22:28, 19 March 2006 (UTC)
- I thought the Juice of Sapho was a pretty important factor. That would explain why there aren't any in real life. —Keenan Pepper 22:40, 19 March 2006 (UTC)
- Try some of the far eastern mystical systems of belief, Zen and Buddism should be good starting points. btw-No writer can write quality without a lot of research. But Dune is more mystical than hard science fiction. Poul Anderson, Arthur C. Clark, Isaac Asimov and Robert A. Heinlein were the patriarchs therein, and writers of today like David Weber, John Ringo, and Tom Clancy. See Science Fictions Golden Age for some other notables. Heinlein's Citizen of the Galaxy refers to 'Renshawing' which is similar to some mentat training, and Asimov wrote a short story using specially trained Kamikazi's in an interplanetary war, as their 'computational ability' was necessary for mid-course correction pioleting their personal missile. (See Baka bomb) FrankB 23:25, 19 March 2006 (UTC)
- Is there anything less religious available? I can't believe Zen monks have a monopoly on this. And no, I don't think the Sapho juice is necessary in the books, it just helps. Like speed. Black Carrot 01:23, 20 March 2006 (UTC)
- As a matter of fact, reading those articles(Zen and stuff), I really really don't think that has anything to do with what I'm talking about. At all. And does anyone disagree with Keenan's assumption that nobody with this kind of memory exists? I know people can to pretty amazing things. I heard awhile ago of some guy breaking the "multiplying really long numbers in his head" record, and the numbers were pretty impressive. Black Carrot 20:10, 20 March 2006 (UTC)
- According to [71] and [72], people exist who might be able to reach the first level of Mentat training without much trouble. Black Carrot 20:33, 20 March 2006 (UTC)
March 20
[edit]is ink harmful?
[edit]canw writing on youself with pen be harmful?--68.169.160.206 00:26, 20 March 2006 (UTC)
- What kind of pen? Most of them are non-toxic, so you should be fine. —Keenan Pepper 00:44, 20 March 2006 (UTC)
- It is if you draw a gang sign. Or, if you're in school, there are other things you could write with it that would get you in trouble. Black Carrot 01:18, 20 March 2006 (UTC)
- Depends, do you use lead ink? LOL--152.163.100.74 03:00, 20 March 2006 (UTC)
- It is if you draw a gang sign. Or, if you're in school, there are other things you could write with it that would get you in trouble. Black Carrot 01:18, 20 March 2006 (UTC)
- Assuming, you are a young schoolgirl, I'm sorry to tell you, but this is another case of scaring you into not doing something. Writing on yourself in pen ink is not harmful by almost any means. By almost any, I am suggesting you haven't covered your whole body or eyes with ink. --
 Mac Davis] ⌇☢ ญƛ. 04:31, 20 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:31, 20 March 2006 (UTC)
- Some markers contain xylene, which is toxic and can be absorbed through the skin. --BluePlatypus 15:42, 20 March 2006 (UTC)
How to splice together .mov files?
[edit]I have some animated clips in QuickTime .mov format and need to splice them together into one long file. Is there free-of-charge software that will do this? Seahen 01:37, 20 March 2006 (UTC)
- Apparently, if you download QuickTime Alternative and install it with QuickTime Player already on your computer, then say "No" when it asks if you want to uninstall QuickTime, it will somehow unlock the QuickTime Pro features (Windows only). See this link. --Canley 02:28, 20 March 2006 (UTC)
Jesus makes me powerful?
[edit]from where did this phrase origionate?--JMP 02:03, 20 March 2006 (UTC)
- I've never heard this phrase before, and it receives no Google hits, so wherever you heard or read it may be the origin of the phrase. Seriously though, it sounds like a overly literal translation of the phrase "Lord, give me strength." which in turn is probably based on Psalm 86:16. --Canley 02:22, 20 March 2006 (UTC)
- How exactly is this a science question?--152.163.100.74 02:58, 20 March 2006 (UTC)
- God works in mysterious ways ;)--inksT 03:52, 20 March 2006 (UTC)
- How exactly is this a science question?--152.163.100.74 02:58, 20 March 2006 (UTC)
That sounds like an advertisement... Jesus...makes you powerful ...--Cosmic girl 00:15, 21 March 2006 (UTC)
- Maybe it is an ad. :) Superm401 - Talk 21:37, 21 March 2006 (UTC)
Superconductors
[edit]I need to clear something up, in the superconductors article it says that superconductors can hold a current for about 100,000 years. So I know this seems really obvious but could a superconductor basically be used as a capacitor for a couple megawatts give or take? Patrick Kreidt
- I'm not sure if I understand the question. A decent capacitor must be able to separate charge, not conduct it. Melchoir 05:42, 20 March 2006 (UTC)
- They don't work like a capacitor, but SMES systems can hold energies in the MW-hour range. That would be a big device, though. --Bob Mellish 06:32, 20 March 2006 (UTC)
- Right. Capacitors store energy in an electric field by separating charges. This is more like an inductor, which stores energy in a magnetic field by keeping a current flowing. —Keenan Pepper 13:24, 20 March 2006 (UTC)
Ignoring the semantics and addressing the implied core question "can energy be stored in superconductors", I would say yes, but it's not the best choice. Superconductors typically must be kept at low temps, which also costs energy. The ideal way to store energy differs by application. For example, water towers are an efficient way to both store clean water and water pressure (in the form gravitational potential energy), thus providing both water and pressure to the homes in a community, even when there is a short disruption in the supply. (Those communities which instead rely on electrical pumps to provide water pressure are vulnerable to both disruptions in the water supply and electrical supply, however.) StuRat 15:23, 20 March 2006 (UTC)
Psychology
[edit]I've looked all over and cannot find the answer to this psychology question.
What are some of the inaccuracies and alterations of memory?
For some reason this question makes no sense to me.
Ashley
- Category:Memory disorders might jog your memory, but you should probably use examples discussed in class. Melchoir 07:07, 20 March 2006 (UTC)
The most publicized and controversial aspects of memory defects are the suppression and recall of traumatic events or the implantation of false memories by a therapist. This type of defect has led to the imprisonment of hundreds of innocent people, especially in the 1980s. See false memory syndrome. alteripse 12:18, 20 March 2006 (UTC)
I'm almost certain you're looking for examples of Memory biases, most of them should be listed in that article. -Obli (Talk)? 23:45, 20 March 2006 (UTC)
Bird flu or H5N1
[edit]what is the name of the vaccination which is developed from the virus of bird flu(H5N1)itself & is used to protect the virus?
- It's hard to tell from your question what you're looking for. According to our flu vaccine article, there have been several vaccines developed against H5N1 but it mutates too quickly for them to be of much use and they'd be of very limited efficacy in any future pandemic, and none seems to have an exciting name. From the "developed from the virus itself" wording, I assume you're not thinking of Oseltamivir (brand name Tamiflu), the antiviral drug that's been labelled as a "flu vaccine" by several underinformed commentators (at least here in the UK). --Bth 12:54, 20 March 2006 (UTC)
THE HUMAN BODY
[edit]Who named the organs and other parts of the human body, such as arms, legs, haart, lungs, etc.------------------
- Ogg did, on a slow evening about 3.7892345 million years ago. He was entertaining his 4 year old but the names stuck and were gradually altered as his descendants separated into various language groups since then. alteripse 12:15, 20 March 2006 (UTC)
- That's pretty accurate. Looks like you got it down to the month. =P —Keenan Pepper 13:22, 20 March 2006 (UTC)
- I don't know...as much as I trust the good doctor, I have to wonder if every single one of those digits is significant. :o) EWS23 | (Leave me a message!) 18:20, 20 March 2006 (UTC)
We have no idea who named each body part, except for more recently discovered organs. Each word has a unique origin, and typically can be traced back to ancient languages, like Latin or Greek, although "haart" sounds Dutch to me. :-) StuRat 15:09, 20 March 2006 (UTC)
Do all DVD-Writers support 8cm discs?
[edit]because mine keeps saying "Illegal disc".
- There are three types of DVD Writers (over simplifying): DVD+ Writers will only accept DVD+R and DVD+RW discs, DVD- Writers will only accept DVD-R and DVD-RW discs, and DVD+/- Writers will accept both DVD+R and DVD+RW discs and DVD-R and DVD-RW discs. Check out whether you have a plus or a minus or a plus/minus writer and then verify which type of discs you are trying to write to. -- CSJoiner (talk) 18:41, 20 March 2006 (UTC)
- Oops... I missed the part about the 8 cm disc... I'm not sure about that, so if someone else knows... -- CSJoiner (talk) 18:43, 20 March 2006 (UTC)
- If it fits in the tray, it probably supports it. But you still have to worry about DVD-R/-RW/+R/+RW/-RAM formats. Do you have a manual? --Optichan 20:14, 21 March 2006 (UTC)
glue, plant tendrils BOTANY - CHEMISTRY
[edit]What is the chemical makeup of the glue that attaches the tendrils of Boston Ivy, Parthenosissus tricuspidata, to walls so permanently? Ants, and possibly other insects or mites, are in the vine when it is in it's rapid growth stage. 24.218.6.39 18:05, 22 March 2006 (UTC)
- We have roots here, not tendrils like in grapewine. "Adventitious roots form along stems of climbing plants and act to stick the stem firmly to the branch or trunk. " [73]. IANABotanist, but I would say the roots are not so much sticky. They hold by contact in micro crevices in walls, bricks, bark ... like you would do climbing. --DLL 21:49, 20 March 2006 (UTC)
Boston - sorry, I can't entirely agree, but thanks. These are not adventitious roots like those found on English ivy, Hedera helix, or Phillodendron spp. etc. They are most definitely tendrils, akin to grape vine tendrils (Parthenocissus is the grape genus) and though they do not wrap around objects for support, they do have five "sub-tendrils" with "sticky"? pads that unfurl and adhere to flat surfaces for, I am quite sure, support, not nourishment or water. Those dry, oven baked bricks facing south they climb are about as nourishing as the surface of Mars and the tendrils stay stuck years after a vine has been pulled away. The idea that micro contact, perhaps akin to a gekko's toe is a very good guess, thanks. The insects I mentioned are probably attracted to an as yet unidentified liquid at the end of the tendril as it is unfurling.
ANY BETTER ANSWERS OUT THERE? thanks.
hi: single ovary fertility ?
[edit](Made title actually useful. StuRat 15:04, 20 March 2006 (UTC))
my question is can you still get pregnant if you have one ovary ? and also how can you know if you are fertile or not?
- 1) Yes, you can; your one ovary is still producing ova (hopefully viable ones, but if they're not it's because of some other, pre-existing problem). 2) Fertility tests are available over the counter that measure the levels of the various hormones involved. Ask your pharmacist. (That's fertility tests in the sense of finding-out-where-you-are-in-your-cycle, if you're wanting to find out whether your eggs are OK you'll need to see a doctor. Our article on infertility may be of interest to you.) --Bth 13:07, 20 March 2006 (UTC)
I would suspect that a woman with one ovary would typically only be fertile once every other month, instead of the usual once a month, so timing would be more critical to achieving pregnancy. StuRat 15:04, 20 March 2006 (UTC)
- This is a popular misconception; it seems like common sense, but it's inaccurate. The ovaries don't alternate or 'take turns' during ovulation. There are various chemical cues which either ovary can pick up leading to the release of a mature egg. (The old wives' tale that boys come from one ovary and girls come from the other is also wrong.)
- In animal studies, there is an indication that the remaining ovary is enlarged after one ovary has been removed; this result hasn't been confirmed in humans, however. The question is moot to an extent, however—the number of potential eggs is fixed by the time a girl is born. Numbering in the millions at birth, the supply is exhausted at menopause. So the removal of an ovary also removes half of the supply of potential ova, potentially hastening menopause. (There are some studies which seem to confirm this.)
- Until the supply of eggs is exhausted, however, there shouldn't be a major reduction in fertility (unless whatever medical condition led to the loss or removal of the first ovary also affects the remaining one or the rest of the reproductive machinery.) TenOfAllTrades(talk) 16:04, 20 March 2006 (UTC)
- There seems to be a discrepancy in your math. Even with only one million ova, used at a rate of say 13 per year, I would expect them to last 1,000,000/13 or around 76,923 years, but they only seem to last about 40 years, which would be 40x13 or 520 ova. I suspect this means that the ova aren't the limiting factor after all, but rather hormonal changes, such as decreased estrogen, cause menopause. Thus, having only one ovary would reduce the amount of estrogen produced prematurely, and therefore bring about an early menopause. StuRat 17:19, 20 March 2006 (UTC)
- Usually when one of a number of glands are removed the others pick up the slack. So removal of an ovary would not necessarily result in early menopause, but result in menopause-like symptoms to a lesser degree than true menopause until the remaining ovary picked up the slack.
Video
[edit]Some hardware encoder has ASI out,what is ASI ? and where is it use ? Explain in detail. Thanks
Moon "tides"?
[edit]NASA recently published an on-line article (http://science.nasa.gov/headlines/y2006/15mar_moonquakes.htm) which stated, "There are at least four different kinds of moonquakes: (1) deep moonquakes about 700 km below the surface, probably caused by tides; ..." I do not understand the reference to tides. Surely not tides on earth; those are a result of lunar position and wouldn't seem to have an effect on the moon itself. The Wikipedia article on the moon makes no reference to any sub-surface water that might be involved in "lunar tides." Is there believed to be a molten core of some material that undergoes "tidal movements" as the earth & moon travel in orbit about the sun? Again, the Wikipedia article on the moon makes reference to the lunar "crust" but does not mention any 'ocean' of liquid beneath this crust (unless I just missed that reference). Thanks in advance, Dennis 207.5.100.27 15:22, 20 March 2006 (UTC)
- Tides can and do occur even in solid bodies, though obviously to a lesser extent than in liquids (or gases). While the moon keeps (almost) the same face towards the earth at all times, and thus doesn't experience "earth tides", it still feels a tidal force from the sun. —Ilmari Karonen (talk) 16:10, 20 March 2006 (UTC)
Ah, of course. "Tidal forces" on a solid object = stress. I guess if the NASA article had used the term tidal forces instead of tides, I would have caught on. Thanks *very* much for your quick response. Dennis.
TVP vs MSG
[edit]Hello!
I have been reading some things about MSG and have found on this site http://www.happyhippie.com/ubb/Forum9/HTML/000102.html - a reference indicating that Textured protein always contains MSG
Would that also go for TVP? or are we sure that it does not include MSG?
Thank you for your response
- All proteins or protein products contain some amount of glutamic acid, whose sodium salt MSG is. Whether it's there as a free amino acid or as part of a peptide chain makes little difference, since the peptide chains will be broken during digestion anyway. So yes, TVP does contain MSG. So do meat and soybeans and any other protein sources. —Ilmari Karonen (talk) 16:34, 20 March 2006 (UTC)
- There should probably be a minor caveat attached to that. While digestion of nearly all proteins will yield some glutamic acid (and hence glutamate), consuming the sodium salt directly may lead to effects related to rapid uptake—a transient spike in the levels of glutamate that one wouldn't see when (comparatively) slowly digesting a meal. Compare the effects of starch and glucose, for example. Starch is made up of chained glucose units, and digestion serves to reduce it to glucose which the body can use. However, consuming one product or the other can have different physiological effects. Ingesting pure glucose will result in a much more rapid spike in blood sugar than ingesting starch.
- However, except for a very small fraction of the population there appears to be no harm caused by a transient increase in blood glutamate levels. A few particularly sensitive individuals seem to experience short-term symptoms after consuming large quantities of MSG. The amount of MSG in TVP should be fairly small. TenOfAllTrades(talk) 00:01, 21 March 2006 (UTC)
- Half the dry weight of the human body is protein. On average, 5.8% of that is glutamic acid. Thus, your average person is walking around with about 3 kg of pure glutamate. The vast majority of all the glutamate you get in your food is from protein, not from MSG. MSG does contain Sodium, which some people need to avoid. However, the amount of Sodium from MSG when used as a flavoring is far less than that in table salt. (Which is used in larger quantities and contains more Sodium by weight) There is no reason to avoid MSG, it's been heavily studied, and the consensus is that it's perfectly safe, and as I'm trying to say: there is little reason to assume it'd be toxic to begin with. That doesn't mean MSG isn't a public health problem.. In the sense that it distracts people from real dietary issues. --BluePlatypus 17:49, 20 March 2006 (UTC)
- I personally have to avoid eating too much food with large amounts of MSG (canned & chain-restaurant soups, Chinese food, ramen, etc.) because the next day I always get severe migraines. Also, there are others who claim "Chinese restaurant syndrome". I wouldn't call it perfectly safe, but for the general public it's generally safe. — TheKMantalk 00:51, 21 March 2006 (UTC)
MRSA in British Hospital Staff
[edit]Has any British National Health Service Hospital ever conducted an MRSA screening programme amongst its staff, both medical and non-medical? If not, should they not do so without further delay? If yes, what were the consequences in terms of allowing/not allowing those found to be infected to continue working in close proximity to patients?
- Time to suitly emphazi again -> MRSA. StuRat 17:05, 20 March 2006 (UTC)
- Thank you for that meaningless comment which was patently unhelpful to me as a new user of Wikpedia. If you have anything of useful interest to add either to your comment or my very serious question I shall be most grateful for it. But please do not indulge yourself in quasi-academic twaddle. It is not appreciated. A site such as this is concerned with the dissemination of knowledge, and not the kind of deliberate fogging behaviour from which you seem to get your rocks off.
- Since you used a technical acronym without bothering to explain what it means, (just like your last question on MRSA), I was nice enough to provide a link where people can find the meaning. We are doing you a favor by answering your questions, and don't forget it. Don't be rude to us or we won't bothering answering your questions at all. StuRat 17:32, 20 March 2006 (UTC)
- Thank you for your prompt response. I now (think) I understand what you meant (both times) by suitly emphazi, a phrase not to be found in the Wikipedia Search Engine nor anywhere else I took the trouble to look. I also (think) I now understand that questionners like myself should not use technical acronyms such as 'MRSA' even though that particular one must be as commonly understood as HIV, or AIDS. I also appreciate your voluntary status and earlier advice on where to find more information on 'MRSA', for which, once again, many thanks, though it did not lead me to the kind of answer I am looking for. But it may be of some use to myself, and others similarly inexpert in using Wikipedia, to have a real Idiots' Guide on the page, which also included Suitly Abbrevies, Must Do's, and Don't Do's. Please accept my apologies for any offence given.
- If you had Googled the words "suitly emphazi", the second result provides the answer, so I guess you didn't bother to take the trouble to look it up on Google. While StuRat was gracious enough to accept your apology, it reads to me like a very condescending way saying sorry for being rude. The question itself looks like it's part of an assignment, or something to harass the Minister of Health with during question time in parliament. To answer it would require a very extensive literature search - you'd be better off asking this question on Google answers.--inksT 21:56, 20 March 2006 (UTC)
- Apology accepted. Perhaps it's as well-know in the UK, but that abbrev is far less well-known in the US than others, like AIDS. You must also bear in mind that not all responses to a question will be answers. We may also need to explain the question, ask what you meant, point out why the question can't be answered in it's current form, etc. StuRat 19:02, 20 March 2006 (UTC)
Now, to get to the actual question: I don't know if there have been any MRSA screening programs in British hospitals, but can list some factors that would go into deciding whether it's wise:
1) The cost of the test, which can be purely economic or can include the medical and non-medical staff's time taken away from their duties.
2) The risk of the test. Is it safe ?
3) How widespread the disease is at present.
4) How transmissible the disease is from the medical and non-medical staff to patients.
5) How dangerous the disease would be if contracted by patients.
So, it would be advisable to perform a cheap, quick, safe test for a widespread, highly transmissible, deadly disease. Does MRSA meet those qualifications ? If so, then testing would be indicated, with the frequency of the testing depending on the answers, as well.
StuRat 19:11, 20 March 2006 (UTC)
- For fun, I looked this up. Firstly, the acronym MRSA is very British, I think we call it something else in Canada (and elsewhere). Secondly, although we appreciate questions from those who think we are superhuman, we can't provide answers that don't exist on the Internet. Even if you shout and yell. These superbugs are an emerging issue, and there isn't much on them. The most important thing is to get the dang doctors to gel their hands between visits. This is apparently very difficult. Also, if I am healthy, and am carrying a few superbugs, what are you going to do? Even if you dip me in a vat of acid, my putrid body will pick up stray bacteria as soon as it's lifted. You could just shoot everybody, but that has its problems, too. --Zeizmic 20:09, 20 March 2006 (UTC)
- The acronym MRSA is also used in Canada. - 216.218.44.237 22:42, 21 March 2006 (UTC)
- And it's also used in the U.S. There's nothing "very British" about it. - Nunh-huh 05:16, 23 March 2006 (UTC)
- The acronym MRSA is also used in Canada. - 216.218.44.237 22:42, 21 March 2006 (UTC)
- I looked this up on Google News. This story contains the following quote: He [an infection control specialist in Colchester] dismisses the idea of routine MRSA screening for practice staff: "For a start, it would miss people who simply carry the organism on their hands - and who are most likely to pass it on." Which reinforces Zeizmic's point about the importance of getting highly trained medical professionals to follow basic hygiene advice. (Which doesn't sound like it should be that difficult, does it?) --Bth 10:45, 21 March 2006 (UTC)
- I suggest a punitive approach, like cutting their pay 1% each time you catch them failing to wash their hands when indicated. StuRat 14:37, 21 March 2006 (UTC)
- Actually the most common screening test for hospital employees is culturing nasal swabs (yes, infection control nurses visit the employees and stick cotton swabs up their nostrils) to test for nasal colonization with MRSA. Those employees found to carry MRSA in their nares are subjected to topical application of mupericin or some other antibiotic ointment up the nose. These measures decrease the rate of nosocomial MRSA infections, and so do handwashing programs (for a time, till everyone gets used to them). See this article (search for MRSA) for references and details. Targetted MRSA surveillance (culturing the nares of employees who work in an area of the hospital where an MRSA has occurred) is a standard infection control practice in U.S. hospitals. But routine periodic culture of all employees is not. - Nunh-huh 05:16, 23 March 2006 (UTC)
- Thanks Nunh-huh for your informative (and supportive) responses. They are much appreciated.
SPDIF
[edit]Can anyone tell me what SPDIF is in relation to computers and sound cards. The article is too elaborate. --Chris 16:20, 20 March 2006 (UTC)
- S/PDIF is a means of sending digital audio from one device to another. If your soundcard has S/PDIF-out, then you can plug that into the S/PDIF-in on a receiver. If you don't have any equipment with an S/PDIF port, you will have no use for it on a soundcard. --Kainaw (talk) 17:00, 20 March 2006 (UTC)
- I see. I think my sound card allows for it, but I don't have anything else to connect it to. Just wondering. Thanks. --Chris 17:05, 20 March 2006 (UTC)
- I've added a request to at least make the introduction of the SPDIF article less technical, see Talk:S/PDIF -- Bovineone 00:10, 21 March 2006 (UTC)
- I see. I think my sound card allows for it, but I don't have anything else to connect it to. Just wondering. Thanks. --Chris 17:05, 20 March 2006 (UTC)
Excel macro: Finding words and neighbors using wildcards
[edit]Hi everyone,
I know code isn't answered much here, but I've got a thesis riding on this, so any help you could give would be REALLY appreciated! :)
I've got some experimental data in an Excel file with two columns: One with words and one with numbers.
What I want to do is to save all the words for which their number is > 100, AND for which there exists word* (ie: the original word with any arbitrary letters following it) with a number < 50.
So for instance, if I have
abc 65 abcd 32 pqr 105 xyz 101 xyza 45 xyzbc 35
I would like an output (anywhere) which shows
xyz 101 xyza 45 xyzbc 35
My knowledge of excel programming is really pretty basic, so I'd really love a detailed response.
Thank you so much for your help — this could save my month!
~Mary M.
- It seems like the easy way to do this would be to select both columns and then choose Data --> Sort. Then sort first by the column with the word and secondly by the column with the numbers. This will arrange your columns in alphabetical order (keeping numbers associated with words). You can then scroll through the list and manually select any data that you want to keep and copy this data into your output range. There is probably a more elegant work-around that does EXACTLY what you're asking for, but this way is almost definately faster if you're just trying to analyze your data. 169.232.140.49 18:29, 20 March 2006 (UTC)
- Oops... I wasn't signed in. -- CSJoiner (talk) 18:36, 20 March 2006 (UTC)
- Dear Csjoiner — the problem is that the list is many thousands of items long. It would take way too long to search by hand.
- Any help really appreciated! ~Mary
- Maybe I'm misunderstanding your problem, so allow me to restate my understanding of it: You're trying to find rows of data in your Excel spreadsheet where the word (in one column of words) starts with some specific string of characters and where the number (in some column of numbers) is greater than some number, is that correct?
- If so, the sort that I described will alphabatize all of the words, so manually selecting the block of words that you are looking for is as simple as scrolling to the first word in the list that starts with the string of characters which you are looking for and then manually selecting every entry that follows until you reach the last word that starts with the string of characters which you are looking for. (Since you're only looking for firsts and lasts, this isn't the same as checking each entry individually.) I realize that this is slightly time consuming, but it should be less time consuming than writing a macro to do it for you.
- If you then need to further analyze your results, you can copy and paste your results onto a second spreadsheet and re-sort this group of results by selecting both the word column and the number column, choosing Data --> Sort, and sorting by the number column. Then, you need to simply eliminate all entries that contain numbers less than or equal to 100. Since you've sorted the list, it's again only a matter of looking for firsts and lasts... just scroll down until all numbers are greater than 100 and you're set! -- CSJoiner (talk) 19:29, 20 March 2006 (UTC)
- Make a copy of your original data. For "anything with >100 in the second column", sort by the second column. Then, delete anything you don't want. I'd use a descending sort. Then on a copy of the original data, for the "anything beginning with word*", sort on the first column. Delete everything you don't want. Resort on the second column to get your >50 if you like and delete anything you don't want. You will now have the original data, a file with the >100 and a file with entries beginning with the word you like. You can cut/paste what you need into one document. --Kainaw (talk) 19:32, 20 March 2006 (UTC)
- Oopses! I think that you have try this. Sort your spreadsheet by the first column.
- Then add formulas there (check yourself the first line results, because one formula refer to the preceding line) :
- second line, third column =IF(AND(B2>100;LEFT(A3;LEN(A2))=A2);1;0)
- second line, fourth column =IF(AND(C1+D1>0;B2<50);1;0)
- Copy the formulas down. Select data that bear "1" in the third or fourth column (freeze results before sorting again : "copy" and "paste special" | values). I can give more precise help but mind my Excel is in french. --DLL 21:30, 20 March 2006 (UTC)
- This makes some assumptions about the data that may produce wrong results. My solution (which works basically the same way follows). -- CSJoiner (talk) 22:47, 20 March 2006 (UTC)
- Okay. I understand now, and this should work.
- Step One: Make a new spreadsheet. In Cell A1 write "Words", in Cell B1 write "numbers" in Cell C1 write "0" in Cell D1 write "Match Words" and in cell E1 write "Match Numbers".
- Step Two: Copy all of the words and numbers into columns A and B beginning with row 2 (do not leave any empty rows).
- Step Three: Select columns A and B and (from the menu) choose Data --> Sort. Ensure that "My Data has a header row" is selected, and choose "Words" in ascending order, then click okay.
- Step Four: In cell C2 write IF(B2>100,A2,C1) and then copy this for every cell in the column
- Step Five: In cell D2 write IF(OR(AND(B2>100,LEFT(A3,LEN(C2))=C2),AND(B2<50,LEFT(A2,LEN(C2))=C2)),A2,0) and then copy this for every cell in the column
- Step Six: In cell E2 write IF(D2=0,"N/A",B2) and then copy this for every cell in the column
- Step Seven: Select columns D and E and then choose "copy" then right click and choose "paste special" --> "values" then click okay
- Step Eight: Select columns D and E and and (from the menu) choose Data --> Sort. Ensure that "My Data has a header row" is selected, and choose "Match Words" in ascending order, then click okay.
- And that's it. The two columns D and E now contain your matches (ignore any rows with "0" and "NA" in them). -- CSJoiner (talk) 22:47, 20 March 2006 (UTC)
Hi everyone.
First, thanks so much for the adviced. It was really helpful reading what you posted, and sorry that I caused confusion by not making clear what it was that I wanted. I ended up writing a macro after all, and it was much shorter than I thought it would have to be. This macro does what I wanted, and lays all the selected words to the side in the next column, where a simple 'sort' can bring them all up to the top:
Sub Find_Words()
' Select cell A2, *first line of data*.
Range("A2").Select
' Set Do loop to stop when an empty cell is reached.
Do Until IsEmpty(ActiveCell)
Dim root As String
root = ActiveCell.Value
If ActiveCell.Offset(0, 1).Value > 100 Then 'If root is high enough
Dim i As Integer
For i = 1 To 10 'Check for derivations in a window of length 10
Dim deriv As String
deriv = ActiveCell.Offset(i, 0).Value
If InStr(deriv, root) = 1 Then
If ActiveCell.Offset(i, 1).Value < 50 Then 'If deriv is low enough
ActiveCell.Offset(0, 2).Value = root 'Copy words to the side
ActiveCell.Offset(i, 2).Value = deriv
End If
End If
Next
End If
ActiveCell.Offset(1, 0).Select
Loop
End Sub
Thanks for all your help! ~Mary
- Thanks, Csjoiner! I expect every one here take our answers cum grano salis. I thought after that my formulas would be based on assumptions about data (and would be refactored, according to WP usage). Also, it is a pity that XL does not offer utilities like grep. --DLL 18:24, 21 March 2006 (UTC)
Variable vs Constant bit rate
[edit]Here's a question for all you computer geeks lurking on the Ref Desk. What are the advantages and disadvantages of constant and variable bit rate for encoding music for storage and playback on a computer, in MP3 format? (I know that, for instance, OGG Vorbis files are almost always VBR) If I understand it correctly, for space-limited applications (storing music on a hard drive), VBR beats CBR because it reduces the bit rate for 'simple' sections, reducing file size. But does it, for instance, impose an extra decoding overhead or something? Why is it not used universally? Thanks! — QuantumEleven | (talk) 17:57, 20 March 2006 (UTC)
- A lot of older decoders get a bit upset with VBR - not much of a problem now, but if you're looking to use one of those MP3-CD players that were around c.2000/1, it can be an issue. Shimgray | talk | 18:44, 20 March 2006 (UTC)
- There isn't really any processing overhead, the mp3 codec specifies each frame to have its own bit rate in the header. Originally, every frame had the same bit rate in their headers, but it was found that the bit rate could be varied in each frame header. So in either case, the decoder has to read the header for every frame, and therfore there's no overhead, though vbr mp3's have the advantage of providing higher sound quality for the same file size. The only problems you'll run into will be with older mp3 decoders that didn't read the headers of every frame.--Krackpipe 22:38, 20 March 2006 (UTC)
Can you determine what this program is for? When I open it up in WordPad, it has the following:
œËË�uÒ�‘X
The rest won't copy and paste here for some reason.
