COVID-19: Difference between revisions
Filled in 3 bare references |
No edit summary Tags: Mobile edit Mobile web edit |
||
| Line 175: | Line 175: | ||
===Mechanical ventilation=== |
===Mechanical ventilation=== |
||
Most cases of COVID-19 are not severe enough to require [[mechanical ventilation]] (artificial assistance to support breathing), but a percentage of cases do.<ref name=murthy>{{cite journal | vauthors = Murthy S, Gomersall CD, Fowler RA | title = Care for Critically Ill Patients With COVID-19 | journal = JAMA |date=11 March 2020 |pmid=32159735 | doi = 10.1001/jama.2020.3633 | url = https://jamanetwork.com/journals/jama/fullarticle/2762996 }}</ref><ref>{{cite web |last=World Health Organization|date=28 January 2020|title=Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected|url=https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf|journal=|volume=|pages=|via=}}</ref> Some Canadian doctors recommend the use of [[mechanical ventilation|invasive mechanical ventilation]] because this technique limits the spread of [[Airborne disease|aerosolized]] transmission [[Vector (epidemiology)|vectors]].<ref name=murthy/> Severe cases are most common in older adults (those older than 60 years<ref name=murthy/> and especially those older than 80 years{{cn|date=March 2020}}). Many developed countries do not have enough [[List of countries by hospital beds|hospital beds per capita]], which limits a [[health system]]'s capacity to handle a sudden spike in the number of COVID-19 cases severe enough to require hospitalization.<ref name="VoxCOVID">{{cite news |last1=Scott |first1=Dylan |title=Coronavirus is exposing all of the weaknesses in the US health system High health care costs and low medical capacity made the US uniquely vulnerable to the coronavirus. |url=https://www.vox.com/policy-and-politics/2020/3/16/21173766/coronavirus-covid-19-us-cases-health-care-system |access-date=18 March 2020 |publisher=Vox |date=16 March 2020}}</ref> This limited capacity is a significant driver of the need to [[flatten the curve]] (to keep the speed at which new cases occur and thus the number of people sick at one point in time lower).<ref name="VoxCOVID"/> One study in China found 5% were admitted to [[intensive care unit]]s, 2.3% needed mechanical support of ventilation, and 1.4% died.<ref name="Guan Ni Hu Liang p.">{{cite journal | vauthors = Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS | display-authors = 6 | title = Clinical Characteristics of Coronavirus Disease 2019 in China | journal = The New England Journal of Medicine | date = February 2020 | pmid = 32109013 | doi = 10.1056/nejmoa2002032 | publisher = Massachusetts Medical Society | doi-access = free }}</ref> An Italian startup employed [[3D printing]] technology to produce valves for life-saving coronavirus treatment due to a broken [[supply chain]] of original manufacturing.<ref>{{cite news |title=[Updating] Italian hospital saves Covid-19 patients lives by 3D printing valves for reanimation devices |url=https://www.3dprintingmedia.network/covid-19-3d-printed-valve-for-reanimation-device/ |access-date=20 March 2020 |work=3D Printing Media Network |date=14 March 2020}}</ref> |
Most cases of COVID-19 are not severe enough to require [[mechanical ventilation]] (artificial assistance to support breathing), but a percentage of cases do.<ref name=murthy>{{cite journal | vauthors = Murthy S, Gomersall CD, Fowler RA | title = Care for Critically Ill Patients With COVID-19 | journal = JAMA |date=11 March 2020 |pmid=32159735 | doi = 10.1001/jama.2020.3633 | url = https://jamanetwork.com/journals/jama/fullarticle/2762996 }}</ref><ref>{{cite web |last=World Health Organization|date=28 January 2020|title=Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected|url=https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf|journal=|volume=|pages=|via=}}</ref> Some Canadian doctors recommend the use of [[mechanical ventilation|invasive mechanical ventilation]] because this technique limits the spread of [[Airborne disease|aerosolized]] transmission [[Vector (epidemiology)|vectors]].<ref name=murthy/> Severe cases are most common in older adults (those older than 60 years<ref name=murthy/> and especially those older than 80 years{{cn|date=March 2020}}). Many developed countries do not have enough [[List of countries by hospital beds|hospital beds per capita]], which limits a [[health system]]'s capacity to handle a sudden spike in the number of COVID-19 cases severe enough to require hospitalization.<ref name="VoxCOVID">{{cite news |last1=Scott |first1=Dylan |title=Coronavirus is exposing all of the weaknesses in the US health system High health care costs and low medical capacity made the US uniquely vulnerable to the coronavirus. |url=https://www.vox.com/policy-and-politics/2020/3/16/21173766/coronavirus-covid-19-us-cases-health-care-system |access-date=18 March 2020 |publisher=Vox |date=16 March 2020}}</ref> This limited capacity is a significant driver of the need to [[flatten the curve]] (to keep the speed at which new cases occur and thus the number of people sick at one point in time lower).<ref name="VoxCOVID"/> One study in China found 5% were admitted to [[intensive care unit]]s, 2.3% needed mechanical support of ventilation, and 1.4% died.<ref name="Guan Ni Hu Liang p.">{{cite journal | vauthors = Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS | display-authors = 6 | title = Clinical Characteristics of Coronavirus Disease 2019 in China | journal = The New England Journal of Medicine | date = February 2020 | pmid = 32109013 | doi = 10.1056/nejmoa2002032 | publisher = Massachusetts Medical Society | doi-access = free }}</ref> Every |
||
