Dihydrotestosterone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 104: | Line 104: | ||
[[Synthetic compound|Synthetic]] derivatives of DHT used as AAS include [[mesterolone]] (1α-methyl-DHT), [[drostanolone]] (2α-methyl-DHT), [[metenolone]] (1-methyl-δ<sup>1</sup>-DHT), [[stenbolone]] (2-methyl-δ<sup>1</sup>-DHT), [[epitiostanol]] (2α,3α-epithio-3-deketo-DHT), [[mepitiostane]] (a 17-[[ether]] [[prodrug]] of epitiostanol), [[1-testosterone]] (dihydroboldenone; Δ<sup>1</sup>-DHT), [[mesabolone]] (a 17-ether prodrug of Δ<sup>1</sup>-DHT), [[prostanozol]] (a 17-ether prodrug of the non-17α-methylated analogue of stanozolol), and [[bolazine]] (an [[azine]] [[dimer (chemistry)|dimer]] prodrug of a drostanolone-like AAS), as well as the [[17α-alkylated anabolic steroid|17α-alkylated]] derivatives [[mestanolone]] (17α-methyl-DHT), [[methasterone]] (2α,17α-dimethyl-DHT), [[oxandrolone]] (2-oxa-17α-methyl-DHT), [[oxymetholone]] (2-hydroxymethylene-17α-methyl-DHT), [[stanozolol]] (a 2,3-[[pyrazole]] A [[bicyclic molecule|ring-fused]] derivative of 17α-methyl-DHT), [[furazabol]] (a 2,3-[[furan]] A ring-fused derivative of 17α-methyl-DHT), [[androisoxazole]] (a 2,3-[[isoxazole]] A ring-fused derivative of 17α-methyl-DHT), [[methylstenbolone]] (2,17α-dimethyl-δ<sup>1</sup>-DHT), [[methyl-1-testosterone]] (methyldihydroboldenone; 17α-methyl-δ<sup>1</sup>-DHT), [[methylepitiostanol]] (2α,3α-epithio-3-deketo-17α-methyl-DHT), [[desoxymethyltestosterone]] (3-deketo-17α-methyl-δ<sup>2</sup>-DHT), and [[mebolazine]] (an azine dimer prodrug of a methasterone-like AAS).<ref name="Llewellyn2011">{{cite book | first= William | last = Llewellyn | name-list-format = vanc | title = Anabolics|url=https://books.google.com/books?id=afKLA-6wW0oC&pg=PT23|year=2011|publisher=Molecular Nutrition Llc|isbn=978-0-9828280-1-4|pages=23–25}}</ref> |
[[Synthetic compound|Synthetic]] derivatives of DHT used as AAS include [[mesterolone]] (1α-methyl-DHT), [[drostanolone]] (2α-methyl-DHT), [[metenolone]] (1-methyl-δ<sup>1</sup>-DHT), [[stenbolone]] (2-methyl-δ<sup>1</sup>-DHT), [[epitiostanol]] (2α,3α-epithio-3-deketo-DHT), [[mepitiostane]] (a 17-[[ether]] [[prodrug]] of epitiostanol), [[1-testosterone]] (dihydroboldenone; Δ<sup>1</sup>-DHT), [[mesabolone]] (a 17-ether prodrug of Δ<sup>1</sup>-DHT), [[prostanozol]] (a 17-ether prodrug of the non-17α-methylated analogue of stanozolol), and [[bolazine]] (an [[azine]] [[dimer (chemistry)|dimer]] prodrug of a drostanolone-like AAS), as well as the [[17α-alkylated anabolic steroid|17α-alkylated]] derivatives [[mestanolone]] (17α-methyl-DHT), [[methasterone]] (2α,17α-dimethyl-DHT), [[oxandrolone]] (2-oxa-17α-methyl-DHT), [[oxymetholone]] (2-hydroxymethylene-17α-methyl-DHT), [[stanozolol]] (a 2,3-[[pyrazole]] A [[bicyclic molecule|ring-fused]] derivative of 17α-methyl-DHT), [[furazabol]] (a 2,3-[[furan]] A ring-fused derivative of 17α-methyl-DHT), [[androisoxazole]] (a 2,3-[[isoxazole]] A ring-fused derivative of 17α-methyl-DHT), [[methylstenbolone]] (2,17α-dimethyl-δ<sup>1</sup>-DHT), [[methyl-1-testosterone]] (methyldihydroboldenone; 17α-methyl-δ<sup>1</sup>-DHT), [[methylepitiostanol]] (2α,3α-epithio-3-deketo-17α-methyl-DHT), [[desoxymethyltestosterone]] (3-deketo-17α-methyl-δ<sup>2</sup>-DHT), and [[mebolazine]] (an azine dimer prodrug of a methasterone-like AAS).<ref name="Llewellyn2011">{{cite book | first= William | last = Llewellyn | name-list-format = vanc | title = Anabolics|url=https://books.google.com/books?id=afKLA-6wW0oC&pg=PT23|year=2011|publisher=Molecular Nutrition Llc|isbn=978-0-9828280-1-4|pages=23–25}}</ref> |
||
==History== |
|||