- You are seeing garbage. You cannot view a program in WordPad. You have to view it in a hex editor. --Kainaw (talk) 18:39, 20 March 2006 (UTC)
- Can't you do this simply in Notepad? Kilo-Lima|(talk) 18:52, 20 March 2006 (UTC)
- You can use a program such as SysInternals' strings utility to view the printable ASCII text within a binary executable. -- Bovineone 19:07, 20 March 2006 (UTC)
- To be VERY brief... Text (like you see in WordPad or Notepad) uses 7 bits. Data is stored in 8 bits. So, you cannot use WordPad, Notepad, Edit, Word, Excel, Paint, or Internet Explorer to view the 8-bit program data. You must use a Hex editor. Even then, the question "What is this program for?" cannot be answered. --Kainaw (talk) 19:17, 20 March 2006 (UTC)
- It is quite likely part of a larger application and will not run on its own. What is the filename? If you Google the filename you will probably find out what it is for. --Shantavira 19:18, 20 March 2006 (UTC)
- Out of curiosity, this only applies to very simple text editors? I mean, MS Word uses unicode, right? Oskar 19:47, 20 March 2006 (UTC)
- Yes. I was being very brief. Unicode is 8-bit. But, you can't make sense of program code by looking at it in Unicode. You'll just get a jumbled mess of foreign characters. They won't even be all of the same language (some Chinese, some Japanese, some Taiwanese, some Swahili...) --Kainaw (talk) 19:55, 20 March 2006 (UTC)
- The question is not 100% clear, but it seems to be talking about an executable file, in which case it's a compiled binary file. The only way to "open" such a file is in a hex editor, and even then it will be gibberish to all but the most expert programmer. The only real way to find out what a program does is to run it - which, if you don't know where it's from, might not be such a good idea!
Unicode is still text, just fancily-encoded text. — QuantumEleven | (talk) 19:54, 20 March 2006 (UTC)
- The question is not 100% clear, but it seems to be talking about an executable file, in which case it's a compiled binary file. The only way to "open" such a file is in a hex editor, and even then it will be gibberish to all but the most expert programmer. The only real way to find out what a program does is to run it - which, if you don't know where it's from, might not be such a good idea!
- You can open binary files in a hex editor, but you won't be able to get much information from it other than a message saying it can't be run in DOS mode and perhaps some other strings. If you really want to know what the program does, you can check if there's a Version tab in its properties dialog box or search for the filename on the Internet. --Optichan 20:08, 21 March 2006 (UTC)
- If it came attached to an email message, it may be a trojan. If you're curious, you can 1. learn machine language and disassemble it. 2. run it on one of your unimportant machines, where it may do something like erase your harddrive or give access to government spooks from unknown country. GangofOne 23:17, 21 March 2006 (UTC)
- According to Magic number (programming), it doesn't look like any "standard" executable file (i.e. no "MZ" at the front). Try changing the encoding in your text editor to see if it makes sense in some other character set.
- Also, try viewing the file in khexedit (or similar) and look for magic numbers, in case your text editor isn't showing you the complete file (it might contain control characters that cause wordpad to not display properly, and the characters you pasted here might not be at the beginning of the file) Ojw 13:42, 25 March 2006 (UTC)
heat
[edit]a silver bar 0.125 meter long is subjected to a temperature change from 200degrees c. to 100degrees c. what will be the length of the bar after the temperature change?
- Is this for your physics or chemistry homework? Either way, there's a note at the top about not asking homework questions. --Kainaw (talk) 23:42, 20 March 2006 (UTC)
- You might find our articles on thermal expansion and the coefficient of thermal expansion useful in doing your homework, however. TenOfAllTrades(talk) 04:05, 21 March 2006 (UTC)
- Rule of thumb : the bar shall be slightly shorter.
- Aditional question : Silver says the coeff. = "(25 °C) 18.9 µm/(m•K)". It cannot be linear between the absolute zero point and the melting one. The links above should explain that the coeffs are approximative, only true around the indicated temperature, and give more examples. This is important when building bridges or any big metallic structures. Where are the "real" data for calculation ? --DLL 18:17, 21 March 2006 (UTC)
Digital Universe
[edit]According to serious accepted (uncrancky) physics... is there a possibility that our universe which seems analog, is digital?...like, subatomic particles are being 'represented' in a 'digital' machine?...what do informatics and physics have to say about this?. --Cosmic girl 23:43, 20 March 2006 (UTC)
There is a possibility.
"serious accepted (uncrancky) physics"; there is no agreement on what that phrase means , so I ignore it.
- Ed Fredkin thought of it
- Stephen Wolfram seem to believe it see A New Kind of Science
- John Walker. Review of Leonard Susskind's The Cosmic Landscape: String Theory and the Illusion of Intelligent Design blog None Dare Call It Reason Makes a case for the idea that a kind of intelligent design where the universe is actually a simulation in a computer is more parsimonius (in the Ocham's Razor sense) than current physical theories.
Lots of physicist question "continuity", the idea that space-time can be infinitely divided, eg Richard Feynman. So there would be some granularity.
- Heim theory talks about such granularity. (not accepted physics, though)
Nobody knows, or if they do, they're not telling. --GangofOne 00:26, 21 March 2006 (UTC)
I suppose one could argue than particles are "digital", meaning discrete packets, whereas waves are "analog", meaning continuous. Quantum mechanics is based on "quanta", or discrete energy packets, as well. StuRat 00:37, 21 March 2006 (UTC)
hmmm...cool I c... but I meant more like... the basic 'thing' being information...like '1s and 0s'...and 'events' like... a particle 'moves' not because it actually mooooooves (lol) ...but because it ceases to exist here and suddenly appears there, but that happens so darn fast that we can't notice...do you think granularity is more plausible than continuity? or the other way around?.--Cosmic girl 00:45, 21 March 2006 (UTC)
- I think maybe this theory you want to know about is called or related to the holographic theory. --
 Mac Davis] ⌇☢ ญƛ. 04:53, 21 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:53, 21 March 2006 (UTC)
- The Wikipedia article about the holographic theory can be found at Holographic principle. --DannyZ 07:50, 22 March 2006 (UTC)
Thanx! :) ...but I think they are kind of different...but I'll take a look at the article.--Cosmic girl 16:41, 22 March 2006 (UTC)
toxic plastic
[edit]If you were to burn a credit card you received in the mail, would it release toxic fumes? Or if you threw a old bottle of Germ-X into a campfire? etc. schyler 23:50, 20 March 2006 (UTC)
- Yes. Unless you burn plastics at very high temperatures (much higher than those you typically have available in a campfire or home fireplace) there will be a number of toxic products of incomplete combustion. TenOfAllTrades(talk) 00:04, 21 March 2006 (UTC)
- I'm not quite sure what type of plastic is used for credit cards, but as a general rule, burning plastics in excess oxygen should yeild water and carbon dioxide, unless the oxygen supply is limited, causing carbon monoxide to form, which is highly toxic. As for the Germ-X; its main components appear to be ethanol, good old alcohol, nothing harmful about burning that, you get water and carbon dioxide there, too. The other compnent, Benzalkonium chloride, is however listed as harmful, so I wouldn't bet on vaporizing it being a good idea, but then agin, it's most likely to be found in a very low concentration of germ-x from what I've gathered. -Obli (Talk)? 00:06, 21 March 2006 (UTC)
- I would go with TenOfAllTrades' answer, not Obli's. You can tell you don't get complete combustion because of the burning plastic smell. If you had complete combustion to carbon dioxide and water, you wouldn't smell anything. StuRat 00:32, 21 March 2006 (UTC)
- I've always heard that burning plastics release dioxin and furan compounds. --Kainaw (talk) 00:27, 21 March 2006 (UTC)
- Yeah, I should probably add that it's a very theorethical answer, the other things in addition to carbon di/monoxide and water depend on what other elements are present in the plastic compound, I've just assumed Carbon and hydrogen in my asnwer, since no specific type of plastic was specified, other than "plastic card plastic". -Obli (Talk)? 14:43, 21 March 2006 (UTC)
- It's more difficult to avoid incomplete combustion than you think. Not only is excess oxygen required, but also a sufficiently high temperature. You don't get those in a normal fire. That's why high temperature incinerators are used, to avoid releasing pollution into the air. An alternative to a high temperature is a 100% oxygen environment. In such an environment, things are much more flammable than in normal air (including people, making this rather dangerous). StuRat 21:12, 22 March 2006 (UTC)
March 21
[edit]Will the PS3 loose the console war? GPU of the PS3?
[edit]What will happen to the video game industry as a whole if Sony looses the console war to Microsoft? It looks as though this could happen since the XBOX 360 has had a head start in sales and the PS3 has to wait till November to play catch up. Also, the PS3 will almost certainly be more expensive than the XBOX 360. I don't know if gamers are willing to pay $500-600 for a "superior product" as Sony claims.
Also, is there any truth to the rumor that Sony was going to develop the graphics for the PS3 but soon discovered that the Cell processor was not powerful enough and Nvidia had to bail them out by stuffing a 7800GTX into the PS3 and renaming it the "RSX"?
- If PS3 turns out to be too expensive and ends up failing miserably, the victory will be going to the Nintendo Revolution. XBox has never been popular in Japan, and most likely never will - and since Japan is probably the most important territory, they'd only be able to win on a national scale.
- I honestly think Revolution is the biggest competitor anyway, though it does depend on them being able to make good use of the controller. If they can do that, though, they stand a good chance of gaining MASSIVE sales.
- As for the rumor, I haven't heard of it, and I can find nothing supporting it here on the Wiki. Of course, that doesn't necessarily mean it isn't true, but I doubt it. The processor is powerful enough to make full-res HDTVs with PLENTY of power, see Cell (microprocessor). --Pidgeot (t) (c) (e) 01:57, 21 March 2006 (UTC)
- Somhow I feel this has nothing to do with Science. More like Sociology or something. Either way, this discussion should not take place on this page. I'm not totally sure, but I think this discussion could go on some other special page like the Wikipedia Millionth Topic Pool. schyler 02:25, 21 March 2006 (UTC)
- I disagree. Which of a list of competing electronic products wins is due, at least in part, to the level of technology each can offer. StuRat 03:12, 21 March 2006 (UTC)
- In that way you are right. I just saw this discussion turning into a XBox 360 fans vs. PS3 fans vs. Revolution fans. But conserning the technological aspects, I can see that fitting on this page. schyler 12:56, 21 March 2006 (UTC)
- Perhaps they'll "lose" the console war, but I'm positive they won't "loose" it. Personally, I couldn't care less which one flops. - 131.211.210.15 12:41, 28 March 2006 (UTC)
elevation
[edit]how is elevation measured?
- The article Altimeter describes a few methods. Melchoir 01:04, 21 March 2006 (UTC)
- And it's typically described as feet or meters above sea level. StuRat 01:27, 21 March 2006 (UTC)
- When measuring altitude, but when measuring elevation it's usually height above the geoid. EricR 02:01, 21 March 2006 (UTC)
- And it's typically described as feet or meters above sea level. StuRat 01:27, 21 March 2006 (UTC)
- There are lots of ways to measure elevation.
- use your GPS receiver
- use a clinometer or theodolite
- by celestial observation
- measure the boiling point of water to get the pressure altitude
- throw something off the top and time the fall
- Elevations on the earth's surface have probably been measured by all those methods. Take a look at Shuttle Radar Topography Mission to see a good way of doing it today. EricR 01:37, 21 March 2006 (UTC)
- And it's shown on a topographical map with elevation contour lines. StuRat 02:05, 21 March 2006 (UTC)
Time to repeat a classic tale: Teacher says Explain how to use a barometer to determine the height of a tall building. The student wrote, Tie a rope around the barometer, lower it from the top of the building until it hits the ground, make a mark on the rope, pull it up, and measure the length of the rope. (There are other ways of doing this.) This put the teacher in a bit of a bind. He called in one of his colleagues, and explained what had happened. The colleague said, In a way that demonstrates your knowledge of physics, explain how to use a barometer to determine the height of a tall building. The student said, Go to the top of the building with a barometer and a stopwatch. Drop the barometer, and measure the time before the barometer splatters on the ground beneath. Then use the formula y = 1/2 at2 to calculate the height of the building. The teachers conferred and gave him almost full credit. The teacher asked what some of the other ways were: Go outside on a sunny day, and measure the height of the barometer, the length of the barometer's shadow, and the length of the building's shadows, and use ratios to determine the height of the building. This probably isn't the best way, but go into the basement, knock on the superintendent's door, and say, 'Mr. Superintendent! I have a fine barometer for you if you will only tell me the height of the building!' WAS 4.250 12:30, 23 March 2006 (UTC)
iTunes Glitch
[edit]Whenever I try to download pod-casts or see what is in the music store, I see this message in a pop-window: "iTunes could not connect to the Music Store. Make sure your network connection is active and try again." My Internet connection is fine and I have been seeing this message for three days. Also, my version is the latest — 6.0.4.2.
Can anyone offer suggestions and advice?
Patchouli 01:43, 21 March 2006 (UTC)
- Windows or Mac? Did the error occur since you updated iTunes to the latest version. Have you installed any other software or utilities in the last week? Have you installed or activated a firewall or any security software like Little Snitch? Are your network settings using your ISP's proxy server? --Canley 02:09, 21 March 2006 (UTC)
- I have Windows XP Media Center Edition. I updated my iTunes yesterday and this hasn't fixed the problem. I only remember having installed the Google Web Accelerator, Shockwave, and everything from the Windows Update site of Microsoft.
My Windows firewall is off and the sole firewall on my computer is Norton Personal Firewall. Since the Norton Firewall is for Internet Explorer browsing, then it shouldn't affect iTunes.
Wait a minute, I just disabled the Norton Firewall, and then it worked.
Patchouli 02:47, 21 March 2006 (UTC)
- According to this Apple support document, you need to download the latest firewall definitions from Symantec to allow the iTunes Music Store to access the internet. --Canley 05:01, 21 March 2006 (UTC)
- Thank you for imparting your knowledge.Patchouli 07:20, 21 March 2006 (UTC)
Just a comment on "Since the Norton Firewall is for Internet Explorer browsing..." The purpose of a firewall is to block unexpected attempts to connect to your computer from outside, and sometimes to stop your computer connecting unexpectly to outside. This isn't really anything to do with Internet Explorer. Typically, the firewall is going to allow Internet Explorer to connect to the internet, because it needs to do that so you can browse. Blocking all other programs is normal, and you may need to manually allow them to do their work, if you want them to access the internet. The idea of a firewall is (a) to stop bad guys reaching your computer and (b) if the bad guys are already there, stop them calling home with all your private information. Notinasnaid 12:18, 21 March 2006 (UTC)
Organic Synthesization
[edit]hello Give four basic products of organic synthesise and three of their application
Plant Fertilizer
[edit]What does "Total Nitrogen" do for house plants? What does "Phosphoric Acid" do for house planets? What does "Soluble Potash" do for house plants?--209.89.235.178 04:28, 21 March 2006 (UTC)
- Check the nitrogen, phosphoric acid, potash, and fertilizer articles and come back if you aren't satisfied. --
 Mac Davis] ⌇☢ ญƛ. 04:50, 21 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:50, 21 March 2006 (UTC)
Confusing
[edit]This is in the cell (microprocessor) article:
Sony's PlayStation 3 video game console will contain the first production application of the Cell processor, clocked at 3.2 GHz and containing seven usable SPEs. An eighth will be manufactured, but one will be disabled at the factory in order to allow Sony to increase the yield on the processor manufacture.
Could someone explain this statement? --Shanedidona 05:01, 21 March 2006 (UTC)
- Defects in a semiconductor IC are typically a bad spot on the die, due either to an irregularity in the silicon crystal on which it was deposited, or a defect in the depositional process. That bad spot makes the circuits around it, and any depending on them (and any on them, etc.) inoperable. It sounds like Sony will put eight SPEs on the die. If one of those SPEs is knocked out by a defect, seven will remain. They've designed the whole chip such that, when they detect one SPE is bad during manufacturing-test, they can set the equivalent of a jumper (in practice I think they'll make or break a solder connection in the ceramic package that hosts the chip). The chip is wired such that it has seven logical SPEs, each of which is wired to one of the eight available physical ones. The setting of the pseudo-jumper makes it rewire slightly, to use the "spare" SPE, rather than the defective one. So long as only one SPE is defective, the whole chip is still operational. This increases yield because it means that lots of defects that would have killed a chip with no redundancy now don't. Some components aren't redundant, and they're still susceptible to defects which span two SPEs, or to multiple defects, but I'm sure they've analysed the typical defects that arise from the process they're using and have calculated that the gains in yield earn them more revenue than the additional costs associated with this redundancy. I think you'll see a lot more of this kind of thing, mostly in ICs where there are many identical subsystems (particularly memory chips); increasing yield by further increasing the quality of the silicon or the cleanliness of the cleanroom is very epensive (as semiconductor companies already do a very good job of that, and those errors that remain are tough to fix), whereas wasting a few percent of the floorspace on each chip (for a spare element that generally isn't needed) is a pretty low cost. -- Finlay McWalter | Talk 11:05, 21 March 2006 (UTC)
Differences between pass extinctions and the current one
[edit]There have been many extinctions in the pass and according to some scientists, there is one going on right now. If there are any differences between pass extinctions and the current one, what are they? (example: pass ones are largely natural, but this one is probably due to human causes)
Thanks! Alex Ng 05:53, 21 March 2006 (UTC)
- The word for things from before is "past", not "pass". Current extinctions are largely the result of human intervention while past extinctions were the result of natural forces, like a new species which outcompetes the old one, giant meteors, climate change, and changes in the percentage of oxygen in the air. StuRat 11:22, 21 March 2006 (UTC)
- Also, I suspect you meant to ask about "mass extinctions", as extinctions of a few species happens all the time. StuRat 11:30, 21 March 2006 (UTC)
- (after edit conflict) I presume you mean "past" extinctions? We have a pretty good article on the subject: extinction. Your question is difficult to answer precisely because extinction happens all the time, usually at a more-or-less constant rate. Exceptions to this rule are mass extinction events, which very suddently clobber an enormous number of species (the most famous is the Cretaceous-Tertiary extinction event, the one that killed the dinosaurs), see Extinction event. Note that "sudden" is in geological time - ie we could be talking hundreds or thousands of years. Over the last several hundred years, the extinction rate has been increasing sharply due to human activity, so sharply in fact that some some are calling it the Holocene extinction event, caused by human activity. — QuantumEleven | (talk) 12:40, 21 March 2006 (UTC)
- The difference is past mass extinctions were not caused by one species taking over in population, modifying land and other eco-niches for its own use and propagation. Before, the extinctions made "living space", in ecological niches, for new developments of new species. The current takeover by one weed species isn't allowing room for new things. Survival of the weediest. So it is a kind of "end of history". Better to bulldoze the rain forest to raise food-cattle for hamburgers, than allow others to live and let-live. It is the information (noosphere) manipulation and transference that makes this possible. The dominant species even has satellites to watch everything, looking for resources to exploit, and inefficiences to correct. It devotes a large part of its life cycle to transfering symbols and symbol-mainpulation tools to the next generation, without also transmitting an overview that would show that continued life-worth-living depends on the continuation of other genelines. In fact, I suspect your very question arose in such an information-transference context, a school, where you are being trained to answer questions posed to you, such as this very homework essay question, where you are being trained to take your place in the machine, just as the internet, and wikipedia, and this very answer you are now reading are part of the process. Carry on, soulless zombie-robots. --GangofOne 23:02, 21 March 2006 (UTC)
- Several mass extinctions in the past have been attributed to human action, though I believe there is now some dispute over this. In particular I mean the extinction of a huge proportion of the large animal species of the Americas when human hunter gatherers first moved there. I think there was a similar occurrance in the Antipodes too. AllanHainey 16:15, 22 March 2006 (UTC)
Location of the Earth's (ellipsoid) center of gravitation
[edit]To a close approximation, the Earth's shape is an oblate spheroid Becauase of this non-spherical shape, the point of grip of the Earth's gravitation does generally not coincide with the Earth's center of mass. For instance, for an object resting on the equator, the point of grip of the Earth's gravitation is about 10 kilometers away from the Earth's center of mass. This is of course related to the fact that the polar radius is about 21 kilometers less than the equatorial radius
Is there a mathematical formula that gives the point of grip of gravitation as a function of latitude? And a more specific question: for an object at rest on one of the poles, does the point of grip of gravitation coincide with the center of mass?
I have done quite a lot of googling to find an answer to the questions above, but no luck. 'point of grip of graviation' is not standard terminology, I suppose. Other variations, such as 'center of gravitation' were unsuccesful too. --Cleonis | Talk 07:31, 21 March 2006 (UTC)
- I'm not sure what you mean by "point of grip", but I do know that in general, the center of mass and the center of gravity do not coincide. The center of gravity of a body is a function of the gravitational field it is in, as well as its mass distribution. So one could figure out the center of gravity of the Earth in the moon's gravitational field, or the sun's field, or the total field. The center of gravity indicates where the net force acts from another body's attraction. But small objects don't create a field enough to attract the Earth, and so this issue doesn't arise. My weight vector points exactly towards the center of mass of the Earth. You're not looking for a difference between the center of mass and the geometric center, are you? That happens when density is not uniform. -lethe talk + 09:40, 21 March 2006 (UTC)
- The Earth (oblate ellipsoid) has cilindrical symmetry, from that it follows logically that the Earth's center of mass coincides with the Earth's geometrical center. (The Earth's density is not uniform, but the density distribution is cilindrically symmetrical.) That is clear.
- I choose to define 'weight vector' as: the vector that is precisely opposite to the Normal force that the Earth exerts on an object that is resting on the Earth's surface. Obviously, the thus defined weight vector does not point towards the Earth's geometrical center.
- I lay down another definition: 'true newtonian gravity': purely the gravitational force that is exerted by the Earth on an object resting on it. I should point out that gravimetric readings by measuring instruments that are co-rotating with the Earth (resting on the Earth's surface), measure a combination of two things: the gravitational acceleration minus the instrument's own centripetal acceleration. (See the Equatorial bulge article). In other words: gravimetric instruments measure the weight vector.
- The weight vector and true newtonian gravity do not point exactly in the same direction (they do point exactly in the same direction on the poles and on the equator, of course). Neither of the two points exactly to the Earth's geometrical center. (Of the two, true newtonian gravity points closer to the Earth's geometrical center.) --Cleonis | Talk 10:55, 21 March 2006 (UTC)
- OK, so the problem is that the graviational force will not always be normal to the surface, which complicates things. Yeah, I don't know nuthin about that. I guess my above comments were not so helpful. Sorry. -lethe talk + 15:07, 21 March 2006 (UTC)
- See Gravity anomaly. This is the difference of actual gravity from the theoretical ellipsoid. This dominated by bumps on the core, ore bodies, etc. We use this in geophysics all the time. --Zeizmic 12:10, 21 March 2006 (UTC)
- I am looking for information about the difference between the theoretical sphere and the theoretical ellipsoid. I suppose information about that difference is so hard to find because in practice geophycisists do not need to consider that difference. --Cleonis | Talk 13:37, 21 March 2006 (UTC)
- Not sure exactly what you are after, but here is a resource which might help[74]. If you are trying to shift the the geodetic coordinates between points on the reference ellipsoid (a spheroid) to points on a sphere with the center offset, you could use a datum conversion. If you just want to translate the center, you use a 3 parameter datum conversion or Molodensky datum conversion. For more complicated transformations you use a 7 parameter datum conversion. Not sure if that's what you want, but something on that colorado.edu page should help. EricR 14:54, 21 March 2006 (UTC)
- Would this diagram help explain what your are after? It sounds like you are trying to find the tangent of a point on a sphere given a point on the ellipsoid. Not sure which sphere you are interested in though. What are the sphere's center and radius relative to the ellipsoid? EricR 16:04, 21 March 2006 (UTC)
- That colorado.edu site uses the word "Datums". Really annoying, I keep trying to find the "Edit this page" button. GangofOne 22:12, 21 March 2006 (UTC)
- Would this diagram help explain what your are after? It sounds like you are trying to find the tangent of a point on a sphere given a point on the ellipsoid. Not sure which sphere you are interested in though. What are the sphere's center and radius relative to the ellipsoid? EricR 16:04, 21 March 2006 (UTC)
- Thanks, EricR for pointing out the diagram There is the reference ellipsoid, and for every point on the reference ellipsoid there is the vector of the normal force. As the diagrams shows, the point of intersection of the normal-to-the-ellipsoid and the plane-of-the equator does not coincide with the center of the Earth. For the rest: see my reply to GangOfOne. --Cleonis | Talk 14:28, 22 March 2006 (UTC)
- About the original question, "point of grip", (which I don't understand yet), you say "for an object resting on the equator, the point of grip of the Earth's gravitation is about 10 kilometers away from the Earth's center", how did you compute that? Is it on the line throught the center? Is this a concept that is intended to include centrifugal effect, or exclude it? Is 'point of grip' intended to be the point where if all the mass of the planet where put as a point particle, gravity would be the same at the test position? GangofOne 22:12, 21 March 2006 (UTC)
- My intend is to not take centrifugal effect into account. You are correct in surmising that I mean: the point where all the mass would have to be concentrated to exert the same gravitational force as the reference ellipsoid does (in the case of an object that is at rest on a non-spinning reference ellipsoid).
- Approximate calculation for object at rest on the equator:
- An object at rest on the equator of a non-rotating celestial body with the shape of the reference ellipsoid, has a higher gravitational potential energy than the same object at rest on one of the poles. I will call the difference in gravitational potential between those two locations the 'pole-equator potential energy'
- An object at rest on the equator of the spinning Earth has a velocity of about 465 m/s. This corresponds to a kinetic energy of about 1.1 x 10^5 joule per kg of mass.
- It is a theorem of the dynamics of rotation that in the case of perfect circular motion the kinetic energy and the potential energy are equal in magnitude. So in this case the pole-equator potential energy equals the kinetic energy.
- the 1.1 x 10^5 joule per kg of mass corresponds to a difference in height of about 10 kilometers.
- About that 'equal in magnitude' of kinetic energy and potential energy. Here, the potential energy is defined as follows: the integral of the total amount of work that the centripetal force would do if it would be allowed to pull an object all the way to the axis of rotation. --Cleonis | Talk 14:28, 22 March 2006 (UTC)
- EricR, GangOfOne, thank you for taking an interest in my question. I have copied my reply to GangOfOne to my talk page. The huge size of the reference desk page is cumbersome, can you please add any further comments on my talk page? --Cleonis | Talk 15:01, 22 March 2006 (UTC)
About PIC16F877 microcontroller
[edit]Iam new to electronics.I need beginers and complete tutorial on PIC16F877 ,its instruction set,its assembly level programming and examples.Request to reply soon to <email removed for the sake of your inbox>
- Here are a few links that may be more or less useful: [75][76] [77]. HTH, HAND. --Bth 10:29, 21 March 2006 (UTC)
relativistic angular momentum of a rotating body
[edit]I would like to figure out what the angular momentum of a rotating rigid body is, if it's rotating at relativistic speeds. Now, I know the formula for angular momentum is the same r×p in relativity. (Or better, xμpν – xνpμ, since there's no cross product in Minkowski space, but whatever). I can use this to calculate the angular momentum of, say, a spinning disk. The problem is that I expect that the binding energy of the disk (which must grow as the rotation increases) makes a nonnegligible contribution to the angular momentum. But I don't know how to go about estimating that contribution. I was sick the day we covered special relativity of rigid bodies. Any help? -lethe talk + 08:38, 21 March 2006 (UTC)
- Quite possibly the concept of a spinning rigid body cannot defined at all in relativistic physics, which then implies that a concept of 'angular momentum of a rigid disk' cannot be defined in relativistic physics. See Ehrenfest paradox --Cleonis | Talk 09:01, 21 March 2006 (UTC)
- Yeah, I have some idea that rigid bodies are tricky in relativity. I think Born made some method for describing rigid bodies under linear acceleration, but I don't know the details, and I don't know about rotating bodies. How about this as a warm-up: two massive point particles connected by a weightless spring, rotating around their center of mass. -lethe talk + 09:14, 21 March 2006 (UTC)
- Black holes have angular momentum. The problem is if a sun has a rapidly rotating outer surface, it is unlikely to collapse into a neutron star as the surface has to be moving slower than the escape velocity of the sun.
grey / white dot in the pupils
[edit]greetings .. my question is regarding a white or grey dot in the pupils . what is that ? and what does it do in there ? is it bad ? is it treatable ? ,,, thank you for your time.
- First, could it be a spot on the contact lens ? If not, I would suggest going to an eye doctor to have it checked out. StuRat 11:16, 21 March 2006 (UTC)
- How long has it been there? I think you should see an optician in case it's a cateract developing. --Username132 (talk) 12:03, 21 March 2006 (UTC)
- This appears to be a BE/AE difference. In the U.S. you would want to see a ophthalmologist or maybe and optometrist. Rmhermen 18:07, 21 March 2006 (UTC)
Computers
[edit]If a USB 2.0 is faster than USB 1.2 then how can one USB 2.0 hub provide four simultaneous access of USB2.0 ports through one USB2.0 port from the computer?
- I guess they wont all be operating at full speed all of the time. Most of the time though, the devices plugged into USB aren't even capable of using up the full bandwidth. What devices plugged into the hub do you use? --Username132 (talk) 11:57, 21 March 2006 (UTC)
I dont have a hub..just asked the question out of sheer curiousity.Also what about FIREWIRE hub?I guess that MAX speed of USB2.0 divided by 4 is the answer.
Physics:-Relativity
[edit]According to relativity(mass dilation),our mass increases as we gain a higer velocity and vice versa.So suppose a particle were to increase its velocity and decrease its velocity(positive and negative acceleration)at regular intervals,would that cause a gravity wave?Also would a body moving at a constant angluar velocity(uniform circular motion)that experiences a constant linear acceleration put out such waves of disturbances in space time?--61.1.131.70 11:54, 21 March 2006 (UTC)
- Well mass doesn't have direction (it's scalar) so I guess it's the magnitude of the velocity which determines the mass and changes in direction (which is why it's said to be 'accelerating') won't have any effect on the size of the mass. The fact that it's moving around and that its gravitational draw on something are inversely proportional to its distance away from that something might cause a "gravity wave"? --Username132 (talk) 12:02, 21 March 2006 (UTC)
- Any system of masses with a time-varying quadrupole moment will produce gravitational radiation ("gravity waves" is a common phrase used to refer to such radiation, but is potentially misleading as that term has a long history in another context; "gravitational waves" is preferred). Note that the size of gravitational waves is absolutely minuscule except for high mass, high acceleration events like neutron stars spiralling in towards each other. So the question becomes to determine whether your examples have a time-varying quadrupole moment. I have a feeling that neither of your examples qualifies as you're talking about point particles, but that if they became prolate or oblate spheroids, or long rods, or some other thing with extent and non-spherically-symmetric shape, they would. However, I haven't done any detailed analysis and my GR is rusty so take these as the hunches they are. See the "Sources of Gravitational Waves" section of the gravitational radiation article for more. --Bth 12:56, 21 March 2006 (UTC)
- As Bth writes, a necessary condition is a time-varying quadrupole moment. So two neutron stars orbiting each other in circular orbits will not lose energy in the form of gravitational radiation, but if their orbits are elliptical then they will. By contrast: a negative electric charge orbiting a positive electric charge will always radiate electromagnetic energy. Another example is that electrically charged particles that are driven to oscillation lose their energy again by radiating electromagnetic waves. Mass that oscillates in the same way does not produces gravitational radiation. --Cleonis | Talk 14:20, 21 March 2006 (UTC)
difference between a sunglass and an anti-reflective coating eyeglass
[edit]What is the difference between a sunglass and an anti-reflective coating eyeglass?