ventilator is worth its weight in gold as a source says a third of all Coronavirus patients could need it.</ref>https://meduza.io/amp/en/feature/2020/03/21/the-ventilator-problem<ref> An Italian startup employed [[3D printing]] technology to produce valves for life-saving coronavirus treatment due to a broken [[supply chain]] of original manufacturing.<ref>{{cite news |title=[Updating] Italian hospital saves Covid-19 patients lives by 3D printing valves for reanimation devices |url=https://www.3dprintingmedia.network/covid-19-3d-printed-valve-for-reanimation-device/ |access-date=20 March 2020 |work=3D Printing Media Network |date=14 March 2020}}</ref> |
|||
===Experimental treatment===<!-- Not Research: should include only confirmed treatment. Experimental Treatments and Research go in Research section --> |
===Experimental treatment===<!-- Not Research: should include only confirmed treatment. Experimental Treatments and Research go in Research section --> |
||
Revision as of 16:19, 25 March 2020
Template:Use Commonwealth English
| Coronavirus disease 2019 (COVID-19) | |
|---|---|
| Other names |
|
 | |
| Symptoms of COVID-19 | |
| Pronunciation | |
| Specialty | Acute respiratory infection[4] |
| Symptoms | Fever, cough, shortness of breath[5] |
| Complications | Pneumonia, acute respiratory distress syndrome, kidney failure |
| Causes | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
| Risk factors | Travel, exposure to the virus |
| Diagnostic method | rRT-PCR testing, immunoassay, CT scan |
| Prevention | frequent hand washing, cough etiquette, quarantine of infected people and at-risk populations, physical distancing |
| Treatment | Symptomatic and supportive |
| Frequency | 676,609,955[6] confirmed cases |
| Deaths | 6,881,955[6] (2.3% globally, various depending on region. Lower when unconfirmed cases are included)[7] |
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[8] The disease was first identified in 2019 in Wuhan, the capital of Hubei, China, and has since spread globally, resulting in the 2019–20 coronavirus pandemic.[9][10] Common symptoms include fever, cough, and shortness of breath. Muscle pain, sputum production, diarrhea, and sore throat are less common.[5][11][12][13] While the majority of cases result in mild symptoms,[14] some progress to pneumonia and multi-organ failure.[9][15] As of 25 March 2020, the rate of deaths per number of diagnosed cases is 4.5 percent; however, it ranges from 0.2 percent to 15 percent, according to age group and other health problems.[16] The fatality rate varies widely from place to place and over time due to variation in how broadly a population is tested, and due to variations in availability of sufficient healthcare facilities and personnel.
The virus is typically spread during close contact and via respiratory droplets produced when people cough or sneeze.[17][18] Respiratory droplets may be produced during breathing but it is not considered airborne.[17] It may also spread when one touches a contaminated surface and then their face.[17][18] It is most contagious when people are symptomatic, although spread may be possible before symptoms appear.[18] The virus can live on surfaces up to 72 hours.[19] Time from exposure to onset of symptoms is generally between two and fourteen days, with an average of five days.[20][21] The standard method of diagnosis is by reverse transcription polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab. The infection can also be diagnosed from a combination of symptoms, risk factors and a chest CT scan showing features of pneumonia.[22][23]
Recommended measures to prevent infection include frequent hand washing, social distancing (maintaining physical distance from others, especially from those with symptoms), and keeping unwashed hands away from the face.[24][25] The use of masks is recommended by some national health authorities for those who suspect they have the virus and their caregivers, but not for the general public, although simple cloth masks may be used by those who desire them.[26][27] There is no vaccine or specific antiviral treatment for COVID-19. Management involves treatment of symptoms, supportive care, isolation, and experimental measures.[28]
The World Health Organization (WHO) declared the 2019–20 coronavirus outbreak a Public Health Emergency of International Concern (PHEIC) on 30 January 2020[29][30] and a pandemic on 11 March 2020.[10] Evidence of local transmission of the disease has been found in many countries across all six WHO regions.[31]
Signs and symptoms
| Symptom[32] | % |
|---|---|
| Fever | 87.9 |
| Dry cough | 67.7 |
| Fatigue | 38.1 |
| Sputum production | 33.4 |
| Loss of smell[33] | 30 to 66 |
| Shortness of breath | 18.6 |
| Muscle or joint pain | 14.8 |
| Sore throat | 13.9 |
| Headache | 13.6 |
| Chills | 11.4 |
| Nausea or vomiting | 5.0 |
| Nasal congestion | 4.8 |
| Diarrhea | 3.7 to 31[34] |
| Haemoptysis | 0.9 |
| Conjunctival congestion | 0.8 |
Although those infected with the virus may be asymptomatic, many develop flu-like symptoms, including fever, cough, and shortness of breath.[5][35][36] Emergency symptoms include difficulty breathing, persistent chest pain or pressure, confusion, difficulty waking, and bluish face or lips; immediate medical attention is advised if these symptoms are present.[37] Less commonly, upper respiratory symptoms, such as sneezing, runny nose, or sore throat may be seen. Symptoms such as nausea, vomiting, and diarrhea have been observed in varying percentages from 3% to 31% of cases depending on the study.[34][38][39] Some cases in China initially presented only with chest tightness and palpitations.[40] In some, the disease may progress to pneumonia, multi-organ failure, and death.[9][15]
As is common with infections, there is a delay from when a person is infected with the virus to when they develop symptoms, known as the incubation period. The incubation period for COVID-19 is typically five to six days but may range from two to 14 days.[41][42] 97.5% of people who develop symptoms will do so within 11.5 days of infection.[43]
Cause
