From Wikipedia, the free encyclopedia
Prochloraz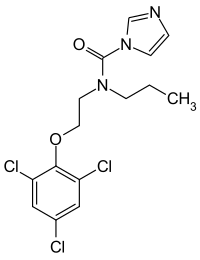 |
|
| Trade names | Abavit, Ascurit, Dibavit, Mirage, Octave, Omega, Prelude, Rival, Sporgon, Sportak, Sprint, Tenor[1][2] |
|---|
|
N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1-carboxamide
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| KEGG | |
|---|
| ChEBI | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
| ECHA InfoCard | 100.060.885  |
|---|
|
| Formula | C15H16Cl3N3O2 |
|---|
| Molar mass | 376.66 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
CCCN(CCOC1=C(C=C(C=C1Cl)Cl)Cl)C(=O)N2C=CN=C2
|
InChI=1S/C15H16Cl3N3O2/c1-10(2)21(15(22)20-4-3-19-9-20)5-6-23-14-12(17)7-11(16)8-13(14)18/h3-4,7-10H,5-6H2,1-2H3 Key:XJABPYGRRIVUOG-UHFFFAOYSA-N
|
Prochloraz, brand name Sportak, is an imidazole fungicide that was introduced in 1978[3] and is widely used in Europe, Australia, Asia, and South America within gardening and agriculture to control the growth of fungi.[4][5] It is not registered for use in the United States.[5] Similarly to other azole fungicides, prochloraz is an inhibitor of the enzyme lanosterol 14α-demethylase (CYP51A1), which is necessary for the production of ergosterol – an essential component of the fungal cell membrane – from lanosterol.[6] The agent is a broad-spectrum, protective and curative fungicide, effective against Alternaria spp., Botrytis spp., Erysiphe spp., Helminthosporium spp., Fusarium spp., Pseudocerosporella spp., Pyrenophora spp., Rhynchosporium spp., and Septoria spp.[5][2]
Like many imidazole and triazole fungicides and antifungal medications, prochloraz is not particularly selective in its actions.[4][6] In addition to inhibition of lanosterol 14α-demethylase, prochloraz has also been found to act as an antagonist of the androgen and estrogen receptors, as an agonist of the aryl hydrocarbon receptor, and as an inhibitor of enzymes in the steroidogenesis pathway such as CYP17A1 and aromatase.[4][6] In accordance, it has been shown to produce reproductive malformations in mice.[4][6] As such, prochloraz is considered to be an endocrine disruptor.[4][6]
See also
References
- ^ [Anonymous AC05372279] (1997). Consolidated list of products whose consumption and/or sale have been banned, withdrawn, severely restricted or not approved by governments / Pharmaceuticals. United Nations Publications. pp. 576–. ISBN 978-92-1-130219-6.
{{cite book}}: CS1 maint: numeric names: authors list (link)
- ^ a b G. W. A. Milne (2 September 2005). Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties. John Wiley & Sons. pp. 517–. ISBN 978-0-471-73661-5.
- ^ Bill Carlile (28 September 2006). Pesticide Selectivity, Health and the Environment. Cambridge University Press. pp. 81–. ISBN 978-1-139-45756-9.
- ^ a b c d e Vinggaard AM, Hass U, Dalgaard M, Andersen HR, Bonefeld-Jørgensen E, Christiansen S, Laier P, Poulsen ME (2006). "Prochloraz: an imidazole fungicide with multiple mechanisms of action". Int. J. Androl. 29 (1): 186–92. doi:10.1111/j.1365-2605.2005.00604.x. PMID 16466539.
- ^ a b c Kalyani Paranjape; Vasant Gowariker; V N Krishnamurthy; Sugha Gowariker (22 December 2014). The Pesticide Encyclopedia. CABI. pp. 406–. ISBN 978-1-78064-014-3.
- ^ a b c d e Philippa D. Darbre (21 March 2015). Endocrine Disruption and Human Health. Elsevier Science. pp. 86–. ISBN 978-0-12-801120-1.
