Choline

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxy-N,N,N-trimethylethan-1-aminium | |
| Other names
2-Hydroxy-N,N,N-trimethylethanaminium
Bilineurine (2-Hydroxyethyl)trimethylammonium | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 1736748 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.487 |
| EC Number |
|
| 324597 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H14NO+ | |
| Molar mass | 104.17 g/mol |
| Appearance | viscous deliquescent liquid (choline hydroxide)[1] |
| very soluble (choline hydroxide)[1] | |
| Solubility | soluble in ethanol,[1] insoluble in diethylether and chloroform (choline hydroxide)[2] |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3–6 g/kg bw, rats, oral[1] |
| Safety data sheet (SDS) | 4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Choline /ˈkoʊliːn/[3] is an essential nutrient for humans and many other animals.[4] Choline occurs as a cation that forms various salts (X− in the depicted formula is an undefined counteranion).[5] To maintain health, it must be obtained from the diet as choline or as choline phospholipids, like phosphatidylcholine.[4] Humans, as well as most other animal species, do make choline de novo; however, production is generally insufficient. Choline is often not classified as a vitamin, but as a nutrient with an amino acid–like metabolism.[2] In most animals, choline phospholipids are necessary components in cell membranes, in the membranes of cell organelles, and in very low-density lipoproteins.[4] Choline is required to produce acetylcholine – a neurotransmitter – and S-adenosylmethionine (SAM), a universal methyl donor. Upon methylation SAM is transformed into homocysteine.
Symptomatic choline deficiency – rare in humans – causes nonalcoholic fatty liver disease and muscle damage.[4] Excessive consumption of choline (greater than 7.5 g/day) can cause low blood pressure, sweating, diarrhea and fish-like body odor due to trimethylamine, which forms in its metabolism.[4][6] Rich dietary sources of choline and choline phospholipids include organ meats and egg yolks, dairy products, peanuts, certain beans, nuts, seeds and vegetables with pasta and rice also contributing to choline intake in the American diet.[4][7]
Chemistry
The cholines are a family of water-soluble quaternary ammonium compounds.[5][8] Choline is the parent compound of the cholines class, consisting of ethanolamine having three methyl substituents attached to the amino function.[9] Choline hydroxide is known as choline base. It is hygroscopic and thus often encountered as a colorless viscous hydrated syrup that smells of trimethylamine (TMA). Aqueous solutions of choline are stable, but the compound slowly breaks down to ethylene glycol, polyethylene glycols, and TMA.[1]
Choline chloride can be made by treating TMA with 2-chloroethanol:[1]
- (CH3)3N + ClCH2CH2OH → (CH3)3N+CH2CH2OH · Cl–
The 2-chloroethanol can be generated from ethylene oxide. Choline has historically been produced from natural sources, such as via hydrolysis of lecithin.[1]
Metabolism
Biosynthesis
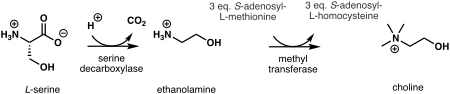
In plants, the first step in de novo biosynthesis of choline is the decarboxylation of serine into ethanolamine, which is catalyzed by a serine decarboxylase.[10] The synthesis of choline from ethanolamine may take place in three parallel pathways, where three consecutive N-methylation steps catalyzed by a methyl transferase are carried out on either the free-base,[11] phospho-bases,[12] or phosphatidyl-bases.[13] The source of the methyl group is S-adenosyl-L-methionine and S-adenosyl-L-homocysteine is generated as a side product.[14]

In humans and most other animals, de novo synthesis of choline is via the phosphatidylethanolamine N-methyltransferase (PEMT) pathway,[6] but biosynthesis is not enough to meet human requirements.[15] In the hepatic PEMT route, 3-phosphoglycerate (3PG) receives 2 acyl groups from acyl-CoA forming a phosphatidic acid. It reacts with cytidine triphosphate to form cytidine diphosphate-diacylglycerol. Its hydroxyl group reacts with serine to form phosphatidylserine which decarboxylates to ethanolamine and phosphatidylethanolamine (PE) forms. A PEMT enzyme moves three methyl groups from three S-adenosyl methionines (SAM) donors to the ethanolamine group of the phosphatidylethanolamine to form choline in the form of a phosphatidylcholine. Three S-adenosylhomocysteines (SAHs) are formed as a byproduct.[6]
Choline can also be released from more complex choline containing molecules. For example, phosphatidylcholines (PC) can be hydrolyzed to choline (Chol) in most cell types. Choline can also be produced by the CDP-choline route, cytosolic choline kinases (CK) phosphorylate choline with ATP to phosphocholine (PChol).[2] This happens in some cell types like liver and kidney. Choline-phosphate cytidylyltransferases (CPCT) transform PChol to CDP-choline (CDP-Chol) with cytidine triphosphate (CTP). CDP-choline and diglyceride are transformed to PC by diacylglycerol cholinephosphotransferase (CPT).[6]