DHT was first prepared by [[Adolf Butenandt]] and his colleagues in 1935.<ref name="Schnitzer1967">{{cite book|author=R Schnitzer|title=Experimental Chemotherapy|url=https://books.google.com/books?id=elAJWRnKqDEC&pg=PA156|date=1 January 1967|publisher=Elsevier Science|isbn=978-0-323-14611-1|pages=156–}}</ref><ref name="Krüskemper2013">{{cite book|author=H.-L. Krüskemper|title=Anabolic Steroids|url=https://books.google.com/books?id=4xIlBQAAQBAJ&pg=PA12|date=22 October 2013|publisher=Elsevier|isbn=978-1-4832-6504-9|pages=12–}}</ref> It was [[chemical synthesis|synthesized]] via [[hydrogenation]] of testosterone,<ref name="Krüskemper2013" /> which had been discovered earlier than year.<ref name="M.D.2002">{{cite book|author=William N. Taylor, M.D.|title=Anabolic Steroids and the Athlete, 2d ed.|url=https://books.google.com/books?id=OGcQ0Tp2AFcC&pg=PA178|date=16 January 2002|publisher=McFarland|isbn=978-0-7864-1128-3|pages=178–}}</ref> DHT was not elucidated as an endogenous substance until 1956, when it was found to be formed from testosterone in rat liver homogenates.<ref name="Krüskemper2013" /><ref name="pmid13323010">{{cite journal | vauthors = RUBIN BL, DORFMAN RI | title = In vitro conversion of testosterone to 17beta-hydroxyandrostan-3-one | journal = Proc. Soc. Exp. Biol. Med. | volume = 91 | issue = 4 | pages = 585–6 | year = 1956 | pmid = 13323010 | doi = | url = }}</ref> The biological importance of DHT was not realized until the early 1960s, when it was found to be produced by 5α-reductase from circulating testosterone in target tissues like the prostate gland and seminal vesicles and was found to be more potent than testosterone in bioassays.<ref name="Agmo2011">{{cite book|author=Anders Agmo|title=Functional and Dysfunctional Sexual Behavior: A Synthesis of Neuroscience and Comparative Psychology|url=https://books.google.com/books?id=mmJjj6UvB9YC&pg=PA196|date=18 April 2011|publisher=Academic Press|isbn=978-0-08-054938-5|pages=196–}}</ref><ref name="OreopoulosMichelis2012">{{cite book|author1=Dimitrios G. Oreopoulos|author2=M.F. Michelis|author3=S. Herschorn|title=Nephrology and Urology in the Aged Patient|url=https://books.google.com/books?id=8BioBgAAQBAJ&pg=PA495|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-94-011-1822-4|pages=495–}}</ref><ref name="WebsterRawlings2007">{{cite book|author1=Guy F. Webster|author2=Anthony V. Rawlings|title=Acne and Its Therapy|url=https://books.google.com/books?id=sx_cua_GYS4C&pg=PA168|date=17 May 2007|publisher=CRC Press|isbn=978-1-4200-1841-7|pages=168–}}</ref><ref name="SmithMitchell2013">{{cite book|author1=Lee B. Smith|author2=Rod T. Mitchell|author3=Iain J. McEwan, PhD|title=Testosterone: From Basic Research to Clinical Applications|url=https://books.google.com/books?id=wH69BAAAQBAJ&pg=PA5|date=1 October 2013|publisher=Springer Science & Business Media|isbn=978-1-4614-8978-8|pages=5–}}</ref> |
|||
==References== |
==References== |
||
| Line 111: | Line 114: | ||
{{Androgens and antiandrogens}} |
{{Androgens and antiandrogens}} |
||
{{Androgen receptor modulators}} |
{{Androgen receptor modulators}} |
||
{{GABAA receptor positive allosteric modulators}} |
|||
[[Category:Alcohols]] |
[[Category:Alcohols]] |
||
| Line 117: | Line 121: | ||
[[Category:Animal reproductive system]] |
[[Category:Animal reproductive system]] |
||
[[Category:Biology of gender]] |
[[Category:Biology of gender]] |
||
[[Category:GABAA receptor positive allosteric modulators]] |
|||
[[Category:Hormones of the hypothalamus-pituitary-gonad axis]] |
[[Category:Hormones of the hypothalamus-pituitary-gonad axis]] |
||
[[Category:Hormones of the testis]] |
[[Category:Hormones of the testis]] |
||
Revision as of 23:47, 30 June 2017
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anaboleen, Anabolex, Anaprotin, Andractim, Androlone, Apeton, Gelovit, Neodrol, Ophtovital, Pesomax, Stanaprol, and Stanolone |
| Other names | DHT; 5α-Dihydrotestosterone; 5α-DHT; Androstanolone; Stanolone |
| Pregnancy category |
|
| Routes of administration | Intramuscular, transdermal |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 0–2%[citation needed] |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.554 |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.442 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
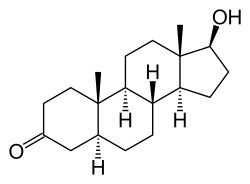
