Estradiol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Numerous |
| Other names | Estra-1,3,5(10)-triene-3,17β-diol |
| AHFS/Drugs.com | Monograph |
| Routes of administration | Oral, sublingual, topical, transdermal, vaginal, intramuscular (as an ester), subcutaneous (as an ester) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: <5%[2] |
| Protein binding | ~98%:[2][3] • Albumin: 60% • SHBG: 38% • Free: 2% |
| Metabolism | Liver (via hydroxylation, sulfation, glucuronidation) |
| Metabolites | Major (90%):[2] • Estrone • Estrone sulfate • Estrone glucuronide • Estradiol glucuronide |
| Elimination half-life | Oral: 13–20 hours[2] Sublingual: 8–18 hours[4] Topical (gel): 36.5 hours[5] |
| Excretion | Urine: 54%[2] Feces: 6%[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.022 |
| Chemical and physical data | |
| Formula | C18H24O2 |
| Molar mass | 272.38 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
This article needs additional citations for verification. (February 2013) |
Estradiol (abbreviated as E2), or 17β-estradiol, also known as estra-1,3,5(10)-triene-3,17β-diol, is a steroid and estrogen sex hormone, and the primary female sex hormone. It is named for and is important in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is essential for the development and maintenance of female reproductive tissues[6] but it also has important effects in many other tissues including bone. While estrogen levels in men are lower compared to women, estrogens have essential functions in men as well. Estradiol is found in most vertebrates as well as many crustaceans, insects, fish, and other animal species.[7][8]
Estradiol or œstradiol (American or British English usages), derives from estra-, Gk. οἶστρος (oistros, literally meaning "verve or inspiration")[9] and -diol, a chemical name and suffix indicating that this form of steroid and sex hormone is a type of alcohol bearing two hydroxyl groups.
Estradiol is produced especially within the follicles of the female ovaries, but also in other endocrine (i.e., hormone-producing) and non-endocrine tissues (e.g., including fat, liver, adrenal, breast, and neural tissues). Estradiol is biosynthesized from progesterone (arrived at in two steps from cholesterol, via intermediate pregnenolone).[10] One principal pathway then converts progesterone to its 17α-hydroxy derivative, 17α-hydroxyprogesterone, and then to 4-androstenedione via sequential cytochrome P450-catalyzed oxidations.[citation needed] Action of aromatase on 4-androstenedione generates estrone, and action of a dehydrogenase on this gives the title compound, 17β-estradiol.[citation needed] Alternatively, 4-androstenedione can be converted into the androgen, testosterone, which in turn can be converted directly into 17β-estradiol.
Effects
Sexual development
The development of secondary sex characteristics in women is driven by estrogens, to be specific, estradiol. These changes are initiated at the time of puberty, most are enhanced during the reproductive years, and become less pronounced with declining estradiol support after the menopause. Thus, estradiol produces breast development, and is responsible for changes in the body shape, affecting bones, joints, and fat deposition. Fat structure and skin composition are modified by estradiol.[citation needed]
Female reproduction
In the female, estradiol acts as a growth hormone for tissue of the reproductive organs, supporting the lining of the vagina, the cervical glands, the endometrium, and the lining of the fallopian tubes. It enhances growth of the myometrium. Estradiol appears necessary to maintain oocytes in the ovary. During the menstrual cycle, estradiol produced by the growing follicle triggers, via a positive feedback system, the hypothalamic-pituitary events that lead to the luteinizing hormone surge, inducing ovulation. In the luteal phase, estradiol, in conjunction with progesterone, prepares the endometrium for implantation. During pregnancy, estradiol increases due to placental production. The effect of estradiol, together with estrone and estriol, in pregnancy is less clear. They may promote uterine blood flow, myometrial growth, stimulate breast growth and at term, promote cervical softening and expression of myometrial oxytocin receptors.[citation needed] In baboons, blocking of estrogen production leads to pregnancy loss, suggesting estradiol has a role in the maintenance of pregnancy. Research is investigating the role of estrogens in the process of initiation of labor. Actions of estradiol are required before the exposure of progesterone in the luteal phase.[citation needed]
Male reproduction
The effect of estradiol (and estrogens in general) upon male reproduction is complex. Estradiol is produced by action of aromatase mainly in the Leydig cells of the mammalian testis, but also by some germ cells and the Sertoli cells of immature mammals.[11] It functions (in vitro) to prevent apoptosis of male sperm cells.[12] While some studies in the early 1990s claimed a connection between globally declining sperm counts and estrogen exposure in the environment,[13] later studies found no such connection, nor evidence of a general decline in sperm counts.[14][15] Suppression of estradiol production in a subpopulation of subfertile men may improve the semen analysis.[16]
Males with certain sex chromosome genetic conditions, such as Klinefelter's syndrome, will have a higher level of estradiol.[17]
Bone
Estradiol has a profound effect on bone. Individuals without it (or other estrogens) will become tall and eunuchoid, as epiphyseal closure is delayed or may not take place. Bone structure is affected also, resulting in early osteopenia and osteoporosis.[18] Also, women past menopause experience an accelerated loss of bone mass due to a relative estrogen deficiency.[19]
Brain
Estrogens can be produced in the brain from steroid precursors. As antioxidants, they have been found to have neuroprotective function.[20]
The positive and negative feedback loops of the menstrual cycle involve ovarian estradiol as the link to the hypothalamic-pituitary system to regulate gonadotropins.[21] (See Hypothalamic–pituitary–gonadal axis.)
Estrogen is considered to play a significant role in women’s mental health, with links suggested between the hormone level, mood and well-being. Sudden drops or fluctuations in, or long periods of sustained low levels of estrogen may be correlated with significant mood-lowering. Clinical recovery from depression postpartum, perimenopause, and postmenopause was shown to be effective after levels of estrogen were stabilized and/or restored.[22][23]
Recently, the volumes of sexually dimorphic brain structures in transgender women were found to change and approximate typical female brain structures when exposed to estradiol over a period of months,[24] suggesting that estradiol has a significant part to play in sex differentiation of the brain, both prenatally and throughout life.
There is also evidence the programming of adult male sexual behavior in many vertebrates is largely dependent on estradiol produced during prenatal life and early infancy.[25] It is not yet known whether this process plays a significant role in human sexual behavior, although evidence from other mammals tends to indicate a connection.[26]
Gynecological cancers
Estradiol has been tied to the development and progression of cancers such as breast cancer, ovarian cancer and endometrial cancer. Estradiol affects target tissues mainly by interacting with two nuclear receptors called estrogen receptor α (ERα) and estrogen receptor β (ERβ).[27][28] One of the functions of these estrogen receptors is the modulation of gene expression. Once estradiol binds to the ERs, the receptor complexes then bind to specific DNA sequences, possibly causing damage to the DNA and an increase in cell division and DNA replication. Eukaryotic cells respond to damaged DNA by stimulating or impairing G1, S, or G2 phases of the cell cycle to initiate DNA repair. As a result, cellular transformation and cancer cell proliferation occurs.[29]
Other effects
Estradiol has complex effects on the liver. In high amounts, it can lead to cholestasis. It affects the production of multiple proteins, including lipoproteins, binding proteins, and proteins responsible for blood clotting.[citation needed]
Estrogen affects certain blood vessels. Improvement in arterial blood flow has been demonstrated in coronary arteries.[30]
Several benign gynecologic conditions are dependent on estrogen, such as endometriosis, leiomyomata uteri, and uterine bleeding.[citation needed]
Medical uses
Hormone replacement therapy
Menopause
If severe side effects of low levels of estradiol in a woman's blood are experienced (commonly at the beginning of menopause or after oophorectomy), hormone replacement therapy (HRT) may be prescribed. Such therapy is usually combined with a progestin to reduce the risk of endometrial cancer.[31]
Hypogonadism
Estradiol is used as an agent of stimulating female puberty induction, as in the treatment of delayed puberty caused by hypogonadism (e.g., in Turner syndrome).