In humans, certain PEMT-enzyme mutations and estrogen deficiency (often due to menopause) increase the dietary need for choline. In rodents, 70% of phosphatidylcholines are formed via the PEMT route and only 30% via the CDP-choline route.[6] In knockout mice, PEMT inactivation makes them completely dependent on dietary choline.[2]
Absorption
In humans, choline is absorbed from the intestines via the SLC44A1 (CTL1) membrane protein via facilitated diffusion governed by the choline concentration gradient and the electrical potential across the enterocyte membranes. SLC44A1 has limited ability to transport choline: at high concentrations part of it is left unabsorbed. Absorbed choline leaves the enterocytes via the portal vein, passes the liver and enters systemic circulation. Gut microbes degrade the unabsorbed choline to trimethylamine, which is oxidized in the liver to trimethylamine N-oxide.[6]
Phosphocholine and glycerophosphocholines are hydrolyzed via phospholipases to choline, which enters the portal vein. Due to their water solubility, some of them escape unchanged to the portal vein. Fat-soluble choline-containing compounds (phosphatidylcholines and sphingomyelins) are either hydrolyzed by phospholipases or enter the lymph incorporated into chylomicrons.[6]
Transport
In humans, choline is transported as a free molecule in blood. Choline–containing phospholipids and other substances, like glycerophosphocholines, are transported in blood lipoproteins. Blood plasma choline levels in healthy fasting adults is 7–20 micromoles per liter (μmol/L) and 10 μmol/L on average. Levels are regulated, but choline intake and deficiency alters these levels. Levels are elevated for about 3 hours after choline consumption. Phosphatidylcholine levels in the plasma of fasting adults is 1.5–2.5 mmol/L. Its consumption elevates the free choline levels for about 8–12 hours, but does not affect phosphatidylcholine levels significantly.[6]
Choline is a water-soluble ion and thus requires transporters to pass through fat-soluble cell membranes. Three types of choline transporters are known:[16]
- SLC5A7
- CTLs: CTL1 (SLC44A1), CTL2 (SLC44A2) and CTL4 (SLC44A4)
- OCTs: OCT1 (SLC22A1) and OCT2 (SLC22A2)
SLC5A7s are sodium- (Na+) and ATP-dependent transporters.[16][6] They have high binding affinity for choline, transport it primarily to neurons and are indirectly associated with the acetylcholine production.[6] Their deficient function causes hereditary weakness in the pulmonary and other muscles in humans via acetylcholine deficiency. In knockout mice, their dysfunction results easily in death with cyanosis and paralysis.[17]
CTL1s have moderate affinity for choline and transport it in almost all tissues, including the intestines, liver, kidneys, placenta and mitochondria. CTL1s supply choline for phosphatidylcholine and trimethylglycine production.[6] CTL2s occur especially in the mitochondria in the tongue, kidneys, muscles and heart. They are associated with the mitochondrial oxidation of choline to trimethylglycine. CTL1s and CTL2s are not associated with the acetylcholine production, but transport choline together via the blood–brain barrier. Only CTL2s occur on the brain side of the barrier. They also remove excess choline from the neurons back to blood. CTL1s occur only on the blood side of the barrier, but also on the membranes of astrocytes and neurons.[16]
OCT1s and OCT2s are not associated with the acetylcholine production.[6] They transport choline with low affinity. OCT1s transport choline primarily in the liver and kidneys; OCT2s in kidneys and the brain.[16]
Storage
Choline is stored in the cell membranes and organelles as phospholipids, and inside cells as phosphatidylcholines and glycerophosphocholines.[6]
Excretion
Even at choline doses of 2–8 g, little choline is excreted into urine in humans. Excretion happens via transporters that occur within kidneys (see transport). Trimethylglycine is demethylated in the liver and kidneys to dimethylglycine (tetrahydrofolate receives one of the methyl groups). Methylglycine forms, is excreted into urine, or is demethylated to glycine.[6]
Function
Choline and its derivatives have many functions in humans and in other organisms. The most notable function is that choline serves as a synthetic precursor for other essential cell components and signalling molecules, such as phospholipids that form cell membranes, the neurotransmitter acetylcholine, and the osmoregulator trimethylglycine (betaine). Trimethylglycine in turn serves as a source of methyl groups by participating in the biosynthesis of S-adenosylmethionine.[18][19]
Phospholipid precursor
Choline is transformed to different phospholipids, like phosphatidylcholines and sphingomyelins. These are found in all cell membranes and the membranes of most cell organelles.[2] Phosphatidylcholines are structurally important part of the cell membranes. In humans 40–50% of their phospholipids are phosphatidylcholines.[6]