Dihydrotestosterone (DHT), or 5α-dihydrotestosterone (5α-DHT), also known as androstanolone or stanolone, is an endogenous androgen sex steroid and hormone. The enzyme 5α-reductase catalyzes the formation of DHT from testosterone in certain tissues including the prostate gland, seminal vesicles, epididymides, skin, hair follicles, liver, and brain. This enzyme mediates reduction of the C4-5 double bond of testosterone. Relative to testosterone, DHT is considerably more potent as an agonist of the androgen receptor (AR).
Biological activity
DHT is a potent agonist of the androgen receptor (AR), and is in fact the most potent known endogenous ligand of the receptor. It has an affinity (Kd) of 0.25 to 0.5 nM for the human AR, which is about 2- to 3-fold higher than that of testosterone (Kd = 0.4 to 1.0 nM)[1] and 15–30 times higher than that of adrenal androgens.[2] In addition, the dissociation rate of DHT from the AR is 5-fold slower than that of testosterone.[3] The EC50 of DHT for activation of the AR is 0.13 nM, which is about 5-fold stronger than that of testosterone (EC50 = 0.66 nM).[4] In bioassays, DHT has been found to be 2.5- to 10-fold more potent than testosterone.[1]
The terminal half-life of DHT in the body (53 minutes) is longer than that of testosterone (34 minutes), and this may account for some of the difference in their potency.[5] A study of transdermal DHT and testosterone treatment reported terminal half-lives of 2.83 hours and 1.29 hours, respectively.[6]
Biological function
Sexual development
During male embryogenesis DHT has an essential role in the formation of the male external genitalia, while in the adult male DHT acts as the primary androgen in the prostate gland, seminal vesicles, skin, and hair follicles.[7]
An example illustrating the significance of DHT for the development of secondary sex characteristics is congenital 5α-reductase type II deficiency. This genetic mutation can result in pseudohermaphroditism.[8] The condition typically presents with underdeveloped male genitalia and prostate. Males with this condition are often raised as girls due to their lack of conspicuous male genitalia.[8] At the onset of puberty, although their DHT levels remain very low,[citation needed] their testosterone levels elevate normally. Their musculature develops like that of other male adults. After puberty, men with this condition have a large deficiency of pubic and body hair and reportedly no incidence of androgenic alopecia (pattern hair loss).[9] They also reportedly have no incidence of prostate cancer.[10]
Unlike other androgens such as testosterone, DHT cannot be converted by the enzyme aromatase into an estrogen like estradiol. Therefore, it is frequently used in research settings to distinguish between the effects of testosterone caused by binding to the AR and those caused by testosterone's conversion to estradiol and subsequent binding to and activation of estrogen receptors.[11]
Pathology
DHT produced locally at the site of hair follicles by 5α-reductase, and not systemic DHT, is the primary causal factor in male androgenic alopecia, although the pathology regarding this phenomenon is poorly understood.[12][13] In the case of female androgenic alopecia, on the other hand, the situation is more complex, and DHT is only one of several possible causes.[14] Women with increased levels of DHT may develop symptoms of hyperandrogenism such as certain androgynous masculine secondary sex characteristics, including a deepened voice and facial hair. In men, prostate growth and differentiation are highly dependent on androgens, especially DHT, and DHT is involved in the pathogenesis of benign prostatic hyperplasia (BPH) and prostate cancer.[15]
Management
5α-Reductase inhibitors like finasteride and dutasteride, which inactivate the 5α-reductase enzyme and block the formation of DHT, are commonly used for the treatment of two DHT-related conditions, androgenic alopecia and BPH. Both finasteride and dutasteride are approved for the treatment of BPH and androgenic alopecia. Dutasteride is three times more potent than finasteride in inhibiting the type II enzyme and 100 times more potent than finasteride in inhibiting the type I form of the DHT-producing enzyme. Both finasteride and dutasteride are potent inhibitors of the third isotype of the enzyme.[16]