Transgender care
Estrogen therapy is also used as part of the hormone replacement therapy for trans women. Oral, transdermal, implanted, or injectible (subdermal and intramuscular) estradiol is used in higher concentrations during initial treatment and transition; estradiol is continued in lower doses to maintain female-level hormones following sex reassignment surgery.[32]
Hormonal contraception
Estradiol is used, in combination with a progestogen and often in ester form, in estradiol-containing oral contraceptives and combined injectable contraceptives. However, a more potent chemical derivative of estradiol, ethinyl estradiol, is by far the most commonly used estrogen in hormonal contraceptives.[33] Combined forms of hormonal contraception contain estrogen and a progestin, which both contribute to the inhibition of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), which accounts for the ability of these birth control methods to prevent ovulation and thus prevent pregnancy. Other types of hormonal birth control contain only a progestin and no estrogen.[34]
Hormone-sensitive cancers
Estrogen therapy is used to treat advanced prostate cancer and breast cancer, though mostly in the past and with more potent estrogens than estradiol like ethinyl estradiol and diethylstilbestrol.[35][36] Estradiol undecylate has been used in high dosages for the treatment of prostate cancer, however.
Other uses
Estrogen therapy may be used in treatment of infertility in women when there is a need to develop sperm-friendly cervical mucus or an appropriate uterine lining.[citation needed] This is often prescribed in combination with clomifene.[citation needed]
Estrogens have been used to limit final height in adolescent girls with tall stature.[citation needed]
Estrogen deprivation
Inducing a state of hypoestrogenism may be beneficial in certain situations where estrogens are contributing to unwanted effects, e.g., certain forms of breast cancer, gynecomastia, premature closure of epiphyses, and inhibiting feminization in hormone replacement therapy for transgender men. Estrogen levels can be reduced by inhibiting production using GnRH analogues or blocking the enzyme aromatase using an aromatase inhibitor, such as anastrozole, or with an estrogen receptor antagonist, such as tamoxifen or fulvestrant.
Medical formulations
Estrogen is marketed in a number of ways to address issues of hypoestrogenism. Thus, there are oral, transdermal, topical, injectable, and vaginal preparations. Furthermore, the estradiol molecule may be linked to an alkyl group at the C17 (sometimes also at C3) position to facilitate the administration. Such modifications give rise to forms such as estradiol acetate (oral and vaginal applications) and to estradiol cypionate (injectable), which behave as prodrugs of estradiol.
Oral preparations are not necessarily predictably absorbed, and are subject to a first pass through the liver, where they can be metabolized, and also initiate unwanted side effects. Therefore, alternative routes of administration that bypass the liver before primary target organs are hit have been developed. Transdermal and transvaginal routes are not subject to the initial liver passage.
Ethinyl estradiol, the most common estrogen ingredient in combined oral contraceptive pills, is a more profound alteration of the estradiol structure.
Not all products are available worldwide. Estradiol is also part of conjugated estrogen preparations, such as Premarin, though it is present only in small quantities in a conjugated form (sodium estradiol sulfate) and is not the major ingredient. (Premarin consists of a large number of estrogen derivatives. As its name alludes to, Premarin is isolated from the urine of pregnant mares.)[37]
List of formulations
- Gel: Estrogel, Estrasorb, Estraderm, Rontagel
- Oral versions: estradiol (Estrace), estradiol hemihydrate (Estrofem), estradiol acetate (Femtrace), estradiol valerate (Progynova)
- Transdermal patches: Alora, Climara, Minivelle, Vivelle-Dot, Menostar, Estraderm
- Topical Spray: EvaMist, Lenzetto
- Ointments: Divigel, Estrasorb Topical, Elestrin
- Injection: estradiol cypionate, estradiol valerate, estradiol enanthate, estradiol benzoate
- Vaginal ointment: Estrace Vaginal Cream
- Vaginal ring: Estring (estradiol), Femring (estradiol acetate)
- Vaginal tablet: Vagifem (estradiol hemihydrate)
- Estradiol combined with a progestin: CombiPatch (transdermal), Activella (oral), AngeliQ (oral)
Estradiol hemihydrate
Estradiol hemihydrate (INN) (brand names Climara, Estraderm, Estralis, Estrasorb, Estreva, Estring, Estrofem, Estrogel, Vagifem, many others), or œstradiol hemihydrate, is the hemihydrate form of estradiol.[38] In terms of activity and bioequivalence, estradiol and its hemihydrate are identical, with the only disparities being an approximate 1% difference in potency by weight (due to the presence of water molecules in the hemihydrate form of the substance) and a slower rate of release with certain formulations of the hemihydrate.[39][40] This is because estradiol hemihydrate is more hydrated than anhydrous estradiol, and for that reason, is highly insoluble in water in comparison, which results in slower absorption rates with specific formulations such as Vagifem, a vaginal tablet form of the drug.[40] Estradiol hemihydrate has also been shown to result in less systemic absorption as a vaginal tablet formulation relative to other topical estradiol formulations such as vaginal creams.[41]
Contraindications
Estradiol should be avoided when there is undiagnosed abnormal genital bleeding, known, suspected or a history of breast cancer, current treatment for metastatic disease, known or suspected estrogen-dependent neoplasia, deep vein thrombosis, pulmonary embolism or history of these conditions, active or recent arterial thromboembolic disease such as stroke, myocardial infarction, liver dysfunction or disease. Estradiol should not be taken by people with a hypersensitivity/allergy or those who are pregnant or are suspected pregnant.[42]
Adverse effects
Adverse effects, which may occur as a result of use of estradiol and have been associated with estrogen and/or progestin therapy, include changes in vaginal bleeding, dysmenorrhea, increase in size of uterine leiomyomata, vaginitis including vaginal candidiasis, changes in cervical secretion and cervical ectropion, ovarian cancer, endometrial hyperplasia, endometrial cancer, nipple discharge, galactorrhea, fibrocystic breast changes and breast cancer. Cardiovascular effects include chest pain, deep and superficial venous thrombosis, pulmonary embolism, thrombophlebitis, myocardial infarction, stroke, and increased blood pressure. Gastrointestinal effects include nausea and vomiting, abdominal cramps, bloating, diarrhea, dyspepsia, dysuria, gastritis, cholestatic jaundice, increased incidence of gallbladder disease, pancreatitis, or enlargement of hepatic hemangiomas. Skin adverse effects include chloasma or melasma that may continue despite discontinuation of the drug. Other effects on the skin include erythema multiforme, erythema nodosum, otitis media, hemorrhagic eruption, loss of scalp hair, pruritus, or rash. Adverse effects on the eyes include retinal vascular thrombosis, steepening of corneal curvature or intolerance to contact lenses. Adverse central nervous system effects may include headache, migraine, dizziness, chorea, nervousness/anxiety, mood disturbances, irritability, and worsening of epilepsy. Other adverse effects may include changes in weight, reduced carbohydrate tolerance, worsening of porphyria, edema, arthralgias, bronchitis, leg cramps, hemorrhoids, changes in libido, urticaria, angioedema, anaphylactic reactions, syncope, toothache, tooth disorder, urinary incontinence, hypocalcemia, exacerbation of asthma, and increased triglycerides.[42][43]
Side effects of exogenous estradiol in women include breast tenderness, breast enlargement, headache, fluid retention, and nausea.[2]
Estrogen combined with medroxyprogesterone acetate is associated with an increased risk of dementia. It is not known whether estradiol taken alone is associated with an increased risk of dementia. Estrogens should only be used for the shortest possible time and at the lowest effective dose due to these risks. Attempts to gradually reduce the medication via a dose taper should be made every three to six months.[42]
Interactions
St. John's wort, phenobarbital, carbamazepine and rifampicin decrease the levels of estrogens, such as estradiol, by speeding up their metabolism, whereas erythromycin, cimetidine, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may slow down metabolism, leading to increased levels in the blood plasma.[42]
Mechanism of action
Estradiol acts primarily as an agonist of the estrogen receptor (ER), a nuclear steroid hormone receptor. There are two subtypes of the ER, ERα and ERβ, and estradiol potently binds to and activates both of these receptors. The result of ER activation is a modulation of gene transcription and expression in ER-expressing cells, which is the predominant mechanism by which estradiol mediates its biological effects in the body. Estradiol also acts as an agonist of membrane estrogen receptors (mERs), such as GPER (GPR30), a recently discovered non-nuclear receptor for estradiol, via which it can mediate a variety of rapid, non-genomic effects.[44] Unlike the case of the ER, GPER appears to be selective for estradiol, and shows very low affinities for other endogenous estrogens, such as estrone and estriol.[45] Additional mERs besides GPER include ER-X, ERx, and Gq-mER.[46][47]
In the E2 classical pathway or estrogen classical pathway, estradiol enters the cytoplasm, where it causes dissociation of heat-shock protein (HSP). Estradiol then binds to HSP and can homodimerise (form structures of two HSP and two estradiol molecules) and then bind to specific domains on the nucleus (estrogen response element, ERE), allowing for gene transcription which can take place over hours and days.