Choline phospholipids also form lipid rafts in the cell membranes along with cholesterol. The rafts are centers, for example for receptors and receptor signal transduction enzymes.[2]
Phosphatidylcholines are needed for the synthesis of VLDLs: 70–95% of their phospholipids are phosphatidylcholines in humans.[6]
Choline is also needed for the synthesis of pulmonary surfactant, which is a mixture consisting mostly of phosphatidylcholines. The surfactant is responsible for lung elasticity, that is for lung tissue's ability to contract and expand. For example, deficiency of phosphatidylcholines in the lung tissues has been linked to acute respiratory distress syndrome.[20]
Phosphatidylcholines are excreted into bile and work together with bile acid salts as surfactants in it, thus helping with the intestinal absorption of lipids.[2]
Acetylcholine synthesis
Choline is needed to produce acetylcholine. This is a neurotransmitter which plays a necessary role in muscle contraction, memory and neural development, for example.[6] Nonetheless, there is little acetylcholine in the human body relative to other forms of choline.[2] Neurons also store choline in the form of phospholipids to their cell membranes for the production of acetylcholine.[6]
Source of trimethylglycine
In humans, choline is oxidized irreversibly in liver mitochondria to glycine betaine aldehyde by choline oxidases. This is oxidized by mitochondrial or cytosolic betaine-aldehyde dehydrogenases to trimethylglycine.[6] Trimethylglycine is a necessary osmoregulator. It also works as a substrate for the BHMT-enzyme, which methylates homocysteine to methionine. This is a S-adenosylmethionine (SAM) precursor. SAM is a common reagent in biological methylation reactions. For example, it methylates guanidines of DNA and certain lysines of histones. Thus it is part of gene expression and epigenetic regulation. Choline deficiency thus leads to elevated homocysteine levels and decreased SAM levels in blood.[6]
Content in foods
Choline occurs in foods as a free molecule and in the form of phospholipids, especially as phosphatidylcholines. Choline is highest in organ meats and egg yolks though it is found to a lesser degree in non-organ meats, grains, vegetables, fruit and dairy products. Cooking oils and other food fats have about 5 mg/100 g of total choline.[6] In the United States, food labels express the amount of choline in a serving as a percentage of daily value (%DV) based on the adequate intake of 550 mg/day. 100% of the daily value means that a serving of food has 550 mg of choline.[21] "Total choline" is defined as the sum of free choline and choline-containing phospholipids, without accounting for mass fraction.[22][23][6]
Human breast milk is rich in choline. Exclusive breastfeeding corresponds to about 120 mg of choline per day for the baby. Increase in a mother's choline intake raises the choline content of breast milk and low intake decreases it.[6] Infant formulas may or may not contain enough choline. In the EU and the US, it is mandatory to add at least 7 mg of choline per 100 kilocalories (kcal) to every infant formula. In the EU, levels above 50 mg/100 kcal are not allowed.[6][24]
Trimethylglycine is a functional metabolite of choline. It substitutes for choline nutritionally, but only partially.[2] High amounts of trimethylglycine occur in wheat bran (1,339 mg/100 g), toasted wheat germ (1,240 mg/100 g) and spinach (600–645 mg/100 g), for example.[22]
| Meats | Vegetables | ||
|---|---|---|---|
| Bacon, cooked | 124.89 | Bean, snap | 13.46 |
| Beef, trim-cut, cooked | 78.15 | Beetroot | 6.01 |
| Beef liver, pan fried | 418.22 | Broccoli | 40.06 |
| Chicken, roasted, with skin | 65.83 | Brussels sprout | 40.61 |
| Chicken, roasted, no skin | 78.74 | Cabbage | 15.45 |
| Chicken liver | 290.03 | Carrot | 8.79 |
| Cod, atlantic | 83.63 | Cauliflower | 39.10 |
| Ground beef, 75–85% lean, broiled | 79.32–82.35 | Sweetcorn, yellow | 21.95 |
| Pork loin cooked | 102.76 | Cucumber | 5.95 |
| Shrimp, canned | 70.60 | Lettuce, iceberg | 6.70 |
| Dairy products (cow) | Lettuce, romaine | 9.92 | |
| Butter, salted | 18.77 | Pea | 27.51 |
| Cheese | 16.50–27.21 | Sauerkraut | 10.39 |
| Cottage cheese | 18.42 | Spinach | 22.08 |
| Milk, whole/skimmed | 14.29–16.40 | Sweet potato | 13.11 |
| Sour cream | 20.33 | Tomato | 6.74 |
| Yogurt, plain | 15.20 | Zucchini | 9.36 |
| Grains | Fruits | ||
| Oat bran, raw | 58.57 | Apple | 3.44 |
| Oats, plain | 7.42 | Avocado | 14.18 |
| Rice, white | 2.08 | Banana | 9.76 |
| Rice, brown | 9.22 | Blueberry | 6.04 |
| Wheat bran | 74.39 | Cantaloupe | 7.58 |
| Wheat germ, toasted | 152.08 | Grape | 7.53 |
| Others | Grapefruit | 5.63 | |
| Bean, navy | 26.93 | Orange | 8.38 |
| Egg, hen | 251.00 | Peach | 6.10 |
| Olive oil | 0.29 | Pear | 5.11 |
| Peanut | 52.47 | Prune | 9.66 |
| Soybean, raw | 115.87 | Strawberry | 5.65 |
| Tofu, soft | 27.37 | Watermelon | 4.07 |
- ^ Foods are raw unless noted otherwise. Contents are "total choline" as defined above.