Acne, hirsutism (excessive hair growth), and seborrhea are also DHT-related conditions, and 5α-reductase inhibitors may be used to treat these conditions as well.[17] In addition, antiandrogens like cyproterone acetate, spironolactone, and bicalutamide, as well as estrogens like ethinylestradiol (which are functional antiandrogens), may also be used to treat these conditions.[17][18]
Biochemistry
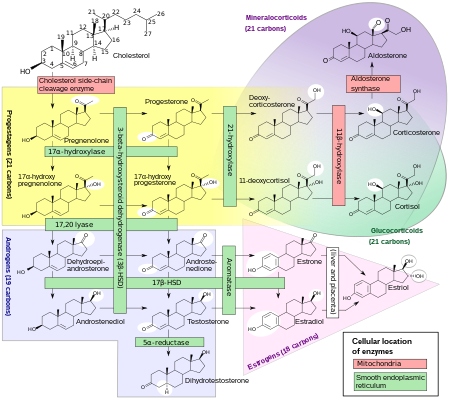
Biosynthesis
DHT is synthesized from testosterone by the enzyme 5α-reductase.[20] In males, approximately 5% of testosterone undergoes 5α-reduction into DHT.[citation needed]
Metabolism
DHT is inactivated in the liver and extrahepatic tissues like the skin into 3α-androstanediol and 3β-androstanediol by the enzymes 3α-hydroxysteroid dehydrogenase and 3β-hydroxysteroid dehydrogenase, respectively.[21] These metabolites are in turn converted, respectively, into androsterone and epiandrosterone, then conjugated (via glucuronidation and/or sulfation), released into circulation, and excreted in urine.
Unlike testosterone, DHT cannot be aromatized into an estrogen, and for this reason, has no propensity for estrogenic effects.[22]
Levels
Serum DHT levels are about 10% of those of testosterone, but levels in the prostate gland are 5- to 10-fold higher than those of testosterone due to a more than 90% conversion of testosterone into DHT by locally expressed 5α-reductase.[23] For this reason, and in addition to the fact that DHT is much more potent as an AR agonist than is testosterone,[1] DHT is considered to be the major androgen of the prostate gland.[23]
Medical use
DHT is available in pharmaceutical formulations for medical use as an androgen or anabolic-androgenic steroid (AAS).[24] When used as a drug, it is referred to as androstanolone (INN) or as stanolone (BAN).[24][25][26] The availability of pharmaceutical DHT is limited; it is not available in the United States or Canada,[27][28] but is available in certain European countries, including the United Kingdom, Germany, France, Spain, Italy, Belgium, and Luxembourg.[26][29] Brand names of DHT include Anaboleen, Anabolex, Anaprotin (UK), Andractim (formerly AndroGel-DHT) (FR, BE, LU), Androlone, Apeton, Gelovit (ES), Neodrol, Ophtovital, (DE), Pesomax (IT), Stanaprol, and Stanolone, among others.[24][25][26][29][30] The available formulations of DHT include buccal or sublingual tablets (Anabolex, Stanolone), topical gels (Andractim, Gelovit, Ophtovital), and, as esters in oil, injectables like dihydrotestosterone propionate (Pesomax) and dihydrotestosterone valerate (Apeton).[24][29][30] Esters of DHT act as prodrugs of DHT in the body and have a long-lasting depot when given via intramuscular injection.[24] Dihydrotestosterone benzoate (Ermalone-Amp, Hermalone, Sarcosan) and dihydrotestosterone enanthate (Anaboleen Depot) are additional DHT esters that are also available for medical use, while a few others, including dihydrotestosterone acetate, dihydrotestosterone butyrate, and dihydrotestosterone formate, were developed but never marketed.[25]
Unlike testosterone and various synthetic AAS, DHT cannot be aromatized, and for this reason, poses no risk of estrogenic side effects like gynecomastia at any dosage.[31] In addition, DHT cannot be metabolized by 5α-reductase (as it is already 5α-reduced), and for this reason, is not potentiated in so-called androgenic tissues like the skin, hair follicles, and prostate gland. This provides exogenous DHT with a greater ratio of anabolic to androgenic effects compared to testosterone, and DHT may be less prone to producing certain skin and hair-related side effects like acne, oily skin, seborrhea, hirsutism (excess facial/body hair growth), and androgenic alopecia (pattern hair loss), as well as prostate enlargement (which can lead to benign prostatic hyperplasia) and an increased risk of prostate cancer.