Estradiol is about 10 times as potent as estrone and about 80 times as potent as estriol in its estrogenic effect.[citation needed]
Pharmacokinetics
| Estrone:Estradiol Ratio[48] | |
|---|---|
| Premenopause | 1:2 |
| Postmenopause | 2:1 |
| Oral estradiol | 5:1 |
| Oral estrone sulfate | 5:1 |
| Transdermal estradiol (patch) | 1:1 |
| Transdermal estradiol (gel) | 1:1 |
| Intranasal estradiol | 2:1 |
| Sublingual estradiol | 1:3 |
| Vaginal estradiol | 1:5 |
| Subcutaneous estradiol (implant) | 1:1.5 |
| Intramuscular estradiol | 1:2 |
Different routes of administration of estradiol produce different effects in the body due to differences in the amount of estradiol that is exposed to the liver as well as different levels of estradiol produced.[49]
Estradiol is plasma protein binding loosely to albumin and tightly to sex hormone-binding globulin (SHBG), with approximately 97 to 98% of estradiol bound to plasma proteins.[50] In the circulation, approximately 38% of estradiol is bound to SHBG and 60% is bound to albumin, with 2–3% free.[51]
Oral administration
Absorption
The oral bioavailability of estradiol is very poor and the hormone must be micronized to be bioavailable to any significant extent.[50][52] This is because estradiol is extensively metabolized during first-pass metabolism in the intestines and liver, and micronization increases the rate of absorption and improves the metabolic stability of estradiol.[51] As micronization is required for significant bioavailability, all oral estradiol tablets are micronized.[52] Estradiol is rapidly and completely absorbed upon oral administration.[51] This is true for oral doses of 2 mg and 4 mg, but absorption was found to be incomplete for a dose of 8 mg.[51] Although micronized estradiol is well-absorbed orally and has greatly improved bioavailability relative to non-micronized estradiol, its bioavailability is still nonetheless very low due to the extensive first-pass metabolism.[50][52] Around 95% of oral estradiol is metabolized prior to entering systemic circulation,[51] and in accordance, the absolute bioavailability of oral micronized estradiol is between 0.1 to 12%.[50]
A dosage of 1 mg/day oral micronized estradiol results in circulating concentrations of 30–50 pg/mL estradiol and 150–300 pg/mL estrone, while a dosage of 2 mg/day produces circulating levels of 50–180 pg/mL estradiol and 300–850 pg/mL estrone.[53]
Metabolism
When taken orally, most estradiol is converted into estrone and estrone sulfate in the liver during first-pass metabolism.[41][54] Oral estradiol administration produces a plasma estradiol-to-estrone ratio of about 1:5 to 1:7.[55]
About 15% of orally administered estradiol is converted into estrone and 65% is converted into estrone sulfate.[56] About 5% of estrone and 1.4% of estrone can be converted back into estradiol.[56] An additional 21% of estrone sulfate can be converted into estrone, whereas the conversion of estrone to estrone sulfate is approximately 54%.[56] This reversible interconversion between estradiol and estrone is mediated by 17β-hydroxysteroid dehydrogenase,[56] whereas the interconversion between estrone and estrone sulfate is mediated by estrogen sulfotransferase and steroid sulfatase.[57][58]
The terminal half-life of orally administered estradiol is a composite parameter that is dependent on interconversion between estrone and sulfate and glucuronide conjugates and enterohepatic recirculation.[56] It has a range of 13 to 20 hours.[56]
Pharmacodynamics
Estrone is approximately 10-fold less potent relative to estradiol as an estrogen,[59] and also has a different binding profile relative to estradiol, for instance, lacking affinity to the GPER.[45] In addition, the resulting supraphysiological levels of estrogen in the liver (4- to 5-fold higher relative to the circulation)[60] increase the risk of blood clots,[61] suppress growth hormone (GH)-mediated insulin-like growth factor 1 (IGF-1) production,[62][63] and increase levels of a variety of binding proteins including thyroid binding globulin (TBG), cortisol binding globulin (CBG), sex hormone binding globulin (SHBG), growth hormone binding protein (GHBP),[64] insulin-like growth factor-binding proteins (IGFBPs),[65] and copper binding protein (CBP),[41][66] but also produce positive blood lipid changes.[54][67]
Sublingual administration
Micronized estradiol tablets can be taken sublingually instead of orally.[68] All estradiol tablets are micronized, as estradiol cannot be absorbed efficiently otherwise.[52] Sublingual ingestion bypasses first-pass metabolism in the intestines and liver.[69] It has been found to result in levels of estradiol and an estradiol-to-estrone ratio that are substantially higher in comparison to oral ingestion.[70] Circulating levels of estradiol are as much as 10-fold higher with sublingual administration relative to oral administration and the absolute bioavailability of estradiol is approximately 5-fold higher.[48] On the other hand, levels of estradiol fall rapidly with sublingual administration, whereas they remain elevated for an extended period of time with oral administration.[48][56] This is responsible for the discrepancy between the maximal estradiol levels achieved and the absolute bioavailability.[48][56]
The rapid and steep fall in estradiol levels with sublingual administration is analogous to the case of intravenous administration of the hormone, in which there is a rapid distribution phase of 6 minutes and terminal disposition phase of only 1 to 2 hours.[48][56][71] In contrast to intravenous and sublingual administration, the terminal half-life of estradiol is 13 to 20 hours with oral administration.[48][56] The difference is due to the fact that, upon oral administration, a large hormonally inert pool of estrogen sulfate and glucuronide conjugates with extended terminal half-lives is reversibly formed from estradiol during first-pass metabolism, and this pool serves as a metabolically resistant and long-lasting circulating reservoir for slow reconversion back into estradiol.[48][56]
Upon sublingual ingestion, a single 0.25 mg tablet of micronized estradiol has been found to produce peak levels of 300 pg/mL estradiol and 60 pg/mL estrone within 1 hour.[48] A higher dose of 1 mg estradiol was found to result in maximum levels of 450 pg/mL estradiol and 165 pg/mL estrone.[48] This was followed by a rapid decline in estradiol levels to 85 pg/mL within 3 hours, whereas the decline in estrone levels was much slower and reached a level of 80 pg/mL after 18 hours.[48]
Although sublingual administration of estradiol has a relatively short duration, the drug can be administered multiple times per day in divided doses to compensate for this.[48] In addition, it is notable that the magnitude of the genomic effects of estradiol (i.e., signaling through the nuclear ERs) appears to be dependent solely on the total exposure as opposed to the duration of exposure.[48] For instance, in both normal human epithelial breast cells and ER-positive breast cancer cells, the rate of breast cell proliferation has been found not to differ with estradiol incubation of 1 nM for 24 hours and incubation of 24 nM for 1 hour.[48] In other words, short-term high concentrations and long-term low concentrations of estradiol appear to have the same degree of effect in terms of genomic estrogenic signaling.[48]
On the other hand, non-genomic actions of estradiol, such as signaling through membrane estrogen receptors like the GPER, may be reduced with short-term high concentrations of estradiol relative to more sustained levels.[48] For instance, although daily intranasal administration of estradiol (which, similarly to sublingual administration, produces extremely high peak levels of estradiol followed by a rapid fall in estradiol levels) is associated in postmenopausal women with comparable clinical effectiveness (e.g., for hot flashes) relative to longer acting routes of estradiol administration, it is also associated with significantly lower rates of breast tension (tenderness and enlargement) relative to longer acting estradiol routes, and this is thought to reflect comparatively diminished non-genomic signaling.[48]
Transdermal administration
Transdermal estradiol is available in the forms of topical gels, patches, sprays, and emulsions.