Daily values
This section may require cleanup to meet Wikipedia's quality standards. The specific problem is: Should be merged to above list. The overlaps are quite large to the extent that the values (when converted to 100g) are virtually identical. DV calculation is quite trivial, so this isn't adding anything useful for now. (September 2022) |
The following table contains updated sources of choline to reflect the new Daily Value and the new Nutrition Facts and Supplement Facts Labels.[21] It reflects data from the U.S. Department of Agriculture, Agricultural Research Service. FoodData Central, 2019.[21]
| Food | Milligrams (mg) per serving | Percent DV* |
| Beef liver, pan fried, 3 oz (85 g) | 356 | 65 |
| Egg, hard boiled, 1 large egg | 147 | 27 |
| Beef top round, separable lean only, braised, 3 oz (85 g) | 117 | 21 |
| Soybeans, roasted, 1⁄2 cup | 107 | 19 |
| Chicken breast, roasted, 3 oz (85 g) | 72 | 13 |
| Beef, ground, 93% lean meat, broiled, 3 oz (85 g) | 72 | 13 |
| Cod, Atlantic, cooked, dry heat, 3 oz (85 g) | 71 | 13 |
| Mushrooms, shiitake, cooked, 1⁄2 cup pieces | 58 | 11 |
| Potatoes, red, baked, flesh and skin, 1 large potato | 57 | 10 |
| Wheat germ, toasted, 1 oz (28 g) | 51 | 9 |
| Beans, kidney, canned, 1⁄2 cup | 45 | 8 |
| Quinoa, cooked, 1 cup | 43 | 8 |
| Milk, 1% fat, 1 cup | 43 | 8 |
| Yogurt, vanilla, nonfat, 1 cup | 38 | 7 |
| Brussels sprouts, boiled, 1⁄2 cup | 32 | 6 |
| Broccoli, chopped, boiled, drained, 1⁄2 cup | 31 | 6 |
| Cottage cheese, nonfat, 1 cup | 26 | 5 |
| Tuna, white, canned in water, drained in solids, 3 oz (85 g) | 25 | 5 |
| Peanuts, dry roasted, 1⁄4 cup | 24 | 4 |
| Cauliflower, 1 in (2.5 cm) pieces, boiled, drained, 1⁄2 cup | 24 | 4 |
| Peas, green, boiled, 1⁄2 cup | 24 | 4 |
| Sunflower seeds, oil roasted, 1⁄4 cup | 19 | 3 |
| Rice, brown, long-grain, cooked, 1 cup | 19 | 3 |
| Bread, pita, whole wheat, 1 large (6+1⁄2 in or 17 cm diameter) | 17 | 3 |
| Cabbage, boiled, 1⁄2 cup | 15 | 3 |
| Tangerine (mandarin orange), sections, 1⁄2 cup | 10 | 2 |
| Beans, snap, raw, 1⁄2 cup | 8 | 1 |
| Kiwifruit, raw, 1⁄2 cup sliced | 7 | 1 |
| Carrots, raw, chopped, 1⁄2 cup | 6 | 1 |
| Apples, raw, with skin, quartered or chopped, 1⁄2 cup | 2 | 0 |
DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for choline is 550 mg for adults and children age 4 years and older.[citation needed] The FDA does not require food labels to list choline content unless choline has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.[21]
The U.S. Department of Agriculture's (USDA's) FoodData Central lists the nutrient content of many foods and provides a comprehensive list of foods containing choline arranged by nutrient content.[21]
Dietary recommendations
Recommendations are in milligrams per day (mg/day). The European Food Safety Authority (EFSA) recommendations are general recommendations for the EU countries. The EFSA has not set any upper limits for intake.[6] Individual EU countries may have more specific recommendations. The National Academy of Medicine (NAM) recommendations apply in the United States,[21] Australia and New Zealand.[25]
| Age | EFSA adequate intake[6] | US NAM adequate intake[21] | US NAM tolerable upper intake levels[21] |
|---|---|---|---|
| Infants and children | |||
| 0–6 months | Not established | 125 | Not established |
| 7–12 months | 160 | 150 | Not established |
| 1–3 years | 140 | 200 | 1,000 |
| 4–6 years | 170 | 250 | 1,000 |
| 7–8 years | 250 | 250 | 1,000 |
| 9–10 years | 250 | 375 | 1,000 |
| 11–13 years | 340 | 375 | 2,000 |
| Males | |||
| 14 years | 340 | 550 | 3,000 |
| 15–18 years | 400 | 550 | 3,000 |
| 19+ years | 400 | 550 | 3,500 |
| Females | |||
| 14 years | 340 | 400 | 3,000 |
| 15–18 years | 400 | 400 | 3,000 |
| 19+ y | 400 | 425 | 3,500 |
| If pregnant | 480 | 450 | 3,500 (3,000 if ≤18 y) |
| If breastfeeding | 520 | 550 | 3,500 (3,000 if ≤18 y) |
Intake in populations
Twelve surveys undertaken in 9 EU countries between 2000 and 2011 estimated choline intake of adults in these countries to be 269–468 milligrams per day. Intake was 269–444 mg/day in adult women and 332–468 mg/day in adult men. Intake was 75–127 mg/day in infants, 151–210 mg/day in 1- to 3-year-olds, 177–304 mg/day in 3- to 10-year-olds and 244–373 mg/day in 10- to 18-year-olds. The total choline intake mean estimate was 336 mg/day in pregnant adolescents and 356 mg/day in pregnant women.[6]
A study based on the NHANES 2009–2012 survey estimated the choline intake to be too low in some US subpopulations. Intake was 315.2–318.8 mg/d in 2+ year olds between this time period. Out of 2+ year olds, only 15.6±0.8% of males and 6.1±0.6% of females exceeded the adequate intake (AI). AI was exceeded by 62.9±3.1% of 2- to 3-year-olds, 45.4±1.6% of 4- to 8-year-olds, 9.0±1.0% of 9- to 13-year-olds, 1.8±0.4% of 14–18 and 6.6±0.5% of 19+ year olds. Upper intake level was not exceeded in any subpopulations.[26]
A 2013–2014 NHANES study of the US population found the choline intake of 2- to 19-year-olds to be 256±3.8 mg/day and 339±3.9 mg/day in adults 20 and over. Intake was 402±6.1 mg/d in men 20 and over and 278 mg/d in women 20 and over.[27]
Deficiency
Signs and symptoms
Symptomatic choline deficiency is rare in humans. Most obtain sufficient amounts of it from the diet and are able to biosynthesize limited amounts of it.[2] Symptomatic deficiency is often caused by certain diseases or by other indirect causes. Severe deficiency causes muscle damage and non-alcoholic fatty liver disease, which may develop into cirrhosis.[28]
Besides humans, fatty liver is also a typical sign of choline deficiency in other animals. Bleeding in the kidneys can also occur in some species. This is suspected to be due to deficiency of choline derived trimethylglycine, which functions as an osmoregulator.[2]
Causes and mechanisms
