Desmethylflunitrazepam
Tools
Actions
General
Print/export
Print/export
In other projects
From Wikipedia, the free encyclopedia
This is an old revision of this page, as edited by Headbomb (talk | contribs) at 14:31, 28 June 2016 (→top: clean up using AWB). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.018.072 |
| Chemical and physical data | |
| Formula | C15H10FN3O3 |
| Molar mass | 299.261 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
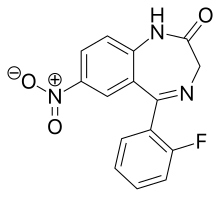
Desmethylflunitrazepam (Ro05-4435, fonazepam) is a benzodiazepine that is a metabolite of flunitrazepam.[1][2][3] It has an IC50 value of 1.499 nM for the GABAA receptor.[4][5]
See also
References
- ^ Ruud W. Busker; Gerard M.J.Beijersbergen van Henegouwen; Brigitta M.C. Kwee; Jos H.M. Winkens (May 1987). "Photobinding of flunitrazepam and its major photo-decomposition product N-desmethylflunitrazepam". International Journal of Pharmaceutics. 36 (2–3): 113–120. doi:10.1016/0378-5173(87)90145-1.
- ^ Janet K Coller; Andrew A Somogyi; Felix Bochner (November 1998). "Quantification of flunitrazepam's oxidative metabolites, 3-hydroxyflunitrazepam and desmethylflunitrazepam, in hepatic microsomal incubations by high-performance liquid chromatography". Journal of Chromatography B. 719 (1–2): 87–92. doi:10.1016/S0378-4347(98)00383-1. PMID 9869368.
- ^ Tansel Kilicarslan; Robert L. Haining; Allan E. Rettie; Usanda Busto; Rachel F. Tyndale; Edward M. Sellers (April 2001). "Flunitrazepam Metabolism by Cytochrome P450s 2C19 and 3A4". Drug Metabolism and Disposition. 29 (4): 460–465. PMID 11259331.
- ^ Desmond J. Maddalena; Graham A. R. Johnston (February 1995). "Prediction of Receptor Properties and Binding Affinity of Ligands to Benzodiazepine/GABAA Receptors Using Artificial Neural Networks". Journal of Medicinal Chemistry. 38 (4): 715–724. doi:10.1021/jm00004a017. PMID 7861419.
- ^ Sung-Sau So; Martin Karplus (December 1996). "Genetic Neural Networks for Quantitative Structure−Activity Relationships: Improvements and Application of Benzodiazepine Affinity for Benzodiazepine/GABAA Receptors". Journal of Medicinal Chemistry. 39 (26): 5246–5256. doi:10.1021/jm960536o. PMID 8978853.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
| Receptor (ligands) |
| ||||
|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||
This pharmacology-related article is a stub. You can help Wikipedia by expanding it. |