Estradiol patches delivering a daily dosage of 0.05 mg (50 µg) achieve estradiol and estrone levels of 30–65 pg/mL and 40–45 pg/mL, respectively, while a daily dosage of 0.1 mg (100 µg) attains respective levels of 50–90 pg/mL and 30–65 pg/mL of estradiol and estrone.[53]
Once daily administration of 1.25 g gel containing 0.75 mg estradiol (brand name EstroGel) for 2 weeks was found to produce mean peak estradiol and estrone levels of 46.4 pg/mL and 64.2 pg/mL, respectively.[72] The time-averaged levels of circulating estradiol and estrone with this formulation over the 24-hour dose interval were 28.3 pg/mL and 48.6 pg/mL, respectively.[72] Levels of estradiol and estrone are stable and change relatively little over the course of the 24 hours following an administration, indicating a long duration of action.[72] Steady-state levels of estradiol are achieved after three daily administrations.[72] A higher dosage of estradiol gel containing 1.5 mg estradiol per daily administration has been found to produce estradiol levels of 40–100 pg/mL and estrone levels of 90 pg/mL, while 3 mg/daily has been found to result in respective estradiol and estrone levels of 60–140 pg/mL and 45–155 pg/mL.[53]
Transdermal estradiol bypasses the liver and hence first-pass metabolism in a similar but more complete manner relative to sublingual administration. Estradiol patches have been found not to increase the risk of blood clots[61] and to not affect hepatic IGF-1, SHBG, GHBP,[64] IGFBP,[65] or other protein production.[62][63][66] Transdermal administration of estradiol via patch or gel results in a ratio of about 1:1.[56]
Vaginal administration
Vaginal micronized estradiol achieves a far higher estradiol-to-estrone ratio in comparison, with a daily dosage of 0.5 mg resulting in estradiol and estrone levels of 250 pg/mL and 130 pg/mL, respectively.[53] Vaginal micronized estradiol has been found to bypass the liver and first-pass metabolism similarly to transdermal estradiol and in accordance does not affect hepatic protein production at menopausal replacement dosages.[73]
Intramuscular administration
Estradiol, in an ester prodrug form such as estradiol cypionate or estradiol valerate, can be administered via intramuscular depot injection.[74][75] In marked contrast to the oral route, the bioavailability of both estradiol and estradiol esters like estradiol valerate is complete (i.e., 100%) via intramuscular administration.[71]
A single 4 mg intramusuclar (i.m.) injection of estradiol cypionate or estradiol valerate results in maximal plasma levels of estradiol of about 250 pg/mL and 390 pg/mL, respectively, with levels declining to 100 pg/mL (which was baseline for estradiol cypionate) by 12–14 days.[76][77] A single 2.5 mg intramuscular injection of estradiol benzoate in individuals being treated with a GnRH analogue (and hence having minimal baseline levels of estrogen) was found to result in serum estradiol levels of >400 pg/mL at 24 hours post-administration.[74] The differences in the serum levels of estradiol achieved with these different estradiol esters may be explained by their different rates of absorption, as their durations and levels attained appear to be inversely proportional. For instance, estradiol benzoate, which has the shortest duration (4–5 days with a single i.m. dose of 5 mg), produces the highest levels of estradiol, while estradiol cypionate, which has the longest duration (~11 days with a single i.m. dose of 5 mg), produces the lowest levels of estradiol).[74] Estradiol valerate was found to have a duration of 7–8 days after a single i.m. dose of 5 mg.[74]
A study of combined high-dose i.m. estradiol valerate and hydroxyprogesterone caproate in peri- and postmenopausal and hypogonadal women (described as a "pseudopregnancy" regimen), with specific dosages of 40 mg weekly and 250 mg weekly, respectively, was found to result in serum estradiol levels of 3028–3226 pg/mL after three months and 2491–2552 pg/mL after six months of treatment from a baseline of 27.8–34.8 pg/mL.[78]
Subcutaneous administration
Subcutaneous and intramuscular injections of estradiol cypionate have been found to show almost identical estradiol levels produced and pharmacokinetics (e.g., duration).[75] However, subcutaneous injections may be easier and less painful to perform, and hence, may result in improved patient satisfaction.[75]
Intravenous administration
The terminal half-life of estradiol administered via intravenous injection is 2 hours in men and 50 minutes in women.[71]
Normal estradiol levels
For comparison, normal menstrual cycle serum levels of estradiol in premenopausal women are 40 pg/mL in the early follicular phase to 250 pg/mL at the middle of the cycle and 100 pg/mL during the mid-luteal phase.[53] Serum estrone levels during the menstrual cycle range from 40 to 170 pg/mL, which parallels the serum levels of estradiol.[53] Mean levels of estradiol in premenopausal women are between 80 and 150 pg/mL according to different sources.[79][80][76] The estradiol-to-estrone ratio in premenopausal women is higher than 1:1.[53] In postmenopausal women, the serum levels of estradiol are below 15 pg/ml and the average levels of estrone are about 30 pg/ml; the estradiol-to-estrone ratio is reversed to less than 1:1.[53]
Biochemistry
Biosynthesis


This section needs additional citations for verification. (June 2014) |
Estradiol, like other steroids, is derived from cholesterol. After side chain cleavage and using the Δ5 or the Δ4- pathway, Δ4-androstenedione is the key intermediary. A portion of the Δ4-androstenedione is converted to testosterone, which in turn undergoes conversion to estradiol by aromatase. In an alternative pathway, Δ4-androstenedione is aromatized to estrone, which is subsequently converted to estradiol.[81]
During the reproductive years, most estradiol in women is produced by the granulosa cells of the ovaries by the aromatization of Δ4-androstenedione (produced in the theca folliculi cells) to estrone, followed by conversion of estrone to estradiol by 17β-hydroxysteroid dehydrogenase. Smaller amounts of estradiol are also produced by the adrenal cortex, and, in men, by the testes.[citation needed]
Estradiol is not produced in the gonads only, in particular, fat cells produce active precursors to estradiol, and will continue to do so even after menopause.[31] Estradiol is also produced in the brain and in arterial walls.
The biosynthesis of estradiol has been observed in various other species, as indicated above, but also in such species as Phaseolus vulgaris.[relevant?][82] More often referred to as "beans", consumption may equate to unintentional ingestion of estradiol. In light of this, consumption can be counterproductive to patients undergoing treatment for breast cancer, which usually includes depriving the cancer cells of estrogens. Soybeans are another bean that contains chemicals that act similarly to estrogen in the human body and also cause such interactions.[citation needed]
Metabolism

In plasma, estradiol is largely bound to SHBG, and also to albumin. Only a fraction of 2.21% (± 0.04%) is free and biologically active, the percentage remaining constant throughout the menstrual cycle.[83] Deactivation includes conversion to less-active estrogens, such as estrone and estriol. Estriol is the major urinary metabolite. Estradiol is conjugated in the liver by sulfate and glucuronide formation and, as such, excreted via the kidneys. Some of the water-soluble conjugates are excreted via the bile duct, and partly reabsorbed after hydrolysis from the intestinal tract. This enterohepatic circulation contributes to maintaining estradiol levels.
In the liver, estradiol is non-specifically metabolized by CYP1A2, CYP3A4, and CYP2C9 via 2-hydroxylation into 2-hydroxyestradiol, and by CYP2C9, CYP2C19, and CYP2C8 via 17β-hydroxy dehydrogenation into estrone,[84] with various other cytochrome P450 (CYP) enzymes and metabolic transformations also being involved.[85] As a result, cimetidine, a known, non-selective inhibitor of CYP450 enzymes, can increase the levels of exogenous, orally-ingested estradiol.[84]
2-Hydroxylation
Addition of a hydroxyl group at C-2 represents the major hepatic pathway for estradiol metabolism, as mediated by CYP1A2, CYP2C8, CYP2C9 and CYP3A4. Extrahepatic 2-hydroxylation is chiefly mediated by CYP1A1 and CYP3A4.
2-hydroxyestradiol (2-OHE2) can experience three metabolic fates: methylation to yield 2-meOHE2, oxidation to form quinones, or dehydrogenation to yield 2-OHE1.
2-OHE2 can bind to estrogen receptors but with markedly lower affinity. This metabolite has several physiological consequences: the ability to influence intracellular signalling, adenohypophyseal hormone secretion, radical and quinone formation, and inhibition of tumor formation. Weak carcinogenic activity has been shown, likely due to radical formation and induction of single-strand DNA breaks.[86]
Inactivation of 2-OHE2 is catalysed by catechol-O-methyltransferase (COMT),[87] with COMT exhibiting a faster rate for the methylation of 2-OHE2 versus 4-OH-E2. COMT, a blood-borne enzyme, mediates the most common form of 2- or 4-hydroxyestradiol inactivation, in addition to glucuronidation and sulfation. However, this inactivation can allow for the accumulation of 4-OHE2, as 2-OHE2 inhibits 4-OHE2 methylation by COMT, but 4-OHE2 does not inhibit 2-OH-E2 methylation in return.