External links
- Prochloraz in the Pesticide Properties DataBase (PPDB)
|
|---|
| ARTooltip Androgen receptor | | Agonists | |
|---|
| SARMsTooltip Selective androgen receptor modulator | |
|---|
| Antagonists | |
|---|
|
|---|
| GPRC6A | |
|---|
|
|
|---|
| ERTooltip Estrogen receptor | | Agonists |
- Steroidal: 2-Hydroxyestradiol
- 2-Hydroxyestrone
- 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
- 3α-Androstanediol
- 3α,5α-Dihydrolevonorgestrel
- 3β,5α-Dihydrolevonorgestrel
- 3α-Hydroxytibolone
- 3β-Hydroxytibolone
- 3β-Androstanediol
- 4-Androstenediol
- 4-Androstenedione
- 4-Fluoroestradiol
- 4-Hydroxyestradiol
- 4-Hydroxyestrone
- 4-Methoxyestradiol
- 4-Methoxyestrone
- 5-Androstenediol
- 7-Oxo-DHEA
- 7α-Hydroxy-DHEA
- 7α-Methylestradiol
- 7β-Hydroxyepiandrosterone
- 8,9-Dehydroestradiol
- 8,9-Dehydroestrone
- 8β-VE2
- 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
- 11β-Chloromethylestradiol
- 11β-Methoxyestradiol
- 15α-Hydroxyestradiol
- 16-Ketoestradiol
- 16-Ketoestrone
- 16α-Fluoroestradiol
- 16α-Hydroxy-DHEA
- 16α-Hydroxyestrone
- 16α-Iodoestradiol
- 16α-LE2
- 16β-Hydroxyestrone
- 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
- 17α-Estradiol (alfatradiol)
- 17α-Dihydroequilenin
- 17α-Dihydroequilin
- 17α-Epiestriol (16α-hydroxy-17α-estradiol)
- 17α-Ethynyl-3α-androstanediol
- 17α-Ethynyl-3β-androstanediol
- 17β-Dihydroequilenin
- 17β-Dihydroequilin
- 17β-Methyl-17α-dihydroequilenin
- Abiraterone
- Abiraterone acetate
- Alestramustine
- Almestrone
- Anabolic steroids (e.g., testosterone and esters, methyltestosterone, metandienone (methandrostenolone), nandrolone and esters, many others; via estrogenic metabolites)
- Atrimustine
- Bolandiol
- Bolandiol dipropionate
- Butolame
- Clomestrone
- Cloxestradiol
- Conjugated estriol
- Conjugated estrogens
- Cyclodiol
- Cyclotriol
- DHEA
- DHEA-S
- ent-Estradiol
- Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
- Epimestrol
- Equilenin
- Equilin
- ERA-63 (ORG-37663)
- Esterified estrogens
- Estetrol
- Estradiol
- Estramustine
- Estramustine phosphate
- Estrapronicate
- Estrazinol
- Estriol
- Estrofurate
- Estrogenic substances
- Estromustine
- Estrone
- Etamestrol (eptamestrol)
- Ethinylandrostenediol
- Ethinylestradiol
- Ethinylestriol
- Ethylestradiol
- Etynodiol
- Etynodiol diacetate
- Hexolame
- Hippulin
- Hydroxyestrone diacetate
- Lynestrenol
- Lynestrenol phenylpropionate
- Mestranol
- Methylestradiol
- Moxestrol
- Mytatrienediol
- Nilestriol
- Norethisterone
- Noretynodrel
- Orestrate
- Pentolame
- Prodiame
- Prolame
- Promestriene
- RU-16117
- Quinestradol
- Quinestrol
- Tibolone
- Xenoestrogens: Anise-related (e.g., anethole, anol, dianethole, dianol, photoanethole)
- Chalconoids (e.g., isoliquiritigenin, phloretin, phlorizin (phloridzin), wedelolactone)
- Coumestans (e.g., coumestrol, psoralidin)
- Flavonoids (incl. 7,8-DHF, 8-prenylnaringenin, apigenin, baicalein, baicalin, biochanin A, calycosin, catechin, daidzein, daidzin, ECG, EGCG, epicatechin, equol, formononetin, glabrene, glabridin, genistein, genistin, glycitein, kaempferol, liquiritigenin, mirificin, myricetin, naringenin, penduletin, pinocembrin, prunetin, puerarin, quercetin, tectoridin, tectorigenin)
- Lavender oil
- Lignans (e.g., enterodiol, enterolactone, nyasol (cis-hinokiresinol))
- Metalloestrogens (e.g., cadmium)
- Pesticides (e.g., alternariol, dieldrin, endosulfan, fenarimol, HPTE, methiocarb, methoxychlor, triclocarban, triclosan)
- Phytosteroids (e.g., digitoxin (digitalis), diosgenin, guggulsterone)
- Phytosterols (e.g., β-sitosterol, campesterol, stigmasterol)
- Resorcylic acid lactones (e.g., zearalanone, α-zearalenol, β-zearalenol, zearalenone, zeranol (α-zearalanol), taleranol (teranol, β-zearalanol))
- Steroid-like (e.g., deoxymiroestrol, miroestrol)
- Stilbenoids (e.g., resveratrol, rhaponticin)
- Synthetic xenoestrogens (e.g., alkylphenols, bisphenols (e.g., BPA, BPF, BPS), DDT, parabens, PBBs, PHBA, phthalates, PCBs)
- Others (e.g., agnuside, rotundifuran)
|
|---|
Mixed
(SERMsTooltip Selective estrogen receptor modulators) | |
|---|
| Antagonists |
- Coregulator-binding modulators: ERX-11
|
|---|
|
|---|
| GPERTooltip G protein-coupled estrogen receptor | | Agonists | |
|---|
| Antagonists | |
|---|
| Unknown | |
|---|
|
|---|
|