Is the eyeglass with anti-reflective coating the one which we use while using a computer?
Is there any type of special eyeglass which can be used while using a computer?
What glass material is the anti-glare screen used before PC monitors made of? Are they made of anti-reflective coating material or are they made of the material used in sunglass?
- Sunglasses limit the amount of light that passes through the glass, while anti-reflective glass limits the amount of light that reflects off the glass. For anti-glare screens the material doesn't matter, so long as the surface is rough. This causes reflections to be diffuse rather than bright and sharp. Diffuse reflections are not as distracting. StuRat 12:58, 21 March 2006 (UTC)
Just to add slightly to StuRats comments. There are three things that can occur to light when it hits the surface of the eyeglass lense (or the computer monitor, or a window, etc). The light can be reflected, transmitted, or absorbed. Sunglasses increase the absorption (and sometimes the reflection) to reduce the transmission. Antireflective coatings (typically a thin layer of titanium dioxide on sunglasses) increase the transmission and reduce the reflection. And antiglare coatings are, as StuRat says, a rough surface (usually a polymer for computer screens) that scatters the reflected light so it isn't so bright. If you are looking for a way to reduce eye strain while using the computer, my suggestion would be short breaks every once and a while with some eye exercises (you can find ideas all over the web). Chapuisat 18:41, 21 March 2006 (UTC)
Question about phishing
[edit]I just received yet another phishing attempt, and there's one thing I don't understand. When I mouse-over the link that purportedly goes to chaseonline.com, where I could put my non-existent Chase bank account number, I see the name of the phishing site, http: //www.chaseonline .chase.3y-cgi. com/redirect.php. My question is: isn't the person who registered the domain easily traceable? When I do a whois for 3y-cgi.com, I get a name and registration info (including the fact the the domain was only created three days ago). Obviously this name could be faked, but at some point doesn't the person need a valid credit card to register a domain? How come these people don't get thrown in prison the very next day after sending out phishing attempts? — Asbestos | Talk (RFC) 14:00, 21 March 2006 (UTC)
- Yes. They are easily traceable. But, there are many things that work in the favor of the criminal. If they are overseas, local police cannot touch them. If they are local, local police have to do an investigation - which takes months and months and months... By the time anyone gets around to trying to arrest them, they've moved. Finally, the name/address you got from the domain is most likely fake. With all the "buy a cheap domain" sites, it is very easy to purchase one with fake information. Then, with all the "get cheap hosting" sites, it is very easy to get hosting with fake information. --Kainaw (talk) 14:06, 21 March 2006 (UTC)
- Think this through one more time... What makes you think they don't use the volumes of stolen information to further their efforts? Not that i'm in that business, but if i were that would seem like a no-brainer. They can use layers upon layers of cracked 'zombie' computers to make their ultimate location untraceable, and with all the personal and financial information they steal they simply use some of it to 'reinvest' in their efforts by buying domains, hosting space, whatever. Who cares if they get shut down a day later and some stranger gets a knock on the door from the FBI (not even likely). They just click a few buttons and have a whole new operation running in a matter of minutes. --Jmeden2000 15:44, 21 March 2006 (UTC)
- Hey I got that Chase one too. And I don't have a Chase account, or have ever seen a Chase. I don't get any spam.Two weeks ago, I had 4 phishing attempts. Now they're gone. I have about 1-per-week now. --
 Mac Davis] ⌇☢ ญƛ. 21:05, 28 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 21:05, 28 March 2006 (UTC)
Akrit Jaswal
[edit]anyone know anything about this guy Akrit Jaswal? look him up on google news
- Erm, I know there was a TV programme about him on Channel Five in the UK last night, "Extraordinary People: The Seven Year Old Surgeon". Unfortunately, I didn't see it (I was at a salsa class), but is their page on the show any use to you? What in particular do you want to know about him? (Our article is sadly only a stub, if you find some interesting material do consider adding it.) --Bth 16:35, 21 March 2006 (UTC)
medical condition
[edit]I have been told that there is a condition where someone can be born with one buttock instead of two. I have looked all over the web and can not find anything on it. Was I misinformed or is it actually a condition?
Thank you Jessica
- I can picture one being smaller than the other, but if one was completely missing, what would that mean ? The hip bone showing right through ? StuRat 16:47, 21 March 2006 (UTC)
We can name it, aplasia glutei maximi, but it appears we have no article on it. alteripse 17:00, 21 March 2006 (UTC)
- And who's going to be first with the obligatory Monty Python (or perhaps Coneheads) reference? —Steve Summit (talk) 19:19, 21 March 2006 (UTC)
- There was an episode of King of the Hill about this. - 216.218.44.237 22:33, 21 March 2006 (UTC)
This is an "assinine" question. Uh, huh huh. Brian G. Crawford, the so-called "Nancy Grace of AfD" 02:43, 22 March 2006 (UTC)
- Back on the farm we had a calf born with no butt-hole, just an oval of pink skin. That is a lot like what Jessica is asking.
As opposed to parallel universes, are there any references to perpendicular universes? Like one universe intersecting another and causing implosions ans explosions and all kinds of space-time anomalies?—Preceding unsigned comment added by 4.19.93.2 (talk • contribs)
- Um, that's not what parallel universe means, universes don't make any sense in 3D, so the idea of orthogonal universes is kind of.... weird--152.163.100.74 17:40, 21 March 2006 (UTC)
-
- Oddly, that google definition sources a non existent en.wikipedia article--152.163.100.74 17:41, 21 March 2006 (UTC)
- The article was deleted January 11. How often does Google update that? --Optichan 20:19, 21 March 2006 (UTC)
- Some ideas in the (highly speculative) field of brane cosmology might qualify as fitting the general idea of "intersecting universes and their consequences". --Bth 17:50, 21 March 2006 (UTC)
- True, but since "parallel" universes have nothing to with geometry, wouldn't the points of intersection still fit the definition of a parallel set of universes, rather than forming the basis of a new term?
- In fact, don't parallel universes actually refer to branch points, making them, mathematically speaking, fractals, which would have to be seen as specifically non-parallel?--152.163.100.74 17:57, 21 March 2006 (UTC)
- Yes, perpendicular universes would be the same thing as parallel, they don't have to do with geometry, and physicists tend to represent them as "bubbles in a foam" anyway. Parallel just means time is the same at both, even though that is not necessarily true anymore. --
 Mac Davis] ⌇☢ ญƛ. 21:05, 21 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 21:05, 21 March 2006 (UTC)
- Hmmm I thought a universe was parallel because actions in one universe do not affect the other, except where transdimensional gateways allowed travel from one to the other. A perpendicular universe, hmmm. Try reading a sci-fi short story called Highway J. This allowed a section of space to be rotated so the time axis was transposed with one of the mundane axes in the normal universe. This allowed a person to move on the time axis by moving along the apparent mundane axis.
I don't know about anybody else, but sometimes I think we're living in a skew universe. —Steve Summit (talk) 17:00, 22 March 2006 (UTC)
Combustion products of nylon 6,6
[edit]There are no references in the MSDS for products given off by polyamides (nylon 6,6) when they are burned. Where might I find this information? Frank W. Croft Safety & Training Coordinator Fenner Drives (email and other identifying information removed as spam and privacy protection)
- According to [78]:
- HAZARDOUS DECOMPOSITION PRODUCTS: Burning may produce CO, CO2, NOx, HCN.
- Here's a Material Safety Data Sheet (MSDS): [79]
Blender problem
[edit]It's a question to people who are writting Blender tutorial: "Blender 3D: Noob to Pro". What should I do, if I cannot make two colours in Blender? I added a new material in "Link and materials" tab. When I want to add another one material, it becomes the same as prior one. I'm waiting for quick reply. Thank's in advance.
- Erm... this is Wikipedia, the tutorial you're talking about is on Wikibooks: Blender 3D. You should go and ask there, probably on the book's talk page. — QuantumEleven | (talk) 07:22, 22 March 2006 (UTC)
Why does this work?
[edit]- While sitting at your desk, lift your right foot off the floor and make clockwise circles.
- Now, while doing this, draw the number "6" in the air with your right hand. Your foot will change direction.
Something to do with the area of the brain that controls movement on that side of the body? Dismas|(talk) 20:40, 21 March 2006 (UTC)
- The brain sends many signals at once on an unconscious level, but only one complex voluntary sequence can typically be sent at a time. If you practiced enough, you could teach your brain to do this, however. StuRat 20:54, 21 March 2006 (UTC)
- Whoa, that is sweeeeet. --
 Mac Davis] ⌇☢ ญƛ. 21:09, 21 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 21:09, 21 March 2006 (UTC)
- Isn't that just a question of coordination? --BluePlatypus 23:00, 21 March 2006 (UTC)
- I can do it now, with about one minute's practice. I suspect it's just the "rub your tummy, pat your head" thing; as someone said, coordination. --George 05:56, 22 March 2006 (UTC)
Gluclose->Galactose
[edit]what happens to glucose or galactose when the Cu+2 in benedict is reduced? Shauna
- Sounds like a homework question (see above). I'll give you a hint: If the copper is reduced, the other reagent must be ___? --BluePlatypus 21:21, 21 March 2006 (UTC)
- Increased?
- This is a specialized chemical use of the word reduce. See Redox. —Keenan Pepper 03:41, 22 March 2006 (UTC)
Data replication
[edit]I'd seen an article an year ago about efficient data replication in lossy networks by means of first "breaking" up the data in several duplicated data chunks (say n chunks) and then these 'n' chunks are transmitted. As long as the receiver is able to get a fraction of those chunks, then the data can be "recreated" from those 'm' chunks. Now, i'm not able to remember where i read this or what it is called. Could anyone please shed some light on this technology? Thanks! Vasu
- possibly an error-correcting code? EricR 21:45, 21 March 2006 (UTC)
- The accurate term is probably Forward Error Correction, but unfortunately the article isn't very thorough. 84.239.128.9 21:50, 22 March 2006 (UTC)
This was not at the level of error correction. It was at a higher layer wherein the data would get divided into chunks which together would exceed the size of the original data. This is due to the duplication of parts of the data across the chunks. So, as long as the receiver would get a minimum number of any data chunks, it would be possible to deduce the original data content.
- If you mean "not at the level of bytewise error correction", no, but what you're talking about here is a sort of "filewise error correction". The basic idea is very simple. For example, I might break a file up into, say, 512-byte chunks, and for each five chunks A, B, C, D, and E in the input, transmit or store an extra, sixth chunk F where
F = A ^ B ^ C ^ D ^ E
- where
^is the XOR operator (applied across every bit in every byte in the chunks). I repeat this with the 6th-10th chunks in the input (resulting in an extra 12th chunk in the output), the 11th-15th, etc. Then, if (for example) chunk C gets damaged in transit (which I'll need some kind of per-chunk checksum scheme to detect) I can reconstruct it asC = A ^ B ^ D ^ E ^ F. (But if two chunks out of any six get damaged, I'm out of luck.) This technique probably goes by various names; I call the groups of chunks XOR redundancy groups (which I see from the red link color we don't have an article on).
- I first heard about this techinique in the VMS BACKUP program, but I'm sure it's been used elsewhere. I keep meaning to write data to my archival CD's this way, since CD's are known not to be a perfectly stable long-term archival medium. —Steve Summit (talk) 23:10, 21 March 2006 (UTC)
- I don't know if there is a specific name for it, but its the same idea that's used in RAID 5. Basically, you decide how much overhead you want and the tolerance of the system to unit failure is equivalent to that overhead, because parity data is dispersed and allows for recreating one element the size of the overhead, if any single element is lost. The Raid 5 article has some insight into techniques and theories. --Jmeden2000 18:16, 22 March 2006 (UTC)
Canadian health care: Availability, waiting times
[edit]I have fairly serious health problems, since I broke my back last year, and I'm considering moving to Canada. The Health care in Canada and Canadian and American health care systems compared didn't give me a good idea of how things compared from a patient's point of view. Is there a longer wait time for services in Canada? (One of the articles alluded to this, but said nothing concrete). Will it be harder to find medical specialists, such as a physiatrist? What differences would I expect as a patient with specialized medical needs? -- Creidieki 23:03, 21 March 2006 (UTC)
- Just hope you don't break your hip too—see [80] (admittedly not the most neutral of sources) --zenohockey 23:34, 21 March 2006 (UTC)
Canada's a big country; health care is provincially administered, and even within a province conditions in one city may be different from another. You really need information about the specific place or places you are thinking of moving to. For that matter, unless we know about conditions wherever it is you are moving from, we can't tell you what's different, can we? --Anonymous, 05:30 UTC, March 22, 2006.
If you have $$$, you can get much faster service in the USA (or India for that matter). Health care services in Canada do vary from province to province. Physical therapy and rehabilitation is one of those things that tends to vary. What Canadian "universal" health insurance means is that you will be covered for most services rendered in a hospital (such as most surgeries) and visits to a GP/family doctor or specialist. Some things are not covered, such as cosmetic surgery. Generally, it is easier to find a specialist in the larger cities than in rural areas. - Cybergoth 02:10, 25 March 2006 (UTC)
- It all depends on what type of health service you're looking for. Personally, I have a lot of health issues and I've never really been bothered by waiting times. One possible myth that should be put to rest concerns emergency services. In Canada, if you need immediate attention in a life or death situation, you'll get it. Call 911 and the ambulance will get to you as fast as is humanly possible for attention. Similarly, if you need an emergency procedure, it'll be done as quickly as necessary. For example, last month my father had a heart attack. The ambulance was at our door within five minutes, and he was in a hospital bed hooked up to all the necessary equipment within the hour. It was determined that he required a triple bypass, and it was performed four days later (it would likely have been performed even earlier had he not been in relatively good condition in the interim). So any horror stories of people literally dying in the waiting rooms of hospitals are pure myth.
- But like I said, it all depends on the service. Waiting lists for some specialists can take up to 8 months. Fortunately, the system is rational. Basically, the longer you can afford to wait (medically speaking) the longer the waiting period tends to be. I hope this helps. Loomis51 03:23, 25 March 2006 (UTC)
Medicine - Hole in one's throat
[edit]Hi there! I would like no know the medical name for the hole that is done in one's throat and allows, for example, to smoke a cigarrete without using your mouth. As seen in the last episode of the X-Files TV series.
Thanks in advance for any help, Ricardo M.
- The irony is that that procedure is often needed as a result of throat cancer, caused by smoking. StuRat 23:21, 21 March 2006 (UTC)
Actually, tracheotomy is the name for the surgical procedure that creates the tracheostomy, the hole in the trachea. Brian G. Crawford 01:10, 22 March 2006 (UTC)
March 22
[edit]C++ to Java or Flash
[edit]Is there any way to convert C++ to a internet format without transliterating it manually? Assume that there is no IDE or complier to work with. --hello, i'm a member | talk to me! 00:24, 22 March 2006 (UTC)
- This is a very strange question, sort of like "Is there any way to convert French to Shakespeare?". By "an internet format" I assume you mean a graphics/multimedia format which web browsers can natively display. And, furthermore, I'm guessing you're talking about a C++ program that outputs some graphics or animation, or otherwise does something visual which you're trying to capture.
- If that is, in fact, what you're trying to do, there probably isn't a simple way of doing it. But if you can be a bit more clear on your situation we might be able to offer some suggestions. —Steve Summit (talk) 02:30, 22 March 2006 (UTC)
There's a open source tank game written in C++ that I want to put online that can load in a web browser. --hello, i'm a member | talk to me! 02:44, 22 March 2006 (UTC)
- So you're trying to convert a program written in C++ to a program written in a web-based format. The short answer is no. The long answer is that it's generally possible to convert it to Java ('cause the languages have similar philosophies and syntaxes), but you'll need to do it by hand. And you'll need the Java compiler. (However, you can always just put up the existing C++ executable for people to download, but you've probably considered that.) --Geoffrey 03:28, 22 March 2006 (UTC)
- It depends very much on what libraries the game uses. —Keenan Pepper 03:40, 22 March 2006 (UTC)
Firefox: SessionSaver
[edit]I use Firefox 1.5.0.1 with the SessionSaver extension, v. 0.2.1.031. To delete a session (under Tools > SessionSaver), you have to press the scroll wheel. But I use a laptop with no mouse -- just a touch pad and left and right buttons. When I try to use Ctrl + left click to simulate the scroll wheel (in Firefox, for nonusers, that opens the selected link in a new tab; this is ordinarily done with the scroll wheel), it doesn't always work (it might be a timing issue on my part). Is there another way to do this? --zenohockey 00:30, 22 March 2006 (UTC)
- Holding Shift and left-clicking works. This site has more information. --Cadaeib 01:29, 22 March 2006 (UTC)
- Perfect. Thanks! --zenohockey 05:12, 22 March 2006 (UTC)
End of the World
[edit]Can anyone provide me with a list of doomsday beliefs that put the date right around this year? I'd expect there to be quite a few of them, and I'd very much like to start Current Events in Government class each day with the announcement, "The world has not ended, and _____ apologizes for the mistake." Black Carrot 02:49, 22 March 2006 (UTC)
- Most of the "prophets for profit" are smart enough not to give a specific date, that way nobody can ever prove them wrong. StuRat 03:28, 22 March 2006 (UTC)
- Legend has it that Cardano (who wasn't dumb) predicted the date of his death exactly years before it happened. He commited suicide. Some people just hate being wrong. --BluePlatypus 04:42, 22 March 2006 (UTC)
- Damn, that's hard core. But it isn't in the article... do you have a source? —Keenan Pepper 05:00, 22 March 2006 (UTC)
- The prediction is mentioned, though. I read it here, in any case. (Worth reading in full, he was quite a character) --BluePlatypus 05:27, 22 March 2006 (UTC)
- That is a very cool idea for a presentation :) It doesn't quite cover the very recent past, but you may want to browse through Timeline of unfulfilled Christian Prophecy. — QuantumEleven | (talk) 07:14, 22 March 2006 (UTC)
Sorry...Today is your last day.
- The Aztecs regulated their history within various 'ages', with each of the carrying an end of the world incorporating a massive disaster and leaving just two humans behind, to start again another generation of the world within the circle of life. This idea might be useful if the presentations is carried out through long cycles. Luthinya 11:24, 28 March 2006 (UTC)
Plant Fertilizer
[edit]I am aware that Nitrogen,Phosphoric Acid and Soluble Potash are all plant fertilizers, but they each have their own purpose. What is the benefit of each? Thank-You.--209.89.235.178 02:53, 22 March 2006 (UTC)
- Since this is a double posting, I have forwarded Mac Davis's reply to the anon's question on their talk page. --Uthbrian (talk) 03:11, 22 March 2006 (UTC)
Planet Demoltion
[edit]After reading the Hitcher Hickers Guide to the Galaxy I sometimes think about the demolition of the Earth. What would be the cleanest and most efficent method for destroying the Earth? Patrick Kreidt
- There's a whole Usenet newsgroup, alt.destroy.the.earth, devoted to this exact question. I haven't read it in years so I have no idea if it's all porn ads now. Anyway their nemesis was alt.pave.the.earth, the contributors to which were radically opposed to your project, because if you destroy the Earth, how are you going to pave it? --Trovatore 03:55, 22 March 2006 (UTC)
- Lol, that's great. Here's the FAQ. —Keenan Pepper 03:59, 22 March 2006 (UTC)
- It all depends on how thoroughly you want it destroyed. Is destroying all life enough, or do you want the physical ball of rock to be broken up as well? —Keenan Pepper 03:58, 22 March 2006 (UTC)
- As our article on the Death Star notes, complete demolition of the Earth or a comparably-sized planet would require ridiculous amounts of energy; there is no known physically-plausible source (even technologically challenging ones like massive lumps of antimatter) for such energy output. As the article says, if you just want to destroy civilization, there are much easier and cheaper options - dropping a sufficiently large asteroid onto Earth, or, even cheaper, some cobalt-salted nuclear weapons. --Robert Merkel 04:35, 22 March 2006 (UTC)
- Yes, a cobalt bomb is probably the most effective method to destroy civilization with current technology. With some advances in genetic engineering, however, a deadly virus might be more efficient. —Keenan Pepper 04:58, 22 March 2006 (UTC)
- Cobalt bombs ar too salty for my taste. --Optichan 16:00, 22 March 2006 (UTC)
- Yes, a cobalt bomb is probably the most effective method to destroy civilization with current technology. With some advances in genetic engineering, however, a deadly virus might be more efficient. —Keenan Pepper 04:58, 22 March 2006 (UTC)
- Haven't read the hitchhiker's guide, but it seems to me that the easiest way to destroy the earth would be to push it out of orbit, preferably into the sun, but into another planet would work. To completely stop the earth's movement around the sun, one would require 2.7X10^33 Joules. Then it would accelerate straight towards the sun (with minor bends towards other planets). Boom! No earth. For refence, if we were to store up all our energy that arrives on earth (primarily through solar radiation), we would have to wait about 4.9x10^8 years before we had enough energy to stop the planet. So it's a lot of power just to stop it. Of couse, I'm completely ignoring the fact that the forces on the earth that would be required to stop it would also rip it apart, but no matter. --AK7 17:08, 22 March 2006 (UTC)
- Haven't read The Hitchhiker's Guide??!? You call yourself an educated, computer-using, sentient galactic being and you haven't read the seminal work in the field? Take a quick break and catch up on your reading, please, and come back when you've achieved a basic level of clutural literacy! :-) —Steve Summit (talk) 04:42, 23 March 2006 (UTC)
- What if you stopped the moon from orbiting and let it crash into the earth? Less energy should be required, though what the result would be I don't know.Markyour words 17:40, 22 March 2006 (UTC)
- Good point. The moon's KE relative to the earth is 3.8X10^18 joules, much much smaller than the earth's KE. It would take a very short time of accumulating power (days or shorter) before we could press the big red button. But I don' think the earth would be completely destroyed; there would certainly be plenty of debris which would clutter the area. Pushing it into the sun deals with this, although if shoemaker-levy 9 is any indication, it could have an interesting effect on the sun...--AK7 17:50, 22 March 2006 (UTC)
- I have not done the math, but my suggestion was to devise a way to get all of the hydrogen in the oceans to undergo fusion. Don't know how that could be done, or how much energy it would yeild.
- Well, if the destructor of Earth is a patient person then he/she could, instead of stopping the Moon in its orbit, slow the Moon's orbit. That would cause the Moon to spiral—slowly or quickly, depending on how much you slow the Moon—into Earth. This is fun! :D —OneofThem(talk)(contribs) 20:37, 22 March 2006 (UTC)
- What if, instead of stopping the Earth entirely (at utterly ruinous energy expenditure) we just adjusted its orbit? Kick it into a more elliptical orbit – maybe slingshot around Venus or something – and fling ourselves into the Sun that way? I'm not really keen to do the astrophysics for it, but I suspect we could save a good chunk of energy with that technique. Maybe save 50, 75%? TenOfAllTrades(talk) 04:18, 23 March 2006 (UTC)
A similar question was asked here in December. One reference I found useful was here. Dmharvey 22:00, 22 March 2006 (UTC)
Thanks all these suggestions have been increasingly amusing.
- No science fiction apparatus is needed to destroy the earth. A single large enough nuclear bomb would suffice. There is no upper limit to the size of a hydrogen bomb. By simply cascading more fission/fusion stages in theory a device of any size can be constructed. Scientists have discussed a 50,000 megaton device which is probably relatively straightforward to make (for a superpower). There are 4.2E15 joules per megaton, so it would release 2.1E20 joules. The best possible yield-to-weight ratio is 166 kg nuclear material to megaton of output, so it would weigh 8300 metric tons, not exactly air-deliverable. Ironically Dr. Strangelove got it right: "Mr. President....When you merely wish to bury bombs, there is no limit to the size". Joema 05:26, 23 March 2006 (UTC)
- Ah, but follow the math through from there. From our Death Star article, the floor on energy for destroying a planet seems to be at least 1032 joules; some estimates run about a million times higher.
- At 2E20 joules per 8300 tons, we get a minimum required mass of about 4E15 tons of hydrogen (even assuming a rather implausible 100% efficiency). Current global production of hydrogen is a paltry 5E7 tons per year, so we'd need to save our industrial output for a hundred million years to have enough.
- The density of liquid hydrogen is about 70 kg/m3, so we'd need about 5E16 m3 to store it. That's roughly 2000 times the volume of the Great Lakes, or – if my math is about right – about twice the volume of the entire Mediterranean Sea.
- On the bright side, we do have enough hydrogen available on Earth. The hydrosphere contains about 1.4E18 tons of water, of which about 1.5E17 tons are hydrogen. So we'd only have to crack about 2.5% of all the water on Earth to make our bomb.
- Note that the above estimates are based on the low end of the Death Star estimates and 100% efficiency. If the high end is correct, then the figures need to be a million times higher—and we don't have enough hydrogen. Pity. TenOfAllTrades(talk) 06:26, 23 March 2006 (UTC)
Digital rm to mp3 converter
[edit]Does anyone know any good freeware programmes that allow digital conversion from rm to mp3? -- Миборовский U|T|C|M|E|Chugoku Banzai! 05:06, 22 March 2006 (UTC)
- First off, do you realize this will degrade the quality of the sound? Conversion from one lossy data compression format to another is never good. So, ask yourself if you really want to do this, and if so, you need something that can decode realaudio into an uncompressed format (e.g. WAV) and something that can encode that into an MP3 (like LAME). There's no way to convert directly from one format to the other. —Keenan Pepper 05:14, 22 March 2006 (UTC)
Science/Environmental Science/Water/Rivers Concept or Theory Question
[edit]Hi, thanks for offering this service. My question might not have an answer, I'm trying to learn if there is a term for a theory that all water on the earth being some how being connected. Like how water form the ocean gets pulled into the air, then becomes cold and falls as snow on a mountain, then the snow melts and runs into a river that flows back into the ocean. I know this isn't scientifically how things work, but I was wondering if there was any informal or perhaps old mystical term that can be used to describe this idea of all water being connected or flowing. I am a poet and I'm exploring the idea of connectedness and thought I would see if there is any such term or concept. Thank you,
Schuyler
- Are you looking for Water cycle or perhaps something more? Melchoir 05:23, 22 March 2006 (UTC)
- I'm a little frightened that anyone, anywhere, could reach this level of literacy without being aware of the water cycle, and indeed thinking the concept of it (when they come up with it themselves) is unscientific. Schools really do neglect the sciences. 57.66.51.165 12:22, 27 March 2006 (UTC)
Transistors
[edit]tell me the theory about insulated gate bipolar transistor and its functioning
- This sounds like homework ... Our article on transistor might contain some useful stuff to get you started, though admittedly it could do with a bit of a cleanup. --Bth 11:39, 22 March 2006 (UTC)
Pollution rates
[edit]I have spoken to strong advocates of renewable energy, and most of them have said a significant decrease in air pollution would ensue if widespread use of them occurred. I was wondering if any of you know of any graphs/charts that would show projected air pollution rates now with the use of conventional energy sources vs. air pollution if alternative or renewable energy (i.e. ethanol, solar power, etc.) was used. Thanks -- Anonymous
- Our articles on greenhouse gas, Global warming and carbon dioxide have some excellent graphs for pollution rates. Hope this helps. Kilo-Lima|(talk) 18:51, 22 March 2006 (UTC)
- Note that some renewable forms of energy, such as burning wood, cause very high rates of air pollution, while some non-renewable sources, like nuclear fission reactors, have almost zero air pollution associated with them. Basically, anything you burn will cause air pollution, whether it's renewable or not, while most non-combustion based forms of energy have low air pollution associated with them. StuRat 19:06, 22 March 2006 (UTC)
- It's also worth thinking about what you want to consider as 'air pollution'. Do you mean carbon dioxide alone? All greenhouse gases? Particulates (soot)? Oxides of nitrogen and sulphur?
- From a CO2 standpoint, biomass (mostly plant matter) is very 'clean', because when you regrow your supply of biomass you pull carbon dioxide back out of the air. (Burning the wood from a tree puts CO2 into the air, which is pulled back out of the air when you grow a replacement tree.) On the other hand, if the wood isn't burned under carefully controlled conditions then you will generate a bunch of soot and smoke that may be undesirable. TenOfAllTrades(talk) 00:09, 23 March 2006 (UTC)
temperature and calorie burn.
[edit]First off, I recommend reading through this. Lots of fun, but the good stuff is at the bottom. Anyway, the basic idea is that the vast, vast majority of the calories we burn are radiated or conducted away from our bodies. And since our bodies will increase metabolism to stay at 98.6 F(37C), decreasing the temperature of the environment should help burn calories. I read somewhere that this is why we get lethargic in the afternoon; it's hot, we're not needing to burn many calories to stay warm, so our bodies slow down the consumption of glucose. I've also read from a couple different sources that shivering burns 400 calories an hour (on par with vigorous exercise) and this seems right since the whole point of shivering is to increase body temperature. Of course, to shiver, you have to cool yourself off considerably, and if you do it for more than an hour or two, you could get hypothermia.
That's obviously a problem. Hypothermia sucks, plus it can kill you when your body temperature drops once your cells run out of energy stores (stored in glucose or glycogen). Which is where part 2 of my future-fad-diet comes in: Caffeine. Stimulates lipolysis and dramatically increases glucose in the bloodstream, which should help keep you from dying of the hypothermia. On the negative side, it reduces the amount of blood near the skin, which means that you won't radiate heat so well. Oh well, it's an acceptable loss. Oh, and just for kicks, caffeine is a diuretic; assuming you're drinking ice-cold water, this will help you burn an extra few calories since every liter of ice water you drink has to rise to 37C before leaving your body. It's only 37 kilocalories a liter, but it's something.
So the fad diet would go something like this. Get pure caffeine (thank you eBay). Put 500mg in an absolutely bitter-tasting concoction. Drink it during the early evening. Stay up all night, nude or mostly so, in a cold room, and take a shower (doesn't have to be cold, just don't make it hot) and don't dry the water if you can stand it. All that evaporation will draw away heat from the skin, and you'll shiver away fat all night while you write about it on Wikipedia (actually, you'll write about it later on wikipedia after finding the research on it in google answers the night of). It's the Next Big Thing!
So, the question. I'm pretty much just wondering if there are any glaring problems in this (besides the obvious ones of killing people who overdo it and freeze themselves to death). Plus the google answers page says "expires 3-25" and I'm not sure if it's going to just dissapear then. In any case I'd like to produce some sort of record for other lazy overweight teenagers like myself who avoid exercise at all costs to look through. Until them scientists perfect diet pills that don't result in death, this may be the only way to lose weight while not leaving our precious computers.