Antitumor activity of 2-meOE2 is thought to be mediated by antiproliferative and antimetastatic effects. Inhibition of cellular proliferation and metastasis appears to be via induction of caspase-8, followed by caspase-3 and eventually DNA fragmentation. Induction of apoptosis by 2-meOE2 may be p53 dependent or independent. 2-meOE2 has also been found to inhibit aromatase activity, thereby lowering the in situ synthesis of E2 in cancer tissue.[88] 2-meOE2 has a higher binding affinity for sex hormone-binding globulin (SHBG) than E2 and 2-OH-E2 and has no affinity for the estrogen receptor.
2-meOE2 is also a potent inhibitor of angiogenesis in tumor tissues. Administration of this estradiol metabolite prevents vascular smooth muscle growth. This inhibition of angiogenesis is eliminated by co-administration with cytochrome P450 and COMT inhibitors, thereby confirming the involvement of cytochrome P450 enzymes in the blockade of tumor blood supply. Further antitumor activity of 2-meOE2 has been identified through immunomodulation. The cytokines IL-6 and TNFα, as well the prostaglandin PGE2, are capable of stimulating aromatase activity. Since macrophages and lymphocytes are present in breast tissue, this provides a concerning means of upregulating in situ estradiol biosynthesis. 2-meOE2 appeared to be able to halve the basal aromatase activity in mammary fibroblasts, possibly through destabilisation of the microtubules that mediate translocation of the cytokine receptors to the plasma membrane. Inhibition of cytokine receptor synthesis and blockade of the autocrine and paracrine actions of cytokines and PGE2 were also observed.[89]
4-Hydroxylation

The enzyme most responsible for estradiol 4-hydroxylation is CYP1B1. In humans, CYP1B1 mRNA and protein exhibit constitutive expression in the lung and kidney, as well as estrogen-regulated tissues such as breast, ovary and uterus. Whereas 4-hydroxylation constitutes the minor pathway in the liver, the greater proportion of CYP1B1 expression in extrahepatic tissues shifts the balance in favor of 4-OH-E2 formation. 4-OH-E2 is thought to be the most carcinogenic of all the estradiol metabolites, especially considering that CYP1B1 exhibits overexpression in breast cancer tumors.
4-OH-E2, like 2-OH-E2, can be physiologically active as well as tumorigenic. 4-OH-E2 is capable of binding ER with a reduced dissociation rate and prolonged activation, thereby inducing cellular growth and proliferation,[90] adenohypophyseal hormone secretion, and prostaglandin production.
Das et al.[91] implicated 4-OH-E2 in the induction of estrogen-responsive genes, a response that exhibited partial or no abrogation by coadministration with an antiestrogen, providing evidence for the ability of 4-OH-E2 to carry out genetic upregulation via a pathway independent of ER signalling. Effects independent of ER binding include breakage of single-stranded DNA, especially when interacting synergistically with nitric oxide in human breast cancer cells and the production of quinones and free radicals.
CYP1B1 can be induced by E2.[92] ERα, after binding to estradiol, interacts with the CYP1B1 ERE to stimulate CYP1B1 expression. Thus, although E2 causes genetic changes conducive to its own inactivation, the decrease in estrogenic activity yields a toxicologically active metabolite that constitutes an additional pathway of estradiol-dependent carcinogenesis.
4-OH-E2 shares the metabolic scheme of 2-OH-E2: methylation to 4-methoxyestradiol (4-meOE2), oxidation to quinones, or dehydrogenation to 4-OH-E1. Conjugation by the ubiquitously present COMT represents the most common extrahepatic pathway of 4-OH-E2 inactivation. However, if estrogen homeostasis is imbalanced by an increase in CYP1B1 and a decrease in COMT, a greater degree of genotoxic quinone formation from 4-OH-E2 will occur.[93] 4-OHE2 can be oxidized by microsomal CYPs or peroxidases to yield estradiol-3,4-semiquinone.[94] This semiquinone can undergo redox cycling with oxygen to form estradiol-3,4-quinone (E2-3,4-Q) and superoxide. E2-3,4-Q can be converted back to 4-OHE2 in a single step by quinone reductase, or in two sequential steps catalysed by P450 reductase via the semiquinone intermediate. GSH / S-transferase activity can abrogate E2-3,4-Q levels via formation of glutathione conjugates.
E2-3,4-Q is a potent nucleophile, and will readily react with electrophilic DNA. This yields the formation of the DNA adducts 4-OHE2-1-N7Gua and 4-OHE2-1-N3Ade via a Michael addition. Destabilization of the glycosyl bond between the nitrogenous base and ribose sugar creates apurinic sites as the unstable adducts are lost from DNA. 4-OHE2-1-N7Gua has a relatively slow depurination half-life of approx. 3 hours, allowing enough time for base excision repair mechanisms to correct the change. However, 4-OHE2-1-N3Ade exhibits instantaneous depurination, leading to error-prone repair and the induction of mutations. Indeed, E2-3,4-Q has been shown to cause A-to-G mutations in the gene coding for H¬-ras, ras being vital to the correct regulation of the cellular response to growth factors. Though 2- and 4-OHE2 have similar redox potentials and thus similar redox cycling activity, the greater carcinogenic capacity of 4-OHE2 can be attributed to its increased reactivity with DNA. Another harmful effect of estrogen redox cycling is the production of superoxide and hydroxyl radicals. P450 reductase catalysis produces superoxide radicals, which can, in the presence of superoxide dismutase and Fe3+, form highly reactive hydroxyl radicals capable of damaging virtually all macromolecules.
16α-Hydroxylation
Through the action of CYP1A1, CYP1A2, CYP2C8, and the CYP3A isoforms, 16α-hydroxyestradiol (16α-OHE2), also known as estriol, is produced in abundance during pregnancy. 16α-OHE2 can be dehydrogenated to 16α-hydroxyestrone (16α-OHE1), a metabolite that has been shown to bind covalently to the estrogen receptor via Schiff base formation(25).[95] This covalent linkage occurs between the steroid carbonyl and the ε-amino group of lysine. In theory, 16α-OHE1 could also bind DNA, although this has not been observed. 16α-OHE2 is a potent ER agonist, capable of levels of cellular proliferation stimulation that near those obtained with E2.[96] Though studies in hamster kidney tumor models showed weak carcinogenicity, the carcinogenic potential of 16α-OHE2 in humans remains unknown.
Other hydroxylations
The function of the remainder of the hydroxylated E2 metabolites (6α-, 6β-, 7α-, 12β-, 15α-, 15β-, and 16β-OHE2) remain to be elucidated. Some of these metabolites, such as 15α-OHE2, are excreted in relatively large amounts in pregnant women, possibly serving as an indicator of good fetal health.
Levels
During the reproductive years of the human female, its serum levels are somewhat higher than that of estrone, except during the early follicular phase of the menstrual cycle; thus, estradiol may be considered the predominant estrogen during human female reproductive years in terms of absolute serum levels and estrogenic activity.[citation needed] During pregnancy, estriol becomes the predominant circulating estrogen, and this is the only time at which estetrol occurs in the body, while during menopause, estrone predominates (both based on serum levels).[citation needed] The estradiol produced by male humans, from testosterone, is present at serum levels roughly comparable to those of postmenopausal women (14-55 versus <35 pg/mL, respectively).[citation needed] It has also been reported that if concentrations of estradiol in a 70-year-old man are compared to those of a 70-year-old woman, levels are approximately 2- to 4-fold higher in the man.[97]
Measurement
In women, serum estradiol is measured in a clinical laboratory and reflects primarily the activity of the ovaries. As such, they are useful in the detection of baseline estrogen in women with amenorrhea or menstrual dysfunction, and to detect the state of hypoestrogenicity and menopause. Furthermore, estrogen monitoring during fertility therapy assesses follicular growth and is useful in monitoring the treatment. Estrogen-producing tumors will demonstrate persistent high levels of estradiol and other estrogens. In precocious puberty, estradiol levels are inappropriately increased.
Ranges
Individual laboratory results should always been interpreted using the ranges provided by the laboratory that performed the test.
