Penfluridol: Difference between revisions
Undid revision 787746676 by 203.205.34.102 (talk)Consensus view is that drugs are inventions; see Wikipedia talk:WikiProject Pharmacology#Are pharmaceutical drugs inventions? |
Lena08041993 (talk | contribs) Added evidence from a Cochrane review |
||
| Line 48: | Line 48: | ||
}} |
}} |
||
'''Penfluridol''' ('''Semap''', '''Micefal''', '''Longoperidol''') is a highly potent, first generation [[diphenylbutylpiperidine]] [[antipsychotic]]. It was discovered at [[Janssen Pharmaceutica]] in 1968. Related to other diphenylbutylpiperidine antipsychotics, [[pimozide]] and [[fluspirilene]], penfluridol has an extremely long elimination half-life and its effects last for many days after single oral dose. Its antipsychotic potency, in terms of dose needed to produce comparable effects, is similar to both [[haloperidol]] and pimozide. It is only slightly [[sedative]], but often causes [[extrapyramidal symptoms|extrapyramidal]] side-effects, such as [[akathisia]], [[dyskinesia]]e and pseudo-[[Parkinsonism]]. Penfluridol is indicated for antipsychotic treatment of chronic [[schizophrenia]] and similar [[psychosis|psychotic disorders]], it is, however, like most typical antipsychotics, being increasingly replaced by the [[atypical antipsychotics]]. Due to its extremely long-lasting effects, it is often prescribed to be taken orally as tablets only once a week (q 7 days). The once-weekly dose is usually 10–60 mg. |
'''Penfluridol''' ('''Semap''', '''Micefal''', '''Longoperidol''') is a highly potent, first generation [[diphenylbutylpiperidine]] [[antipsychotic]]. It was discovered at [[Janssen Pharmaceutica]] in 1968. Related to other diphenylbutylpiperidine antipsychotics, [[pimozide]] and [[fluspirilene]], penfluridol has an extremely long elimination half-life and its effects last for many days after single oral dose. Its antipsychotic potency, in terms of dose needed to produce comparable effects, is similar to both [[haloperidol]] and pimozide. It is only slightly [[sedative]], but often causes [[extrapyramidal symptoms|extrapyramidal]] side-effects, such as [[akathisia]], [[dyskinesia]]e and pseudo-[[Parkinsonism]]. Penfluridol is indicated for antipsychotic treatment of chronic [[schizophrenia]] and similar [[psychosis|psychotic disorders]], it is, however, like most typical antipsychotics, being increasingly replaced by the [[atypical antipsychotics]]. Due to its extremely long-lasting effects, it is often prescribed to be taken orally as tablets only once a week (q 7 days). The once-weekly dose is usually 10–60 mg. A 2006 [[systematic review]] examined the use of penfluridol for people with schizophrenia: |
||
{| class="wikitable" |
|||
|+ Penfluridol compared to typical antipsychotics (oral) for schizophrenia<ref name=Soa2006>{{cite journal|last1=Soares| first1=B| last2=Silva de Lima|first2=M| first3=| last3=|title=Penfluridol for schizophrenia|journal=Cochrane Database of Systematic Reviews|date=2006|volume=2|url=http://www.cochrane.org/CD002923/SCHIZ_penfluridol-for-schizophrenia|pages=CD002923.pub2 |DOI=10.1002/14651858.CD002923.pub2}}</ref> |
|||
|- |
|||
! Summary |
|||
|- |
|||
|Although there are shortcomings and gaps in the data, there appears to be enough overall consistency for different outcomes. The [[Efficacy|effectiveness]] and adverse effects profile of penfluridol are similar to other typical [[Antipsychotic|antipsychotic]]s; both oral and depot. Furthermore, penfluridol is shown to be an adequate treatment option for people with schizophrenia, especially those who do not respond to oral medication on a daily basis and do not adapt well to depot drugs. One of the results favouring penfluridol was a lower drop out rate in medium term when compared to depot medications. It is also an option for people with long-term schizophrenia with residual psychotic symptoms who nevertheless need continuous use of [[Antipsychotic|antipsychotic]] medication. An additional benefit of penfluridol is that it is a low-cost intervention.<ref name=Soa2006/> |
|||
|- |
|||
| style="padding:0;" | |
|||
{| class="wikitable collapsible collapsed" style="width:100%;" |
|||
|- |
|||
! scope="col" style="text-align: left;"| Outcome |
|||
! scope="col" style="text-align: left;"| Findings in words |
|||
! scope="col" style="text-align: left;"| Findings in numbers |
|||
! scope="col" style="text-align: left;"| Quality of evidence |
|||
|- |
|||
! colspan="4" style="text-align: left;"| Global state |
|||
|- |
|||
| No marked improvement (CGI)<br>Follow-up: 3 to 12 months || Penfluridol does not clearly change the chance of experiencing 'no marked improvement' when compared with receiving typical antipsychotic drugs. These findings are based on data of low quality. |
|||
|| [[Relative risk|RR]] 0.92 (0.68 to 1.24) || [[The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach|Low]] |
|||
|- |
|||