Estrogen production is a relevant factor which predisposes individuals to deficiency along with low dietary choline intake. Estrogens activate phosphatidylcholine producing PEMT enzymes. Women before menopause have lower dietary need for choline than men due to women's higher estrogen production. Without estrogen therapy, the choline needs of post-menopausal women are similar to men's. Some single-nucleotide polymorphisms (genetic factors) affecting choline and folate metabolism are also relevant. Certain gut microbes also degrade choline more efficiently than others, so they are also relevant.[28]
In deficiency, availability of phosphatidylcholines in the liver are decreased – these are needed for formation of VLDLs. Thus VLDL-mediated fatty acid transport out of the liver decreases leading to fat accumulation in the liver.[6] Other simultaneously occurring mechanisms explaining the observed liver damage have also been suggested. For example, choline phospholipids are also needed in mitochondrial membranes. Their inavailability leads to the inability of mitochondrial membranes to maintain proper electrochemical gradient, which, among other things, is needed for degrading fatty acids via β-oxidation. Fat metabolism within liver therefore decreases.[28]
Excess intake
Excessive doses of choline can have adverse effects. Daily 8–20 g doses of choline, for example, have been found to cause low blood pressure, nausea, diarrhea and fish-like body odor. The odor is due to trimethylamine (TMA) formed by the gut microbes from the unabsorbed choline (see trimethylaminuria).[6]
The liver oxidizes TMA to trimethylamine N-oxide (TMAO). Elevated levels of TMA and TMAO in the body have been linked to increased risk of atherosclerosis and mortality. Thus, excessive choline intake has been hypothetized to increase these risks in addition to carnitine, which also is formed into TMA and TMAO by gut bacteria. However, choline intake has not been shown to increase the risk of dying from cardiovascular diseases.[29] It is plausible that elevated TMA and TMAO levels are just a symptom of other underlying illnesses or genetic factors that predispose individuals for increased mortality. Such factors may have not been properly accounted for in certain studies observing TMA and TMAO level related mortality. Causality may be reverse or confounding and large choline intake might not increase mortality in humans. For example, kidney dysfunction predisposes for cardiovascular diseases, but can also decrease TMA and TMAO excretion.[30]
Health effects
Neural tube closure
Some human studies showed low maternal intake of choline to significantly increase the risk of neural tube defects (NTDs) in newborns.[4] Folate deficiency also causes NTDs. Choline and folate, interacting with vitamin B12, act as methyl donors to homocysteine to form methionine, which can then go on to form SAM (S-adenosylmethionine).[4] SAM is the substrate for almost all methylation reactions in mammals. It has been suggested that disturbed methylation via SAM could be responsible for the relation between folate and NTDs.[31] This may also apply to choline.[citation needed] Certain mutations that disturb choline metabolism increase the prevalence of NTDs in newborns, but the role of dietary choline deficiency remains unclear, as of 2015.[update][4]
Cardiovascular diseases and cancer
Choline deficiency can cause fatty liver, which increases cancer and cardiovascular disease risk. Choline deficiency also decreases SAM production, which partakes in DNA methylation – this decrease may also contribute to carcinogenesis. Thus, deficiency and its association with such diseases has been studied.[6] However, observational studies of free populations have not convincingly shown an association between low choline intake and cardiovascular diseases or most cancers.[4][6] Studies on prostate cancer have been contradictory.[32][33]
Cognition
Studies observing the effect between higher choline intake and cognition have been conducted in human adults, with contradictory results.[4][34] Similar studies on human infants and children have been contradictory and also limited.[4]
Perinatal development
This section needs additional citations for verification. (December 2016) |
Both pregnancy and lactation increase demand for choline dramatically. This demand may be met by upregulation of PEMT via increasing estrogen levels to produce more choline de novo, but even with increased PEMT activity, the demand for choline is still so high that bodily stores are generally depleted. This is exemplified by the observation that Pemt −/− mice (mice lacking functional PEMT) will abort at 9–10 days unless fed supplemental choline.[35]
While maternal stores of choline are depleted during pregnancy and lactation, the placenta accumulates choline by pumping choline against the concentration gradient into the tissue, where it is then stored in various forms, mostly as acetylcholine. Choline concentrations in amniotic fluid can be ten times higher than in maternal blood.[35]
Functions in the fetus
Choline is in high demand during pregnancy as a substrate for building cellular membranes (rapid fetal and mother tissue expansion), increased need for one-carbon moieties (a substrate for methylation of DNA and other functions), raising choline stores in fetal and placental tissues, and for increased production of lipoproteins (proteins containing "fat" portions).[36][37][38] In particular, there is interest in the impact of choline consumption on the brain. This stems from choline's use as a material for making cellular membranes (particularly in making phosphatidylcholine). Human brain growth is most rapid during the third trimester of pregnancy and continues to be rapid to approximately five years of age.[39] During this time, the demand is high for sphingomyelin, which is made from phosphatidylcholine (and thus from choline), because this material is used to myelinate (insulate) nerve fibers.[40] Choline is also in demand for the production of the neurotransmitter acetylcholine, which can influence the structure and organization of brain regions, neurogenesis, myelination, and synapse formation. Acetylcholine is even present in the placenta and may help control cell proliferation and differentiation (increases in cell number and changes of multiuse cells into dedicated cellular functions) and parturition.[41][42]