The disease is caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously referred to as the 2019 novel coronavirus (2019-nCoV).[44] It is primarily spread between people via respiratory droplets from coughs and sneezes.[45] A study investigating the rate of decay of the virus found no viable viruses after 4 h on copper, 24 h on cardboard, 72 h on stainless steel, and 72 h on plastic. However, detection rates did not reach 100% and varied between surface type (limit of detection was 3.33×100.5 TCID50 per liter of air for aerosols, 100.5 TCID50 per milliliter of medium for plastic, steel, and cardboard, and 101.5 TCID50 per milliliter of medium for copper). Estimation of the rate of decay with a Bayesian regression model suggests that viruses may remain viable up to 18 h on copper, 55 h on cardboard, 90 h on stainless steel, and over 100 h on plastic. The virus remained viable in aerosols throughout the time of the experiment (3 h).[46] The virus has also been found in faeces, and transmission through faeces is being researched.[12][47]
The lungs are the organs most affected by COVID-19 because the virus accesses host cells via the enzyme ACE2, which is most abundant in the type II alveolar cells of the lungs. The virus uses a special surface glycoprotein called a "spike" (peplomer) to connect to ACE2 and enter the host cell.[48] The density of ACE2 in each tissue correlates with the severity of the disease in that tissue and some have suggested that decreasing ACE2 activity might be protective,[49][50] though another view is that increasing ACE2 using angiotensin II receptor blocker medications could be protective and that these hypotheses need to be tested.[51] As the alveolar disease progresses, respiratory failure might develop and death may follow.[50]
The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelium[12] as well as endothelial cells and enterocytes of the small intestine.[52] The virus has been found in the faeces of as many as 53%[12] of hospitalised people and more anal swab positives have been found than oral swab positives in the later stages of infection.[53] The virus was found in faeces from 1 to 12 days and 17% of patients continued to present the virus in faeces after no longer presenting them in respiratory samples, indicating that the viral gastrointestinal infection and the potential fecal-oral transmission can last even after viral clearance in the respiratory tract.[12] Reoccurrence of the virus has also been detected through anal swabs suggesting a shift from more oral positive during the early stages of the disease to more anal positive during later periods.[53]
The virus is thought to be natural and have an animal origin,[54][55] through spillover infection.[56] The origin is unknown but by December 2019 the spread of infection was almost entirely driven by human-to-human transmission.[57][58] The earliest known infection occurred on 17 November 2019 in Wuhan, China.[59]
-
Microscopy image showing SARS-CoV-2. The spikes on the outer edge of the virus particles resemble a crown, giving the disease its characteristic name.
-
Schematic diagram of the coronavirus particle. S, spike protein; M, membrane protein; E, envelope protein; N, nucleocapsid protein; structural proteins of coronavirus. Coronavirus virion structure.
Diagnosis

The WHO has published several testing protocols for the disease.[61] The standard method of testing is real-time reverse transcription polymerase chain reaction (rRT-PCR).[62] The test can be done on respiratory samples obtained by various methods, including a nasopharyngeal swab or sputum sample.[63] Results are generally available within a few hours to two days.[64][65] Blood tests can be used, but these require two blood samples taken two weeks apart and the results have little immediate value.[66] Chinese scientists were able to isolate a strain of the coronavirus and publish the genetic sequence so that laboratories across the world could independently develop polymerase chain reaction (PCR) tests to detect infection by the virus.[9][67][68] As of 19 March 2020,[69] there were no antibody tests though efforts to develop them are ongoing.[70] The FDA approved the first point-of-care test on 21 March 2020 for use at the end of that month.[71]
Diagnostic guidelines released by Zhongnan Hospital of Wuhan University suggested methods for detecting infections based upon clinical features and epidemiological risk. These involved identifying people who had at least two of the following symptoms in addition to a history of travel to Wuhan or contact with other infected people: fever, imaging features of pneumonia, normal or reduced white blood cell count, or reduced lymphocyte count.[22]
One study in China found that CT scans showed ground-glass opacities in 56%, but 18% had no radiological findings.[72] Bilateral and peripheral ground glass opacities are the most typical CT findings, though they are non-specific.[73] Consolidation, linear opacities and reverse halo sign are other radiological findings.[73] Initially, the lesions are confined to one lung, but as the disease progresses, indications manifest in both lungs in 88% of so-called "late patients" in the study group (the subset for whom time between onset of symptoms and chest CT was 6–12 days).[73] Ground glass opacities are also a common feature in children's disease.[74]
-
Typical CT imaging findings
-
CT imaging of rapid progression stage
Prevention



Preventive measures to reduce the chances of infection include staying at home, avoiding crowded places, washing hands with soap and warm water often and for at least 20 seconds, practicing good respiratory hygiene and avoiding touching the eyes, nose, or mouth with unwashed hands.[80][81][82] The CDC recommends covering the mouth and nose with a tissue when coughing or sneezing, or using inside of the elbow if no tissue is available.[80] They also recommend proper hand hygiene after any cough or sneeze.[80] Social distancing strategies aim to reduce contact of infected persons with large groups by closing schools and workplaces, restricting travel, and canceling mass gatherings.[83] Social distancing also includes that people stay at least 6 feet apart (about 1.80 meters).[84]