Oh, and another crazy/dangerous idea: exercise through electric shocks. The major risk is, of course, if your device malfunctions, you're a dead man... but mucles tense in the presense of an electric current, no? So by regularly applying and un-applying an electric current (say at 1 or 2 Hz), could we get some fair exercise for muscles? The next logical step is to make it so that this happens when one is hurt in a game. It takes a rumble pack to a whole new level. --AK7 18:40, 22 March 2006 (UTC)
- Well, those methods sound like typical fad diets, in that they are unhealthy and less effective than the proper advice to "eat healthy food, consume fewer calories, and exercise". Specific problems:
- Cold puts a stress on the immune system, making the subjects more vulnerable to disease.
- Any method that causes you to burn more calories will just make you hungrier, too. So you will eat as many calories as you burn, and then some.
- The more calories you burn, the more free radicals are released. The aging effect of those can be reduced by anti-oxidants, but not eliminated.
- Caffeine has a number of health problems associated with it.
- Electro-shock is dangerous, and has also already been marketed (briefly, I assume due to lawsuits) for strengthening facial muscles. The only legit use of this method I see is to keep the muscles of comatose patients in shape.
- I've seen ads for muscle stimulators for abdominal muscles so you don't need situps. Don't know if they take more time than situps. Plus , it must be very annoying.
- For typical person, basal metabolic rate is 40 watts == 825 kcal/day. That is , if you lie in bed all day, you still use 825 kcal. If you move around, than they typically say, typical person uses 2000-2500kcal/day. If you're an Olympica athelete in training, you might eat and use 6000kcal/day (according to what I read). If you want to lose weight, just reduce intake below usage. (obviously) Energy is stored in different ways in the body, long term storage is fat. To "burn" fat most effectively, use long (more than 1/2 hour) aerobic exercise, that's what stimulates fat utilization. Plus you'll be more buffed than if you lie in bed and eat only 400kcal/day. I suggest, a bicycle and an MP3 player/FM radio with headphones, and a few cups of coffee before starting out. It's fun. (Ignore all fads, dietary, philosophical, political.) GangofOne 21:58, 22 March 2006 (UTC)
- Caffeine is already found in most diet pills. 500mg is a monstrous amount of caffeine to take in one dose, as a can of Coke has 40mg of caffeine in it. An overdose of a stimulant like caffeine can cause heart arrythmia and extreme anxiety. As for lowering the room temperature, I knew people in college in New Hampshire who would keep their house at 50 degrees (Fahrenheit) to keep weight off. According to them, it worked. Hypothermia is a dangerous medical condition and should never be induced outside of an operating room. Brian G. Crawford, the so-called "Nancy Grace of AfD" 01:33, 23 March 2006 (UTC)
- Of course, I also knew wrestlers in college who would jog around in black plastic garbage bags and then cool down by going to the sauna for an hour, also to keep their weight off. I guess what all these ideas have in common is that you have to be utterly deranged to subject your body to such risk for the sake of a few pounds. ~Mary
Nuclear Bomb
[edit]Is it possible to make a non-radioactive nuclear bomb, or something as stong as a nuclear bomb without the radioactivity?
- For example, the Hiroshima bomb was the equivalent of 13 kilotons of TNT. How about 13 kilotons of TNT? Are there more design specifications you wish to mention? GangofOne 20:45, 22 March 2006 (UTC)
- If you don't have a size limit, then yes. The power of nuclear weapons is measured in kilotons or megatons of TNT, so, by definition, thousands or millions of tons of TNT would have the same explosive force. If you want it to be as small as a nuclear weapon, then we would need some future technology, say an antimatter bomb, which should actually be over 10,000 times more powerful given an equal weight of antimatter versus uranium or plutonium. StuRat 20:54, 22 March 2006 (UTC)
- Actually, it's not completely obvious that you could simulate the blast of a big nuclear weapon by stacking millions of tons of TNT or other conventional explosive. Your bomb would be the size of a large building and you would need to make sure that all parts of the explosive actually contribute to the blast without being destroyed first. I imagine pyrotechnics experts could deal with that by using many detonators and suitable control circuits, but I'm not one and I don't know. Another points is that there clearly wouldn't be any single place that would get the equivalent of the extreme blast forces very close to the nuke, although at say a half-mile from the center or further out, you might not be able to tell the difference. --Anonymous, 22:40 UTC, March 22, 2006.
- I understand that the larger the volume of the explosive, the less damping effect distance has. E.g, a 1MT nuke may expend a lot of energy in its immediate vicinity, but the larger volume of a 1MT TNT bomb will expand much further, projecting force much further away. The principle behind FAEs is similiar, in that you don't need quite as much explosives to do damage over a larger area. Tzarius 07:41, 24 March 2006 (UTC)
- This has already been done. FAE (Fuel Air Explosive) bombs exist that are .02 Megatons, that is about 20 Kilotons.
- Bullshit. Pardon my french, but the biggest Thermobaric weapons are still only in the tens of tons. .02 Kilotons, not .02 megatons. However, some of the smallest nuclear weapons have warheads that can make a blast as small as tens of tons. You should keep in mind, though, that a small nuclear weapon unleashes blast effects in a way very different from a larger weapon, since most of the ionizing radiation impacts the target directly, rather than being converted to a blast wave and overpressure through atmospheric heating. A Davy Crockett nuclear weapon will be more likely to fry you with radiation than blast you apart, especially if you're inside a tank. Night Gyr 22:21, 22 March 2006 (UTC)
- My source told me it was .02 MT, but I am entirely willing to believe he may have misstated. It is true, though that FAE devices have yeilds approaching Nuclear Devices.
- Please note that a "ton of TNT" or "kiloton of TNT" is a unit of measure, and does not directly correspond to a certain weight or mass of TNT.[81] Johntex 23:29, 22 March 2006 (UTC)
- Interesting. I certainly think it was intended to correspond with the weight, but I suppose variations in the explosive power of TNT and it's weight might throw it off a bit. This reminds me of "horsepower", which seems to be considerably less than the power of a typical horse. A small car, for example, might have an anemic 50 HP engine and have rather slow acceleration. However, if you could hook that same car up to 50 actual horses, I would expect to get quite impressive acceleration. StuRat 23:50, 22 March 2006 (UTC)
- The original bombs were calibrated against test detonations of TNT, to give some sense of scale, so you're not wrong. However I imagine that one can really change things around with how you detonate TNT to get different types of effects, which is why they eventually developed a "ton of TNT equivalent" as a fixed expression of just energy. --Fastfission 01:48, 23 March 2006 (UTC)
- In theory, yes. A hydrogen-hydrogen or deuterium-deuterium fusion bomb, without a fission detonator, would have no radioactive fallout. The problem is finding a replacement for that detonator: there's nothing else known that can produce the combination of pressure and temperature needed. --Serie 00:29, 23 March 2006 (UTC)
- Wouldn't subatomic particles coming from the deuterium convert some elements they strike into radioactive isotopes ? StuRat 00:39, 23 March 2006 (UTC)
- You'd probably generate a lot of neutrons and from that some artificial radioactivity, as I understand it. But you would lack fission products which make up a lot of the really nasty stuff that comes out of a bomb. We have an article on pure fusion weapons for those who are interested. --Fastfission 01:48, 23 March 2006 (UTC)
- You can accomplish the same damage as a "nominal" nuclear weapon easily with conventional weapons. The firebombings of Tokyo destroyed more area and killed more people than the bomb of Hiroshima. The difference is that the former case took a few hundred bombers each loaded to the brim with tons of explosives, while the latter took one bomber with one bomb. There's an economics of scale there, if you are interested in just destroying things. --Fastfission 01:48, 23 March 2006 (UTC)

- Oh hey, dig this article I just stumbled across while trying to look up the "Minor Scale" non-nuclear nuclear simulation (which I had just remembered): List of the largest artificial non-nuclear explosions. --Fastfission 01:52, 23 March 2006 (UTC)
A Nap
[edit]Whenever I take a nap during the day, I will wake up feeling light-headed and have a bad taste in my mouth. I will continue feeling so for about 30 minutes afterwards. Is there any way to keep this from happening? — Ilyanep (Talk) 23:55, 22 March 2006 (UTC)
- I find that after a nap you end up with something very similar to morning breath. The light-headedness is pretty common as far as I know. It's usually your position and the blood rushing through your head (sometimes you can see it as points of light and darkness during the dizziness). As for a cure or a preventative? I don't know if there is any. Keep a mint or some minty dental floss around and chew on that to cure the breath. The dizziness can be lessened by standing up slower. M@$+@ Ju ~ ♠ 00:05, 23 March 2006 (UTC)
- Hmm...well the thing is that I start feeling lightheaded before I even sit up or stand up. — Ilyanep (Talk) 00:44, 23 March 2006 (UTC)
- I think part of it is also caused by a combination of dehydration and congestion - if your nose is blocked you breathe through your mouth, which dries out. I haven't found a decent cure for it myself, sadly enough. Confusing Manifestation 00:11, 23 March 2006 (UTC)
- As for the dizziness, try sitting up for a couple minutes before you stand up. The bad taste could also be a minor case of acid reflux disease, which is typically worse when lying down. Try chewing some antacids before your nap, that might lessen the effect. StuRat 00:31, 23 March 2006 (UTC)
- Acid reflux appearing at 14? — Ilyanep (Talk) 00:44, 23 March 2006 (UTC)
- It's possible. Do you having a burning or sore feeling in your throat or mouth ? StuRat 01:00, 23 March 2006 (UTC)
- Not really...it's really only a bad taste. And brushing my teeth gets rid of it. — Ilyanep (Talk) 02:20, 23 March 2006 (UTC)
- In that case the cause might be a lack of air circulating through your mouth when you sleep (assuming you breathe through your nose). This lack of ventilation allows odors and tastes (from decomposition of food) that would otherwise be ventilated, to accumulate. You might also try brushing your teeth before each nap. StuRat 09:57, 23 March 2006 (UTC)
Galaxies
[edit]What is a common feature among galaxies?
- They all contain students who expect others to do their homework for them. StuRat 01:22, 23 March 2006 (UTC)
- I wish you'd think. Click on the word "galaxy" now in your question. If you haven't discovered that many galaxies in outer space including our own feature stars. Because God lives in heaven, which is in the sky, in the clouds, galaxies are all higher than God. Galaxies all have the letter q in their middle name. I hyperlinked the word "galaxies" to a Wikipedia article about galaxies. It took me a while to learn wikipedia's programming language, and on the way, one of the first things I learned was how to link words to Wikipedia articles. I learned this many years ago. It was spring 2001 and the muggy amazon air was wearing heavy in my lungs and covered my body with a cloak of sweat. It was getting dark and I knew my Kogi tribal guide was trembling with fear and wouldn't help me for long as we near the hidden temple. --
 Mac Davis] ⌇☢ ญƛ. 08:37, 23 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 08:37, 23 March 2006 (UTC)
- I wish you'd think. Click on the word "galaxy" now in your question. If you haven't discovered that many galaxies in outer space including our own feature stars. Because God lives in heaven, which is in the sky, in the clouds, galaxies are all higher than God. Galaxies all have the letter q in their middle name. I hyperlinked the word "galaxies" to a Wikipedia article about galaxies. It took me a while to learn wikipedia's programming language, and on the way, one of the first things I learned was how to link words to Wikipedia articles. I learned this many years ago. It was spring 2001 and the muggy amazon air was wearing heavy in my lungs and covered my body with a cloak of sweat. It was getting dark and I knew my Kogi tribal guide was trembling with fear and wouldn't help me for long as we near the hidden temple. --
- Weird question! They must have dozens of common features i.e those features that are common to galaxies!! They are all spiral, elliptical or irregular. Each has on average 100,000,000,000 stars (roughly). They are all made up of stars (and gas and debris). They all can't be seen with the naked eye (except Andromeda and one other). They rotate. The spirals have spiral arms but the ellipticals don't. They are all travelling ever so fast through space. They like to to clump together into groups of galaxies. I wrote all this from memory without reading the article so you can check me out then invent other similarities for yourself (note: I am not an astronomer) - Adrian Pingstone 20:50, 23 March 2006 (UTC)
- I think perhaps if you were more specific with the question, we would be able to help you better. Luthinya 11:26, 28 March 2006 (UTC)
Methamphetamine Production - Odorous or not?
[edit]Does the illegal "cooking" of Methamphetamine cause a noticeable or describable odor or scent?
What does it smell like? Is the smell a strong, pervasive odor or a vague, indistinct fragrance?
- There are several different synthetic pathways that can be used, each with its own associated smells. See Methamphetamine#Production. —Keenan Pepper 06:14, 23 March 2006 (UTC)
- [82] (PDF) lists ether-like, solvent-like, vinegar-like, and ammonia-like odors among the possible smells of a clandestine meth lab. Are you wondering if your neighbors are running a meth lab? Are you planning to set one up yourself? In either case be advised that possible meth lab odors may be probable cause for a search. —Charles P._(Mirv) 06:26, 23 March 2006 (UTC)
- The odors also tend to be toxic and explosive, so I would call the police and evacuate, if you suspect a meth lab. StuRat 09:51, 23 March 2006 (UTC)
- As someone whose occupation involves carrying out organic chemical reactions much like those associated with "cooking" methamphetamines, I can say that the odors associated with it are definitely noticeable and in most cases very distinctive; the fumes are also often toxic and highly flammable. There is very little chance that anyone could get away with it for long without being noticed unless they are quite isolated from neighbors. Organic chemists work in fume hoods that carry solvent and other chemical vapor away from them; otherwise it would be too dangerous (and unpleasant) to be exposed to them. --Ed (Edgar181) 12:46, 23 March 2006 (UTC)
Impact on Rotation
[edit]Has the combined effect of all human and animal activities since life began on Earth had any appreciable impact on the rotation of the Earth? JackofOz 04:12, 23 March 2006 (UTC)
- I once heard, from someone in a position to know, that the mass of water held at elevation behind dams had increased the earth's angular moment of inertia and therefore, ever so slightly (but measurably) decreased its rotational speed, in the manner of a skater extending their arms. But I'm not sure I believe it. —Steve Summit (talk) 04:35, 23 March 2006 (UTC)
- No. Have you been to that dumb "jump" site? --
 Mac Davis] ⌇☢ ญƛ. 04:58, 23 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:58, 23 March 2006 (UTC)
- No, what's the url? --GangofOne 01:19, 24 March 2006 (UTC)
- See the Polar motion article. If you beleive humans are contributing to melting of the Greenland ice sheet then, yes. EricR 04:59, 23 March 2006 (UTC)
- And, if you believe its melting ;) --
 Mac Davis] ⌇☢ ญƛ. 08:21, 23 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 08:21, 23 March 2006 (UTC)
- And, if you believe its melting ;) --
- Bearing in mind the inclusion of the word "appreciable", I would say no. Any changes brought about by life on Earth are completely insignificant. StuRat 09:48, 23 March 2006 (UTC)
- Polar motion is significant for those trying to accurately measure positions on the earth's surface. The contribution of isostatic rebound seems to be "appreciable", as in possible to estimate, measure or perceive. Whether or not humans have had an appreciable impact on off-axis glacial melt depends on who you ask i guess. EricR 17:54, 23 March 2006 (UTC)
- According to Resnick and Halliday, Physics 3rd ed, 1977, p11, cites Essen Physics Today July 1970, shows annual variation in Earth's rotation of 160 parts out of 1010, and decreasing every year. (Cesium clocks in use then are good for 1 part in 1012.) Explanations mentioned: tidal friction between water and land. Seasonal motion of the winds. Melting and refreezing of polar icecaps. Something I read somewhere else: deciduous trees losing/regrowing their leaves seasonally. Have no doubt that the comings-and-goings of HuMan have an effect, but it will never so great as the tides and flows of nonhuman nature. GangofOne 01:06, 24 March 2006 (UTC)
Graphs - all pairs shortest path
[edit]Many of the APSP algorithms highlighted specify that they are for directed graphs. eg. the Floyd-Warshall algorithm. Can they also be used on undirected (all edges positively weighted) graphs?
- Yes. Transforming an undirected graph into an equivalent directed graph is very easy, if you think about it. With an appropriate data structure implementation the algorithm doesn't even have to care whether the graph is directed or undirected. 130.188.8.13 09:51, 23 March 2006 (UTC)
Many thanks for the help.
DNA to organism
[edit]Say someone works out the complete DNA sequence (including mitochondrial DNA and whatever else) for some organism, for example a wombat. You've given a CD-ROM of the sequence but not told what the organism is (maybe you're told it's a mammal). Of course maybe you could match it against some database but let's not count that. Is it possible in principle to run the DNA sequence through a computer program and simulate the growth of the organism to the point that you can figure out what it looked like? Obviously nobody knows how to do this today, but is it plausible? What kind of knowledge and technology needs to be developed for it to happen? Thanks. Phr 13:21, 23 March 2006 (UTC)
- I'd say that it isn't plausible. That would basically assume that the environment (womb) that the thing grows in is of no great significance to the result, which it is. --BluePlatypus 13:40, 23 March 2006 (UTC)
- One of the big challenges would be in figuring out how to convert a DNA gene sequence into a fully folded three-dimensional protein. Proteins are synthesized as long chains of amino acid residues; these chains are folded into complex shapes to give them their final structure and function. Modelling this process is extremely difficult, and we aren't anywhere near being able to do it yet.
- Of course, once you fold those proteins, you then have to model how they interact with other complex molecules in their surroundings—particularly other proteins. Also nontrivial, given the number and variety of different molecular species in a cell. TenOfAllTrades(talk) 13:57, 23 March 2006 (UTC)
- There is a big gap in our current knowledge about how to get from genotype to phenotype in a complete organism; "development" is still a big question mark a majority of the time (the number of places in which we understand clearly how one gets from DNA to a given phenotype is dashingly small in comparison with the amount of DNA an entire organism uses). In any case, one would probably have more efficient ways to figure out what sort of animal any piece of DNA was than trying to model the whole organism -- certain genes are likely to only be present in mammals, for example, and you could just look for those if that's what you want to know. --Fastfission 14:26, 23 March 2006 (UTC)
- Since he seemed to be asking "would it ever be plausible", not, "is it plausible today", I would say yes, given maybe a century of new discoveries, we could do all of that. I agree with Fastfission that it would hardly be the most efficient mechanism for identifying a strand of DNA, however. StuRat 17:53, 23 March 2006 (UTC)
- Even if it were technically possible (which I highly doubt) to model a whole organism at the level of detail required, I don't see how any discovery or technology would provide information that simply isn't there; DNA alone just does not suffice to grow a complex organism. You could guess at the environmental factors, and work by trial-and-error, but that would always just be a guess which you wouldn't be able to verify. --BluePlatypus 00:03, 24 March 2006 (UTC)
- Yeah, I meant was it possible even in principle. I was imagining two kinds of applications: 1) You get hold of some dinosaur DNA and you want to know what dinosaurs really looked like, and it's much more appealing to just drop the DNA sample into a sequencing machine and get a nice dinosaur picture on your computer a few minutes later, than spend years doing some Jurassic Park process of growing an actual dinosaur and ending up with a huge dangerous beast clomping around eating your lab assistants. 2) You want to genetically engineer a flying wombat, so you sequence the DNA of a regular wombat and model the effects of different possible gene splices on your computer til you get what looks like flight capabability. Again, this is much faster and less messy than waiting through a whole wombat gestation cycle for each splice that you want to test. Anyway, the answers given are very informative. Thanks again. Phr 02:33, 24 March 2006 (UTC)
- Even if it were technically possible (which I highly doubt) to model a whole organism at the level of detail required, I don't see how any discovery or technology would provide information that simply isn't there; DNA alone just does not suffice to grow a complex organism. You could guess at the environmental factors, and work by trial-and-error, but that would always just be a guess which you wouldn't be able to verify. --BluePlatypus 00:03, 24 March 2006 (UTC)
- Thanks, everyone. The quality of answers found on this page, not just to this question but to most of the others that I've looked at, is absolutely astonishing. What an amazing resource this is. Phr 22:14, 23 March 2006 (UTC)
Even if such a technology could be developed, many more would return with arguments against its ethnical correctness, in humans effectively playing god with genotypes. The creation and giving of life needs to be considered with greater care than the frenzied curiosity of one experimentation, and the consequences of a thoughtless creation of life would not bear to be thought about. In many respects people would argue that we are not yet ready to take this sort of aborted life seriously- that we can only ever ruthlessly sport with the works of our own hands, no matter how precious. Such actions bear a heavy burden, and ere we should even attempt to develope the technologies that will enable us upon this task, we must choose between what we theoretically could do and what we humanely should do. Luthinya 11:31, 28 March 2006 (UTC)
Finding Easter Eggs
[edit]How easter eggs (hidden inside any software) can be found? Whether it is possible or not? If possible then how? Is their any tools (softwares) for this?
- Poeple who find these things (rather than getting inside tips) spend hours or days trying every combination of keystrokes and everything, in the hope of the fame and riches that come from finding easter eggs. Automation would not be possible. Notinasnaid 15:23, 23 March 2006 (UTC)
- "Fame and riches"? That's a joke, right? I think some keystroke automation might be possible, but I'm not sure. I suspect nearly all easer eggs are leaked from inside the team that made the software. Johntex\talk 15:34, 23 March 2006 (UTC)
In many cases it can be done by looking at the code, and searching for things that look odd. For great justice. 16:36, 23 March 2006 (UTC)
- Another way which seems to work is looking on google for sites which list easter eggs and cookies :) Grutness...wha? 00:56, 24 March 2006 (UTC)
The Easter egg (virtual) article might give you the information you want. --Halcatalyst 03:34, 24 March 2006 (UTC)
- A Debugger program can look inside the code and see what variables are being used, then you can change these variables and see what happens. A GameShark type device does this on consoles, but it can be done in software too, and then it's just a matter of finding the corresponding code to trigger it inside the program. Night Gyr 18:26, 24 March 2006 (UTC)
- Which is a bit more difficult than it may sound. --Optichan 18:34, 24 March 2006 (UTC)
explorer
[edit]Suppose for a second that for whatever reason you've been using IE as a browser (why? who knows, sadism? bored? whatever..) and your browser freezes, why the hell is it that iexplorer.exe itself, the program that is actually freezing, never crashes per se, but rather explorer.exe is always the one that crashes, I mean right now, explorer is totally dead, yet I'm using iexplorer to type this question, even though I have no access to windows itself as explorer is quite dead, why does one crash, but not the other?--64.12.116.74 17:23, 23 March 2006 (UTC)
- One problem that can cause this is memory overflow. If one application allocates a 100 Kb block for writing data, then proceeds to write 150 Kb of data to that block, the extra 50 Kb will overwrite whatever was in the next memory location. This could be the same application, another application in the same suite, or a completely unrelated application, even the operating system. These type of "memory leaks" can be extremely difficult to locate, as the source is not immediately apparent. If properly designed, an operating system would not permit any application to write to memory it has not explicitly allocated. However, the common operating systems have no such protection. StuRat 17:46, 23 March 2006 (UTC)
- Um, no, all recent versions of Microsoft Windows have memory protection, which means a program can only screw up its own data. If it tries to access the memory of a different process it will crash with a segmentation fault (aka "General Protection Fault"?). A memory leak is a different thing, when a program keeps allocating memory but not freeing it, so the memory cannot be reclaimed by the OS. —Keenan Pepper 17:58, 23 March 2006 (UTC)
- How recently ? My (admittedly old) computer doesn't have this feature, and I'm guessing the person who asked the question doesn't have this feature either. StuRat 18:43, 23 March 2006 (UTC)
- I don't know much about Windows, but Microsoft Windows 3.0 said it had memory protection. —Keenan Pepper 00:50, 24 March 2006 (UTC)
- Which doesn't really make it so. Windows NT/XP/2000 has true memory protection, in the sense that it catches any and all such overstepping of bounds. Windows 95/98/ME did not really have memory protection in that sense, although it did have safeguards in place to stop programs from writing to obviously wrong places (like memory address 0), which Microsoft touted as "memory protection". --BluePlatypus 13:20, 24 March 2006 (UTC)
- There's a much more likely problem here. explorer.exe uses Internet Explorer (iexplore.exe) to display many of the things it displays. For example, when you press F5 in folder view, notice how it refreshes the folder view? That's because F5 refreshes the page in Internet Explorer, and Windows Explorer uses certain iexplore components. So when iexplore.exe crashes, explorer.exe likes to crash as well, since it depends on parts of iexplore to run. -- Daverocks (talk) 08:07, 24 March 2006 (UTC)
Tension Tensor, Forces...
[edit]You're given a tension tensor:
If there are no volume forces, how can I prove this fluid is in balance? How can I calculate overall surface forežce that acts on the upper part of the sphere
- Is this a homework problem? If so, you should do your own homework. If not, I haven't a clue. :-) Johntex\talk 20:30, 23 March 2006 (UTC)
- No, it's not. It's just a problem and I don't have a clue how to solve it.
- This looks like a matrix. Would using matrix arithmetic help? --Halcatalyst 03:06, 24 March 2006 (UTC)
- Tensors are represented with matrices. You have to know at least the basics of matrix arithmetic, yes. But that's not the problem here.
- If by tension you mean force, then there is no such thing as a tension tensor. You must be meaning stress tensor. In that case, the equilibrium ("balance") is given by:
are the components of your tensor. In your case, there's no body force ("volume force"), so b1 , b2 , and b3 are zero. It's very easy to apply these conditions in your case, and find the answer. Hope this helps! deeptrivia (talk) 01:21, 25 March 2006 (UTC)
- For the second part, you can use Cauchy's principle (will create article soon) to find out the net force on the top surface of the cylinder. The principle states that the traction on a surface element whose area normal is defined by is given by the vector . You will have to consider an infinitesimal area element of the surface and find its area normal (convert to spherical coordinates, and this should be a cakewalk), and integrate over the top surface. There might be some intuitive arguments (symmetry, etc.) which could be used to avoid this integration, but this general procedure is extremely straightforward too. deeptrivia (talk) 01:40, 25 March 2006 (UTC)
Apollo missions & orbital mechanics
[edit]I have a question about a technical aspect of the Apollo missions. From my understanding, the ship would launch, go through staging of the first and second stages, orbit the Earth, go through a systems checkout and then initiate the trans-lunar injection (TLI) burn which would take the ship out of Earth orbit and send it towards the moon at some high rate of speed.
During the TLI, the CSM would detach from the 3rd stage, rotate and maneuver in order to dock with the Lunar Module. The CSM would extract the LM from the 3rd stage and then use its thrusters to separate from the 3rd stage. The 3rd stage would then be sent to crash into the moon.
The thing I don't understand is this: how was the CSM able to travel backwards towards the 3rd stage while still speeding towards the moon? I know that once you start talking about vacuums, space and zero-gravity that all the things that usually make sense go out the window, but I just can't get my head around the fact that ostensibly it's a vehicle that manages to continue travelling east while at the same time heading west. It's like being on a train that's heading east and walking west down the car. But what is the force that represents the train?
My only guess is that it's something to do with gravity: does the TLI merely change the shape of the orbit of the CSM such that it is elongated enough to take the CSM to the point where the lunar gravity takes over?
Did I just answer my own question?
- Rotational velocity is differnt from linear velocity. Imagine a car that "spins out" on a wet road. The car starts out traveling east, goes into a spin, and is now facing west, but overall, the car is still traveling east. The low friction of the wet road makes this easier. In space, with zero friction (ignoring space dust) this is even easier. Johntex\talk 20:29, 23 March 2006 (UTC)
- If I understand you correctly, it's just the case of both objects moving in direction X, but with different velocities, and by changing the relative velocities of each object they can converge or separate as they please.--inksT 20:56, 23 March 2006 (UTC)
- Ahh -- so it's not so much that the CSM would actually start travelling towards stage 3 so much as it would slow down slightly such that stage 3 would meet up with it. I imagine that at the speeds at which they were travelling such minor adjustments to their velocity would have little overall effect.... Thanks!Xous 22:23, 23 March 2006 (UTC)
- That's right, from the frame of reference of Earth. From the frame of reference of the either the CSM or LM you would describe it differently, but I don't want to confuse you more by going into that. StuRat 22:36, 23 March 2006 (UTC)
It's worth noting that the separation of the CSM from stage three was not accomplished by the main propulsion engine of the CSM (doing that would fry the LEM). It was done with reaction control engines placed at four places around the circumference of the service module. These engines had small jets pointing in several directions and allowed for the turning of the CSM to face the LEM and also for the slight thrust necessary to slow the CSM down relative to the third stage and LEM. They were also used to "reverse the LEM out of the garage" of the third stage. Only once the craft was clear of the then obsolete third stage were the main engines fired. The same jets were used to jettison the service module from the command module prior to earth re-entry. Grutness...wha? 01:04, 24 March 2006 (UTC)
RSS program
[edit]Is there any RSS program which download main page of a particular web-sites? roscoe_x 20:36, 23 March 2006 (UTC)
- Um, could you clarify your question further? RSS is live feeds, which update frequently. Downloading the main page of a particular website is as simple as pointing your web browser to its URL. -- Daverocks (talk) 07:50, 24 March 2006 (UTC)
- Also, you might want to check out our article on RSS (file format). -- Daverocks (talk) 07:52, 24 March 2006 (UTC)
Making a homepage with RSS feeds, Gmail, Yahoo Calander, etc
[edit]I want to make a one-stop home page for myself which includes things like my Gmail inbox, my calander, my Remember The Milk to-do list, RSS feeds, recent Flikr pics and so on. Is (any of) this possible, and what kinds of tools exist for such a page?
Any advice greatly appreciated, Thanks, ~Mary
- Have you tried personalising your Google homepage? There are modules for displaying your Gmail inbox, event calendar (although not Yahoo! calendar), to-do list, flickr feeds and any RSS feed (click Add Content in the top left and paste the URL into the text field under 'Create a Section'). Bewildebeast 12:45, 24 March 2006 (UTC)
- Wow, thanks!
computer internal hard drive
[edit]Is it possible to use Windows Explorer to divide my internal hard drive so that there is a new drive to then create 26 new files labled A-Z? My plan is to install a scanner to copy each subject's information and save this to that file (in the new drive, for example, drive G) for quicker subject reference.
My goal is to eventually do away with "paper" files and have the files on the hard drive.
I appreciate any information you may give.
Sincerely,
--205.188.116.74 21:42, 23 March 2006 (UTC)
- Why would this be any faster or easier than just having 26 folders labled A-Z in your C: drive? It seems rather that it would be unnecessarily complicated — it would be more difficult to transfer documents, or do global changes on your file set, not to mention that it would just require an extra step of navigating to each partition. Anyway, in answer to your question, there are a number of utilities listed at Partition (computing). — Asbestos | Talk (RFC) 21:49, 23 March 2006 (UTC)
- Also, Windows Explorer cannot alter drive partitions, which is what you are asking to do. Also also, putting 26 partitions on a single drive will waste a lot of the drive. Not only do you need overhead for each partition, you have to hard-set the partition size when you make it. How much bigger should the S partition be than the X partition? --Kainaw (talk) 23:27, 23 March 2006 (UTC)
- The easiest idea is not to remember approximately where you stored some information, but to find it quickly. Try Copernic or Google, they have home research utilities to index your data on your drive and find them from any word(s). --DLL 17:34, 24 March 2006 (UTC)
Chemistry help
[edit]I've been trying to work this problem out, but I'm hopelessly stuck. Could someone explain to me how to do this?