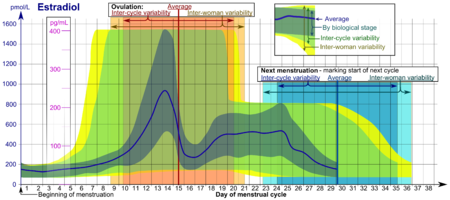
- The ranges denoted By biological stage may be used in closely monitored menstrual cycles in regard to other markers of its biological progression, with the time scale being compressed or stretched to how much faster or slower, respectively, the cycle progresses compared to an average cycle.
- The ranges denoted Inter-cycle variability are more appropriate to use in unmonitored cycles with only the beginning of menstruation known, but where the woman accurately knows her average cycle lengths and time of ovulation, and that they are somewhat averagely regular, with the time scale being compressed or stretched to how much a woman's average cycle length is shorter or longer, respectively, than the average of the population.
- The ranges denoted Inter-woman variability are more appropriate to use when the average cycle lengths and time of ovulation are unknown, but only the beginning of menstruation is given.
| Reference ranges for serum estradiol | |||
|---|---|---|---|
| Patient type | Lower limit | Upper limit | Unit |
| Adult male | 50[98] | 200[98] | pmol/L |
| 14 | 55 | pg/mL | |
| Adult female (follicular phase, day 5) |
70[98] 95% PI (standard) |
500[98] 95% PI |
pmol/L |
| 110[99] 90% PI (used in diagram) |
220[99] 90% PI | ||
| 19 (95% PI) | 140 (95% PI) | pg/mL | |
| 30 (90% PI) | 60 (90% PI) | ||
| Adult female (preovulatory peak) |
400[98] | 1500[98] | pmol/L |
| 110 | 410 | pg/mL | |
| Adult female (luteal phase) |
70[98] | 600[98] | pmol/L |
| 19 | 160 | pg/mL | |
| Adult female - free (not protein bound) |
0.5[100][original research?] | 9[100][original research?] | pg/mL |
| 1.7[100][original research?] | 33[100][original research?] | pmol/L | |
| Post-menopausal female | N/A[98] | < 130[98] | pmol/L |
| N/A | < 35 | pg/mL | |
In the normal menstrual cycle, estradiol levels measure typically <50 pg/ml at menstruation, rise with follicular development (peak: 200 pg/ml), drop briefly at ovulation, and rise again during the luteal phase for a second peak. At the end of the luteal phase, estradiol levels drop to their menstrual levels unless there is a pregnancy.
During pregnancy, estrogen levels, including estradiol, rise steadily toward term. The source of these estrogens is the placenta, which aromatizes prohormones produced in the fetal adrenal gland.
Chemistry
Estradiol is an estrane (C18) steroid.[48] It is also known as 17β-estradiol or as estra-1,3,5(10)-triene-3,17β-diol. As its common name implies, estradiol, sometimes abbreviated as E2, has two hydroxyl groups in its molecular structure; its relatives estrone (E1) and estriol (E3) have one and three, respectively, and estetrol (E4) has four.[citation needed]
See also
- Estrogen ester
- Phytoestrogen
- Estrogen insensitivity syndrome
- Aromatase deficiency
- Hyperestrogenism
- Aromatase excess syndrome
- Estrogen-dependent condition
- List of steroidal estrogens
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f g Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824.
- ^ Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22–. ISBN 0-323-03309-1.
- ^ Price, T; Blauer, K; Hansen, M; Stanczyk, F; Lobo, R; Bates, G (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17-estradiol". Obstetrics & Gynecology. 89 (3): 340–345. doi:10.1016/S0029-7844(96)00513-3. ISSN 0029-7844.
- ^ Naunton, Mark; Al Hadithy, Asmar F. Y.; Brouwers, Jacobus R. B. J.; Archer, David F. (2006). "Estradiol gel". Menopause. 13 (3): 517–527. doi:10.1097/01.gme.0000191881.52175.8c. ISSN 1072-3714.
- ^ Ryan KJ (August 1982). "Biochemistry of aromatase: significance to female reproductive physiology". Cancer Res. 42 (8 Suppl): 3342s–3344s. PMID 7083198.
- ^ Mechoulam R, Brueggemeier RW, Denlinger DL (September 1984). "Estrogens in insects" (PDF). Cellular and Molecular Life Sciences. 40 (9): 942–944. doi:10.1007/BF01946450.
- ^ Ozon R (1972). "Estrogens in Fishes, Amphibians, Reptiles, and Birds". In Idler DR (ed.). Steroids In Nonmammalian Vertebrates. Oxford: Elsevier Science. pp. 390–414. ISBN 032314098X.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ "Greek Word Study Tool: oistros". Perseus Digital Library. Retrieved 2011-12-28.
- ^ Saldanha, Colin J., Luke Remage-Healey, and Barney A. Schlinger. "Synaptocrine signaling: steroid synthesis and action at the synapse." Endocrine reviews 32.4 (2011): 532-549.
- ^ Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S (2003). "Aromatase expression and role of estrogens in male gonad : a review". Reproductive Biology and Endocrinology. 1: 35. doi:10.1186/1477-7827-1-35. PMC 155680. PMID 12747806.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L (2000). "Estradiol acts as a germ cell survival factor in the human testis in vitro". The Journal of Clinical Endocrinology and Metabolism. 85 (5): 2057–67. doi:10.1210/jcem.85.5.6600. PMID 10843196.
- ^ Sharpe RM, Skakkebaek NE (1993). "Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract?". Lancet. 341 (8857): 1392–5. doi:10.1016/0140-6736(93)90953-E. PMID 8098802.
- ^ Handelsman, DJ (2001). "Estrogens and falling sperm counts". Reproduction, Fertility and Development. 13 (4): 317–24. PMID 11800170.
- ^ Fisch, Harry; Goldstein, Robert (2003). "Environmental estrogens and sperm counts" (PDF). Pure Applied Chemistry. 75 (11–12): 2181–2193. doi:10.1351/pac200375112181.
- ^ Raman JD, Schlegel PN (2002). "Aromatase inhibitors for male infertility". The Journal of Urology. 167 (2 Pt 1): 624–9. doi:10.1016/S0022-5347(01)69099-2. PMID 11792932.
- ^ Visootsak J, Graham JM (2006). "Klinefelter syndrome and other sex chromosomal anueploidies". Orphanet Journal of Rare Diseases. 1 (42): 42. doi:10.1186/1750-1172-1-42. PMC 1634840. PMID 17062147. Retrieved 20 November 2013.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER (1997). "Effect of testosterone and estradiol in a man with aromatase deficiency". The New England Journal of Medicine. 337 (2): 91–5. doi:10.1056/NEJM199707103370204. PMID 9211678.
- ^ Albright, Fuller; Smith Patricia H.; Richardson Anna M. (31 May 1941). "Postmenopausal Osteoporosis: Its Clinical Features". JAMA. 116 (22): 2465–2474. doi:10.1001/jama.1941.02820220007002. Retrieved 20 November 2013.
- ^ Behl C, Widmann M, Trapp T, Holsboer F (November 1995). "17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro". Biochem. Biophys. Res. Commun. 216 (2): 473–82. doi:10.1006/bbrc.1995.2647. PMID 7488136.
- ^ Meethal, S. V.; Liu, T.; Chan, H. W.; Ginsburg, E.; Wilson, A. C.; Gray, D. N.; Bowen, R. L.; Vonderhaar, B. K.; Atwood, C. S. (2009). "Identification of a regulatory loop for the synthesis of neurosteroids: A steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors". Journal of Neurochemistry. 110 (3): 1014–1027. doi:10.1111/j.1471-4159.2009.06192.x. PMC 2789665. PMID 19493163.
- ^ Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK (2005). "Estrogen-related mood disorders: reproductive life cycle factors". Adv Nurs Sci. 28 (4): 364–75. doi:10.1097/00012272-200510000-00008. PMID 16292022.
- ^ Lasiuk GC, Hegadoren KM (October 2007). "The effects of estradiol on central serotonergic systems and its relationship to mood in women". Biol Res Nurs. 9 (2): 147–60. doi:10.1177/1099800407305600. PMID 17909167.
- ^ Hulshoff HE, Cohen-Kettenis PT, Van Haren NE, Peper JS, Brans RG, Cahn W, Schnack HG, Gooren LJ, Kahn RS (July 2006). "Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure". European Journal of Endocrinology. 155 (suppl_1): 107–114. doi:10.1530/eje.1.02248.