Because a vaccine against SARS-CoV-2 is not expected to become available until 2021 at the earliest,[85] a key part of managing the COVID-19 pandemic is trying to decrease the epidemic peak, known as flattening the epidemic curve through various measures seeking to reduce the rate of new infections.[76] Slowing the infection rate helps decrease the risk of health services being overwhelmed, allowing for better treatment of current cases, and delaying additional cases until therapeutics or a vaccine become available.[76]
According to the WHO, the use of masks is recommended only if a person is coughing or sneezing or when one is taking care of someone with a suspected infection.[86] Some countries also recommend healthy individuals to wear face masks, particularly China,[87] Hong Kong[88] and Thailand.[89] In order to meet the need for masks, the WHO estimates that global production will need to increase by 40%. Hoarding and speculation have worsened the problem, with the price of masks increasing sixfold, N95 respirators tripled, and gowns doubled.[90] Some health experts consider wearing non-medical-grade masks and other face coverings like scarves or bandanas a good way to prevent people from touching their mouths and noses, even if non-medical coverings would not protect against a direct sneeze or cough from an infected person.[91]
Those diagnosed with COVID-19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, wear a face mask when exposed to an individual or location of a suspected infection, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.[92][93] The CDC also recommends that individuals wash hands often with soap and water for at least 20 seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose, coughing, or sneezing. It further recommends using an alcohol-based hand sanitizer with at least 60% alcohol, but only when soap and water are not readily available.[80]
For areas where commercial hand sanitizers are not readily available, the WHO suggested two formulations for the local production. In both these formulations the antimicrobial activity of ethanol or isopropanol is enhanced by a low concentration of hydrogen peroxide while glycerol acts as a humectant.[94]
Management
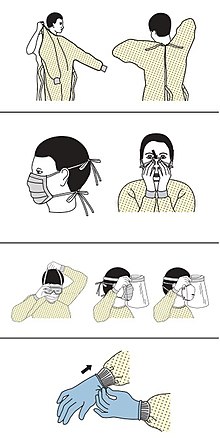
People are managed with supportive care, which may include fluid, oxygen support, and supporting other affected vital organs.[96][97][98] Steroids such as methylprednisolone are not recommended unless the disease is complicated by acute respiratory distress syndrome.[99][100]
The CDC recommends that those who suspect they carry the virus wear a simple face mask.[26] Extracorporeal membrane oxygenation (ECMO) has been used to address the issue of respiratory failure, but its benefits are still under consideration.[72][101] While the WHO does not oppose the use of non-steroidal anti-inflammatory drugs such as ibuprofen for symptoms,[102] some recommend paracetamol (acetaminophen) for first-line use.[103] There are four reported cases of children who developed severe symptoms after taking ibuprofen.[104] While theoretical concerns have been raised about ACE inhibitors and Angiotensin receptor blockers, as of 19 March 2020 these are not sufficient to justify stopping these medications.[105][106][107]
The WHO and Chinese National Health Commission have published recommendations for taking care of people who are hospitalised with COVID-19.[108][109] Intensivists and pulmonologists in the US have compiled treatment recommendations from various agencies into a free resource, the IBCC.[110][111]
Personal protective equipment
Precautions must be taken to minimize the risk of virus transmission, especially in healthcare settings when performing procedures that can generate aerosols, such as intubation or hand ventilation.[112] Some public health officials have complained that the limited supply of protective gear should not be used to expand outpatient testing. New York City has departed from CDC guidelines and is recommending that testing be limited to hospitalized patients to conserve supplies.[113]
CDC outlines the specific guidelines for the use of personal protective equipment (PPE) during the pandemic. The recommended gear is as follows:
- gown
- mask or respirator,[114][115]
- goggles or a face shield,[116]
- medical gloves[117][118]
The N95 respirators are approved for industrial settings but the FDA has authorized the masks for use under an Emergency Use Authorization (EUA). They are designed to protect from airborne particles like dust but effectiveness against a specific biological agent is not guaranteed for off-label uses.[119] When masks are not available the CDC recommends using face shields, or as a last resort homemade masks.[120]
Mechanical ventilation
Most cases of COVID-19 are not severe enough to require mechanical ventilation (artificial assistance to support breathing), but a percentage of cases do.[121][122] Some Canadian doctors recommend the use of invasive mechanical ventilation because this technique limits the spread of aerosolized transmission vectors.[121] Severe cases are most common in older adults (those older than 60 years[121] and especially those older than 80 years[citation needed]). Many developed countries do not have enough hospital beds per capita, which limits a health system's capacity to handle a sudden spike in the number of COVID-19 cases severe enough to require hospitalization.[123] This limited capacity is a significant driver of the need to flatten the curve (to keep the speed at which new cases occur and thus the number of people sick at one point in time lower).[123] One study in China found 5% were admitted to intensive care units, 2.3% needed mechanical support of ventilation, and 1.4% died.[72] Every
ventilator is worth its weight in gold as a source says a third of all Coronavirus patients could need it.</ref>https://meduza.io/amp/en/feature/2020/03/21/the-ventilator-problemCite error: A <ref> tag is missing the closing </ref> (see the help page).