50.0 Liters of hydrogen gas and some oxygen gas mix to form water. Calculate the volume of oxygen needed to completely use up the hydrogen. What mass of water forms? (The reaction is kept at STP).
I have no idea what to do unless I can convert Liters to grams...please help.
- This sounds like a homework problem. Hint: the problem tests your knowledge of Avogadro's law, part of the ideal gas law. Phr 22:09, 23 March 2006 (UTC)
- The formula for water is H2O. So you need half a many atoms of Oxygen as Hydrogen to make water. Find the number of atoms of Hydrogen in 50.0 Liters. Take half that number and find the volume of Oxygen. Find out the mass of the number of Hydrogen atoms and the mass of the number of Oxygen atoms and add them together. You do not really need to calculate the actual number of atoms, just know that 6.02 * 10^23 atoms is equal to the atomic weight in grams This is called one Mole. From the
density of Hydrogen you can find out the mass of 50.0 Liters and thus how many Moles it is. Take half that number of Moles and find the mass of Oxygen. Now using the density of Oxygen you can find how many Liters that is.
- Thank you! But how do I find the number of atoms in Liters?
- These guys below are making it too complicated in my opinion. You need to find the density of hydrogen, that is the mass of Hydrogen in a Liter. You can look this up on the web in many places. You do not really need to find the number of atoms in a Liter. All you need is to know that 6.02 * 10^23 atoms is a 'mole' and this is equal to the atomic weight in grams. So using the density find the number of grams in 50.0 Liters and divide that by the grams in a 'mole', this will give you the number of 'moles'. You will need half as many moles of oxygen.
- You make the not-unreasonable assumption that the gasses are ideal and use the ideal gas law to calculate the number of moles of H2 in 50L. You then work out how many moles of O2 are used in the reaction which gives you the volume of Oxygen needed. You also know how many moles of H2O are created from the reaction, which together with the molar mass of water gives you the mass of water formed. -- AJR | Talk 00:50, 24 March 2006 (UTC)
- So is it safe to assume taht 2.23 moles of hydrogen is about 2.23 grams since H has 1 gfm?
- 2.23 moles of hydrogen atoms, yes. But Avogadro's law is about the volume in liters per mole of gas molecules. Remember that hydrogen molecules at STP are H2 (splitting them apart takes a tremendous amount of heat), so each hydrogen molecule has two hydrogen atoms. It's the same way with oxygen molecules. Anyway, I think the point of the exercise is to understand how to use Avogadro's law, so please click on the link and see if it explains what you need. Phr 02:17, 24 March 2006 (UTC)
- Right. Thanks!
- 2.23 moles of hydrogen atoms, yes. But Avogadro's law is about the volume in liters per mole of gas molecules. Remember that hydrogen molecules at STP are H2 (splitting them apart takes a tremendous amount of heat), so each hydrogen molecule has two hydrogen atoms. It's the same way with oxygen molecules. Anyway, I think the point of the exercise is to understand how to use Avogadro's law, so please click on the link and see if it explains what you need. Phr 02:17, 24 March 2006 (UTC)
- So is it safe to assume taht 2.23 moles of hydrogen is about 2.23 grams since H has 1 gfm?
- You make the not-unreasonable assumption that the gasses are ideal and use the ideal gas law to calculate the number of moles of H2 in 50L. You then work out how many moles of O2 are used in the reaction which gives you the volume of Oxygen needed. You also know how many moles of H2O are created from the reaction, which together with the molar mass of water gives you the mass of water formed. -- AJR | Talk 00:50, 24 March 2006 (UTC)
You are all missing the easiest solution, which is to use Gay-Lussac's Law directly. Volume ratio equals mole ratio, hence half of 50 = 25 litres. Then, for the mass of water, moles hydrogen = moles water = 50/22.4. The molar volume of hydrogen (and any other gas]] at s.t.p. = 22.4 litres. Then simply multiply by 18 to get the mass of water formed.G N Frykman 19:47, 24 March 2006 (UTC)
MicroATX motherboards
[edit]I'm thinking of getting a MicroATX board to save £20 compared to a similarly-featured ATX board. Could I install a MicroATX motherboard in my ATX case? Also, is it wise to get a MicroATX board just because it's cheaper? Is there anything I'd be missing out on (other than fewer PCI slots)? Thanks. Sum0 22:36, 23 March 2006 (UTC)
- Well, the ATX article says A full size ATX board is 12" wide by 9.6" deep (305 mm x 244 mm). This allows many ATX form factor chassis to accept microATX boards as well. And the MicroATX article says MicroATX towers are a lot smaller than typical ATX towers. This means that although the same standard hardware is supported, it is supported in lower quantities. So you can install one, but you may not be able to shove as much hardware in there. Perhaps you should check one out to be sure that you can fit all the components you want in there. --Optichan 14:44, 24 March 2006 (UTC)
- You may also lose out on one DIMM slot with an mATX board, although this won't be a problem unless you want more than 2GB of RAM. Obviously the small form factor of the board will mean that things are very close together, so component installation and connections (particularly once the board is in the case) are likely to be fiddlier than with full ATX. Out of interest, which boards are you considering? I'd recommend taking a look at the AsRock range if you haven't already; my experience with them has been good and they cater well for smaller budgets. Yummifruitbat 05:30, 26 March 2006 (UTC)
Airport Extreme Compatability
[edit]Will my Netgear Wirless router with wireless g format work with airport extreme, or do I have to Buy anything? Seamus215 22:53, 23 March 2006 (UTC)
- I'm assuming you have a Mac with an Airport Extreme card, yes? They will work very well together.
- Yup, Airport Extreme and wireless 802.11g are completely compatible. Sum0 09:31, 24 March 2006 (UTC)
- However -- some combinations of non-Apple 802.11g gear are not completely happy with Airport Express -- so if you add an Express to the mix (for the lovely purpose of playing your iTunes through your stereo), you might find yourself suffering from dropouts, in which case the only solution is to force the wireless router to only use b rather than g. (Linksys in particular has this problem; I don't know about others.) --jpgordon∇∆∇∆ 20:19, 26 March 2006 (UTC)
March 24
[edit]the moon
[edit]Why is our moon the only one in the universe without a name?
- It has a name, Luna. There just isn't much call to use it's name, since everyone knows which moon you're talking about when you say "the moon". StuRat 01:22, 24 March 2006 (UTC)
- There are billions of moons in the universe that humans haven't given names to, because humans have not yet discovered them. It's only the tiny, infinitesimal proportion of moons that exist in our Solar System that have ever been named.
- Our Moon has different names in different languages. Luna is its name in Latin, Russian and some other languages. It's "der Mond" in German, "la lune" in French, and "the Moon" (upper case) in English. The word "moon" (lower case) is also used generically in English to mean a natural satellite of any planet, just as "sun" is used generically to mean any star. But the original and primary meanings of "moon" and "sun" were the specific bodies the Moon and the Sun. The Moon is sometimes referred to as Luna or Selene by English speakers, but these are poetic or dramatic uses, just as England is sometimes referred to as Albion, or the USA is sometimes personified as Uncle Sam. JackofOz 02:01, 24 March 2006 (UTC)
- Similarly, the Sun is just "the Sun," despite the fact there are many suns out there. I think it should have a name of its own, and I propose Halcatalyst 03:02, 24 March 2006 (UTC)!
- Isn't the Sun called Sol in some language?
- I have various 4-letter names for the Sun, which I employ when I wake up and open my eyes, only to discover the Sun is burning a hole in my retina. (I have mini-blinds on the window, and the Sun has an amazing talent for finding the little holes in the blinds for the cord, and shining directly into my eye.) StuRat 03:23, 24 March 2006 (UTC)
- Our sun's name is Sol, and our moon's name is Luna. They are both borrowed from Latin. —Keenan Pepper 03:40, 24 March 2006 (UTC)
- I disagree. Those are the names in the Latin language, but they have never entered the English language as formal names for these places. Back when scientific papers were all written in Latin, these words would have been used. Their names in the English language are "the Moon" and "the Sun" - and our articles back me up on this. JackofOz 00:57, 25 March 2006 (UTC)
A similar point can be made about the planet we're presently living on. Unlike every other planet in our solar system, and indeed in others, our planet has no REAL name. It's simply been called "Earth" (or an equivalent in whatever other language...Terre in French, Terra in Latin,) because all those centuries ago, before we even realized it was a sphere, we basically bent down and examined what the ground was made of and named it after that. Loomis51 04:42, 24 March 2006 (UTC)
- Which means that is the name of the planet. Any map of the solar system will show all the planets with their names. Ours is "Earth", or "the Earth". And of course it is a "real" name. JackofOz 00:57, 25 March 2006 (UTC)
- Didn't I see the same question some weeks ago ? --DLL 17:30, 24 March 2006 (UTC)
The two questions here are based on the same false premise: Why is our moon the only one in the universe without a name and Similarly, the Sun is just "the Sun," despite the fact there are many suns out there. The Moon is a natural satellite. All the known natural satellites have names - our one is called "The Moon". However, that name has become like a generic trademark - just as all ballpoint pens are (incorrectly) called Bics and Biros, so all natural satellites are (incorrectly) called "moons". The second question makes this false premise even more obvious: "There are many suns out there"? No, there are many stars out there - often poetically referred to as suns because they resemble our own star, whose name is "The Sun". Grutness...wha? 10:59, 25 March 2006 (UTC)
- I think of the word "sun" (lowercase) as meaning "the star which the observer orbits". Thus, to us, Sol is our sun, and the rest are just stars. However, to someone on a planet orbiting Alpha-Centauri, Alpha-Centauri is their sun and Sol is just an ordinary star. StuRat 12:49, 25 March 2006 (UTC)
antique phonograph motors
[edit]i am searching for information concering an antique phonograph motor manufactored by the united mfg. and dist. company. model no. 7 thank you, richard
- Do you have any other info, such as the approximate date and location of manufacture ? StuRat 10:58, 24 March 2006 (UTC)
The location of the manufacture is Chicago, Ill. It gives no date on the motor. Any information could help locate which type of phonograph this is.Tks
- I haven't had much luck. The most I could find out is that the United Manufacturing Company of Chicago switched to making pinball machines in the early 1950's, so your phonograph motor is likely older than that, but you probably already knew that. StuRat 22:55, 24 March 2006 (UTC)
Opioid Pain-Killers
[edit]I suffer from extreme chronic pain. Unfortunately two of my brothers are heroin addicts and the idea of taking a narcotic scares me to death. I've seen the misery it can cause. Yet I have never taken a street drug in my life and I believe that I would be incapable of resorting to the streets for drugs.
Does there exist an opioid-type drug that is extremely long-lasting, in the sense that I could take a pill, an injection or whatever once a week or so, administered by a nurse and monitored by my doctor or do all opiods have a fairly short term effect? If it could be administered to me once a week or so it would greatly relieve my fears of potential abuse, since I know myself, and I know that if administered this way there would be zero possibility of me resorting to street drugs to feed my habit, just because I know that I'm incapable of ever doing that.
I'd appreciate any info I can get.Loomis51 04:28, 24 March 2006 (UTC)
- We are not doctors, and can therefore not give medical advice. Especially in a difficult situation such as this one, I would strongly urge you to consult your doctor, even if he/she can't help you directly, they will certainly know someone who can advise you. — QuantumEleven | (talk) 08:34, 24 March 2006 (UTC)
- I appreciate your restraint on giving medical advice, but what I'm asking for is not medical advice per se, I'd just like to know if there exists a long lasting or extended release form of opioid-type drug. It's actually more of a chemistry/pharmaceutical question than a request for medical advice. Surely, as I said, I would only do this under the strict direction of my doctor, it's just that my next appointment is several months away and I wouldn't like to have this hanging over my head for all that time. Loomis51 11:04, 24 March 2006 (UTC)
- Oxycontin is specifically designed to be a time-release drug, but it relies on its shell to be intact and slowly let out the drug into the body. Any break in the shell makes it no better than older drugs as far as a sudden dose of narcotic, and it's become a popular street drug since it can be ground up and snorted for a quick high. It takes a lot of precautions for use, but for some patients it's a lifesaver. Night Gyr 18:35, 24 March 2006 (UTC)
I don't think any of that class of meds are long lasting, and also think they are all addictive, just as you fear. To avoid the risk, I'm afraid you will need to use a different class of pain killers. Aspirin can be effective against some types of pain, especially those accompanied by inflammation, such as arthritis. Ibuprofen and other over-the-counter drugs are another alternative. Some steroids can also work as pain relievers, but have some serious side effects, too. StuRat 10:46, 24 March 2006 (UTC)
- Aspirin and Ibuprofen are completely innefective in my case. Obviously, given my fear of narcotics, I would have tried them first. Loomis51 02:06, 25 March 2006 (UTC)
Also note that pain may be an indication of a physical problem which needs to be addressed. I assume you and your doctors have already looked into this. StuRat 10:46, 24 March 2006 (UTC)
- Yes they have. Loomis51 02:06, 25 March 2006 (UTC)
The drug you are describing is methadone, which is sometimes used (in the US at least) for chronic pain. alteripse 11:36, 24 March 2006 (UTC)
- Unfortunately, methadone is also addictive. Our article on it says: "some heroin addicts feel that it is actually harder to quit methadone than heroin itself". StuRat 20:41, 24 March 2006 (UTC)
- Why would it have to be an opiate?`--BluePlatypus 13:16, 24 March 2006 (UTC)
- By the way, if the pain you are experiencing is neuropathy (commonly caused by shingles or long-term diabetes), then opioids might not be the best choice. --Uthbrian (talk) 16:26, 24 March 2006 (UTC)
- I'm asking about opiates because everything else has been tried on me and my doctor, who's a pain specialist told me that in a few months' time we'll be faced with the difficult decision of whether to use them on me.
- Special thanks to Night Gyr. I was not aware that oxycontin was a time-release drug. How frequently must it be taken to be effective? If it's about once a week that perfect. I'd just go to the hospital and take it under the supervision of a nurse (no crushing and snorting!)
- No, the usual dosing of Oxycontin is every 12 hours. You're not going to find an opiate with a dosage interval much longer than that. - Nunh-huh 03:03, 25 March 2006 (UTC)
- Sorry buddy, sounds like you are in a real difficult fix. If you have talked to your doctor already, I do not think there will be much help forthcoming here. The only other pain med I can think of is Novocaine, which dentists use for local anesthetic, it is not long lasting, but at least not addictive.
- Hmmm... since it sounds like you've tried most everything, another option for long-lasting relief from severe pain may be Duragesic transdermal patch; each patch can be worn for 72 hours of pain relief. However, this option is also addictive. Plus, it's only for use in people who've already tried other opioid options and are tolerant to opioids. There are also many other black box warnings for Duragesic: see Rxlist.com for more details.
- If you haven't done so, inform the doctor about your family history for opioid dependence; s/he can take some precautions such as prescribing a limited quantity in a given timeframe. --Uthbrian (talk) 06:32, 28 March 2006 (UTC)
Hypnotism or acupuncture may be much better solutions than drugs...hotclaws**==
Unless you have a proper diagnosis of the underlying cause of your chronic pain, your best choice for pain relief cannot be known. Don't rely on WIkipedia or any other on-line outfit for pharmacological advice. You should consult with a chronic pain specialist. Unfortunately, they are few and far between unless you live in Florida or California, where the population mix provides a large number of patients. Even neuropathic pain, which sounds like a single diagnosis, responds differently to various classes of medication depending on the underlying cause. So neuropathy from diabetes responds differently than neuropathy from herpes or trauma or from MS or other neurodegenerative diseases. The location of the pain makes a difference, too. Localized, regional, and generalized pain each demand different approaches. So does pain originating from internal organs, skeletal muscles, bones and joints, the peripheral nervous system or the central nervous system. Someone mentioned the Duragesic patch. Well, that uses Fentanyl, otherwise known as China White and much stronger than heroin. Same with Methadone, it is just a slow acting acetylated morphine, another name for pharmaceutical grade heroin. Oxycontin is just slow released codeine. These are all highly addictive. I'm not against taking narcotic medication for serious chronic pain, but you've really got to get a lot of information about your condition and your options because, unfortunately, chronic pain is poorly understood and seldom gets the medical attention it needs. There are a number of chronic pain support groups on-line. Look into a few of them, but don't base your decisions on a bunch of anecdotes. You may get some relief by finding a well experienced physical therapist who works specifically with the type of pain you have: if you have neuropathic pain, soft tissue therapies will probably make it worse. Ande B. 06:31, 30 March 2006 (UTC)
gallbladder
[edit]I have a question about the gallbladder. I am in Anatomy and Physiology so I understand the functions of the gallbladder. My question is: what happens to the bile the gallbladder normally stores after you have a cholisystectomy? Do other organs compensate for the functions of the gallbladder once it is removed or what happens to the bile it otherwise would store? Your help is appreciated. Thank you, Pat Burt
- I'm not a professional surgeon, but the gall bladder just gets its bile from the liver, and dumps into the intestine when food comes along. When the gall bladder is removed the duct from the liver is connected directly to the intestine. GangofOne 07:46, 24 March 2006 (UTC)
- I would think that would mean that much of the bile is wasted by dumping it into the intestines when there is no food and therefore the amount available when food does need digestion would be reduced. Thus, digestion, especially of fats, would be less effective. Most likely the person would compensate for the missing calories by eating more. StuRat 10:36, 24 March 2006 (UTC)
- People who have had the gall bladder removed are advised to eat a low-fat diet. They can't digest fats as well as they could with a gall bladder, and overindulgence in fats can give them diarrhea. Brian G. Crawford, the so-called "Nancy Grace of AfD" 18:01, 24 March 2006 (UTC)
- And steatorrhea, which isn't pleasant. In response to the OP: Yes, other organs compensate after cholecystectomy (note proper spelling). In particular, the liver increases bile synthesis since it can't be stored. Much of this bile isn't wasted in fact: the vast majority of bile (some 90%) that enters the small intestine is recycled at the end of the ileum. Even still, the liver can't produce bile fast enough to cope with a high-fat diet, so low-fat diets are recommended as Brian suggested. --David Iberri (talk) 21:31, 27 March 2006 (UTC)
Spectra in psychology
[edit]Spectrum (disambiguation) lists two psychological spectra: the bipolar spectrum and the autistic spectrum. Are there any others? --Smack (talk) 06:48, 24 March 2006 (UTC)
- After the work by Alfred Kinsey, many psychologists (including most of the reputable ones) consider sexual orientation to be part of a spectrum. Kinsey denoted it on a 0 to 6 scale - see Kinsey scale. Raul654 07:44, 24 March 2006 (UTC)
What's it like to be blind?
[edit]What is it like to be blind? By this I mean, does a blind person see blackness like a sighted person would if he closed his eyes in an unlit room? Do the optic nerves still function at all? What neural stimulation does he receive?
- I went temporarily blind while undergoing LASIK (they have to use vacuum sunction to pull the eye forward in order to operate on it. Doing so causes blindness in that eye while they operate). Yes, all a blind person sees is blackness. Cup your hand over your eye, and that's what a blind person sees. Raul654 06:52, 24 March 2006 (UTC)
- I don't think that's a valid analogy. For a normally sighted person to be temporarily blind, or in a dark room, or just with their eyes closed, has no necessary relationship to the experience of a person who has never been able to see. Sighted people who temporarily can't see often imagine that they can (I "see" all sorts of patterns when I cup my hands over my eyes), and in any case they know that they can see and will be able to again. I can imagine that profoundly blind people don't have this particular imagination, or that they have something completely different (and unimaginable to me) to replace it with.
- We saw the same fallacy in a discussion on the Miscellaneous Reference Desk earlier this week over the question Can blind people jump?. Someone mused about blindfolding people and having them try to jump, and reasoned that if they could, presumably blind people could, too. But the blindfolded people could rely on the muscle memory of a skill learned with their eyes open, an opportunity denied to a blind person. (Now, mind you, I'm not saying that blind people can't jump, just that this wasn't a valid argument.)—Steve Summit (talk) 16:47, 24 March 2006 (UTC)
- Although, for the record, I suppose there are other forms of blindness that this might not necessariy be true for. In the situation I describe, my optic nerve and the visual cortex of my brain were working fine; it was the eye that wasn't doing its job. I suppose there are other forms of blindness that this is not true for, so my description might not necessarily apply. Raul654 06:54, 24 March 2006 (UTC)
- I imagine that for someone who has been blind since birth, the answer would be (Null), not (Zero (or RGB 0,0,0)). It's like me asking you what you feel from your EMF sensing organ - you don't have one so you can't accurately say "I detect no EMF field" (the correct answer would be along the lines of Mu.) Tzarius 08:09, 24 March 2006 (UTC)
- It might be like that, except my (and presumably the OP's as well) EMF sensing organ is fully functional. Do remember that light is an electromagnetic field. -lethe talk + 14:02, 24 March 2006 (UTC)
- Ah, you got me. Perhaps I should have specified radio-frequency EMF. Tzarius 23:27, 25 March 2006 (UTC)
- The term "blind" is used to describe people who cannot see at all. But it is also used with people who have something like a functional system for sight, but cannot see clearly enough for practical purposes. For them, the world has light, colour and contrast, but not detail. I hope that isn't oversimplifying too much. Notinasnaid 08:58, 24 March 2006 (UTC)
- In the US, the term "legally blind" is used to denote those with poor vision, particularly meaning so poor that they can't get a driver's license. Note that the humorous phrase, and "Legally Blonde" movies, are take-offs on this term. StuRat 10:21, 24 March 2006 (UTC)
- I do not know the name of it, but there is a form of blindness where the outer rods start to form and then stop. No cones form and the person can only sense light and dark. They cannot see any images. A friend of mine was born that way. He can tell if a light is turned on, but that is about it. It surprises people to learn that he is a programmer for Microsoft now. He's actually very good, but it always leads to jokes about blind programmers programming Windows' GUIs (which is not what he works on there). --Kainaw (talk) 14:38, 24 March 2006 (UTC)
It depends on why a person is blind. If they are blind because the back of the brain is damaged, but the eyes still work, the subconcious still gets some information cause visual data is also sent elsewhere. WAS 4.250 16:42, 24 March 2006 (UTC)
- If I recall a cognitive scientist I once heard speak, the answer is something like: If the brain hardware for thinking visually is still there, then it can be done to some degree though lacking a referent it is probably not the same thing as how someone who can actually see does it. There are also issues of blindsight which is what WAS is referring to. --Fastfission 22:52, 24 March 2006 (UTC)
- Forgive me for getting a bit off track for the moment, but I once heard of a fascinating "brain-teaser"-type question concerning blindness.
- If a person is born blind, but subsequently his blindness is somehow "cured" and he acquires regular vision, would he be able to tell, from sight alone, the difference between say, a cube and a sphere? He knows what the two feel like, but will his brain be able to make the connection between how the objects feel and how they look? Most people I've asked immediately assume the answer is yes, just out of instinct (a sphere feels round so it must look round). But I believe the answer is no. Having never seen roundness, the formerly blind person would have no real way of recognizing how roundness looks. Any ideas? Loomis51 02:20, 25 March 2006 (UTC)
- I kinda agree, but... There seems to be a natural "standard of perception" for humans. An excellent example is the Booba and Kiki experiment (see synaesthesia for more on that.) So, perhaps our brain has some sort of pre-programmed way of dealing with certain forms of input from the senses, and this could be easily applied to the case you described. What I mean is, "roundness" might have a pre-defined, common meaning for our various senses. ☢ Ҡi∊ff⌇↯ 10:39, 25 March 2006 (UTC)
- I guess blind people have the same impression like you do for everything outside your field of view... Cacycle 11:03, 25 March 2006 (UTC)
Gsm network
[edit]I want information about Motorola GSM 1800Mhz type designation-ctu2 Device
List of manufacturers who have supplied Refineries for Bio Disel in Brazil
[edit]Kindly inform me the contact address and phone numbers/ Mail ID of the manufacturers who have supplied and Commissioned Refineries for Bio Diesel Production in Brazil.
Representations of species of pre-humans
[edit]Hi, I realised that whenever we see artists representations of Neanderthals, Homo erectus, & all the other types of pre-homo sapien human species they are white. Is this a purely cultural construct & do African/Chinese/etc representations of these species show black & chinese versions of neanderthals? AllanHainey 12:26, 24 March 2006 (UTC)
- Usually they're neither "black" nor "white", and the skin coloring and features such as eye shape vary quite a bit.. just do a Google image search for Homo Erectus and you'll see quite a bit of variation, at least in the eye of this beholder, they don't fit into any modern racial category. (Why would they?) As for Neanderthals, it wouldn't be a purely cultural construct to assume they had relatively light skin - they lived in cold climates. --BluePlatypus 13:13, 24 March 2006 (UTC)
Most primates, and indeed mammals in general, either have black skin (usually in exposed areas, like noses), or pink skin (usually under hair or fur), so it would be reasonable to assume one of those two possibilities, or some combination. Of those, I would expect black skin to be more likely, as that is the more common in current primates most closely related to us. This is speculation, of course, until we find well preserved skin frozen in a glacier or DNA from which we are able to read the skin pigmentation genes. And as BluePlatypus noted, those in the tropics would be expected to have darker skin, and those in temperate zones would have lighter skin, due to the vitamin D synthesis/genetic damage optimization based on UV exposure. If you need this explained, just ask. StuRat 13:40, 24 March 2006 (UTC)
- There is also a theory accepted by very few scientists that humans are an ape-like animal that took to water (like dolphins and whales) and then came back on land. Hence, the extreme lack of hair, a nose shape that keeps water from going up it, and the little hair that does exist grows downward instead of outward (like fur) so we can move through the water easier. If that were true (a mighty big "if"), it would be highly likely that early humans had similar skin tone to the other aquatic mammals - mostly gray. --Kainaw (talk) 14:30, 24 March 2006 (UTC)
- That's one wacky theory. We are quite poor swimmers compared with aquatic mammals. We don't have grey skin. We don't have blow-holes. We don't have fins. The hair we do have is frequently curly, which makes for poor fluid dynamics. We also have arched feet for climbing trees. Would those be underwater trees ? StuRat 15:28, 24 March 2006 (UTC)
- I'll say it's a wacky theory. To StuRat's points I'll add: aquatic mammals all have thick blubber or very thick fur for insulation. Humans (modern ones, at least) have neither, and suffer hypothermia after an extended period in water that's at all cooler than their body temperature. —Steve Summit (talk) 16:21, 24 March 2006 (UTC)
- Hmm, but wouldn't one also expect then that we'd be lighter on the belly and darker on the back too, like all aquatic mammals and most fish? --BluePlatypus 15:14, 24 March 2006 (UTC)
- Or you can really go out on a limb and claim that is evidence our ancestors used the backstroke half the time.. :) --BluePlatypus 15:15, 24 March 2006 (UTC)
You might what to understand the Aquatic ape hypothesis before you misrepresent it and the evidence both for and against it. WAS 4.250 16:52, 24 March 2006 (UTC)
- Yea, it's not saying that pre-humans were aquatic mammals like whales or dolphins, but only waded on shores and in riverbeds looking for food. I can buy that, but see no reason why that would effect our skin color. StuRat 17:14, 24 March 2006 (UTC)
- Wow. That's actually on Wikipedia!? I only saw it very late one night on PBS. They had very poor computer graphics of humanoids with elongated fingers and feet flapping through the water. I found it rather humorous, which is why I remembered it. I never studied it in detail. The hypothesis in the article here is much more believable than the documentary I saw. I can believe that early humans spent a lot of time in the water, but I can't see them living in it. --Kainaw (talk) 19:08, 24 March 2006 (UTC)
- Dude! This is Wikipedia. We got a millyun articles; we got articles on everything.
- (Just kidding. I was impressed, too. And I take back my armchair comments above about blubber, which they explicitly address.) —Steve Summit (talk) 21:39, 24 March 2006 (UTC)
- Just one query. Leaving the wacky bits aside, isn't it the case that humans are not just "ape-like animals" as Kainaw said, but are actually apes? JackofOz 00:32, 25 March 2006 (UTC)
- No. The closest living relatives of man are the chimpanzees/bonobos, not the apes. You have to go back through many extinct species to find a common ancestor even with chimpanzees, however, so we aren't even particularly closely related to them. If we were more closely related, you'd expect greater similarity in speech, walking posture, and intelligence. The reason that more closely related species are all extinct is that modern humans outcompeted them. More distant relatives, however, like chimps, occupy different ecological niches (the jungle canopy, in the case of chimps), so did not compete directly with modern humans, and thus survived. StuRat 02:16, 25 March 2006 (UTC)
- Are you sure of that? According to our articles (Human, Ape, and Great Ape), humans are indeed classified as apes. Black Carrot 04:25, 27 March 2006 (UTC)
- You've got it right, Black Carrot. Humans are a type of ape. Apes are simply tailless primates, so that's us. Its also chimps, gorillas and orangutans. (Primates with tails include monkeys.) Confusion arises because for quite a long period of time most people, even those in the sciences, could not bear the notion of having humans in a non-exclusive category. So they created the Hominidae taxonomic family and only let humans join. The other great apes were put into the Pongidae family. Although the bio-science community moved away from that attitude quite some time ago and dropped the Pongidae label, many commentators have not. Ande B. 10:29, 30 March 2006 (UTC)
Sound disturbance in computer speaker when call on mobile is incoming.
[edit]I am sure many of you must have noticed this that when the computer speaker is turned on, and if a "call" or "message" is coming to a mobile kept nearby, there is a peculiar disturbance noticed in the speaker. Why does this happen? And, moreover, why does this disturbance does not occur in the mobile's "speaker" or "sound system" (If I am listening to songs on my mobile, and if call comes, then no disturbance is there on a mobile). So why does this does not take place in this case?
- It's radio interference between the transmissions of the phone and the signal in the speaker cable. The broadcast from the phone's antenna as it talks to the tower is creating a current in the speaker cable which is mistaken for an audio signal and creates a sound in the speaker. The phone's own speaker doesn't have such a long cable to pick up signals and so would not be affected by the transmissions of the antenna. Night Gyr 19:01, 24 March 2006 (UTC)
- It's due to time division multiple access. "A disadvantage of TDMA systems is that they create interference at a frequency which is directly connected to the timeslot length. This is the irritating buzz which can sometimes be heard if a GSM phone is left next to a radio or speakers." Apparently. Sum0 20:01, 24 March 2006 (UTC)
- It also occurs before the call comes, at least in my case. Titoxd(?!? - help us) 20:04, 24 March 2006 (UTC)
- It's due to time division multiple access. "A disadvantage of TDMA systems is that they create interference at a frequency which is directly connected to the timeslot length. This is the irritating buzz which can sometimes be heard if a GSM phone is left next to a radio or speakers." Apparently. Sum0 20:01, 24 March 2006 (UTC)
- It sounds like the speakers are picking up the negotiations between the tower and the handset before the call is connected. I imagine that this is similar in principle to the irritating whine of a modem connecting. For great justice. 21:59, 24 March 2006 (UTC)
- It is often audible to me in the phone earpiece. It seems as if the better constructed the device is the more it will resist it. I imagine the phone itself has been designed for the most resistance to this. Its not the signal going to the speaker wire that causes it, its the RF getting into the signal wire going to the audio amplifier, that then gets pumped up and sent out via the speakers.