Pharmaceutical DHT is used mainly in the treatment of male hypogonadism.[29] It was under development in a topical formulation for the treatment of cachexia in cancer patients, and reached phase III clinical trials for this indication, but ultimately was not introduced for this purpose.[29] Although DHT itself has not been approved for the treatment of cachexia, an orally active synthetic derivative of DHT, oxandrolone (2-oxa-17α-methyl-DHT), is approved and used for this indication.[32][33]
Chemistry
DHT, also known as 5α-androstan-17β-ol-3-one, is an androstane steroid with a ketone group at the C3 position and a hydroxyl group at the C17β position. It is the derivative of testosterone in which the double bond between the C4 and C5 positions has been reduced or hydrogenated.
Derivatives
Several C17β ester prodrugs of DHT, including androstanolone benzoate, androstanolone enanthate, androstanolone propionate, and androstanolone valerate, have been developed and introduced for medical use as AAS.[25][34]
Synthetic derivatives of DHT used as AAS include mesterolone (1α-methyl-DHT), drostanolone (2α-methyl-DHT), metenolone (1-methyl-δ1-DHT), stenbolone (2-methyl-δ1-DHT), epitiostanol (2α,3α-epithio-3-deketo-DHT), mepitiostane (a 17-ether prodrug of epitiostanol), 1-testosterone (dihydroboldenone; Δ1-DHT), mesabolone (a 17-ether prodrug of Δ1-DHT), prostanozol (a 17-ether prodrug of the non-17α-methylated analogue of stanozolol), and bolazine (an azine dimer prodrug of a drostanolone-like AAS), as well as the 17α-alkylated derivatives mestanolone (17α-methyl-DHT), methasterone (2α,17α-dimethyl-DHT), oxandrolone (2-oxa-17α-methyl-DHT), oxymetholone (2-hydroxymethylene-17α-methyl-DHT), stanozolol (a 2,3-pyrazole A ring-fused derivative of 17α-methyl-DHT), furazabol (a 2,3-furan A ring-fused derivative of 17α-methyl-DHT), androisoxazole (a 2,3-isoxazole A ring-fused derivative of 17α-methyl-DHT), methylstenbolone (2,17α-dimethyl-δ1-DHT), methyl-1-testosterone (methyldihydroboldenone; 17α-methyl-δ1-DHT), methylepitiostanol (2α,3α-epithio-3-deketo-17α-methyl-DHT), desoxymethyltestosterone (3-deketo-17α-methyl-δ2-DHT), and mebolazine (an azine dimer prodrug of a methasterone-like AAS).[35]
History
DHT was first prepared by Adolf Butenandt and his colleagues in 1935.[36][37] It was synthesized via hydrogenation of testosterone,[37] which had been discovered earlier than year.[38] DHT was not elucidated as an endogenous substance until 1956, when it was found to be formed from testosterone in rat liver homogenates.[37][39] The biological importance of DHT was not realized until the early 1960s, when it was found to be produced by 5α-reductase from circulating testosterone in target tissues like the prostate gland and seminal vesicles and was found to be more potent than testosterone in bioassays.[40][41][42][43]
References
- ^ a b c Mozayani, Ashraf; Raymon, Lionel (18 September 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Hemat RA (2004). Principles Of Orthomolecularism. Urotext. p. 426. ISBN 1-903737-05-2.