Experimental treatment
No medications are approved to treat the disease by the WHO although some are recommended by individual national medical authorities.[124] Research into potential treatments started in January 2020,[125] and several antiviral drugs are in clinical trials.[126][127] Although new medications may take until 2021 to develop,[128] several of the medications being tested are already approved for other uses, or are already in advanced testing.[124] Antiviral medication may be tried in people with severe disease.[96] The WHO recommended volunteers take part in trials of the effectiveness and safety of potential treatments.[129]
Information technology
In February 2020, China launched a mobile app to deal with the disease outbreak.[130] Users are asked to enter their name and ID number. The app is able to detect 'close contact' using surveillance data and therefore a potential risk of infection. Every user can also check the status of three other users. If a potential risk is detected, the app not only recommends self-quarantine, it also alerts local health officials.[131]
Big data analytics on cellphone data, facial recognition technology, mobile phone tracking and artificial intelligence are used to track infected people and people whom they contacted in South Korea, Taiwan and Singapore.[132][133] In March 2020, the Israeli government enabled security agencies to track mobile phone data of people supposed to have coronavirus. The measure was taken to enforce quarantine and protect those who may come into contact with infected citizens.[134] Also in March 2020, Deutsche Telekom shared private cellphone data with the German federal government agency, Robert Koch Institute, in order to research and prevent the spread of the virus.[135] Russia deployed facial recognition technology to detect quarantine breakers.[136] Italian regional health commissioner Giulio Gallera said that he has been informed by mobile phone operators that "40% of people are continuing to move around anyway".[137] German government conducted a 48 hours weekend hackathon with more than 42.000 participants.[138][139] Also the president of Estonia, Kersti Kaljulaid, made a global call for creative solutions against the spread of coronavirus.[140]
Psychological support
Infected individuals may experience distress from quarantine, travel restrictions, side effects of treatment, or fear of the infection itself. To address these concerns, the National Health Commission of China published a national guideline for psychological crisis intervention on 27 January 2020.[141][142]
Prognosis
The severity of COVID-19 varies. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the common cold. Mild cases typically recover within two weeks, while those with severe or critical disease may take three to six weeks to recover. Among those who have died, the time from symptom onset to death has ranged from two to eight weeks.[32]
Children of all ages are susceptible to the disease, but are likely to have milder symptoms and a much lower chance of severe disease than adults; in those younger than 50 years, the risk of death is less than 0.5%, while in those older than 70 it is more than 8%.[74][144] Pregnant women are at particular risk for severe infection.[145][146]
In some people, COVID-19 may affect the lungs causing pneumonia. In those most severely affected, COVID-19 may rapidly progress to acute respiratory distress syndrome (ARDS) causing respiratory failure, septic shock, or multi-organ failure.[147][148] Complications associated with COVID-19 include sepsis, abnormal clotting, and damage to the heart, kidneys, and liver. Clotting abnormalities, specifically an increase in prothrombin time, have been described in 6% of those admitted to hospital with COVID-19, while abnormal kidney function is seen in 4% of this group.[149] Liver injury as shown by blood markers of liver damage is frequently seen in severe cases.[150]
Some studies have found that the neutrophil to lymphocyte ratio (NLR) may be helpful in early screening for severe illness.[151]
Many of those who die of COVID-19 have pre-existing (underlying) conditions, including hypertension, diabetes mellitus, and cardiovascular disease.[152] The Istituto Superiore di Sanità reported that 68.9% of deaths from the disease in the country had at least one preexisting condition, with patients that had preexisting conditions having an average of 2.7 conditions.[153][154] According to the same report, the median time between onset of symptoms and death was eight days, with half that time being spent hospitalized. However, patients transferred to an ICU had a median time of five days between hospitalization and death.[154] In a study of early cases, the median time from exhibiting initial symptoms to death was 14 days, with a full range of six to 41 days.[155] In a study by the National Health Commission (NHC) of China, men had a death rate of 2.8% while women had a death rate of 1.7%.[156] Histopathological examinations of post-mortem lung samples show diffuse alveolar damage with cellular fibromyxoid exudates in both lungs. Viral cytopathic changes were observed in the pneumocytes. The lung picture resembled acute respiratory distress syndrome (ARDS).[32] In 11.8% of the deaths reported by the National Health Commission of China, heart damage was noted by elevated levels of troponin or cardiac arrest.[40]
Availability of medical resources and the socioeconomics of a region may also affect mortality.[157] Estimates of the mortality from the condition vary because of those regional differences,[158] but also because of methodological difficulties. The under-counting of mild cases can cause the mortality rate to be overestimated.[159] However, the fact that deaths are the result of cases contracted in the past can mean the current mortality rate is underestimated.[160][161]
It is unknown if past infection provides effective and long-term immunity in people who recover from the disease.[162] Immunity is likely, based on the behaviour of other coronaviruses,[163] but cases in which recovery from COVID-19 have been followed by positive tests for coronavirus at a later date have been reported.[164][165] It is unclear if these cases are the result of reinfection, relapse, or testing error.[citation needed]