Choline uptake into the brain is controlled by a low-affinity transporter located at the blood–brain barrier.[43] Transport occurs when arterial plasma choline concentrations increase above 14 μmol/L, which can occur during a spike in choline concentration after consuming choline-rich foods. Neurons, conversely, acquire choline by both high- and low-affinity transporters. Choline is stored as membrane-bound phosphatidylcholine, which can then be used for acetylcholine neurotransmitter synthesis later. Acetylcholine is formed as needed, travels across the synapse, and transmits the signal to the following neuron. Afterwards, acetylcholinesterase degrades it, and the free choline is taken up by a high-affinity transporter into the neuron again.[44]
Uses
Choline chloride and choline bitartrate are used in dietary supplements. Bitartrate is used more often due to its lower hygroscopicity.[2] Certain choline salts are used to supplement chicken, turkey and some other animal feeds. Some salts are also used as industrial chemicals: for example, in photolithography to remove photoresist.[1] Choline theophyllinate and choline salicylate are used as medicines,[1][45] as well as structural analogs, like methacholine and carbachol.[46] Radiolabeled cholines, like 11C-choline, are used in medical imaging.[47] Other commercially used salts include tricholine citrate and choline bicarbonate.[1]
Antagonists and inhibitors
Hundreds of choline antagonists and enzyme inhibitors have been developed for research purposes. Aminomethylpropanol is among the first ones used as a research tool. It inhibits choline and trimethylglycine synthesis. It is able to induce choline deficiency that in turn results in fatty liver in rodents. Diethanolamine is another such compound, but also an environmental pollutant. N-cyclohexylcholine inhibits choline uptake primarily in brains. Hemicholinium-3 is a more general inhibitor, but also moderately inhibits choline kinases. More specific choline kinase inhibitors have also been developed. Trimethylglycine synthesis inhibitors also exist: carboxybutylhomocysteine is an example of a specific BHMT inhibitor.[2]
The cholinergic hypothesis of dementia has not only lead to medicinal acetylcholinesterase inhibitors, but also to a variety of acetylcholine inhibitors. Examples of such inhibiting research chemicals include triethylcholine, homocholine and many other N-ethyl derivates of choline, which are false neurotransmitter analogs of acetylcholine. Choline acetyltransferase inhibitors have also been developed.[2]
History
Discovery
In 1849, Adolph Strecker was the first to isolate choline from pig bile.[48][49] In 1852, L. Babo and M. Hirschbrunn extracted choline from white mustard seeds and named it sinkaline.[49] In 1862, Strecker repeated his experiment with pig and ox bile, calling the substance choline for the first time after the Greek word for bile, chole, and identifying it with the chemical formula C5H13NO.[50][15] In 1850, Theodore Nicolas Gobley extracted from the brains and roe of carps a substance he named lecithin after the Greek word for egg yolk, lekithos, showing in 1874 that it was a mixture of phosphatidylcholines.[51][52]
In 1865, Oscar Liebreich isolated "neurine" from animal brains.[53][15] The structural formulas of acetylcholine and Liebreich's "neurine" were resolved by Adolf von Baeyer in 1867.[54][49] Later that year "neurine" and sinkaline were shown to be the same substances as Strecker's choline. Thus, Bayer was the first to resolve the structure of choline.[55][56][49] The compound now known as neurine is unrelated to choline.[15]
Discovery as a nutrient
In the early 1930s, Charles Best and colleagues noted that fatty liver in rats on a special diet and diabetic dogs could be prevented by feeding them lecithin,[15] proving in 1932 that choline in lecithin was solely responsible for this preventive effect.[57] In 1998, the US National Academy of Medicine reported their first recommendations for choline in the human diet.[58]
References
- ^ a b c d e f g h i j Kirk RE, et al. (2000). Kirk-Othmer encyclopedia of chemical technology. Vol. 6 (4th ed.). John Wiley & Sons. pp. 100–102. ISBN 9780471484943.
- ^ a b c d e f g h i j k l m n Rucker RB, Zempleni J, Suttie JW, McCormick DB (2007). Handbook of vitamins (4th ed.). Taylor & Francis. pp. 459–477. ISBN 9780849340222.
- ^ "Choline". Lexico Dictionaries. Archived from the original on 24 October 2019. Retrieved 9 November 2019.
- ^ a b c d e f g h i j k l "Choline". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. February 2015. Retrieved 11 November 2019.
- ^ a b Choline. The Metabolomics Innovation Centre, University of Alberta, Edmonton, Canada. 17 August 2016. Retrieved 13 September 2016.
{{cite encyclopedia}}:|website=ignored (help) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae "Dietary reference values for choline". EFSA Journal. 14 (8). 2016. doi:10.2903/j.efsa.2016.4484.
In this Opinion, the Panel considers dietary choline including choline compounds (e.g. glycerophosphocholine, phosphocholine, phosphatidylcholine, sphingomyelin).
- ^ "Office of Dietary Supplements - Choline".
- ^ Britannica, The Editors of Encyclopaedia. "choline". Encyclopedia Britannica, 11 Dec. 2013, https://www.britannica.com/science/choline. Accessed 17 February 2022.
- ^ National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 305, Choline. Retrieved February 17, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/Choline.
- ^ Rontein D, Nishida I, Tashiro G, Yoshioka K, Wu WI, Voelker DR, Basset G, Hanson AD (September 2001). "Plants synthesize ethanolamine by direct decarboxylation of serine using a pyridoxal phosphate enzyme". The Journal of Biological Chemistry. 276 (38): 35523–9. doi:10.1074/jbc.M106038200. PMID 11461929.
- ^ Prud'homme MP, Moore TS (November 1992). "Phosphatidylcholine synthesis in castor bean endosperm : free bases as intermediates". Plant Physiology. 100 (3): 1527–35. doi:10.1104/pp.100.3.1527. PMC 1075815. PMID 16653153.