- ^ Grino PB, Griffin JE, Wilson JD (February 1990). "Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone". Endocrinology. 126 (2): 1165–72. doi:10.1210/endo-126-2-1165. PMID 2298157.
- ^ Wilderer, Peter A. (1 September 2010). "Bioassays for Estrogenic and Androgenic Effects of Water Constituents". Treatise on Water Science, Four-Volume Set. Newnes. pp. 1805–. ISBN 978-0-444-53199-5.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Diamanti-Kandarakis E (1999). "Current aspects of antiandrogen therapy in women". Current Pharmaceutical Design. 5 (9): 707–23. PMID 10495361.
- ^ von Deutsch, Daniel A.; Abukhalaf, Imad K.; Lapu-Bula, Rigobert (15 October 2003). "Anabolic Doping Agents". In Mozayani, Ashraf; Raymon, Lionel (eds.). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 510–. doi:10.1007/978-1-61779-222-9_15. ISBN 978-1-59259-654-6.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Amory JK, Anawalt BD, Matsumoto AM, Page ST, Bremner WJ, Wang C, Swerdloff RS, Clark RV (June 2008). "The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men". The Journal of Urology. 179 (6): 2333–8. doi:10.1016/j.juro.2008.01.145. PMC 2684818. PMID 18423697.
- ^ a b Imperato-McGinley J, Peterson RE, Gautier T, Sturla E (May 1979). "Androgens and the evolution of male-gender identity among male pseudohermaphrodites with 5alpha-reductase deficiency". The New England Journal of Medicine. 300 (22): 1233–7. doi:10.1056/NEJM197905313002201. PMID 431680.
- ^ Marks LS (2004). "5alpha-reductase: history and clinical importance". Reviews in Urology. 6 Suppl 9: S11–21. PMC 1472916. PMID 16985920.
- ^ Jain, N. K.; Siddiqi, Maqsood; Weisburger, J. H. (2006). Protective Effects of Tea on Human Health. CABI. pp. 95–. ISBN 978-1-84593-113-1.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Swerdloff RS, Wang C (October 1998). "Dihydrotestosterone: a rationale for its use as a non-aromatizable androgen replacement therapeutic agent". Baillière's Clinical Endocrinology and Metabolism. 12 (3): 501–6. doi:10.1016/s0950-351x(98)80267-x. PMID 10332569.
- ^ Nordqvist C (2012-02-23). "What Is DHT (Dihydrotestosterone)? What Is DHT's Role In Baldness?". Medical News Today.
- ^ "Male Pattern Baldness Causes". Hair Loss Health Center. WebMD, LLC.
- ^ McAndrews PJ. "Women's Hair Loss / Causes of Hair Loss". American Hair Loss Association.
- ^ Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, Presti JC, Kane CJ (October 2005). "Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study". Journal of Clinical Oncology. 23 (30): 7546–54. doi:10.1200/JCO.2005.05.025. PMID 16234520.
- ^ Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS (December 2006). "The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride". Journal of the American Academy of Dermatology. 55 (6): 1014–23. doi:10.1016/j.jaad.2006.05.007. PMID 17110217.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ a b Lotti, Francesco; Maggi, Mario (28 April 2015). "Hormonal Treatment for Skin Androgen-Related Disorders". In Katsambas, Andreas; Lotti, Torello; Dessinioti, Clio; D'Erme, Angelo Massimiliano (eds.). European Handbook of Dermatological Treatments. Springer. pp. 1451–1464. ISBN 978-3-662-45139-7.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Benvenga, Salvatore (27 February 2009). "Therapy of Hirsutism". In Farid, Nadir R.; Diamanti-Kandarakis, Evanthia (eds.). Diagnosis and Management of Polycystic Ovary Syndrome. Springer Science & Business Media. pp. 233–242. doi:10.1007/978-0-387-09718-3_19. ISBN 978-0-387-09718-3.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Messenger, Andrew (26 June 2008). "Male Androgenetic Alopecia". In Blume-Peytavi, Ulrike; Whiting, David A.; Trüeb, Ralph M. (eds.). Hair Growth and Disorders. Springer Science & Business Media. pp. 161–. ISBN 978-3-540-46911-7.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM (July 2003). "Human type 3 3alpha-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells". Endocrinology. 144 (7): 2922–32. doi:10.1210/en.2002-0032. PMID 12810547.