Concerns have been raised about long-term sequelae of the disease. The Hong Kong Hospital Authority found a drop of 20% to 30% in lung capacity in some people who recovered from the disease, and lung scans suggested organ damage.[166]
| Case fatality rates (%) by age and country | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ |
| China as of 11 February[57] | 0.0 | 0.2 | 0.2 | 0.2 | 0.4 | 1.3 | 3.6 | 8.0 | 14.8 |
| Italy as of 19 March[153] | 0.0 | 0.0 | 0.0 | 0.4 | 0.6 | 1.2 | 4.9 | 15.3 | 23.6 |
| South Korea as of 23 March[167] | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.4 | 1.6 | 6.3 | 11.6 |
| Spain as of 22 March[168] | 0.0 | 0.5 | 0.3 | 0.1 | 0.3 | 0.6 | 2.2 | 5.2 | 17.9 |
| Case fatality rates (%) by age in the United States | |||||||
|---|---|---|---|---|---|---|---|
| Age | 0-19 | 20-44 | 45-54 | 55-64 | 65-74 | 75-84 | ≥85 |
| United States as of 16 March[169] | 0.0 | 0.1-0.2 | 0.5-0.8 | 1.4-2.6 | 2.7-4.9 | 4.3-10.5 | 10.4-27.3 |
| Note: The lower bound includes all cases. The upper bound excludes cases that were missing data. | |||||||
Epidemiology
The case fatality rate (CFR) depends on the availability of healthcare, the typical age and health problems within the population, and the number of undiagnosed cases.[170][171] Preliminary research has yielded case fatality rate numbers between 2% and 3%;[16] in January 2020 the WHO suggested that the case fatality rate was approximately 3%,[172] and 2% in February 2020 in Hubei.[173] Other CFR numbers, which adjust for differences in time of confirmation, death or remission but are not peer reviewed, are respectively 7%[174] and 33% for people in Wuhan 31 January.[175] An unreviewed preprint of 55 deaths noted that early estimates of mortality may be too high as asymptomatic infections are missed. They estimated a mean infection fatality ratio (IFR, the mortality among infected) ranging from 0.8% to 0.9%.[176] A peer-reviewed article published on 19 March estimated the overall symptomatic case fatality risk as 1.4% (IQR 0.9–2.1%).[177] The outbreak in 2019–2020 has caused at least 676,609,955Template:Edit sup confirmed infections and 6,881,955Template:Edit sup deaths.[6]
The epidemic spreads faster where people are close together and/or travel to other areas. Researchers found that travel restrictions can reduce the basic reproduction number from 2.35 to 1.05, allowing the epidemic to be manageable.[178]
An observational study of nine people found no vertical transmission from mother to the newborn.[179] Also, a descriptive study in Wuhan found no evidence of viral transmission through vaginal sex (from female to partner), but authors note that transmission during sex might occur through other routes.[180]
-
Total confirmed cases over time
-
Total deaths over time
-
Total confirmed cases of COVID-19 per million people, 20 March 2020[181]
-
Total confirmed deaths due to COVID-19 per million people, 24 March 2020[182]
Terminology
The World Health Organization announced in February 2020 that COVID-19 is the official name of the disease. World Health Organization chief Tedros Adhanom Ghebreyesus explained that CO stands for corona, VI for virus and D for disease, while 19 is for the year that the outbreak was first identified; 30 December 2019. The name had been chosen to avoid references to a specific geographical location (i.e. China), animal species, or group of people, in line with international recommendations for naming aimed at preventing stigmatisation.[183][184]
While the disease is named COVID-19, the virus that causes it is named severe acute respiratory syndrome coronavirus 2 or SARS-CoV-2.[185] The virus was initially referred to as the 2019 novel coronavirus or 2019-nCoV.[186] The WHO additionally uses "the COVID-19 virus" and "the virus responsible for COVID-19" in public communications.[185]
Coronaviruses were named in 1968 for their appearance in electron micrographs which was reminiscent of the solar corona, corona meaning crown in Latin.[187][188][189]
Research
Because of its key role in the transmission and progression of the disease, ACE2 has been the focus of a significant proportion of research and various therapeutic approaches have been suggested.[50]
Vaccine
There is no available vaccine, but research into developing a vaccine has been undertaken by various agencies. Previous work on SARS-CoV is being utilised because SARS-CoV-2 and SARS-CoV both use the ACE2 receptor to enter human cells.[190] There are three vaccination strategies being investigated. First, researchers aim to build a whole virus vaccine. The use of such a virus, be it inactive or dead, aims to elicit a prompt immune response of the human body to a new infection with COVID-19. A second strategy, subunit vaccines, aims to create a vaccine that sensitises the immune system to certain subunits of the virus. In the case of SARS-CoV-2, such research focuses on the S-spike protein that helps the virus intrude the ACE2 enzyme receptor. A third strategy is that of the nucleic acid vaccines (DNA or RNA vaccines, a novel technique for creating a vaccination). Experimental vaccines from any of these strategies would have to be tested for safety and efficacy.[191]
On 16 March 2020, the first clinical trial of a vaccine started with four volunteers in Seattle. The vaccine contains a harmless genetic code copied from the virus that causes the disease.[192]
One difficulty with vaccine development is that older people, who are more vulnerable to the disease, are often poorly vaccinated due to age-related degradation of the thymus. Therefore, alternative methods will need to be developed to increase immunity in this population. One method being considered is treatment with recombinant interleukin 7, which plays an extremely important role in the maturation and reproduction of lymphoid cells. Using interleukin 7 along with vaccines can boost the immune system's response to infections and increase the growth of restoration cells, thus lowering the risk of death in older people.[193][194]
Antivirals