- ^ Harding CF (June 2004). "Hormonal Modulation of Singing: Hormonal Modulation of the Songbird Brain and Singing Behavior". Ann. N.Y. Acad. Sci. 1016. The New York Academy of Sciences: 524–539. doi:10.1196/annals.1298.030. PMID 15313793. Retrieved 2007-03-07.
- ^ Simerly RB (2002-03-27). "Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain" (pdf). Annu. Rev. Neurosci. 25: 507–536. doi:10.1146/annurev.neuro.25.112701.142745. PMID 12052919. Retrieved 2007-03-07.
- ^ Bulzomi P, Bolli A, Galluzzo P, Leone S, Acconcia F, Marino M (January 2010). "Naringenin and 17β-estradiol coadministration prevents hormone-induced human cancer cell growth". IUBMD Life. 62 (1): 51–60. doi:10.1002/iub.279. PMID 19960539.
- ^ Sreeja S, Santhosh Kumar TR, Lakshmi BS, Sreeja S (17 March 2011). "Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation". Journal of Nutritional Biochemistry. 23 (7): 725–32. doi:10.1016/j.jnutbio.2011.03.015. PMID 21839626.
- ^ Thomas CG, Strom A, Lindberg K, Gustafsson JA (22 June 2010). "Estrogen receptor beta decreases survival of p53-defective cancer cells after DNA damage by impairing G2/M checkpoint signaling". Breast Cancer Research and Treatment. 127 (2): 417–427. doi:10.1007/s10549-010-1011-z. PMID 20623183.
- ^ Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, McNeill JG, Poole-Wilson PA (1995). "17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease". Circulation. 92 (1): 24–30. doi:10.1161/01.CIR.92.1.24. PMID 7788912.
- ^ a b Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 434, 444. ISBN 3-8047-1763-2.
- ^ http://www.wpath.org/publications_standards.cfm
- ^ Evans G, Sutton EL (May 2015). "Oral contraception". Med Clin North Am. 99 (3): 479–503. doi:10.1016/j.mcna.2015.01.004. PMID 25841596.
- ^ Glasier, Anna (2010). "Contraception". In Jameson, J. Larry; De Groot, Leslie J. (eds.). Endocrinology (6th ed.). Philadelphia: Saunders Elsevier. pp. 2417–2427. ISBN 978-1-4160-5583-9.
- ^ Ockrim JL; Lalani el-N; Kakkar AK; Abel PD (August 2005). "Transdermal estradiol therapy for prostate cancer reduces thrombophilic activation and protects against thromboembolism". J. Urol. 174 (2): 527–33, discussion 532–3. doi:10.1097/01.ju.0000165567.99142.1f. PMID 16006886.
- ^ Carruba G, Pfeffer U, Fecarotta E, Coviello DA, D'Amato E, Lo Castro M, Vidali G, Castagnetta L (March 1994). "Estradiol inhibits growth of hormone-nonresponsive PC3 human prostate cancer cells". Cancer Res. 54 (5): 1190–3. PMID 8118804.
- ^ Drugs.com: Premarin Official FDA information, side effects and uses.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. pp. 406–. ISBN 978-3-88763-075-1. Retrieved 13 September 2012.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research On Cancer (2007). Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. World Health Organization. p. 384. ISBN 978-92-832-1291-1. Retrieved 13 September 2012.
- ^ a b Archana Desai; Mary Lee (7 May 2007). Gibaldi's Drug Delivery Systems in Pharmaceutical Care. ASHP. p. 337. ISBN 978-1-58528-136-7. Retrieved 13 September 2012.
- ^ a b c Rebekah Wang-Cheng; Joan M. Neuner; Vanessa M. Barnabei (2007). Menopause. ACP Press. pp. 91–. ISBN 978-1-930513-83-9.
- ^ a b c d Barr Laboratories, Inc. (March 2008). "ESTRACE TABLETS, (estradiol tablets, USP)" (PDF). wcrx.com. Retrieved 27 January 2010.
- ^ Pfizer (August 2008). "ESTRING (estradiol vaginal ring)" (PDF).
- ^ Prossnitz ER, Barton M (May 2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Mol. Cell. Endocrinol. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ^ a b Prossnitz ER, Arterburn JB, Sklar LA (2007). "GPR30: A G protein-coupled receptor for estrogen". Mol. Cell. Endocrinol. 265–266: 138–42. doi:10.1016/j.mce.2006.12.010. PMC 1847610. PMID 17222505.
- ^ Soltysik K, Czekaj P (April 2013). "Membrane estrogen receptors - is it an alternative way of estrogen action?". J. Physiol. Pharmacol. 64 (2): 129–42. PMID 23756388.
- ^ Micevych PE, Kelly MJ (2012). "Membrane estrogen receptor regulation of hypothalamic function". Neuroendocrinology. 96 (2): 103–10. doi:10.1159/000338400. PMC 3496782. PMID 22538318.
- ^ a b c d e f g h i j k l m n o p q Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ^ na. Newnes. pp. 486–. ISBN 978-0-444-54286-1.
- ^ a b c d O'Connell MB (1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9 Suppl): 18S–24S. PMID 8530713.
- ^ a b c d e Kuhnz, W.; Blode, H.; Zimmermann, H. (1993). "Pharmacokinetics of Exogenous Natural and Synthetic Estrogens and Antiestrogens". 135 / 2: 261–322. doi:10.1007/978-3-642-60107-1_15. ISSN 0171-2004.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d A. Wayne Meikle (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 380–. ISBN 978-1-59259-700-0.
- ^ a b c d e f g h Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 771–. ISBN 978-0-08-055309-2.
- ^ a b John J.B. Anderson; Sanford C. Garner (24 October 1995). Calcium and Phosphorus in Health and Disease. CRC Press. pp. 215–216. ISBN 978-0-8493-7845-4.
- ^ Eef Hogervorst (24 September 2009). Hormones, Cognition and Dementia: State of the Art and Emergent Therapeutic Strategies. Cambridge University Press. pp. 82–. ISBN 978-0-521-89937-6.
- ^ a b c d e f g h i j k l Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 268, 271. ISBN 978-3-642-60107-1.
- ^ Jose Russo; Irma H. Russo (28 June 2011). Molecular Basis of Breast Cancer: Prevention and Treatment. Springer Science & Business Media. pp. 92–. ISBN 978-3-642-18736-0.
- ^ Sue E. Huether; Kathryn L. McCance (27 December 2013). Understanding Pathophysiology. Elsevier Health Sciences. pp. 845–. ISBN 978-0-323-29343-3.
- ^ Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". Journal of Midwifery & Women's Health. 47 (3): 130–8. doi:10.1016/s1526-9523(02)00233-7. PMID 12071379.
- ^ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 753–. ISBN 978-1-4511-4847-3.
- ^ a b Alkjaersig N, Fletcher AP, de Ziegler D, Steingold KA, Meldrum DR, Judd HL (1988). "Blood coagulation in postmenopausal women given estrogen treatment: comparison of transdermal and oral administration". J. Lab. Clin. Med. 111 (2): 224–8. PMID 2448408.
- ^ a b Weissberger AJ, Ho KK, Lazarus L (1991). "Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women". J. Clin. Endocrinol. Metab. 72 (2): 374–81. doi:10.1210/jcem-72-2-374. PMID 1991807.
- ^ a b Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E (2007). "Effects of the route of oestrogen administration on IGF-1 and IGFBP-3 in healthy postmenopausal women: results from a randomized placebo-controlled study". Clin. Endocrinol. (Oxf). 66 (5): 626–31. doi:10.1111/j.1365-2265.2007.02783.x. PMID 17492948.
- ^ a b Nugent AG, Leung KC, Sullivan D, Reutens AT, Ho KK (2003). "Modulation by progestogens of the effects of oestrogen on hepatic endocrine function in postmenopausal women". Clin. Endocrinol. (Oxf). 59 (6): 690–8. doi:10.1046/j.1365-2265.2003.01907.x. PMID 14974909.
- ^ a b Isotton, A. L.; Wender, M. C. O.; Casagrande, A.; Rollin, G.; Czepielewski, M. A. (2011). "Effects of oral and transdermal estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in patients with hypopituitarism during GH treatment: a randomized study" (PDF). European Journal of Endocrinology. 166 (2): 207–213. doi:10.1530/EJE-11-0560. ISSN 0804-4643. PMID 22108915.