- ^ Nuccio ML, Ziemak MJ, Henry SA, Weretilnyk EA, Hanson AD (May 2000). "cDNA cloning of phosphoethanolamine N-methyltransferase from spinach by complementation in Schizosaccharomyces pombe and characterization of the recombinant enzyme". The Journal of Biological Chemistry. 275 (19): 14095–101. doi:10.1074/jbc.275.19.14095. PMID 10799484.
- ^ McNeil SD, Nuccio ML, Ziemak MJ, Hanson AD (August 2001). "Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase". Proceedings of the National Academy of Sciences of the United States of America. 98 (17): 10001–5. Bibcode:2001PNAS...9810001M. doi:10.1073/pnas.171228998. PMC 55567. PMID 11481443.
- ^ "Superpathway of choline biosynthesis". BioCyc Database Collection: MetaCyc. SRI International.
- ^ a b c d e Zeisel SH (2012). "A brief history of choline". Annals of Nutrition & Metabolism. 61 (3): 254–8. doi:10.1159/000343120. PMC 4422379. PMID 23183298.
- ^ a b c d Inazu M (September 2019). "Functional Expression of Choline Transporters in the Blood-Brain Barrier". Nutrients. 11 (10): 2265. doi:10.3390/nu11102265. PMC 6835570. PMID 31547050.
- ^ Barwick KE, Wright J, Al-Turki S, McEntagart MM, Nair A, Chioza B, et al. (December 2012). "Defective presynaptic choline transport underlies hereditary motor neuropathy". American Journal of Human Genetics. 91 (6): 1103–7. doi:10.1016/j.ajhg.2012.09.019. PMC 3516609. PMID 23141292.
- ^ Glier MB, Green TJ, Devlin AM (January 2014). "Methyl nutrients, DNA methylation, and cardiovascular disease". Molecular Nutrition & Food Research. 58 (1): 172–82. doi:10.1002/mnfr.201200636. PMID 23661599.
- ^ Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ (June 1993). "Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration". Alcoholism: Clinical and Experimental Research. 17 (3): 552–5. doi:10.1111/j.1530-0277.1993.tb00798.x. PMID 8333583.
- ^ Dushianthan A, Cusack R, Grocott MP, Postle AD (June 2018). "Abnormal liver phosphatidylcholine synthesis revealed in patients with acute respiratory distress syndrome". Journal of Lipid Research. 59 (6): 1034–1045. doi:10.1194/jlr.P085050. PMC 5983399. PMID 29716960.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g h i "Choline". Office of Dietary Supplements (ODS) at the National Institutes of Health. Retrieved 19 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c Zeisel SH, Mar MH, Howe JC, Holden JM (May 2003). "Concentrations of choline-containing compounds and betaine in common foods". The Journal of Nutrition. 133 (5): 1302–7. doi:10.1093/jn/133.5.1302. PMID 12730414.
- ^ "USDA Database for the Choline Content of Common Foods, Release 2". USDA Ag Data Commons. January 2008.
Total choline content was calculated as the sum of Cho, GPC, Pcho, Ptdcho, and SM.
- ^ "21 CFR 107.100: Infant formula; Nutrient requirements; Nutrient specifications; Choline content". Code of Federal Regulations, Title 21; Food and Drug Administration. 1 April 2019. Retrieved 24 October 2019.
- ^ Choline (17 March 2014). "Choline". www.nrv.gov.au. Retrieved 22 October 2019.
- ^ Wallace TC, Fulgoni VL (2016). "Assessment of Total Choline Intakes in the United States". Journal of the American College of Nutrition. 35 (2): 108–12. doi:10.1080/07315724.2015.1080127. PMID 26886842. S2CID 24063121.
- ^ "What We Eat in America, NHANES 2013-2014" (PDF). Retrieved 24 October 2019.
- ^ a b c Corbin KD, Zeisel SH (March 2012). "Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression". Current Opinion in Gastroenterology. 28 (2): 159–65. doi:10.1097/MOG.0b013e32834e7b4b. PMC 3601486. PMID 22134222.
- ^ DiNicolantonio JJ, McCarty M, OKeefe J (2019). "Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: is TMAO serving as a marker for hepatic insulin resistance". Open Heart. 6 (1): e000890. doi:10.1136/openhrt-2018-000890. PMC 6443140. PMID 30997120.
- ^ Jia J, Dou P, Gao M, Kong X, Li C, Liu Z, Huang T (September 2019). "Assessment of Causal Direction Between Gut Microbiota-Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis". Diabetes. 68 (9): 1747–1755. doi:10.2337/db19-0153. PMID 31167879.
- ^ Imbard A, et al. (2013). "Neural tube defects, folic acid and methylation". International Journal of Environmental Research and Public Health. 10 (9): 4352–4389. doi:10.3390/ijerph10094352. PMC 3799525. PMID 24048206.
- ^ Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Zeisel SH, Willett WC, Chan JM (October 2012). "Choline intake and risk of lethal prostate cancer: incidence and survival". The American Journal of Clinical Nutrition. 96 (4): 855–63. doi:10.3945/ajcn.112.039784. PMC 3441112. PMID 22952174.
- ^ Han P, Bidulescu A, Barber JR, Zeisel SH, Joshu CE, Prizment AE, et al. (April 2019). "Dietary choline and betaine intakes and risk of total and lethal prostate cancer in the Atherosclerosis Risk in Communities (ARIC) Study". Cancer Causes & Control. 30 (4): 343–354. doi:10.1007/s10552-019-01148-4. PMC 6553878. PMID 30825046.
- ^ Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD (October 2018). "Dietary Choline Intake: Current State of Knowledge Across the Life Cycle". Nutrients. 10 (10): 1513. doi:10.3390/nu10101513. PMC 6213596. PMID 30332744.