- we're talking about computer speakers here, though, so there's no significant length of cable in front of the amp. Night Gyr 22:05, 24 March 2006 (UTC)
- My computer speakers have about one meter of cable before the inbuilt amplifier. --Username132 (talk) 20:08, 25 March 2006 (UTC)
- we're talking about computer speakers here, though, so there's no significant length of cable in front of the amp. Night Gyr 22:05, 24 March 2006 (UTC)
- It is often audible to me in the phone earpiece. It seems as if the better constructed the device is the more it will resist it. I imagine the phone itself has been designed for the most resistance to this. Its not the signal going to the speaker wire that causes it, its the RF getting into the signal wire going to the audio amplifier, that then gets pumped up and sent out via the speakers.
Airplane Engines
[edit]My mother was on an airplace recently in which the engine caught on fire when it sucked in a bird. Aren't airplace engines supposed to be able to withstand birds being sucked into thje engine?
- Depends on the size of the bird and the engine. An F-22 recently suffered over six million dollars in damage when its engine was destroyed by ingesting a five-inch metal pin. See our articles on foreign object damage and bird strike. Night Gyr 22:00, 24 March 2006 (UTC)
- Somewhat, but you can never design for all eventualities, for example, what about a roc? Seriously, airplane engines are designed to withstand this, but there will always be problems if foreign objects are sucked into the turbines. For great justice. 22:02, 24 March 2006 (UTC)
After edit conflict:
- Also, the standard for the engine is not that it is able to survive impacts, but that the engine housing can contain all the pieces so that there are no pieces of shattered turbine impacting the fuselage or other parts of the plane. Night Gyr 22:03, 24 March 2006 (UTC)
- Who knew that there were birdstrike simulators? For great justice. 22:05, 24 March 2006 (UTC)
I've been edit-conflicted out the wazoo, but I can still quote the most relevant section of bird strike for the question:
- Most large commercial jet engines include design features that ensure they can safely shut-down after "ingesting" a bird of weighing up to 1.8 kg (4 lb). Multiple or large strikes require emergency action to control damage. This limit is also applied to the rest of a modern commercial aircraft—it must be able to safely land after taking a 1.8 kg strike.
So, as Night Gyr says, the engine isn't supposed to survive, but the plane is. Melchoir 22:07, 24 March 2006 (UTC)
- I've taken a class on aviation, and our instructor showed us some quite spectacular pictures of the results of a small piston-engined plane's encounter with a flock of birds. The propeller cap and much of the leading sheetmetal was dented, and there were feathers and blood everywhere. They were small birds, though, so the plane survived. Night Gyr 22:15, 24 March 2006 (UTC)
- Impressive. Well, now I have to post this link for the single jet-engine case, and I'll be done: birdsuck.wmv CT155202.pdf Melchoir 22:27, 24 March 2006 (UTC)
- I feel the need to mention this case... Shimgray | talk | 01:05, 25 March 2006 (UTC)
- I've seen the video mentioned on that page. One of my instructors at college showed it to the class. It's really rather interesting to watch. Dismas|(talk) 02:36, 25 March 2006 (UTC)
- I feel the need to mention this case... Shimgray | talk | 01:05, 25 March 2006 (UTC)
- Impressive. Well, now I have to post this link for the single jet-engine case, and I'll be done: birdsuck.wmv CT155202.pdf Melchoir 22:27, 24 March 2006 (UTC)
- The questioner did say AIRPLANE, not JET. I would be interested to know how a bird got sucked into and airplane engine.
March 25
[edit]Cleanrooms for personal use
[edit]Though cleanrooms are used in semiconductor manufactoring,biotech and the life sciences due to my desire to live in a "clean" enviornment I seek to have one installed in my house. Though I run under a limited budget I would like to know if it is possible to have it installed in a normal house and if so how much would it cost for me if I wanted a sterilized class 10 cleanroom. Though this is indeed a very odd question it is of utmost importance to my well being and as such I ask you to reply seriously. Thank you very much for your time.--Ishikawa Minoru
- Sorry if this seems dismissive, but you should consider seeing a psychiatrist instead. It would be much cheaper, and better for you in the long run. —Keenan Pepper 01:14, 25 March 2006 (UTC)
- He might have an actual need, such as severe, life-threatening allergies to just about everything. StuRat 02:04, 25 March 2006 (UTC)
- Something like that, yea. StuRat 02:41, 25 March 2006 (UTC)
- I think you should also see multiple chemical sensitivity. - Cybergoth 04:23, 25 March 2006 (UTC)
- I would suggest that to combat MCS, a filtered, positive-pressure system would be more effective than a clean-room-like environment, and much cheaper.--inksT 04:45, 25 March 2006 (UTC)
- I think you need to see somebody working at IBM, or contact somebody at Rice Univesity [83] for something like this. Check [84] for some modular cleanrooms. Try asking a question on PhysicsForums's engineering forum. However, Keenan Pepper is right, seriously, try seeing a psychologist, I'm sure you've heard it before, but that is because its what you should do. What makes you think living in an environment with no dust or bacteria in the air is better? Please, reply on my talk page at User talk:Mac_Davis. I am interested.--
 Mac Davis] ⌇☢ ญƛ. 02:24, 25 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 02:24, 25 March 2006 (UTC)
- By stating that you'd like a "Class 10" cleanroom, you seem to be going by the FED STD 209E scale as opposed to the ISO standards which only go to 9. You may be interested in this link. You should probably know that this may be prohibitively expensive for you to actually do unless you can get some sort of medical insurance to pay for the costs of the assembly of the room, the hepa filters that would be required to clean the room, the cost of the electricity for the filtration, etc. And getting used to wearing the clean room suit can be rather annoying especially if you're going to wear it for 24 hours a day. Some of the new hires that we get at work just can't stand the suit and quit shortly after getting into the suit. Dismas|(talk) 03:06, 25 March 2006 (UTC)
- Just from Google, I don't think you could attain a Class 10 clean room in a normal residence - particulates from people, pets, bedding, cooking would contaminate the area far faster than you could filter them out. Temporary sterilisation could be done if you have two clean rooms, and UV sterilised one while staying in the other - but it would be very short lived sterilisation. Touching any surface would contaminate it (although perhaps your own biological material is acceptable). UV would play merry hell with the furniture, fabric, and appliances though. Don't forget the airlocks and constant changing of the filters - I'd say the cost would be at least USD$100,000 just for plant (fans, filters, UV, seals, air pumps - scientific equipment is not cheap), possibly more, and it would be fairly expensive to maintain and run.--inksT 02:56, 25 March 2006 (UTC)
- I don't think its gonna happen. --
 Mac Davis] ⌇☢ ญƛ. 12:07, 25 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 12:07, 25 March 2006 (UTC)
- I don't think its gonna happen. --
I don't know about level 10, but I sure would like to be able to keep insects and arachnids out of my bedroom. I once woke up with a spider crawling on my face, which was quite distressing. I found it impossible to get back to sleep that night. StuRat 12:35, 25 March 2006 (UTC)
- How fortunate that you do not live around Solifugae... >:) Tzarius 23:35, 25 March 2006 (UTC)
The Elder Scrolls' fnt+tex files... LaTeX?
[edit]I'm attempting to edit or create fonts compatible with The Elder Scrolls III: Morrowind and The Elder Scrolls IV: Oblivion. The existing fonts appear to be sourced from two matching files, one a .fnt and the other a .tex. You can see an example pair here (.fnt) and here (.tex). However so far I haven't come up with much. Are these LaTeX (or a variation) or are they merely an in-house format?
Thanks in advance. :) GarrettTalk 00:39, 25 March 2006 (UTC)
- The .tex file is definitely not LaTeX. LaTeX is rather human readable. enochlau (talk) 02:56, 25 March 2006 (UTC)
- Yeah, I thought as much. In the meantime I've discovered someone else who it seems DOES know what the heck this format is and how to edit it, so at least there's a happy ending. Thanks anyway. :) GarrettTalk 11:30, 25 March 2006 (UTC)
- .tex being texture for a texture-mapped font? (i.e. an image, probably RGBA or DDS, containing all the glyphs in a grid, with the .fnt file being a list of coordinates for each character) Ojw 15:18, 25 March 2006 (UTC)
- Ah, that makes sense... the search continues! Thanks! :) GarrettTalk 11:39, 27 March 2006 (UTC)
PAL 50 (Europe) to PAL-M on PlayStation 2
[edit]A friend of mine has a friend who bought a PS2 in Europe and brought it here to Brazil, only to find out it doesn't work. My friend said that his friend got another PS2 then, and this European one is pretty much collecting dust right now. Also, he said that if we made it work, we could have the PS2 for ourselves. So I was wondering, is it possible to make the conversion, and what would it take? I have some electronics skills and equipment, though, so I could try some stuff. ☢ Ҡi∊ff⌇↯ 03:25, 25 March 2006 (UTC)
are men smarter than women?
[edit]Are men smarter than women?
- This question doesn't make much sense because we can't even rigidly define what defines something as "smart", and therefore, we cannot give a absolute level of "smartness" of anyone. ☢ Ҡi∊ff⌇↯ 10:27, 25 March 2006 (UTC)
- Men seem to be better specializers than women. i.e. more famous physicists, chemists, historians, neurosurgeons, philosophers, psychiatrists. --
 Mac Davis] ⌇☢ ญƛ. 11:57, 25 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 11:57, 25 March 2006 (UTC)
- Men seem to be better specializers than women. i.e. more famous physicists, chemists, historians, neurosurgeons, philosophers, psychiatrists. --
- That's only due to the culture that previously discouraged women from such fields. An anarchist culture ([[Emma Goldman *cough* cough*) would certainly destroy those barriers. Elle vécut heureuse à jamais (Be eudaimonic!) 15:15, 25 March 2006 (UTC)
- You can't say that, unless you have an anarchist culture to compare to. Cause and effect my dear :) --Blowdart 13:01, 26 March 2006 (UTC)
- Men are better at some skills, like math and 3D visualization, and women are better at others, like communication skills. StuRat 12:23, 25 March 2006 (UTC)
- *cough cough splutter* what a load of bull#$%&. Dmharvey 12:38, 25 March 2006 (UTC)
- Just curious: why did Blowdart and Dmharvey use "*cough cough*"? --Bowlhover 04:46, 27 March 2006 (UTC)
- I'm guesing, based on your lack of communication skills, that you aren't female. :-) StuRat 13:16, 25 March 2006 (UTC)
- I AHVE EXCELLENT COMMUNICATION SKILLS Dmharvey 13:37, 25 March 2006 (UTC)
- You might want to check out our article on it: Sex and intelligence. --Ed (Edgar181) 12:51, 25 March 2006 (UTC)
Woman: "It has been shown, in numerous double-blind scientific studies, that women's communication skills, on average, surpass those of men."
Man: "Nuh-uh !"
StuRat 13:16, 25 March 2006 (UTC)
"No" - Cybergoth 14:40, 25 March 2006 (UTC)
The answers above give you most of whole spectrum of the issue. I can think of at least three additional theories for the apparent overrepresentation of men in many intellectual activities.
- Average intelligence is no different between the sexes, but for biological reasons related to single X chromosome and different reproductive functions (e.g., males are more reprocdutively disposable), males as a group express a wider range of intellectual abilities and are overrepresented at both the high and low ends of the spectrum.
- Intelligence is a vague and abstract concept like "athleticism" comprised of a variety of brain functions, personality attributes, and behaviors reflecting both innate capacity and social development, and the way it is defined puts a higher value on some of the aspects expressed more often in males. alteripse 15:58, 26 March 2006 (UTC)
- Note that going further, and claiming that innate differences in intelligence are majorly responsible for, say, the overrepresentation of men in university science faculties, is extremely controversial. See Larry Summers. --Robert Merkel 00:57, 27 March 2006 (UTC)
- Mens brains are a little larger, and score a bit higher on IQ tests. Women have one the average, less seritonin than men do, which makes it harder to control moods, and thus harder to concentrate.
- But brain size is proportional to the size of the body in general, and it may be noted that men generally have larger bodies in build than that of women. Luthinya 11:40, 29 March 2006 (UTC)
- Cite?
One thing which has puzzled me is why nearly all of the civilizations in the world tended to be patriarchal? Despite famous anomalies such as the Amazons and the American Indians who managed to place weight at least equal to or more than upon women just as that of men, in most civilized nations one tends to find the women excluded from matters of true intellectual capacity. Suppression, I think, in this case, is not sufficient to explain the matter. Every suppression requires a start, and the starting reason is not in all cases totally devoid of proper thinking. What is it that first made man conceive of the inferiority of the women, and began the tradition of misogyny under which many valiant women later suffered? It may also be observed that though perhaps quite a number of women were discontent, very few of them actually decided to take action upon their predicament, seemingly as if it is in the female nature to be passive. An investigation held within my school showed that if a statement was delivered to the class and none of its members understood it, boys are more likely to ask outright than girls, who feared the humiliation and suppressed their curiosity without a thought. As historically argued the suppression of women is generally deficient to society, yet in many developed countries where now women are no longer discriminated against, they still seemed lacking to interest themselves on fields of intense intellect, lacking in intellectual ambition, so to speak, within conformity. But then again it may be a psychological question this poses- women don't ultimately have a lower IQ, but their general behavior and modes of thought sometimes devalue its reason. Luthinya 11:40, 29 March 2006 (UTC)
- I always assumed the original suppression was due to physical inferiority (i.e less bodily strength). Superm401 - Talk 03:25, 31 March 2006 (UTC)
Pyrazole in cetirizine
[edit](Moved here from Talk:Pyrazine)
i am a yr 12 student and am currently doing an assignment on the structure and function of the active ingredient in zyrtec antihistamine called ceterizine hydrochloride. it contains a pyrazine monomer as far as i can tell but i am having alot of difficulty relating it to what its function is in relation to the human body. It is due in two weeks, but i have most of the research and a draft. the problem is the teachers cannot directly help as it is an assignment so i am calling on outside sources. if you have any info on ceterzine hcl or on zyrtec in general in relations to how it works within the human body please email me at xxx@xxx.xxx {address removed to prevent abuse}. any information would be greatly appreciated.
- It's called cetirizine. Cacycle 10:47, 25 March 2006 (UTC)
- Note that it contains a piperazine ring, not a pyrazine. --Ed (Edgar181) 11:22, 25 March 2006 (UTC)
What happens if you drop a rock into a white hole?
[edit](Cosmology and general relativity)
A black hole is a large gravitating object in space that objects can fall into but nothing can get out of. The opposite, a gravitating white hole, that objects can get out of but cannot get into is also therefore possible in theory.
What happens if a brave but determined person finds a white hole and drops a rock into it, or even pushes a rock into it with a stick?
- I would assume the same thing as if you try to drop something into an erupting geyser, it would be blown away from the white hole. StuRat 12:08, 25 March 2006 (UTC)
- You couldn't put a rock into the white hole. Just like how you are not going to be able to go in and pull out a piece of matter out of a black hole's singularity you aren't going to do the opposite, to the opposite of the black hole, and put a piece of matter into a white hole's singularity. See exotic matter and negative energy. --
 Mac Davis] ⌇☢ ญƛ. 12:51, 25 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 12:51, 25 March 2006 (UTC)
- Is that a question or a statement? Seems more like a statement to me. And your reasoning is flawed- just because a black hole exists does not mean the opposite is possible. (Also, things can get out of a black hole, see Hawking Radiation) --BluePlatypus 14:14, 25 March 2006 (UTC)
- So, if white holes are the opposite of black holes, you are saying that white holes suck up radiation. So, all we need to handle all our nuclear waste problems is a white hole in the middle of the storage facility. Illogical reasoning is fun! --Kainaw (talk) 17:10, 25 March 2006 (UTC)
- See White hole. It's news to me, but it suggests it is indeed a theoretical possibility. --Quasipalm 17:13, 25 March 2006 (UTC)
- White hole. Spewing time. Engines dead. Air supply low. Advise please. --GraemeL (talk) 17:19, 25 March 2006 (UTC)
Please, we are dealing with a serious inquiry here and it is not joke time. In answer to general relativity the white whole was posed as the diametric opposite of the black hole, bringing out again what one has take in. It has, of course, never really been verified, but it is certainly a theoretical possibility considering the violence of the radiation issuing from the quasars. Answering to a previous point, technically matter may escape from a black whole via the Hawking Radiation and Quantum Tunnelling, but the possibilities are extremely vague considering the far greater chances of capture within the enormous gravitational field. It is rather strange though if one considers the fall into the quasar- it might be something akin to experience to landing into an erupting volcano- you get rent to pieces then spewed out again!
However we have not yet considered the gravitational field of the quasar itself. If it is indeed the diametric opposite of the Black Hole, with the tremendous amount of matter constantly clustered within it, the quasar must require a yet greater force than the gravitation of the Black Hole in order to drive the pieces of matter away from itself and each other at all! This now poses an even greater theoretical problem, but it does not deny the existence of such a possibility. At the moment no- one can really give the specifics or say such a thing as 'cannot', for we can never fully explore the total wonders of nature. Luthinya 11:52, 29 March 2006 (UTC)
free energy?
[edit]If I have my equations correct, to accelerate an object to a given speed, the energy needed is 1 / 2mv2, so there is a minimum amount of energy needed (if you can eliminate all drag and inefficiencies) to accelerate an object to the previously mentioned given speed. However, Lorentz force law, when made applicable to easily measured phenomenon states that the force produced, and therefore the acceleration is equal to the current, times the magnetic field, times the distance traveled by the charges. Since there are three variables here, doesn’t this contradict the first energy equation, I could theoretical engine I could increase the magnetic fields with permanent magnets and get and increase in thrust without changing the energy input. Any comments would be appreciated, thanks.
- No, F = m*a. --BluePlatypus 19:25, 25 March 2006 (UTC)
I realize that F= m*a, but perhaps I have poorly phrased my question. Since the Lorentz force law allows for an increase in force (by strenghthening the magnetic field) without adding any aditional current (or energy), dons't this violate the laws of physics? I say this wit because if two engines were compared, one using a 1 tesla field and a 5 amp current, and another that uses a 0.5 tesla field and a 10 amp current, both having the same distance traveled by the charges, they would have the same effect, but one would use twice as much energy. So is the only limiting factor hove strong a permenat or superconducting magnet is possible, or is the whole second equation wrong?
- You seem to be asking why a charged particle moving in a magnetic field does not violate the principle of conservation of energy. Take a look at: magnetism#Charged particle in a magnetic field. It's not enough to calculate the force on an object, you must also calculate the work that force does. A magnetic field does not do work on a charged particle. EricR 20:52, 25 March 2006 (UTC)
Thanks, does this mean that after the magnetic field has redirected all of the possible charges, that increasing the strenght of the magnet will have no more effect?
- Now, a changing magnetic field can do work, which means whatever you're doing to change the strength of the magnetic field must use energy. That's how a generator works. —Keenan Pepper 06:49, 26 March 2006 (UTC)
The Lorentz force law does say that force is current times field times distance. However you're neglecting to consider that that is a vector equation, and it also tells you the direction of the force. The direction is perpendicular to the length and to the field, and because of this, the Lorentz force never does any work (work is given by the parallel projection of force and distance. If they're perpendicular, this projection is 0). -lethe talk + 11:35, 26 March 2006 (UTC)
- So is no work is being done by the lorentz force, the reason a engine based on this is that it is just rederecting the work done by the electric charges. When building an engine the strenght of the magnetic field matters because if it is not strong enough some of the charges will not be redircted? Thanks.
Just a clarification = the Lorentz force is the sum of electric and magnetic forces on a charge - a constant magnetic field does not do work on a charge, but the electric field can, therefore the Lorentz force in general can do work. I'm a little unclear on the original question as well. A quick read of http://en.wikipedia.org/wiki/Lorentz_force is probably helpful. I don't agreeh the original question's statement that "[According to the] Lorentz force law....the acceleration is equal to the current, times the magnetic field, times the distance traveled by the charges." I would say that that the force on a current due to a magnetic field is equal to current X magnetic field where both quantities are vectors, and X is a cross product.
Relation between light rays and distance between source and object.
[edit]Well, the question I have to ask is very simple and straight : why do objects which are far away, appear small? It's a very simple question, but still I have not got a simple answer. And dont just say that because the objects are far, they would appear far, give a scientific reson. To put it other way, why do objects which are nearer, seem smaller when taken farther away?
- The reason that objects appear smaller when they are farther away is actually related to how they reflect light. We only see objects that reflect light and then only the percentage of the light that reaches our eyes. If the object you are looking at reflects all of the light that hits it, like a very good mirror, then on earth on a sunny day it is reflecting 1000 watts of sunlight energy. Because the radiated in beams for a single point (the sun) it has a tendancy to spread out. This spreading out icreases the area you can see the sun, and the mirror, from, but the amount of light is staying the same (1000 watts) because of this, the farther you are from the objet reflecting or emmiting light, the less light energy is actually reaching your eyes because it is spread out over a larger area. This dimming of the objet is translated by your eyes into appearing smaller. This is also why a currved out mirror on corners makes people look unusially smaller, the curvature is spreading the light out more than nomal making people look smaller than normal. cc24.137.78.34 19:50, 25 March 2006 (UTC)
- Wow. That is a lot of BS. "This dimming of the objet is translated by your eyes into appearing smaller." I'm speechless. —Keenan Pepper 05:15, 26 March 2006 (UTC)
- I guess this just goes to show how little you should trust the Ref Desk... possibly even less than the rest of Wikipedia! ;o) — QuantumEleven | (talk) 10:28, 26 March 2006 (UTC)
- This question has been asked before--see this. --Bowlhover 19:45, 25 March 2006 (UTC)
- The link given by Bowlhover explains it very well, but I just wanted to point out that the answer given by 24.137.78.34 explains why object appear dimmer at greater distances (for instance, why a lightbulb looks a lot dimmer from 100m than if you're standing right next to it), not why they appear smaller. — QuantumEleven | (talk) 20:22, 25 March 2006 (UTC)
Well, no offense, but I don't think it has anything to do with the amount of light reflected. This question bothered me for a while, because it seems so damn simple, but it isn't immediately obvious. This question is difficult to answer without diagrams, but I'll try. The answer lies in thinking about perceived size as angular distance: Think of an observer as a point receiver of light (forget about the fact that we have two eyes - it's irrelevant in this discussion; just provides stereoscopic vision). The observed size of an object is clearly the angle between the line from one side of an object to the observer and the line from the opposite side to the observer (speaking in 2D for the moment). This angle spanning the object, or the angular size of the object, decreases as the object moves away from the observer, and increases. This explains the diminishing perceived "size" of the object as it moves away from an observer. If you aren't convinced, take a piece of paper, draw a dot for the observer, then draw two identical blobs on the paper, one farther away from the observer than the other. Now draw lines from the observer to each side of the first object and second objects (4 lines total). Witness that the angular deviation between the lines for the closer object is smaller than that of the further object. Hope this helps!
vegetative indices
[edit]hi, i do need to know what vegetative indices means and how are they used to estimate biophysical variables of the earth's surface.
- Try searching for Normalized Difference Vegatative Index (NDVI). EricR 23:48, 25 March 2006 (UTC)
- I hope the real article is spelled Normalized Difference Vegetative Index. alteripse 15:41, 26 March 2006 (UTC)
- According to the article, It's actually Normalized Difference Vegetation Index --Hughcharlesparker 14:08, 30 March 2006 (UTC)
- I hope the real article is spelled Normalized Difference Vegetative Index. alteripse 15:41, 26 March 2006 (UTC)
"First Generation" in Biomedical Science
[edit]I don't really understand the term 'first generation' when applied to adenoviral gene vectors. Is a first generation virus just a modification of a wild-type virus and a second generation virus, a modification of a first generation virus? --Username132 (talk) 20:01, 25 March 2006 (UTC)
this page may be useful to you. The first generation vectors were altered from the "wild-type" so as to allow expression of an inserted gene while limiting the expression of viral genes. Second generation vectors had more of the viral genes removed, making it less likely that laboratory-produced viruses could replicate (they would just shuttle a desired gene into a target cell). --JWSchmidt 15:54, 26 March 2006 (UTC)
POSTing an empty array (HTML forms, PHP)
[edit]I know this will sound a little strange but I realized that I couldn't figure out how to do this (whether I actually need to do this for the little application I am making, I'm not 100% sure yet, but that's another question).
Can you POST an empty array variable with HTML forms? For example, I can use a <INPUT TYPE=HIDDEN NAME="r[5]" VALUE="hello"> in order to send an array (named "r") with an element with a key of 5 and a value of "hello" in the POST action. But can I send "r" as an array with no elements in it? If I used "r[]" it puts in an element with a key of 0 and a value of nothing, but that's not the same thing as an empty array element (it technically does have one entry).
Is there any way to do this? If I just send r without the brackets, my POST processor won't know that it is supposed to be an empty array.
I know it's sort of a silly and somewhat strange question, but it was just one that came up. There are some workarounds I can do (i.e. I have have the script receiving the POST data look for that element and, if it doesn't find it, assume that it should be there but empty), but it was just something that came up that I was curious about. --Fastfission 20:39, 25 March 2006 (UTC)
- I do not know about older versions of PHP, but newer version will convert input names, such as "value[0]" and "value[1]" into an array called "value" with the elements [0] and [1]. It looks for the square brackets to do this automatically. Also, I've never used it with GET, only POST. --Kainaw (talk) 21:18, 25 March 2006 (UTC)
One thing to keep in mind is that HTML forms don't actually send arrays. All that get sent across in the HTTP request are name/value pairs.
<form method="post"> <input type="hidden" name="foo" value="bar"/> <input type="hidden" name="r[5]" value="hello"/> ...
gets sent across in an HTTP request like this:
foo=bar&r[5]=hello
and PHP builds the arrays on the server side when you import the request variables. So why couldn't you do something like this in PHP:
$p_r = array();
import_request_variables('p','p_');
? You start out w/ an empty array, and the post vars can add mappings to it. If you want to actually send complex datatypes across in an HTTP request, you need to use XForms. EricR 01:47, 26 March 2006 (UTC)
Drinking from the Dehumidifier
[edit]Is the water extracted by my dehumidifier safe to drink? --Username132 (talk) 22:18, 25 March 2006 (UTC)
- It's probably safe enough, but I bet it's prone to mold and bacteria if it is allowed to sit around long enough. — TheKMantalk 22:26, 25 March 2006 (UTC)
- If the dehumidifier in question uses a toxic desiccant, I wouldn't drink the water. Melchoir 22:38, 25 March 2006 (UTC)
No, it acts as a filter and collects all sorts of mold and bacteria from the air, making the water a nasty culture that could be quite dangerous to drink. StuRat 23:04, 25 March 2006 (UTC)
- It doesn't use a dessicant so I'll just water the plants with it... --Username132 (talk) 23:11, 25 March 2006 (UTC)
Approximate brickwork date
[edit]This is certainly not old enough to be an archaeology question, but it is along those lines... I was digging a hole for a bush in my backyard. I dug up a cement pad and brickwork. I began to wonder what the age and use of it all was. Perhaps someone here may have a guess. Here are the clues:
- The cement pad is almost exactly 3 feet wide and 6 feet long.
- The cement pad has a single 5-inch hole going down through the center of it.
- The corner of the brickwork foundation is about three feet from the cement pad and a good two feet deeper.
- The foundation is made of a single row of cinderblocks laid so that they are 16 inches long, 4 inches wide, and 8 inches tall.
- The cinderblocks have two oval holes going through them.
- The cinderblocks are surrounded with tiny gravel.
- The bricks are 3-hole bricks on top of the cinderblocks, but there is no mortar.
- Between the pad and the cinderblocks, I found a brick tube that is about 6 inches wide, a circlular hole in the middle, and a hexagon shape on the outside.
- The house was built in the 50's and it a good 40 feet from all of this.
- There was once a railroad about 20 feet away in the opposite direction.
Any guesses are welcome. I plan to dig more tomorrow to see if anything else turns up. --Kainaw (talk) 22:56, 25 March 2006 (UTC)
- Hmm, sounds rather substantial, with special efforts made to ensure proper drainage. From the cinder blocks, it can't be all that old, as they are a relatively modern thing. One guess is a rather well built foundation for a tool shed. The hole in the center may have been for a support pole or might just be for drainage. Perhaps a previous owner kept a lawnmower in there and didn't want it to get rusty from standing water in the shed (after a big rain), so made sure to have proper drainage. I would guess the walls and roof were metal or wood and he likely took that part with him when he moved out. StuRat 23:19, 25 March 2006 (UTC)
- After another day of digging, I can safely say that it is a stable-house. I found broken horseshoes and a lot of nails. There are rotted beams running in from the cinderblock and brick about 8 feet apart - big enough for a horse to stand in. It goes all the way to the house, where it merges with the foundation. I assume the house originally had a stable attached to the back and it was torn down long ago. --Kainaw (talk) 15:34, 27 March 2006 (UTC)
- Sounds likely, especially considering that "modern" cinder blocks appear to have come into common use around the 1930's (though I haven't been able to verify that just yet). Very interesting find, though, StuRat! – ClockworkSoul 13:32, 28 March 2006 (UTC)
- It's actually in Kainaw's yard. Superm401 - Talk 03:32, 31 March 2006 (UTC)
- Sounds likely, especially considering that "modern" cinder blocks appear to have come into common use around the 1930's (though I haven't been able to verify that just yet). Very interesting find, though, StuRat! – ClockworkSoul 13:32, 28 March 2006 (UTC)
Bones and Healing
[edit]I know that fractured bones can be reset and healed, but what about "dented" bone (having a depression)? Thanks. -- Win777 23:40, 25 March 2006 (UTC)
- I don't think it would heal, but I'm not sure that would need to be healed, either. It sounds like the bone would continue to function as before. StuRat 02:22, 26 March 2006 (UTC)
- I suspect that an indentation in the surface of a long bone would be a stress point - so any load/force applied to the bone would be magnified at the indentation to some extent. Bones respond to load by laying down more matrix and generally building up structure and strength. Thus I would expect that a bone with a "dent" would eventually heal (ignoring any associated pathologies or trauma that aggravates the original injury).--inksT 02:37, 26 March 2006 (UTC)
- I was a skateboarder in the late 70's to early 80's and got plenty of dents in my shins. I still have them - all of them to the best that I can tell. --Kainaw (talk) 04:20, 26 March 2006 (UTC)
- I am no doctor, but I bet the dents became stress points and denser bone eventually developed around the dents, rather than fill in the dents.
March 26
[edit]NH3 + NaClO = ?
[edit]I know its a dangerous thing to do, but what exactly happens when you mix ammonia and bleach? --Chris 06:05, 26 March 2006 (UTC)
- It depends on the pH. In alkaline conditions chloramine is produced; in acid dichloramine and nitrogen trichloride. —Keenan Pepper 06:32, 26 March 2006 (UTC)
- A warning also. Chloramine is a toxic gas, Nitrogen Trichloride is a highly sensitive explosive.