- ^ a b Jasonni VM, Bulletti C, Naldi S, Ciotti P, Di Cosmo D, Lazzaretto R, Flamigni C (1988). "Biological and endocrine aspects of transdermal 17 beta-oestradiol administration in post-menopausal women". Maturitas. 10 (4): 263–70. doi:10.1016/0378-5122(88)90062-x. PMID 3226336.
- ^ Dansuk R, Unal O, Karageyim Y, Esim E, Turan C (2004). "Evaluation of the effect of tibolone and transdermal estradiol on triglyceride level in hypertriglyceridemic and normotriglyceridemic postmenopausal women". Gynecol. Endocrinol. 18 (5): 233–9. doi:10.1080/09513590410001715199. PMID 15346658.
- ^ Casper RF, Yen SS (1981). "Rapid absorption of micronized estradiol-17 beta following sublingual administration". Obstet Gynecol. 57 (1): 62–4. PMID 7454177.
- ^ Pines A, Averbuch M, Fisman EZ, Rosano GM (1999). "The acute effects of sublingual 17beta-estradiol on the cardiovascular system". Maturitas. 33 (1): 81–5. doi:10.1016/s0378-5122(99)00036-5. PMID 10585176.
- ^ Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17 beta-estradiol". Obstet Gynecol. 89 (3): 340–5. doi:10.1016/S0029-7844(96)00513-3. PMID 9052581.
- ^ a b c Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. PMID 7169965.
- ^ a b c d "EstroGel® 0.06% (Estradiol Gel) for Topical Use FDA Label" (PDF). Food and Drug Administration. 2014. Retrieved 17 October 2016.
- ^ Hall G, Blombäck M, Landgren BM, Bremme K (2002). "Effects of vaginally administered high estradiol doses on hormonal pharmacokinetics and hemostasis in postmenopausal women". Fertil. Steril. 78 (6): 1172–7. doi:10.1016/s0015-0282(02)04285-1. PMID 12477507.
- ^ a b c d Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
- ^ a b c Sierra-Ramírez JA, Lara-Ricalde R, Lujan M, Velázquez-Ramírez N, Godínez-Victoria M, Hernádez-Munguía IA, Padilla A, Garza-Flores J (2011). "Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg)". Contraception. 84 (6): 565–70. doi:10.1016/j.contraception.2011.03.014. PMID 22078184.
- ^ a b M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28–November 2, 1984. Springer Science & Business Media. pp. 397, 399. ISBN 978-94-009-4145-8.
[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/ml to only 13 pg/ml.
- ^ Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 416–418. ISBN 978-93-5090-575-3.
- ^ Ulrich U, Pfeifer T, Lauritzen C (1994). "Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report". Horm. Metab. Res. 26 (9): 428–31. doi:10.1055/s-2007-1001723. PMID 7835827.
- ^ C. Christian; B. von Schoultz (15 March 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 60–. ISBN 978-1-85070-545-1.
The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/ml.
- ^ Eugenio E. Müller; Robert M. MacLeod (6 December 2012). Neuroendocrine Perspectives. Springer Science & Business Media. pp. 121–. ISBN 978-1-4612-3554-5.
[...] [premenopausal] mean [estradiol] concentration of 150 pg/ml [...]
- ^ Walter F. Boron; Emile L. Boulpaep (2003). Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. p. 1300. ISBN 1-4160-2328-3.
- ^ Young, I. J.; Hillman, J. R.; Knights, B. A. (1978). "Endogenous Estradiol-17 β in Phaseolus vulgaris". Zeitschrift für Pflanzenphysiologie. 90: 45–50. doi:10.1016/S0044-328X(78)80223-2.
- ^ Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (August 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". J. Clin. Endocrinol. Metab. 43 (2): 436–45. doi:10.1210/jcem-43-2-436. PMID 950372.
- ^ a b Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (February 2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacol. Sin. 22 (2): 148–54. PMID 11741520.
- ^ Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT (August 2003). "Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms". Endocrinology. 144 (8): 3382–98. doi:10.1210/en.2003-0192. PMID 12865317.
- ^ Liehr, J. G. (1 February 2000). "Is Estradiol a Genotoxic Mutagenic Carcinogen?". Endocrine Reviews. 21 (1): 40–54. doi:10.1210/EDRV.21.1.0386. PMID 10696569.
- ^ Li, K.-M. (24 October 2003). "Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo". Carcinogenesis. 25 (2): 289–297. doi:10.1093/carcin/bgg191. PMID 14578156.
- ^ Lakhani, NJ; Sarkar, MA; Venitz, J; Figg, WD (February 2003). "2-Methoxyestradiol, a promising anticancer agent". Pharmacotherapy. 23 (2): 165–172. doi:10.1592/phco.23.2.165.32088. PMID 12587805.
- ^ Purohit, A.; Singh, A.; Ghilchik, M.W.; Reed, M.J. (July 1999). "Inhibition of Tumor Necrosis Factor α-Stimulated Aromatase Activity by Microtubule-Stabilizing Agents, Paclitaxel and 2-Methoxyestradiol". Biochemical and Biophysical Research Communications. 261 (1): 214–217. doi:10.1006/bbrc.1999.1010. PMID 10405348.
- ^ Cheng, Z. N.; Shu, Y.; Liu, Z. Q.; Wang, L. S.; Ou-Yang, D. S.; Zhou, H. H. (2001). "Role of cytochrome P450 in estradiol metabolism in vitro" (PDF). Acta pharmacologica Sinica. 22 (2): 148–154. PMID 11741520.
- ^ Das, SK; Taylor, JA; Korach, KS; Paria, BC; Dey, SK; Lubahn, DB (25 November 1997). "Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway". Proceedings of the National Academy of Sciences of the United States of America. 94 (24): 12786–91. doi:10.1073/pnas.94.24.12786. PMC 24216. PMID 9371753.
- ^ Tsuchiya, Y. (1 May 2004). "Human CYP1B1 Is Regulated by Estradiol via Estrogen Receptor". Cancer Research. 64 (9): 3119–3125. doi:10.1158/0008-5472.CAN-04-0166. PMID 15126349.
- ^ Lu, Fang; Zahid, Muhammad; Saeed, Muhammad; Cavalieri, Ercole L.; Rogan, Eleanor G. (June 2007). "Estrogen metabolism and formation of estrogen-DNA adducts in estradiol-treated MCF-10F cells". The Journal of Steroid Biochemistry and Molecular Biology. 105 (1–5): 150–158. doi:10.1016/j.jsbmb.2006.12.102. PMC 1986824. PMID 17582757.
- ^ Liehr, JG; Ulubelen, AA; Strobel, HW (25 December 1986). "Cytochrome P-450-mediated redox cycling of estrogens". The Journal of Biological Chemistry. 261 (36): 16865–70. PMID 3782146.
- ^ Swaneck, GE; Fishman, J (November 1988). "Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization". Proceedings of the National Academy of Sciences of the United States of America. 85 (21): 7831–5. doi:10.1073/pnas.85.21.7831. PMC 282290. PMID 3186693.
- ^ Gupta, Mona; McDougal, Andrew; Safe, Stephen (December 1998). "Estrogenic and antiestrogenic activities of 16α- and 2-hydroxy metabolites of 17β-estradiol in MCF-7 and T47D human breast cancer cells". The Journal of Steroid Biochemistry and Molecular Biology. 67 (5–6): 413–419. doi:10.1016/S0960-0760(98)00135-6. PMID 10030690.
- ^ Sayed Y, Taxel P (2003). "The use of estrogen therapy in men". Curr Opin Pharmacol. 3 (6): 650–4. PMID 14644018.
- ^ a b c d e f g h i j GPNotebook — reference range (oestradiol) Retrieved on September 27, 2009
- ^ a b Values taken from day 1 after LH surge in: Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R (2006). "Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer". Clin. Chem. Lab. Med. 44 (7): 883–7. doi:10.1515/CCLM.2006.160. PMID 16776638. as PDF
- ^ a b c d Total amount multiplied by 0.022 according to 2.2% presented in: Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (August 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". J. Clin. Endocrinol. Metab. 43 (2): 436–45. doi:10.1210/jcem-43-2-436. PMID 950372.[original research?]
External links
- Estradiol MS Spectrum
- Estrogens - Lab Tests Online