- ^ a b Zeisel SH (2006). "Choline: critical role during fetal development and dietary requirements in adults". Annual Review of Nutrition. 26: 229–50. doi:10.1146/annurev.nutr.26.061505.111156. PMC 2441939. PMID 16848706.
- ^ Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for Thiamine, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline. Washington, DC: National Academies Press. 1998.
- ^ Allen LH (2006). "Pregnancy and lactation". In Bowman BA, Russle RM (eds.). Present Knowledge in Nutrition. Washington DC: ILSI Press. pp. 529–543.
- ^ King JC (May 2000). "Physiology of pregnancy and nutrient metabolism". The American Journal of Clinical Nutrition. 71 (5 Suppl): 1218S–25S. doi:10.1093/ajcn/71.5.1218s. PMID 10799394.
- ^ Morgane PJ, Mokler DJ, Galler JR (June 2002). "Effects of prenatal protein malnutrition on the hippocampal formation". Neuroscience and Biobehavioral Reviews. 26 (4): 471–83. doi:10.1016/s0149-7634(02)00012-x. PMID 12204193. S2CID 7051841.
- ^ Oshida K, Shimizu T, Takase M, Tamura Y, Shimizu T, Yamashiro Y (April 2003). "Effects of dietary sphingomyelin on central nervous system myelination in developing rats". Pediatric Research. 53 (4): 589–93. doi:10.1203/01.pdr.0000054654.73826.ac. PMID 12612207.
- ^ Sastry BV (June 1997). "Human placental cholinergic system". Biochemical Pharmacology. 53 (11): 1577–86. doi:10.1016/s0006-2952(97)00017-8. PMID 9264309.
- ^ Sastry BV, Sadavongvivad C (March 1978). "Cholinergic systems in non-nervous tissues". Pharmacological Reviews. 30 (1): 65–132. PMID 377313.
- ^ Lockman PR, Allen DD (August 2002). "The transport of choline". Drug Development and Industrial Pharmacy. 28 (7): 749–71. doi:10.1081/DDC-120005622. PMID 12236062. S2CID 34402785.
- ^ Caudill MA (August 2010). "Pre- and postnatal health: evidence of increased choline needs". Journal of the American Dietetic Association. 110 (8): 1198–206. doi:10.1016/j.jada.2010.05.009. PMID 20656095.
- ^ Rutter P (2017). Community pharmacy: symptoms, diagnosis, and treatment (4th ed.). Elsevier. p. 156. ISBN 9780702069970.
- ^ Howe-Grant M, Kirk RE, Othmer DF, eds. (2000). "C2-Chlorocarbons to Combustion Technology". Kirk-Othmer encyclopedia of chemical technology. Vol. 6 (4th ed.). John Wiley & Sons. pp. 100–102. ISBN 9780471484943.
- ^ Guo Y, Wang L, Hu J, Feng D, Xu L (2018). "Diagnostic performance of choline PET/CT for the detection of bone metastasis in prostate cancer: A systematic review and meta-analysis". PLOS ONE. 13 (9): e0203400. Bibcode:2018PLoSO..1303400G. doi:10.1371/journal.pone.0203400. PMC 6128558. PMID 30192819.
- ^ Strecker A (1849). "Beobachtungen über die galle verschiedener thiere". Justus Liebigs Ann Chem (in German). 70 (2): 149–197. doi:10.1002/jlac.18490700203.
- ^ a b c d Sebrell WH, Harris RS, Alam SQ (1971). The vitamins. Vol. 3 (2nd ed.). Academic Press. pp. 4, 12. doi:10.1016/B978-0-12-633763-1.50007-5. ISBN 9780126337631.
- ^ Strecker A (1862). "Üeber einige neue bestandtheile der schweinegalle". Justus Liebigs Ann Chem (in German). 123 (3): 353–360. doi:10.1002/jlac.18621230310.
- ^ Gobley T (1874). "Sur la lécithine et la cérébrine". J Pharm Chim (in French). 19 (4): 346–354.
- ^ Sourkes TL (2004). "The discovery of lecithin, the first phospholipid" (PDF). Bull Hist Chem. 29 (1): 9–15. Archived (PDF) from the original on 13 April 2019.
- ^ Liebreich O (1865). "Üeber die chemische beschaffenheit der gehirnsubstanz". Justus Liebigs Ann Chem (in German). 134 (1): 29–44. doi:10.1002/jlac.18651340107. S2CID 97165871.
- ^ Baeyer A (1867). "I. Üeber das neurin". Justus Liebigs Ann Chem (in German). 142 (3): 322–326. doi:10.1002/jlac.18671420311.
- ^ Dybkowsky W (1867). "Üeber die identität des cholins und des neurins" [On the identity of choline & neurin]. J Prakt Chem (in German). 100 (1): 153–164. doi:10.1002/prac.18671000126.
- ^ Claus A, Keesé C (1867). "Üeber neurin und sinkalin". J Prakt Chem (in German). 102 (1): 24–27. doi:10.1002/prac.18671020104.
- ^ Best CH, Hershey JM, Huntsman ME (May 1932). "The effect of lecithine on fat deposition in the liver of the normal rat". The Journal of Physiology. 75 (1): 56–66. doi:10.1113/jphysiol.1932.sp002875. PMC 1394511. PMID 16994301.
- ^ Institute of Medicine (US) Standing Committee on the scientific evaluation of dietary reference intakes and its panel on folate, other B. vitamins, and choline. National Academies Press (US). 1998. pp. xi, 402–413. ISBN 9780309064118.