Several existing antiviral medications are being looked at to treat COVID-19 and some are moving into clinical trials.[124] In March 2020, WHO has launched a multi-country trial involving 10 countries called "Solidarity" in response to COVID-19 pandemic. Remdesivir, chloroquine and hydroxychloroquine, ritonavir/lopinavir and ritonavir/lopinavir combined with interferon beta are the experimental treatments currently being researched under Solidarity Trial.[195][196]
There is tentative evidence for remdesivir as of March 2020.[197] Remdesivir inhibits SARS-CoV-2 in vitro.[198] Phase 3 clinical trials are being conducted in the US, in China, and in Italy.[124][199][200]
Chloroquine, previously used to treat malaria, was being studied in China in February 2020, with positive preliminary results.[201] Chloroquine and hydroxychloroquine effectively inhibit SARS-CoV-2 in vitro,[198] with hydroxychloroquine proving to be more potent than chloroquine and with a more tolerable safety profile.[202] Preliminary results from a trial suggested that chloroquine is effective and safe in treating COVID-19 associated pneumonia, "improving lung imaging findings, promoting a virus-negative conversion, and shortening the disease course".[201] However, there are calls for more review of the research to date.[203] The Guangdong Provincial Department of Science and Technology and the Guangdong Provincial Health and Health Commission issued a report stating that chloroquine phosphate "improves the success rate of treatment and shortens the length of person's hospital stay" and recommended it for people diagnosed with mild, moderate and severe cases of novel coronavirus pneumonia.[204]
On 17 March, the Italian Pharmaceutical Agency included chloroquine and hydroxychloroquine in the list of drugs with positive preliminary results for treatment of COVID-19.[205] Korean and Chinese Health Authorities recommend the use of chloroquine.[206][207] However, the Wuhan Institute of Virology, while recommending a daily dose of one gram, notes that twice that dose is highly dangerous and could be lethal. As of 20 March 2020[update] the treatment has not yet been approved by the U.S. Food and Drug Administration.[208]
The Chinese 7th edition guidelines also include interferon, ribavirin, or umifenovir for use against COVID-19.[207]
In 2020, a trial found that lopinavir/ritonavir was ineffective in the treatment of severe illness.[209] Nitazoxanide has been recommended for further in vivo study after demonstrating low concentration inhibition of SARS-CoV-2.[198]
Studies have demonstrated that initial spike protein priming by transmembrane protease serine 2 (TMPRSS2) is essential for entry of SARS-CoV-2 via interaction with the ACE2 receptor.[210][211] These findings suggest that the TMPRSS2 inhibitor camostat approved for use in Japan for inhibiting fibrosis in liver and kidney disease might constitute an effective off-label treatment.[210]
In February 2020, Favipiravir was being studied in China for experimental treatment of the emergent COVID-19 disease.[212][213]
Anti-cytokine storm
Cytokine storm, a life-threatening medical condition, can be a complication in the later stages of severe COVID-19. There is evidence that hydroxychloroquine has anti-cytokine storm properties.[214]
Tocilizumab has been included in treatment guidelines by China's National Health Commission after a small study was completed.[215][216] It is undergoing a phase 2 non randomized test at the national level in Italy after showing positive results in people with severe disease.[167][217][218][unreliable medical source?] Combined with a serum ferritin blood test to identify cytokine storms, it is meant to counter such developments, which are thought to be the cause of death in some affected people.[219][220][221] The interleukin-6 receptor antagonist was approved by the FDA for treatment against cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017.[222][unreliable medical source?]
The Feinstein Institute of Northwell Health announced in March a study on "a human antibody that may prevent the activity" of IL-6.[223]
Enhanced innate immunity
The eicosanoid signaling molecule leukotriene B4 (LTB4) is a possible (as yet untested) candidate drug for treatment of COVID-19. In the lungs, LTB4 is produced in alveolar macrophages, and it recruits neutrophil white blood cells.
A 1989 study in humans [224] reported an approximately 17-fold (1700%) increase in neutrophil white blood cells in recovered human lung fluid 4 hours after LTB4 injection into the human lung (10 ml LTB4 diluted to 5 x 10-7 M in sterile saline). Notably, there was no significant change in lung epithelial permeability associated with LTB4-induced neutrophil recruitment. Therefore, it was suggested that LTB4 can recruit neutrophils without initiating an inflammatory cascade within the lungs.
Several subsequent studies have reported that LTB4 exerts potent anti-viral (and general anti-microbial) effects in the lung. For example, a 2008 study in mice [225] reported that daily i.v. treatment with LTB4 led to a significant decrease in lung viral loads of influenza A at day 5 post-infection. A follow-up study in 2009 [226] reported that LTB4 potentiates cytokine signaling by human white blood cells (neutrophils in particular), and that this action is not sensitive to chloroquine (raising the possibility that chloroquine and LTB4 could be used in combination to combat viral infection).
More recently, a 2019 study in mice [227] reported that a single dose of LTB4 as late as five days post-infection with influenza A virus (at peak viral load) reduces proliferation of inflammatory monocyte-derived macrophages, controls tissue damage and enhanced significant survival against lethal influenza A infection. With regard to the mechanism of LTB4 action, it was found that LTB4 promotes production of interferon-α (IFN-α) (involved in innate immunity in viral infection) by pulmonary interstitial macrophages, resulting in inhibition of inflammatory monocyte-derived macrophages (the excessive accumulation of which is associated with ‘cytokine storm’ and mortality). However, in influenza A infected mice, LTB4 did not significantly alter neutrophil number.