| Global state - needing additional antipsychotic<br>Follow-up: less than 3 months || There is no clear difference between people given penfluridol and those receiving typical antipsychotics. These findings are based on data of low quality. |
|||
|| [[Relative risk|RR]] 1.35 (0.90 to 2.01) || [[The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach|Low]] |
|||
|- |
|||
! colspan="4" style="text-align: left;"| [[Mental health|Mental state]] |
|||
|- |
|||
| Average score ([[Brief Psychiatric Rating Scale|BPRS]])<br>Follow-up: 3 to 12 months|| On average, people receiving penfluridol scored higher than people treated with typical antipsychotics (oral) but there was no clear difference between the groups and this finding is based on data of low quality. The meaning of this in day-to-day care is unclear. || [[Mean absolute difference|MD]] 1.24 higher (4.4 lower to 6.88 higher) || [[The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach|Low]] |
|||
|- |
|||
! colspan="4" style="text-align: left;"| [[Adverse event|Adverse events]] |
|||
|- |
|||
| Needing antiparkinsonism medication<br>Follow-up: less than 3 months || There is no clear difference between people given penfluridol and those receiving typical antipsychotics (oral). These findings are based on data of low quality. |
|||
|| [[Relative risk|RR]] 1.09 (0.61 to 1.97) || [[The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach|Low]] |
|||
|- |
|||
| Insomnia<br>Follow-up: less than 3 months || There is no clear difference between people given penfluridol and those receiving typical antipsychotics (oral). These findings are based on data of low quality. |
|||
|| [[Relative risk|RR]] 1.07 (0.51 to 2.24) || [[The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach|Low]] |
|||
|- |
|||
| || No study reported any data on outcomes such as [[Quality of life|quality of life]] and information relating to time in services|| || |
|||
|- |
|||
|} |
|||
|} |
|||
== See also == |
== See also == |
||
Revision as of 16:31, 27 July 2017
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.689 |
| Chemical and physical data | |
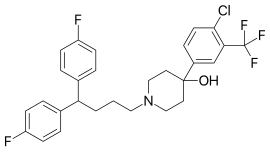
| Formula | C28H27ClF5NO |
| Molar mass | 523.965 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Penfluridol (Semap, Micefal, Longoperidol) is a highly potent, first generation diphenylbutylpiperidine antipsychotic. It was discovered at Janssen Pharmaceutica in 1968. Related to other diphenylbutylpiperidine antipsychotics, pimozide and fluspirilene, penfluridol has an extremely long elimination half-life and its effects last for many days after single oral dose. Its antipsychotic potency, in terms of dose needed to produce comparable effects, is similar to both haloperidol and pimozide. It is only slightly sedative, but often causes extrapyramidal side-effects, such as akathisia, dyskinesiae and pseudo-Parkinsonism. Penfluridol is indicated for antipsychotic treatment of chronic schizophrenia and similar psychotic disorders, it is, however, like most typical antipsychotics, being increasingly replaced by the atypical antipsychotics. Due to its extremely long-lasting effects, it is often prescribed to be taken orally as tablets only once a week (q 7 days). The once-weekly dose is usually 10–60 mg. A 2006 systematic review examined the use of penfluridol for people with schizophrenia:
| Summary | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Although there are shortcomings and gaps in the data, there appears to be enough overall consistency for different outcomes. The effectiveness and adverse effects profile of penfluridol are similar to other typical antipsychotics; both oral and depot. Furthermore, penfluridol is shown to be an adequate treatment option for people with schizophrenia, especially those who do not respond to oral medication on a daily basis and do not adapt well to depot drugs. One of the results favouring penfluridol was a lower drop out rate in medium term when compared to depot medications. It is also an option for people with long-term schizophrenia with residual psychotic symptoms who nevertheless need continuous use of antipsychotic medication. An additional benefit of penfluridol is that it is a low-cost intervention.[1] | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
See also
References
- ^ a b Soares, B; Silva de Lima, M (2006). "Penfluridol for schizophrenia". Cochrane Database of Systematic Reviews. 2: CD002923.pub2. doi:10.1002/14651858.CD002923.pub2.
- Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Verbruggen FJ, Van Nueten JM, Schaper WK., The pharmacology of penfluridol (R 16341) a new potent and orally long-acting neuroleptic drug, Eur J Pharmacol. 1970 July 15;11(2):139-54.
- van Praag HM, Schut T, Dols L, van Schilfgaarden R., Controlled trial of penfluridol in acute psychosis, Br Med J. 1971 December 18;4(5789):710-3.
- Benkert O, Hippius H.: Psychiatrische Pharmakotherapie, Springer-Verlag, 1976, 2. Auflage. ISBN 3-540-07916-5