- ^ Weiner, Irving B.; Gallagher, Michela (2003). Handbook of Psychology, Biological Psychology. John Wiley & Sons. pp. 333–. ISBN 978-0-471-38403-8.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Hay, Ian D.; Wass, John A. H. (26 January 2009). Clinical Endocrine Oncology. John Wiley & Sons. pp. 37–. ISBN 978-1-4443-0023-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d e Hyde, Thomas E.; Gengenbach, Marianne S. (2007). Conservative Management of Sports Injuries. Jones & Bartlett Learning. pp. 1100–. ISBN 978-0-7637-3252-3.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d Elks, J. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 640–. ISBN 978-1-4757-2085-3.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 63–. ISBN 978-3-88763-075-1.
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
- ^ "Drug Product Database - Health Canada". Health Canada. Retrieved 13 November 2016.
- ^ a b c d e "Androstanolone Drug Profile". Adis Insight. 4 December 2006.
- ^ a b List, Paul Heinz; Hörhammer, Ludwig (12 March 2013). Chemikalien und Drogen: Teil B: R, S. Springer-Verlag. pp. 523–. ISBN 978-3-642-66377-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Malven, Paul V. (12 January 1993). Mammalian Neuroendocrinology. CRC Press. pp. 228–. ISBN 978-0-8493-8757-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Nelms, Marcia; Sucher, Kathryn P.; Lacey, Karen; Roth, Sara Long (16 June 2010). Nutrition Therapy and Pathophysiology. Cengage Learning. pp. 766–. ISBN 1-133-00809-7.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Mantovani, Giovanni (6 October 2007). Cachexia and Wasting: A Modern Approach. Springer Science & Business Media. pp. 673–. ISBN 978-88-470-0552-5.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Morton, I.K.; Hall, Judith M. (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 261–. ISBN 978-94-011-4439-1.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Llewellyn, William (2011). Anabolics. Molecular Nutrition Llc. pp. 23–25. ISBN 978-0-9828280-1-4.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ R Schnitzer (1 January 1967). Experimental Chemotherapy. Elsevier Science. pp. 156–. ISBN 978-0-323-14611-1.
- ^ a b c H.-L. Krüskemper (22 October 2013). Anabolic Steroids. Elsevier. pp. 12–. ISBN 978-1-4832-6504-9.
- ^ William N. Taylor, M.D. (16 January 2002). Anabolic Steroids and the Athlete, 2d ed. McFarland. pp. 178–. ISBN 978-0-7864-1128-3.
- ^ RUBIN BL, DORFMAN RI (1956). "In vitro conversion of testosterone to 17beta-hydroxyandrostan-3-one". Proc. Soc. Exp. Biol. Med. 91 (4): 585–6. PMID 13323010.
- ^ Anders Agmo (18 April 2011). Functional and Dysfunctional Sexual Behavior: A Synthesis of Neuroscience and Comparative Psychology. Academic Press. pp. 196–. ISBN 978-0-08-054938-5.
- ^ Dimitrios G. Oreopoulos; M.F. Michelis; S. Herschorn (6 December 2012). Nephrology and Urology in the Aged Patient. Springer Science & Business Media. pp. 495–. ISBN 978-94-011-1822-4.
- ^ Guy F. Webster; Anthony V. Rawlings (17 May 2007). Acne and Its Therapy. CRC Press. pp. 168–. ISBN 978-1-4200-1841-7.
- ^ Lee B. Smith; Rod T. Mitchell; Iain J. McEwan, PhD (1 October 2013). Testosterone: From Basic Research to Clinical Applications. Springer Science & Business Media. pp. 5–. ISBN 978-1-4614-8978-8.
- Alcohols
- Androgens and anabolic steroids
- Androstanes
- Animal reproductive system
- Biology of gender
- GABAA receptor positive allosteric modulators
- Hormones of the hypothalamus-pituitary-gonad axis
- Hormones of the testis
- Human hormones
- Ketones
- Sex hormones
- Testosterone
- World Anti-Doping Agency prohibited substances