Okay then, another question arises. How can two bases be acidic?
- Neither ammonia nor sodium hypochlorite is a strong base, so when mixed with an acid the solution can become acidic. —Keenan Pepper 15:57, 27 March 2006 (UTC)
- Ah...thanks.
Homosexuality and AIDS
[edit]I've heard that homosexuals are more likely to get AIDS.Why?
- A question that is hard to answer, and most answers are open to dispute. Some general theories include:
- They are more likely to have have more sexual partners than the average person.
- They are more likely to have unprotected intercourse.
- They are more likely to be exposed to intravenous drug use.
- There is a higher proportion of HIV positive individuals in a homosexual population then a heterosexual population, thus more people become HIV positive in a feed-forward manner.
I'm sure there are other theories that I have left out, but I want to say that I'm not claiming that the theories just mentioned are true. I am just stating that these are commonly mentioned, and you should evaluate the accuracy of each theory yourself. A good starting point is Pubmed[85].--inksT 07:59, 26 March 2006 (UTC)
- Another theory is that anal sex has a higher transmission rate, and is practiced by a larger percentage of homosexual men than heterosexuals. StuRat 08:09, 26 March 2006 (UTC)
- This is all a bit outdated now anyway, since heterosexuals have overtaken homosexuals as the largest group infected with HIV. Looks like God didn't think this one through... --Username132 (talk) 13:15, 26 March 2006 (UTC)
- Maybe that's just Intelligent Design! (That was a joke.) - Cybergoth 15:06, 26 March 2006 (UTC)
- Or, God doesn't play statistics. A higher percentage of men who have anal sex with other men have HIV than men who do not have anal sex with other men. In the "everybody has AIDS" campaign, the statistics are skewed through many tricks. First, not all men who have anal sex with other men are homosexual. So, that reduces the number of "homosexual" men with HIV. Then, none of the women are homosexual "men", so that increases the number of non-homosexual men with HIV. Finally, homosexual men are a very small percentage of the population. If 100% of them had HIV, that would still be smaller than the percentage of the rest of the population of humans with HIV - even though most people do not have HIV. In my opinion, the "everybody has AIDS" campaign is doing a terrible thing. Instead of making people aware of AIDS, it is making those at high risk of AIDS feel that they are not at high risk because everyone is at the same risk. Then, they don't protect themselves. --Kainaw (talk) 17:06, 26 March 2006 (UTC)
Writing as one who was recently rebuked in Wikipedia for using acronyms, could someone please say what the acronyms for AIDS and HIV stand for and why the questioner here wasn't also rebuked??
- Because they are ridiculously common acronyms; in fact they are better known by their acronyms than they are by their fully spelled-out forms (I'm betting that there are more people who know what "AIDS" is than can spell "immunodeficiency"). You'll notice this reflected in the fact that on Wikipedia the articles are at the acronym names. In contrast MSRA redirects to its long-form name, and neither its long or short forms are generally well known to non-specialists. --Fastfission 19:55, 26 March 2006 (UTC)
- Except in Britain, at least, where newspapers will commonly use 'MRSA' without expanding, because nobody I know could tell you the long name, but all of them know roughly what MRSA is. 57.66.51.165 13:15, 27 March 2006 (UTC)
- As an example of this take a look at this week's Oban Times. The Oban Times largely deals with farming and fishing issues, reports of Highland Games, and people reprimanded for letting of fireworks after 9 pm: but it nevertheless feels its audience will know what MRSA means. By next week the headline will be gone, so here it is: "Dunstaffnage leads war on MRSA". Dunstaffnage is a local place. Notinasnaid 13:23, 27 March 2006 (UTC)
- If MRSA means 'Methicillin resistant staphylococcus aureus', what does MSRA stand for? HenryFlower 16:07, 27 March 2006 (UTC)
- It means "four-letter acronyms often get letters switched around by accident" ;-) Shimgray | talk | 15:37, 28 March 2006 (UTC)
- If MRSA means 'Methicillin resistant staphylococcus aureus', what does MSRA stand for? HenryFlower 16:07, 27 March 2006 (UTC)
- As an example of this take a look at this week's Oban Times. The Oban Times largely deals with farming and fishing issues, reports of Highland Games, and people reprimanded for letting of fireworks after 9 pm: but it nevertheless feels its audience will know what MRSA means. By next week the headline will be gone, so here it is: "Dunstaffnage leads war on MRSA". Dunstaffnage is a local place. Notinasnaid 13:23, 27 March 2006 (UTC)
- Except in Britain, at least, where newspapers will commonly use 'MRSA' without expanding, because nobody I know could tell you the long name, but all of them know roughly what MRSA is. 57.66.51.165 13:15, 27 March 2006 (UTC)
I've heard that homosexuals are more likely to get AIDS ( Acquired Immuno Deficiency Syndrome). Why?
- In case you skimmed over the discussion above... Being homosexual does not increase a person's risk of AIDS. Performing actions that put a person at high risk of getting AIDS increases a person's risk of AIDS. One of those risky actions is anal sex. Note: condoms have a much higher rate of breaking during anal sex - so even protected anal sex is a higher risk than other forms of protected sex. Because anal sex is more common among homosexual men than others, homosexual men have a higher risk of getting AIDS - but, that is only one risky action. Sharing needles and any form of unprotected sex are two other common risky actions. --Kainaw (talk) 02:55, 30 March 2006 (UTC)
- psst, Kainaw ... I think he/she was just posting the question with the fully spelled out name of the syndrome to make sure there was no confusion or complaints. :-) --LarryMac 03:07, 30 March 2006 (UTC)
- No confusion or complaints? It confused me and now I'm complaining! --Kainaw (talk) 03:18, 30 March 2006 (UTC)
- Scroll up a tad, looking particularly for the word "rebuked." --LarryMac 04:07, 30 March 2006 (UTC)
I think Kainaw described the transmissability picture rather well. As it turns out, outside of Europe and North America, the virus is just as likely, if not more likely, to afflict women compared to men. In North America, HIV was first encountered in the gay population and that's where it grew and stayed for a while, simply because women were not being exposed to it in large numbers. Ande B. 07:04, 30 March 2006 (UTC)
Motherboard / Graphics card question
[edit]How do I know what graphics cards fit in my PC? Where can I find out?
- First you must find out what type of graphics_card your motherboard will accept. There are 3 common types, from "newest" to "oldest" they are PCI-E, AGP, and PCI. To find out which one you have, open the case and take a look (the articles I liked to have pictures of what the different types look like), refer to your motherboard/computer manual, or use a diagnostics program like SiSoft Sandra [86]. Once you know which one you need, just look out for that type of graphics card - it will say on the box or in it's name what type it is, e.g. "Leadtek GeForce A6600 GT TDH Video Card, GeForce 6600 GT, 128MB, DDR3, 8XAGP, TV out, DVI, HDTV", which is an AGP card.--inksT 08:09, 26 March 2006 (UTC)
- To see if the motherboard has an AGP slot is easy, as there's usually only a single one, while there are usually multiple PCI slots, even if there's an AGP port too. This is because only video cards use AGP, but PCI is for other extension cards too. However, I think there are also two kinds of AGP cards too, and you can't use the newer one if the motherboard supports only for the newer one. I don't really know much of PCI-express other that that it exists too. – b_jonas 22:17, 27 March 2006 (UTC)
- As detailed in the Accelerated Graphics Port article, there are two main types of AGP slot: 3.3 volt slots supporting AGP 1x and 2x, and 1.5 volt slots supporting the faster AGP 4x and 8x. --Serie 00:14, 28 March 2006 (UTC)
- For even older computers, there are some additional slot types you might encounter, such as VESA local bus, EISA, MCA, and ISA. --Serie 00:14, 28 March 2006 (UTC)
- Opening up the computer is great, if you know what you're looking for. Even with pictures, though, the average person probably isn't all that comfortable trying to figure out what's what inside their computer. Definately check your computer manual – they usually have whole sections about what sort of hardware is compatible. You could also ask the company you bought it from. --68.146.186.92 02:38, 2 April 2006 (UTC)
RISB scale by Rotter
[edit]i want the history and development, scoring ,interpretation and criticism on RISB scale. Kindly, help me... iwill be grateful
- The Rotter Incomplete Sentence Blank, developed by Julian Rotter, seems to be a topic not currently covered in a Wikipedia article. You might try searching on Google. If you know enough about it to write an article, please feel free to start one. -- Rick Block (talk) 19:41, 26 March 2006 (UTC)
Weight of Hydrogen
[edit]Hi there,
I have a figure stating that 10 trillion cubic feet of hydrogen are produced each year. How do you convert that figure into kg, or with other words what is the mass of 10 000 000 000 000 cubic feet of Hydrogen.
Thank you.
- The hydrogen article states that it has a density of 0.08988 g/L at 0 °C and according to Google's calculator 1 liter = 0.0353146667 cubic feet. That's enough info to figure it out. --Ed (Edgar181) 12:03, 26 March 2006 (UTC)
- In addition to 0 °C, that conversion is also at standard pressure of one atmosphere (the air pressure at sea level). StuRat 17:09, 26 March 2006 (UTC)
- Yeah, depends on the density of the hydrogen. Or as stated the temperature and pressure can be used as well.
shape of the Earth 4.5 billion yrs. ago
[edit]What was the shape of the Earth at the early stage before about 4.5 billions yrs? Dulal Acharjee
- (email removed) enochlau (talk) 10:45, 26 March 2006 (UTC)
- Before accretion, it would have been a rotating cloud of gas and dust. After accretion, but while still molten at the surface, it would have been an almost perfect oblate spheroid, slightly wider at the equator than from pole to pole, due to centripetal force. It would have been more oblate than today, due to a faster rotation rate. After the surface solidified, it would start to acquire volcanic cones, fissures, impact craters, etc. One particularly large impact sometime after this solidification probably caused the bulge in the area just north of the equator. StuRat 11:02, 26 March 2006 (UTC)
- Although the general form would have been as StuRat outlines, the infant Earth may have had a significantly different mass and size - especially if you adhere to the theory that the Moon was formed when a large planetary body collided with the still-forming Earth. That would have had to have happened in this very early stage of development. Grutness...wha? 06:24, 27 March 2006 (UTC)
- I am sorry to carry contradiction here but I feel compelled to. Everyone must know that Earth was flat until very recently (some centuries, compared to billion years.) --DLL 16:59, 29 March 2006 (UTC)
- Don't be daft. The Earth didn't even exist until 4004 BC. Billions...ha! TenOfAllTrades(talk) 18:43, 29 March 2006 (UTC)
- The above comment was added with the edit summary "Earth didn't exist until 4004 BC, remember?" ... Dude, some of us may feel a little old sometimes, but we're not that old. O_o Femto 19:26, 29 March 2006 (UTC)
shape of the Earth 4.5 billion yrs. ago
[edit]I don't remember. If it was not in one of it's inhabitation cycles, the surface would have
been ice covered. Fluid saturation gives planetary bodies their stability. During land habitation cycles, land masses are formed as needed ( size and shape ). Organic life is fairly new, cosmically. Nevertheless, 4.5 billion years is not a long time ago, ( speaking beginning of time wise ).
about the carrier
[edit]iam really confused with the courses between biotechnology and biomedical engineering.what is biomedical engineering and what is biochemistry i will be very greatful to you? thnk you
- Biotechnology = new biology related technology, such as genetic engineering, cloning, etc.
- Biomedical engineering = creating and maintaining mechanical and electrical devices used for medical purposes, such as pacemakers, insulin pumps, dialysis machines, etc.
- Biochemistry = the chemistry of the body, such as blood pH and salinity, HCl used in the stomach for digestion, etc.
- Better yet, see biotechnology, biomedical engineering, and biochemistry. TenOfAllTrades(talk) 16:07, 26 March 2006 (UTC)
Easy Particle Physics book
[edit]I am a graduate student in Computer Science. I want an easy introduction to Particle Physics, Theory of Everything, and related topics. I am currently looking at Greene's The Elegant Universe. Kindly recommend suitable books, preferably with a description of each. Thank you. —Masatran 11:13, 26 March 2006 (UTC)
- I liked Griffiths' particle physics book when I was an undergraduate, and it might meet your needs. You might find it a bit hard if you don't have a strong math/physics background, but it doesn't assume any quantum mechanics or quantum field theory, so it's easier than it could be. That is to say, it's probably the easiest particle physics book you can find without resorting to a layman's book such as Brian Greene's (and about layman's books I can make no recommendations). Also, Griffiths is a very talented author with a flare for explaning hard topics in a way that makes them seem easy, and with a flow that makes him fun to read. Another book of similar difficulty (maybe a little more sophisticated and without the flare) is that by Halzen and Martin. If you really want to know about particle physics, then you have to learn quantum field theory though. A more advanced particle physics book that will make use of concepts of quantum field theory is Kerson Huang's book. But actually, any quantum field theory text will also have at least a little particle physics. -lethe talk + 11:21, 26 March 2006 (UTC)
- I think Parallel Worlds, Hyperspace, and The Universe in a Nutshell would suit you well. --
 Mac Davis] ⌇☢ ญƛ. 04:28, 27 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:28, 27 March 2006 (UTC)
Adhesives and fumes
[edit]adhesives, epoxy, etc. Is it necessary that the things that stick & dry well are toxic? Something about the molecular mechanics that makes it inevitable that they're also bad for us to inhale the fumes? --Quiddity 11:14, 26 March 2006 (UTC)
- I don't think they all are. But you don't want adhesives to be either water- or oil-soluble, as that would make them fall apart when they get wet or get oils on them (from people touching them). So, that leaves chemical solvents, which tend to be toxic. You also want them to dry quickly, so you need to use a highly volatile solvent. This combination of toxicity and volatility makes for dangerous, and potentially flammable, fumes. StuRat 11:25, 26 March 2006 (UTC)
- I remember using PVA glue at school. I think that's fairly non-toxic. -Fangz (not logged in)
- No, not really. The simplest glues are just some polymer and a solvent. Paste and wood glue usually use water, and they're usually pretty safe. However, it's desireable for a glue to dry quickly, which means using a more volatile solvent, hence the fumes. Most organic solvents are toxic and flammable. (as are most organic substances) The toxicity varies widely between solvents though. --BluePlatypus 20:13, 26 March 2006 (UTC)
- Thank you all :-) --Quiddity 21:30, 28 March 2006 (UTC)
Boot From Diskette, Install From CD
[edit]If I want to install Linux on a wrinkly old (80 MHz) system without an option to boot from CD in the BIOS, can I use a floppy diskette to enable install from a CD? Installing OS from floppies is no fun :( --Username132 (talk) 14:15, 26 March 2006 (UTC)
- As much as I searched, I could not find a way to boot a CD from a bootable floppy. I would suggest asking at a more technical forum, perhaps one that deals with Linux or boot disks. --Optichan 19:30, 26 March 2006 (UTC)
- There are bootable floppy images that boot into DOS and have common CD drivers. That should be enough to get you access to the CD and then you can run the install program from there - assuming there is a DOS compatable install program on the CD. Just google for "cd boot floppy image". When I used to need them, I had one that had about 30 CD drivers on it. --Kainaw (talk) 21:52, 26 March 2006 (UTC)
- The answer to the original question is most definitely YES. For instance, see http://debian.org installation docs. The boot floppy boots a linux kernel, not DOS, however. The bootable floppy image comes on the CD, or download separately. GangofOne 01:04, 27 March 2006 (UTC)
- There are bootable floppy images that boot into DOS and have common CD drivers. That should be enough to get you access to the CD and then you can run the install program from there - assuming there is a DOS compatable install program on the CD. Just google for "cd boot floppy image". When I used to need them, I had one that had about 30 CD drivers on it. --Kainaw (talk) 21:52, 26 March 2006 (UTC)
- Yes, I haven't tried this myself, but most distros have an initial boot floppy image, containing Linux itself and basic (inc. CD-ROM) drivers, which should be able to use the CD as the source for the rest of the installation (even if there is no BIOS support).
- You might also consider using a network or serial connection to another PC to download the files. Also, Smart Boot Manager/ (which you can find floppy disk images of all over the place) is a bootloader that supports booting from CD-ROM, but I think it may require BIOS support.
- It might help if you said which distro you wanted to install.
- Joe Llywelyn Griffith Blakesley talk contrib 16:17, 27 March 2006 (UTC)
- Not all that important. Username just wants to use a boot floppy to boot from a CD. So it really doesn't matter which distro is on the floppy, as long as it can run the installer from the CD. --Optichan 16:26, 27 March 2006 (UTC)
Computer Servers
[edit]From HP, you can get a server, I think high-end, with a 1.6 GHz processor, which is slower than what I'm using. Theirs beat my PCI slots with upto 146(!!) but apart from that, what makes these computers so special?
- Servers often don't do stuff that uses much of the processor. Much more important in a server are very fast hard disks, lots of memory (to avoid using the disk if at all possible), and above all high reliability. People often buy PCs on the basis of how many GHz they are using, but do they ever check if their current PC is running at 100% CPU? If it isn't, it's like upgrading from a car with top speed 160 mph to one with top speed 170 mph, then driving it at 30 mph anyway. Notinasnaid 16:16, 26 March 2006 (UTC)
- 's a good point, Notinasnaid. The fun part of it all being, people tend to buy top-of-the-line pc's [think 2 or 3 GHz], with something round 256MB [*laughs*] of memory, in order for their offspring to write school assignments and the like. Myself, I operate on a P3/550 with a half a gig of RAM, which allows me to switch off the virtual memory in win98se 8) all this with a quick Matrox HDD and MX400 graphics. And I wouldn't trade it for anything. On the other hand, I had a hard time understanding the original question [Theirs beat my PCI slots with upto 146]. --Ouro 17:12, 26 March 2006 (UTC)
- He may not be referring to a single stand alone server, but to a blade server. That might have a low end configuration of a single processor, but by adding boards to the chassis, you could end up with lots of processors with multiple PCI slots on each blade. --GraemeL (talk) 17:18, 26 March 2006 (UTC)
enegy diagram
[edit]why we represent energy band in parabolic form in E-K diagram? and can we change the state of a partical or material by applying a very high electro magnetic field?61.1.92.181 18:05, 26 March 2006 (UTC)
- This is clearly homework, but the fact that you were posting questions from it on Sunday night means it was probably due in by now.
- This subsection from the online textbook by Yuri Galperin (linked at the bottom of our Solid state physics) article explains why.
- What do you mean by "change the state"? At a first guess, yes, applying an EM field will change the potentials involved and thus the properties of the material. --Bth 11:52, 27 March 2006 (UTC)
Space Research & Energy Development
[edit]I am looking for information on what space research is all about and how it can be applied to energy development (i.e. the developmen of energy resources). Thanks much.
- Could you be a little bit clearer - what do you mean by "space research"? If you're asking about how space travel can affect research into new kinds of energy, that is a vast field - but you can start by looking at orbiting solar panels (which can be lit by the sun continuously without clouds or weather in the way, the problem is then getting the energy down to Earth). — QuantumEleven | (talk) 08:15, 27 March 2006 (UTC)
UTL33 Digital Multimeter
[edit]What is this meter used for?What can be combined with it? How is it used?What do all the symbols stand for?What is NPN , PNP , 10ADC , COM , VoMA ?I would greatly apreciate all the information on this product.Thank you for all your help.
- Multimeter should be quite helpful. --jpgordon∇∆∇∆ 20:12, 26 March 2006 (UTC)
- NPN and PNP are two types of transistor. If you want to measure the hfe (gain) of the transistor, you would identify its three leads (base, collector, and emitter), plug them into the small sockets marked B and C and E, select either NPN or PNP as appropriate, and make the reading.
- 10A DC is used to measure a high (up to 10A) DC current.
- COM is the Common socket, normally black, in which you would plug one of your leads for all voltage and current measurements.
- I don't know what VoMA means. Maybe you're reading it wrongly. Multimeters would often have a low-current range, labelled something like 50mA.
- Rwxrwxrwx 21:36, 28 March 2006 (UTC)
- I think you mean "VΩmA" instead of "VoMA". This is the socket (normally red) where you would plug the red lead when you are measuring voltage (V - volts), resistance (Ω - ohms), or low currents (mA - milliamps).
- Rwxrwxrwx 10:35, 29 March 2006 (UTC)
i386 OS on 486 Architecture?
[edit]Can I install a i386 operating system (Fedora Core 5) on PII 333 computer? --Username132 (talk) 20:25, 26 March 2006 (UTC)
- The "i386" as it is used with Fedora does not mean a 386 chip. It means "32-bit Intel/AMD PC". The Pentium II is a 32-bit Intel, so it will install. However, at 333MHz, it will run awfully slow if you try to run Gnome or KDE for a graphics environment. I have a shell(text)-only install of Fedora on a 333MHz computer and it runs just fine. --Kainaw (talk) 21:15, 26 March 2006 (UTC)
Deadly affair?
[edit]Could it be possible to perform a successfull surgical operation (eg. appendicectomy) on oneself without the help of others? Are there any known cases? Does it have any kind of name? Askeles456 21:46, 26 March 2006 (UTC)
- There have been numerous examples of both successful and unsuccessful surgical operations. The most common examples of major procedures have been amputations of an appendage and trephining. Examples of invasive abdominal or thoracic procedures are uncommon. One of the most striking and bizarre was a report in JAMA about 20-30 years ago that described a college student who performed two major procedures on himself in his dorm room on two separate occasions: if I recall correctly, the castration was successful, but the attempted adrenalectomy was not, because the hand holding the retractor got tired. I may be misremembering details but would love to have the original citation again if someone is inspired to search it out. It was a regular JAMA article in the late 1970s or 1980s. As far as a name for it, it would be termed an "auto-whatever", like an "auto-appendectomy". alteripse 21:58, 26 March 2006 (UTC)
- Case I heard of, no documentation, so may be bogus, guy tried an auto-appendectomy but underestimated the pain, so he called an ambulance to take him to emergency room part way through. For what it's worth. GangofOne 00:53, 27 March 2006 (UTC)
- See self-surgery. - Cybergoth 03:01, 29 March 2006 (UTC)
- The reference for the article mentioned by alteripse is JAMA, May 1979; 241: 2188 - 2189 - Cybergoth 03:08, 29 March 2006 (UTC)
- Case I heard of, no documentation, so may be bogus, guy tried an auto-appendectomy but underestimated the pain, so he called an ambulance to take him to emergency room part way through. For what it's worth. GangofOne 00:53, 27 March 2006 (UTC)
- If you're in London, the traditional venue for auto-appendectomy is the Bakerloo Line. A report by noted physician G. Chapman can be found here, or if you can get ahold of this book there's an annotated tube map and other visual aids. —Steve Summit (talk) 22:03, 27 March 2006 (UTC)
Compaq Presario 3565 Wont Power-On
[edit]I've got an old Compaq Presario 3565 a friend gave me to fix (I failed). The problem is that it wont switch on. When I plug the PSU into the mobo, an orange light next to the supply comes on, but the on switch does nothing. Apparently one morning it just wouldn't switch on. Trying another PSU did nothing. Shorting out the on-switch pins with a pair of pliers does nothing. What should I do? --Username132 (talk) 21:55, 26 March 2006 (UTC)
- If it's still under warrenty you should take it back to Compaq/HP ASAP, if not you could go at it with a voltage meter. If it's not powering on i suggest looking for broken, dodgy or chewed (:P) wires as well as any apparent damage to PCB track or components. Because of the very small nature of the components i doubt you'll be able to change much. Also, i had a similar problem with a desktop computer that was due to loose RAM DIMMS - if the laptop has removable RAM try removing it, cleaning (blowing on it) and re-insterting. Also try asking google to see if anyone else has had a similar experiance :) -Benbread 22:07, 26 March 2006 (UTC)
- Three things have fixed that for me in the past. First, make sure it isn't a broken device (unplug all drives, memory, cards, etc... you don't need those to hear the beep when it turns on). Second, make sure the power button is attached properly. Many times, it is a switch connector on the board and someone plugs it in one-pin over. Finally, replace the fan in the power supply. If the fan doesn't turn, the power supply normally will not turn on. --Kainaw (talk) 18:09, 27 March 2006 (UTC)
Accurate clock for Windows and Linux
[edit]Is there a free program that, when the user clicks a button or presses a key, displays the exact time the button was clicked or the key was pressed? This program has to display at least two digits after the decimal place (e.g. 10:43:34.39), and it has to be accurate to at least 20 milliseconds. Thanks. --Bowlhover 23:12, 26 March 2006 (UTC)
- On Linux, I use ntpdate to sync up with an ntp server like time.nist.gov, then run "date +%T.%N" to show the nanoseconds. "time" indicates the whole command only takes about 10 milliseconds to run, so it should be accurate to 20 milliseconds. —Keenan Pepper 01:04, 27 March 2006 (UTC)
- Is there an equivalent of "date" for Windows? It seems strange that nobody wants a program that can display the exact time. --Bowlhover 00:27, 28 March 2006 (UTC)
- I don't know if there's a windows specific version of the utility, but you might want to look at cygwin. It's a linux-like environment with a bunch of utilities that you can run on windows .. --68.146.186.92 23:25, 2 April 2006 (UTC)
Mystery Computer Port
[edit]The following images show a weird port on a computer I bought. Its card is connected to the motherboard via some sort of IDE cable. It's long an thin and the computer is a server. What is it?
http://username132.tasminslair.com/mysteryport1.jpg http://username132.tasminslair.com/mysteryport2.jpg http://username132.tasminslair.com/mysteryport3.jpg
- Most likely a SCSI interface built into the motherboard. Especially servers tend to use SCSI instead of IDE for the hard drive interface. There are external SCSI harddrives and other devices, so that's why you'd want an external connector. --BluePlatypus 23:39, 26 March 2006 (UTC)
- Yeah, it looks like a 68 pin SCSI connector. It's hard to know which electrical SCSI standard the computer supports for it (but both windows and linux are likely to correctly identify the SCSI controller and tell you in their respective device-info screens. -- Finlay McWalter | Talk 23:49, 26 March 2006 (UTC)
Global Warming
[edit]I am confused on the two different stances that the planetary management worldview takes vs the environmental wisdom world view. Can anyone help a little?
- Dr. Connolley is our resident climate expert, and knows a lot about global warming. I suggest you talk to him; I'm sure he can help you out. Good luck! :) --Ashenai 00:25, 27 March 2006 (UTC)
- But, it is also suggested you talk to somebody on the other side, the people who think global warming is a lot of hoopla. They are more numerous than you think. --
 Mac Davis] ⌇☢ ญƛ. 04:24, 27 March 2006 (UTC)
Mac Davis] ⌇☢ ญƛ. 04:24, 27 March 2006 (UTC)
- This glossary has pretty good definitions for both, which should help you get a handle on the concepts. And as for 'the other side' of the argument mentioned by Mac Davis, you should of course make up your own mind, but I wanted to note that the arguments against human-induced climate change are almost nil, and the vast majority of climatologists agree that humans are at least in part responsible for the climate change we are experiencing today. See our very good article on climate change. — QuantumEleven | (talk) 08:00, 27 March 2006 (UTC)
- I personally agree with QuantumEleven. While there is certainly still valid debate ongoing about how much we're effecting the global climate, the idea that we're not effecting it at all is a rare and hard to support argument. I'd also like to point out that the latest edition of Time (magazine) has a big cover story on global warming, and I'm planning on picking that up very soon. EWS23 | (Leave me a message!) 08:18, 27 March 2006 (UTC)
- Most everyone agrees that the earth is warming up gradually. What they can't agree on is the reason. My opinion is the discussion is too infected with politics to be objective, and even without that, the climate is much more complex than anyone can fathom.
- That's not really true - most everyone who knows anything about it agrees on the reasons (apart from some of the fossil fuels industry). There is debate about the rate of change, but the debate about the reasons is pretty much over. For great justice. 16:43, 29 March 2006 (UTC)
Obtaining Windows Vista CTP's
[edit]Does anyone know how, short of using BitTorrent to pirate one, I can get a copy of a recent Windows Vista Community Technology Preview release? The phrase "Community" in the acronym implies a level of availability, and I've so far been unable to find such availability anywhere.
So how, exactly, does one preview Vista?
Thanks,
Suntiger 23:37, 26 March 2006 (UTC)
- Recent news implies that you might not want to, except on an 'easy to reformat' test machine. --Zeizmic 01:16, 27 March 2006 (UTC)
According to this you have to join the Microsoft beta testing program. --JWSchmidt 02:29, 27 March 2006 (UTC)
- Thanks; I'll look into that! Suntiger 15:10, 29 March 2006 (UTC)
First chemistry analyzer used in the clincal laboratory
[edit]Could you please tell me what the first chemistry analyzer was used in the clinical laboratory? I am looking for the name and methodolgy of the analyzer. I believe that the analyzer could on perform one assay at a time (for example, you would have to run all your patient's potassiums first, and then their Sodiums).
Thank you.
- Seems like a loosely defined question. People have been doing clinical analysis since time immemorial, simply by looking at the color/smell of feces/blood/urine/etc. Most of these tests were useless of course, but some turned out to work. So you'll have to specify what you want to know; What was the first analysis that had some kind of diagnostic value? What was the first analysis performed by a machine? What was the first analysis using a chemical reagent? Etc. Also, there is no such thing as a "chemistry analyzer", even today most clinical tests check for one specific thing. --BluePlatypus 15:35, 27 March 2006 (UTC)
- As someone who has used blood analysis equipment in a lab, I'm going to have to disagree with the comment above. I don't know the answer for sure, but I think it's a Coulter Counter. Check the article on Beckman Coulter Corporation. Brian G. Crawford 15:59, 27 March 2006 (UTC)
- As someone who disagrees with appeals to authority: What do you disagree with? --BluePlatypus 17:46, 27 March 2006 (UTC)
- A Coulter counter counts blood cells: it's not a chemistry analyzer. I suspect what the questioner's looking for is the first automated blood chemistry device, which would probably be one of the colorimetric tests on serum. Coulter Counters were introduced in 1954. According to [87] the first successful automated system used for chemical pathology was the AutoAnalyzer, introduced by Technicon Instruments Corporation in 1957. This was a "single-channel, continuous flow, batch analyser that provided one result per analyte for each specimen at a rate of 40-60 specimens per hour." - Nunh-huh 04:31, 28 March 2006 (UTC)
March 27
[edit]Proliant 3000 Graphics
[edit]I want to upgrade the graphics card in a Proliant 3000 server. I've got PCI and EISA slots to use but I need a decent graphics card to play games. :) Would PCI graphics be better? --Username132 (talk) 00:18, 27 March 2006 (UTC)
- Many manufacturers offer PCI models of their graphics cards, but if your computer is old enough that it still has EISA slots, you'd probably be better off getting a whole new machine if you want to play any recent game. You'll be limited by processor and memory if you get any sort of quality graphics card. Night Gyr 00:42, 27 March 2006 (UTC)
- If I have 666 Mhz and 800+ Mb EDO 60ns, then what would be the best graphics card worth going for? --Username132 (talk) 01:13, 27 March 2006 (UTC)
- Since you say that you have 666MHz, I'm assuming that you have the Proliant 3000 with Dual Pentium II 333Mhz. This was top-of-the-line about 8 years ago. It's a nice little workhorse for perhaps an Apache web server, but I don't think you're going to get what you're dreaming of in terms of a gaming machine. First out, you're not going to get the benefit of those dual processors unless you install a multiprocessor capable OS like Windows XP Professional. Even then it's only dual 333Mhz, and most games are not going to multithread, so you're generally going to get the performance of a 333Mhz machine. Second, this machine has a 100MHz bus - even if you purchased 60ns EDO RAM for it, it's not getting the benefit of that RAM (is likely performing like 100ns RAM). Third, you might have difficulty finding a new PCI accelerated card which will work with this architecture. I wouldn't guarantee anything would work if you get it off-the-shelf, will probably need to go to a small computer shop which is like a "pick-and-pull" and get a Voodoo or early GeForce (Geforce2 MX PCI). I think the long and the short of it is that if you do manage to get something to work, you'll have the equivalent of a great game machine - from about 6-8 years ago. KWH 22:20, 29 March 2006 (UTC)
- A computer like that would be considered a gaming machine about five or six years ago, so something like a GeForce 3 or 4 would have more than enough capability for anything that your processor can handle. You shouldn't spend more than $50, and might be best off buying a used one off of somebody upgrading their machine. Night Gyr 03:38, 27 March 2006 (UTC)
- Bear in mind that if this machine is still a server in more than name, you shouldn't consider playing graphically intensive games on it: it will seriously damage the serving. There's a reason why servers aren't sold with state of the art video cards... Notinasnaid 13:25, 27 March 2006 (UTC)
- Well I've always got my *proper* computer to play games. I've currently got Fedora Core 4 on this machine and I've not got two questions;
- - when you say playing games will hurt the serving, do you mean it would reduce the ability of the server to serve at the same time, or do you mean permanent damage?
- - regarded the multithreading, will I need to find another Linux distro or is there some download that will modify the kernel of this one?
The main point of this machine is for me to learn things about Linux. I just need to upgrade the graphics beyond this god-awful 2mb flickerthon, but preferably to the maxmium before other components become a bottleneck. --Username132 (talk) 01:53, 2 April 2006 (UTC)
mammalian tissue culture
[edit]I need to find infomation on contamination in tissue culture. The 3 main types on contamination are, bacterial, fungi, microplasms and viruses. Where can I find out what I would do in the event of contamination? Thank you, as any help is very much appreciated--Dani27 04:36, 27 March 2006 (UTC)
- It depends. The easiest solution – and the only one I'm willing to guarantee will work 100% of the time – is to throw out your petri dishes and start fresh with clean stock from the freezer. If you can't do that (you don't have any more cells, it's a one of a kind line that you have to save, your frozen stock are contaminated too, etc.) then there are a number of things you can attempt. None are guaranteed to work; I would tend to rate mycoplasma and viral contamination as the most challenging to treat.
- Corning Life Sciences provide a number of handy protocols and technical guides which you might find useful. I recommend "Understanding And Managing Cell Culture Contamination" as an excellent starting point. TenOfAllTrades(talk) 05:24, 27 March 2006 (UTC)
MPEG to Animated GIF
[edit]
Does anyone know of a free software to convert MPEGs to animated GIFs. Actually, I have a matlab function that can be used inside a matlab for loop along with a plotting command, to capture frames and make an MPEG out of it. If someone can tell me how I can do something similar to make a gif, or a way to convert an MPEG to a GIF for free, I can contribute to wikipedia more informative animations like the one on the right. The process I use right now is extremely laborous. deeptrivia (talk) 05:37, 27 March 2006 (UTC)
PS: In fact, I'll strongly prefer a matlab code that can help make an animated gif, because the path through MPEG would seriously deteriorate quality. deeptrivia (talk) 05:39, 27 March 2006 (UTC)
- I know Matlab pretty well... so if someone can tell me what's required to make an animated gif, then I can help deeptrivia. For instance, if I had a gif for each frame, could I make an animated gif? Because I do know how to make an arbitrary series of gifs in Matlab. moink 06:18, 27 March 2006 (UTC)
- Sorry, I just checked, the default drivers that come with Matlab make jpgs and pngs and not gifs. My apologies for getting it wrong. moink 06:21, 27 March 2006 (UTC)
- ImageMagick can join frames into an animated gif. EdC 06:33, 27 March 2006 (UTC)
convert -delay 20 -loop 0 frame-* animation.gif
- ImageMagick can also convert mpeg to gif: "convert -crop 0x0 -geometry 25% -loopback 0 movie_file.mpg movie_file.gif" --Kainaw (talk) 18:12, 27 March 2006 (UTC)
- I have the software to join gif frames into animated gifs. The trouble is, I have to first make a bmp of each frame manually in matlab by changing the parameters, then use MS Paint to convert bmps into gifs, and then join them. This is quite cumbersome. Technically, it must be possible to write a matlab function to save a figure as gif, or as a matlab movie (can use getframe). It must also be possible to write a function that will convert the array of frames obtained using a for loop into an animated gif. Somebody might have done this already. Does anyone know any such functions available somewhere? deeptrivia (talk) 15:04, 27 March 2006 (UTC)
- The imagemagick line above will work for bmp sourcefiles, so that'll save you one step. If you can't figure out how to do the automation in Matlab, is it possible to have Matlab run inside a script (say from perl or python) to generate a single frame. You'd have the containing script generate the file Matlab reads, call matlab, regenerate the input, etc., and then finally run imageMagick to make the gif. -- Finlay McWalter | Talk 15:12, 27 March 2006 (UTC)
- Another program that can create animated GIFs is gifsicle. (But it doesn't do direct conversion from MPEG, either, so it'd be just part of the puzzle.) —Steve Summit (talk) 21:46, 27 March 2006 (UTC)
- Thanks everyone. I figured out an easy way. I didn't know that you can make AVIs in Matlab. I found out a software that can convert AVIs into GIFs. So it's easy now. Cheers :) deeptrivia (talk) 04:45, 29 March 2006 (UTC)
i know image ready will convert a folder of numbered tiffs into a gif.
cell and tissue culture
[edit]Hi i am after some info about powder and liquid media. The info i am after is the chemical difference between a liquid media and powder media? what chemical is missing? and by leaving out this chemical what does it allow the investigator to do when making up the media?. I have looked everywhere for this information and can't seem to find it anywhere, any help would be greatly appreciated. Thank you for your time. janine.
- Your best bet is to phone the manufacturer of your medium. However, if you're looking for something more global, there's an excellent book that I reference quite often that I find invaluable, and that you may find useful as well: Method in Enzymology, Volume LVIII: Cell Culture. It's a number of years old (pub 1979), but the techniques and media discussed within are still in use today. I know that Sigma-Aldrich sells copies of the book here. Good luck! – ClockworkSoul 15:35, 27 March 2006 (UTC)
Mass and Energy - final smackdown
[edit]I propose we settle this controversy once and for all - it seems to come up semi-regularly (such as here), and we hardworking Reference Desk volunteers can't seem to come up with a coherent answer.
So: E=mc² gives the equivalence between mass and energy. Does this mean that when a reaction emits energy, it must lose the corresponding amount of mass? For instance, 2 H2 + O2 = 2 H2O . This reaction is exothermic - does this mean that if you were to carefully measure the mass of the products and the reagents, you would find the latter to be heavier than the former by the energy emitted divided by c²? Which would imply that the principle of the conservation of mass is technically incorrect, but since the mass change for ordinary chemical reactions is so small, it's a useful approximation? (our article on conservation of mass is ambiguous on this point).
Similar principle, different situation: A coil spring which is compressed stores energy. Per the energy-matter equivalence, does this mean that the spring's mass increases very very slightly when it's compressed?
Thanks for all clarification! Hopefully, we can put this somewhat thorny subject to rest... — QuantumEleven | (talk) 11:41, 27 March 2006 (UTC)
- Chemistry and conservation of mass: your understanding accords with mine -- there is a difference in mass between chemical reagents on either side of a reaction due to binding energy, it's just so teeny tiny that it's usually not worth bothering with.
- Coiled spring: same principle applies, I think. Even more difficult to measure the difference here, though.
- In general, the only problems I can see in that earlier discussion relate to the definitions of various terms. As long as you're clear in your mind about what various symbols refer to, the maths all holds together for any consistent set of definitions.
- (Though of course we should remember that "E=mc^2" is only the reduced form of for the special case of zero momentum.) --Bth 12:19, 27 March 2006 (UTC)
- Yes, mass is not conserved, energy is. However, your average chemical reaction energy is on the scale of 100 kJ/mol, which means the mass difference between reactants and products is on the order of 10^-39 grams; totally insignificant and practically immeasureable. Only nuclear reactions emit enough energy to give a measureable difference in mass. So while not strictly true, conservation of mass works for just about anything on the ordinary scale of things. However, relativistic effects are significant in the chemistry of heavy elements, where it has a large effect on the atoms' electronic structure. --BluePlatypus 15:27, 27 March 2006 (UTC)
- Ever since Einstein, neither mass nor energy is necessarily conserved -- that's why the two old conservation laws were replaced with the shiny new Law of Conservation of Mass-Energy.
- The last sentence of that article is incorrect and misleading. (The article is also redundant.. why are there 1,2,3 articles on essentially the same subject?). Energy is conserved, and exactly. The Uncertainty Principle only means that there are time-dependent fluctuations in E (as time is the complementary property). As t goes to infinity, the uncertainty in energy goes to zero, so the total energy of the universe is indeed conserved exactly. The energy can fluctuate by a given amount for a finite amount of time, but in the long run, it will always be conserved. --BluePlatypus 00:46, 28 March 2006 (UTC)
- Be careful: there is no such quantity as "the total energy of the universe". Total energy can only be defined in a universe with a global timelike Killing vector. And that number will only be conserved if the universe is static, like Minkowski space. Our universe isn't like that, so there is no such quantity. Good thing too, otherwise it would be pretty hard to explain accelerating expansion without violating conservation of energy. Also, it's not really accurate to say that the uncertainty principle allows energy to fluctuate. People like to talk about "borrowing energy for a short time as allowed by the uncertainty principle", but it's just a way of speaking. Energy is always conserved. -lethe talk + 00:56, 28 March 2006 (UTC)
- You're right BluePlatypus, that article is terrible! Not only are its comments about the HUP misleading, its characterization of the titular law is wrong! The sum of mass and energy is not the conserved quantity! -lethe talk + 09:50, 28 March 2006 (UTC)
- I've changed that terrible article into a redirect to conservation of energy. -lethe talk + 11:58, 28 March 2006 (UTC)
- The last sentence of that article is incorrect and misleading. (The article is also redundant.. why are there 1,2,3 articles on essentially the same subject?). Energy is conserved, and exactly. The Uncertainty Principle only means that there are time-dependent fluctuations in E (as time is the complementary property). As t goes to infinity, the uncertainty in energy goes to zero, so the total energy of the universe is indeed conserved exactly. The energy can fluctuate by a given amount for a finite amount of time, but in the long run, it will always be conserved. --BluePlatypus 00:46, 28 March 2006 (UTC)
- My understanding of this is that, yes, any time you store potential energy in a system -- by separating two compounds that would rather react, by compressing a spring, by charging a battery, etc -- you increase its mass ever so slightly, and that when you release energy, the mass goes back down again. I have a nice, verifiable reference to cite on this, but it's packed away in a box right now. When it finally surfaces, I'll definitely post some excerpts here. (The book is The Ring of Truth, by Philip and Phylis Morrison, companion to the PBS TV series.)
- Now, a lot of people seem to have a different take on this. (I'm surprised none of them have chimed in here yet.) A lot of people believe that e=mc² applies only to nuclear reactions, but not to "ordinary" chemical, mechanical, or electrical reactions. Or sometimes they'll concede that while it might apply, the effect is always so minuscule that it's "meaningless" or "misleading" to even talk about it.
- Mass-energy equivalence applies to kinetic energy. What people underestimate is how much stuff is kinetic energy. Chemical energy is mostly the (difference in) kinetic energy of the electrons of molecules. --BluePlatypus 00:46, 28 March 2006 (UTC)
- (Note, I'm not saying it only applies to kinetic energy. But I do think most people would agree on at least that much..) --BluePlatypus 00:52, 28 March 2006 (UTC)
- Mass-energy equivalence applies to kinetic energy. What people underestimate is how much stuff is kinetic energy. Chemical energy is mostly the (difference in) kinetic energy of the electrons of molecules. --BluePlatypus 00:46, 28 March 2006 (UTC)
- E=mc^2 applies everywhere and always, perfectly, according to the theory, as Steve Summit and others say above, (well, ok, when the more general case is not relevant.) What you don't want to be confused by is conservation of particles. H2 + 1/2 O2 -> H2O, number of atoms matches both sides of equation. What has changed is chemical binding energy, in this case 68.3 kcal emitted per mole of H2O. (change of enthalpy is almost the same as energy, I ignore the difference here) 68.3 kcal = 3x10^-9 gram. A mole of H2O is 18 gram. So mass difference is 1 part in 10^10. In the case of nuclear rnx, say a neutron hits a U nucleus, it splits, the fragments will have just as many neutron and protons as it started with. The neuclear binding energy is much more than chemical binding energy. The same applies at even higher energies in colliders where subnuclear particles are reacted. Still, certain particle counts are preserved, baryon number, lepton number, charge, etc. Anyway, boiling water. Boiling water increases the mass of the water. If it weren't so, then you could use the boiling water to run turbines and generate electricity , generate gamma rays with that, and have the gamma rays generate electron-positron pairs, which definitely DO have mass, and you would have created mass from "nothing" i.e. violated the conservation of mass rule you were trying to preserve. (Maybe there are better examples.) For a good time, read Spacetime Physics, Taylor and John Archibald Wheeler ISBN 0-7167-0336-X, chapter on "Equivalence of energy and rest mass", wherein it's all explained, with problems like "Two freight trains, each of 10^6 kg mass, travel in opposite direction on the same track with equal speed of 45 m/s (about 100mph). They collide and come to rest. The rest mass of the trains plus the rest mass of the track plus the rest mass of the roadbed is increased immediately after the collison by what number of micrograms?" answer - 20 micrograms. --GangofOne 09:03, 28 March 2006 (UTC)
- As a caveat, while Taylor and Wheeler is very good (I was reading it just last night), their solution to the definitional confusions is to come up with a bunch of ugly neologisms for the four vector quantities involved (like "momenergy", a term which occurs precisely once in Wikipedia in the Aether drag hypothesis article). --Bth 12:16, 28 March 2006 (UTC)
- Wow - you're all amazing. Thank you very much guys and girls for all your indepth knowledge, now I can lay this subject to rest. Cheers! *raises beer glass* — QuantumEleven | (talk) 15:50, 30 March 2006 (UTC)
COCA - COLA
[edit]I have read the article on Coca-cola but was not able to find my answer to my question.
My question is, what is the easiest way to removing the sugar in coke? forgetting the fact the there is the secret substance in the coke.
Thank you
The easiest way to remove it would be with an enzyme that metabolizes it to something else. Adding some live yeast would do it by fermentation. alteripse 12:18, 27 March 2006 (UTC)
- Interesting. I wonder if anyone has made moonshine out of Coke. —Keenan Pepper 13:03, 27 March 2006 (UTC)
- No doubt: people in prison, for example, ferment whatever is available. – ClockworkSoul 15:37, 27 March 2006 (UTC)
Perhaps the easiest way is to buy the Coke that doesn't have sugar added in the first place; just buy some Diet Coke. Of course, if you want to get rid of the Aspartame, then we're back to square one. I admit to being intrigued (and a little bit frightened) by the thought of Coca-Cola moonshine. I guess the question we should be asking is, why do you want to remove the sugar? That might give us some idea of the sorts of processes that could help answer your question. TenOfAllTrades(talk) 15:33, 27 March 2006 (UTC)
- There is no easy way. Sugar is a polar molecule in a polar solvent, and thus pretty hard to seperate by using methods like extraction. The traditional way would be to add a chemical reagent that reacts with the sugar to form an insoluble product. --BluePlatypus 15:42, 27 March 2006 (UTC)
- I suggest allowing the water to evaporate, adding alcohol and dissolving the sugar that way. Another way would be to add ether to it and dissolve the sugar out that way. Both methods will probably also take out other organic components also, but you might try it anyway.
- Most of the sweetness in Coke comes from high-fructose corn syrup, so you're already on your way.TheSPY 21:27, 1 April 2006 (UTC)
Force Vibration, What is it?
[edit]Hello there,
I'm looking for a description of "force vibration", with regards to a system which is said to show simple harmonic motion. Any ideas?
- Your terminology isn't quite correct. See Harmonic oscillator to bone up on the topic. --Zeizmic 20:47, 27 March 2006 (UTC)
Information about Radiology
[edit]I am a high school student and I am doing a research paper on the field of radiology, i woule greatly appreciate if someone in this profession or someone who knows about it to tell me all about the aspects of your job. Things like work enviornment, benefits, ethics, education, etc. If you could get back to me as soon as possible it would greatly help me. Send your answer to [email removed] Thank you soo much
- Not sure if there are any radiologists among the regular RD contributors, so you may get only limited responses (like this one :). For starters, have a look at radiology, medical imaging, and medical ethics; more information can be found at [88], [89], and [90]. Offhand (and by no means should you take this information as authoritative), the often-touted benefits of a career in radiology (at least in the U.S.) include 1) an income that is substantially higher (at $200k-$300k per year) than the average national physician salary and 2) an average weekly workload that is considerably less (at 50-60 hrs/week, I hear) than the national average. Hope that helps, David Iberri (talk) 20:58, 27 March 2006 (UTC)
- Although I cannot comment on the specifics of the specialty of radiology, you should know that it has been in the news recently as one of those vocations that can be easily outsourced to other countries where the fees commanded are, to put it charitably, very economical. Of course, the popularity of "medical tourism" may ultimately affect all medical specialties, but will no doubt affect radiology sooner than those areas where direct patient contact plays a more prominent role.--Mark Bornfeld DDS 21:03, 27 March 2006 (UTC)
- Other benefits of a career in Radiology might include NPC ("no patient contact"), and working at home via telemedicine. - Cybergoth 03:18, 29 March 2006 (UTC)
Changing the effect of a browser's back button
[edit]I have a web page where the my menu links simply replace the contents of one of my div's using innerHTML in javascript. This means that the users are staying within the same web page while they are navigating around the site. However, the consequence of this is that when the user clicks the Back Button, they don't see the last page in my site, they see the page before they arrived at my site.
Is there any way I can set things up so that I can have the users see the contents of the last div, instead of seeing the past page? Any help appreciated! — Asbestos | Talk (RFC) 17:28, 27 March 2006 (UTC)
- Anything you try will fail. There are too many (expletive deleted) websites that try to trap users by disabling the back button. So, browsers are designed to get around their tricks. Your best option is to back to the drawing board and use some kind of cookie/session handling to allow users to recover from hitting the back button. --Kainaw (talk) 18:15, 27 March 2006 (UTC)
Ok, so is there some workaround? This site seems to use #'s to make a new url without needing to re-load the page, but I can't work out what's going on in his source code. Can anyone explain, or suggest something different? — Asbestos | Talk (RFC) 20:33, 27 March 2006 (UTC)
- Well, that's no workaround; the script merely changes the src="image.jpg" attribute in the img tag; the back button doesn't work here either. I know people are trying to get away from framesets, but you're bending over backwards to avoid the obvious. Simply put your navigation buttons in one frame, and change the source html in the other content frame-- your back buttons will work then...--Mark Bornfeld DDS 20:53, 27 March 2006 (UTC)
- The "# workaround" has nothing to do with the back button. It is a workaround for broken web browsers that try to load a URL after an "onclick" event has properly handled the mouse click. What the # does it move down the page to the first bookmark (an <a name='something'> tag - note the lack of an href option). Since it is moving to a bookmark (that usually doesn't exist) on the same page, nothing is loaded. --Kainaw (talk) 03:13, 28 March 2006 (UTC)
- Is it possible to detect a new visitor to the page (from either off-site/blank referrer or non-existing session cookie) and first serve up a non-cached Automatic Redirect page that takes the user straight to your naughty page, then when the Redirect page is re-requested (via the Back button), it serves up a simple page with something like "Do you want to go back to the page you came from, or stay on $name_of_site?"? It doesn't sound impossible... Tzarius 08:41, 28 March 2006 (UTC)
Well, I guess as Mark said I really should be using frames. I think the who think was a bit pointless, though, as the pages make more sense separated. Thanks for all your advice! — Asbestos | Talk (RFC) 21:46, 28 March 2006 (UTC)
Prime numbers in nature
[edit]I don't know if this is the appropriate place to bring this up, but I have questions about a science article: Can anyone verify the two facts in Prime numbers in nature that I added a citation template to? For example, it states that 11 is the "number of ribs in mongoloid children." I can't imagine that either meaning of the word mongoloid would somehow make that line true (or unique to "mongoloids"). And, one of those usages is racially offensive. The source of this factoid seems to be the first "external link", which itself looks very unprofessional, and which itself cites Wikipedia (that's a little problematic too, no?). Also, there is no support for the 2% figure, and my gut tells me that it has been plain fabricated JianLi 21:01, 27 March 2006 (UTC)
This is ridiculus! The number of ribs any human have is a FIXED CONSTANT. The "race" does not matter.
Proof by absurdity:
- Let's say race A has X ribs. Race B has Y ribs. And X not equal to Y.
- So A marries B and have a kid C.
- How many ribs does C have?
Ohanian 01:38, 28 March 2006 (UTC)
- That doesn't prove a thing. Dominant and recessive genes work just like that: C has the trait corresponding to the dominant gene. —Keenan Pepper 04:40, 28 March 2006 (UTC)
- "Mongolism" is the obsolete and offensive terminology for Down's syndrome or trisomy 21, so it has nothing to do with dominance (or race), it's a variation in chromosome number. And while some trisomies (trisomy 8, for example) can cause an abnormal number of ribs, they don't do so consistently enough for it to be a useful example. I'd suggest it just be removed. - Nunh-huh 04:50, 28 March 2006 (UTC)
- Furthermore, is the article implying that "mongoloid adults" have a different number of ribs than "mongoloid children"? Either way, I've removed the example. — Asbestos | Talk (RFC) 21:54, 28 March 2006 (UTC)
- I'm trying to get rid of the article, so I put an "afd" up. Most of the examples given are so trivial and irrelevant, in my opinion, that they do not merit a whole article. What are your opinions on the matter? JianLi 00:07, 29 March 2006 (UTC)
assessment methods of spectral vegetative indices
[edit]please can someone explain how the Tiers 4-5: remote sensing optical measurements and the Tiers 1-3: field radiance measurements work on Spectral vegetation greenness indices. i cant seem to find any explaination of how it works only that used as Assessment methods of spectral vegetation indices. an explanation no matter how brife would be duely appreciated.
Promethium/Technetium
[edit]Note that Technetium has an odd atomic number, in fact it is a prime number, as opposed to Manganese, above it in the Periodic table. It also has no stable isotopes. Now start putting the Rare Earths in place of the Transition Elements are in Period 6, note that Promethium occurs right under Technetium and also has a prime atomic number. Seems to me there ought to be a story there.
- Have you read Technetium#Stability of technetium isotopes? I don't think it has anything to do with prime numbers as such, just with non-round numbers. See also Magic number (physics). —Keenan Pepper 22:46, 27 March 2006 (UTC)
- For an interesting and possibly relevant read, take a look at the article PRIME NUMBERS GET HITCHED, which discusses a possible link between prime numbers and quantum physics. – ClockworkSoul 12:49, 28 March 2006 (UTC)
- I know that odd atomic numbered elements are more likely to be unstable, and those with odd numbers of neutrons likewise, and this is due to forces that cause pairs of like particles to be in a lower energy state than they are seperately. I had a wild idea that this may be true (to a lesser extent) of particles in threes or fives etc. The higher the prime gets, the less true this would be. Hmmm, the articles you posted here do not go that far. Wonder if any studies have been done on this?
human sexual response cycle
[edit]Can you describe to me what the human sexual response cycle is and the impact of both hormones and the psychological factors on sexual motivation. My son was told in health class to find the answer to this and get your parents help, well I have no idea and he has nothing to go by. Thanks!
- Looks like we have an article by that name, human sexual response cycle. Hope that helps — TheKMantalk 21:57, 27 March 2006 (UTC)
- You may also have a look at libido, though the discussion is kind of brief. --Robert Merkel 22:04, 27 March 2006 (UTC)
Polyurethane foam
[edit]Is standard Polyurethane foam considered either an expanded or unexpanded plastic?--64.222.9.2
- But seriously, "foam" means it's filled with tiny gas bubbles, so it's expanded, right? —Keenan Pepper 22:23, 27 March 2006 (UTC)
- Seems "expanded plastic" refers to closed-cell materials (as opposed open-cell materials, e.g. sponges). Apparently, polyurethane comes in both types, so I'm not sure what "standard" polyurethane would be. If it's important that it has one or the other property, I would ask the manufacturer of the foam in question. --BluePlatypus 01:06, 28 March 2006 (UTC)
- See if it's got air bubbles. If it has, the polyurethane was expanded to when the air was put in. _ 131.211.210.15 12:47, 28 March 2006 (UTC)
- Polyurethane foam is expanded. Any foam is an expanded polymer. Other forms of polyurethane (Not foams) would be unexpanded. Any dense (no air bubbles) polymer is unexpanded. Chapuisat (not signed in)12.106.14.201 20:17, 29 March 2006 (UTC)
- Hah! I was right! —Keenan Pepper 06:07, 30 March 2006 (UTC)
sodium usnate/usnic acid!?
[edit]I am doing a chemistry investigation involving usnic acid and I have produced the sodium salt. Waht I want to know is the structural formula of Sodium Usnate as there are 2 hydroxyl groups on the usnic acid, and sodium usnate is a monosodium salt, so which OH does the Na+ attach to? thanks!
- The two hydroxyl groups are both phenols which are in similar chemical environments. They are likely to be very similar in acidity. Therefore each will be approximately equally deprotonated and the sodium cation will associate with one oxygen in half the molecules and with the other oxygen in the other half of the molecules. --Ed (Edgar181) 22:14, 27 March 2006 (UTC)
- After thinking about it a moment, I'm changing my answer. The pKa of a phenol is about 10, but the pKa of a CH group adjacent to three carbonyl groups has a pKa that is lower (~5). So the most acidic hydrogen is the one in the non-aromatic ring that has three adjacent ketones. The resulting anion is going to be distributed between the three carbonyl oxygens and the carbon between them, and the sodium cation will associate with that distrubted negative charge. --Ed (Edgar181) 22:23, 27 March 2006 (UTC)
- I'm looking at this structure of usnic acid and I can't see what you're talking about. There are three carbonyl groups, but they're not all near each other. It would sure help if we had an article on this stuff... —Keenan Pepper 22:54, 27 March 2006 (UTC)
- That structure is drawn as an enol tautomer of the one I was looking at. It's probably a more accurate depiction, too. In either case though, the anion produced upon deprotonation will have the same distribution as I described above. --Ed (Edgar181) 00:30, 28 March 2006 (UTC)
- I'm looking at this structure of usnic acid and I can't see what you're talking about. There are three carbonyl groups, but they're not all near each other. It would sure help if we had an article on this stuff... —Keenan Pepper 22:54, 27 March 2006 (UTC)
- Okay, I see now. Darn those keto-enol tautomers! —Keenan Pepper 04:34, 28 March 2006 (UTC)
Animating a bunch of pictures drawn in gnuplot
[edit]I have a bunch of test cases which print pictures out in Gnuplot. Is there a way to put all the postcript files together to animate them? --HappyCamper 22:29, 27 March 2006 (UTC)
- I've never heard of animated PostScript.
- [Deleted two of my own rambling, unhelpful answers; see history if curious.]
- See if you can get gnuplot to output each frame as a GIF, then use gifsicle to assemble them into an animated GIF. Or, if your copy of gnuplot doesn't output GIF any more, you could output PNG, then use NetPBM to convert from PNG (via PPM) to GIF. Or see "MPEG to Animated GIF" above for other ideas; in particular, ImageMagick can do this sort of thing, too. —Steve Summit (talk) 23:38, 27 March 2006 (UTC)
- While we're at it, is there a way to make a little *.plt file so that I can draw different pictures in different windows? I was able to get something to "animate" by simply drawing different pictures over and over the same one in the same window. I'm interested have a new window pop up whenever I draw a new frame in my animation. Is this possible to do? --HappyCamper 04:42, 28 March 2006 (UTC)
No Internet On Fedora Core 4/Proliant 3000
[edit]Fedora Core 4 recognises my network interface card, but plugging the ethernet of my modem into my RJ45 does not give internet?
Here are the clues;
On start-up: Determining IP information for eth0 = FAILED (everything else = OK)
However when the ethernet cable is plugged in, the three lights on the network card light up, with one of them flashing. But when I try to access the internet with Firefox, I get nothing.
Network controller: Compaq Computer Corporation Netelligent 10/100 TX PCI UTP (rev 10) --Username132 (talk) 23:33, 27 March 2006 (UTC)
- Are you trying to use DHCP to get the network configuration, and if so, do you have a DHCP server somewhere on the network? --Serie 00:02, 28 March 2006 (UTC)
- No, I didn't know what DHCP was. There is no network - just a modem and a computer. :/ --Username132 (talk)
- DHCP is something that the modem might do, to set up the networking on your computer. If it doesn't do it, you will need an IP address. The cable provider will need to tell you which of these methods is needed – or if you can't figure it out you could let us know the provider. By the way, have you previously had a different computer connected to the same modem? Some providers will block you from connecting a second one. Notinasnaid 13:03, 28 March 2006 (UTC)
- How rude! My provider is NTL and I've used the modem with two different computers sucessfully, but this is a third machine. If NTL limits to two computers, how about if I connect one computer to the internet and use my space NIC to connect said computer to the Proliant? --Username132 (talk) 20:00, 28 March 2006 (UTC)
- So connect just two computers to the modem, and use one of them to provide connectivity for the third. And you're lucky if this is the worst experience you've had with ntl. Ojw 19:50, 29 March 2006 (UTC)
- Are you sure this is due to limits imposed by NTL? How do I find out if the company has such a policy? --Username132 (talk) 20:40, 29 March 2006 (UTC)
- Uhm, this sounds like a PPPoE connection to the modem. Is the point to point connection up and running? EricR 21:26, 29 March 2006 (UTC)
Relegating WMP To Tasktray
[edit]Can I force Windows Media Player into the task tray whilst it plays? It gets in the way whilst I'm flipping between programs whilst working. --Username132 (talk) 23:45, 27 March 2006 (UTC)
- You can enable the Windows Media Player toolbar (right-click taskbar --> Toolbars --> Windows Media Player) and WMP will minimize to there. (I make the taskbar multiple rows and autohide it to make room for everything.) You can also use The Wonderful Icon to put it in the tray. --AySz88^-^ 05:51, 28 March 2006 (UTC)






























![{\displaystyle n=[n_{1},n_{2},n_{3}]^{T}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5ebc15d46326604b0e8783291bd4b93b6dd9845e)