Furthermore, a 2011 study in humans [228] reported that nasal fluids recovered from healthy subjects who received nasally-administered LTB4, efficiently killed human coronavirus, respiratory syncytial virus, and influenza B virus.
Passive antibody therapy
Transfering donated blood containing antibodies produced by the immune systems of those who have recovered from COVID-19 to people who need them is being investigated as a nonvaccine method of immunization.[229] This strategy was tried for SARS.[229] Viral neutralization is the anticipated mechanism of action by which passive antibody therapy can mediate defense against SARS-CoV-2. Other mechanisms, such as antibody-dependent cellular cytotoxicity and/or phagocytosis, may however be possible.[229] Other forms of passive antibody therapy, for example, using manufactured monoclonal antibodies, are in development.[229] Production of 'convalescent serum', which consists of the liquid portion of the blood from recovered patients and contains antibodies specific to this virus, could be increased for quicker deployment.[230]
See also
- Coronavirus diseases, a group of closely related syndromes
- Li Wenliang, a doctor at Central Hospital of Wuhan, who later contracted COVID-19 and died of it after raising awareness of the spread of the virus.
- Disease X, a World Health Organisation term
References
- ^ 国家卫生健康委关于新型冠状病毒肺炎暂命名事宜的通知 (in Chinese (China)). National Health Commission. 7 February 2020. Archived from the original on 28 February 2020. Retrieved 9 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Campbell, Charlie (20 January 2020). "The Wuhan Pneumonia Crisis Highlights the Danger in China's Opaque Way of Doing Things". Time. Retrieved 13 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Lucey, Daniel; Sparrow, Annie (14 January 2020). "China Deserves Some Credit for Its Handling of the Wuhan Pneumonia". Foreign Policy. Retrieved 13 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ See SARS-CoV-2 for more.
- ^ a b c "Coronavirus Disease 2019 (COVID-19) Symptoms". Centers for Disease Control and Prevention. United States. 10 February 2020. Archived from the original on 30 January 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)". ArcGIS. Johns Hopkins University. Retrieved 10 March 2023.
- ^ "Global Covid-19 Case Fatality Rates". CEBM.
- ^ "Naming the coronavirus disease (COVID-19) and the virus that causes it". World Health Organization (WHO). Archived from the original on 28 February 2020. Retrieved 28 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. (February 2020). "The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China". Int J Infect Dis. 91: 264–66. doi:10.1016/j.ijid.2020.01.009. PMID 31953166.
- ^ a b "WHO Director-General's opening remarks at the media briefing on COVID-19". World Health Organization (WHO) (Press release). 11 March 2020. Retrieved 12 March 2020.
{{cite press release}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: url-status (link) - ^ "Q&A on coronaviruses (COVID-19)". World Health Organization (WHO). Retrieved 11 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c d e Gu, Jinyang; Han, Bing; Wang, Jian (27 February 2020). "COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission". Gastroenterology. doi:10.1053/j.gastro.2020.02.054. ISSN 0016-5085. PMID 32142785.
- ^ Miri, Seyyed Mohammad; Roozbeh, Fatemeh; Omrani Rad, Ali; Alavian, Seyed Moayed (16 March 2020). "Panic of Buying Toilet Papers: A Historical Memory or a Horrible Truth? Systematic Review of Gastrointestinal Manifestations of COVID-19". Hepatitis Monthly. In Press (In Press). doi:10.5812/hepatmon.102729. ISSN 1735-143X.
- ^ Wang, Vivian (5 March 2020). "Most Coronavirus Cases Are Mild. That's Good and Bad News". The New York Times.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b "Q&A on coronaviruses". World Health Organization (WHO). Archived from the original on 20 January 2020. Retrieved 27 January 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b "Coronavirus (COVID-19) Mortality Rate". www.worldometers.info. 5 March 2020. Retrieved 23 March 2020.
- ^ a b c "Q&A on coronaviruses". World Health Organization. 11 February 2020. Retrieved 24 February 2020.
- ^ a b c "Coronavirus Disease 2019 (COVID-19) - Transmission". Centers for Disease Control and Prevention. 17 March 2020. Retrieved 23 March 2020.
- ^ "New coronavirus stable for hours on surfaces". National Institutes of Health. Retrieved 23 March 2020.
- ^ "Symptoms of Novel Coronavirus (2019-nCoV)". www.cdc.gov. 10 February 2020. Archived from the original on 30 January 2020. Retrieved 11 February 2020.
- ^ Velavan TP, Meyer CG (March 2020). "The COVID-19 epidemic". Tropical Medicine & International Health. n/a (n/a): 278–80. doi:10.1111/tmi.13383. PMID 32052514.
- ^ a b Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. (February 2020). "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)". Military Medical Research. 7 (1): 4. doi:10.1186/s40779-020-0233-6. PMC 7003341. PMID 32029004.
- ^ "CT provides best diagnosis for COVID-19". ScienceDaily. 26 February 2020. Retrieved 2 March 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ "Advice for public". World Health Organization (WHO). Archived from the original on 26 January 2020. Retrieved 25 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Guidance on social distancing for everyone in the UK". GOV.UK. Retrieved 25 March 2020.
- ^ a b CDC (11 February 2020). "2019 Novel Coronavirus (2019-nCoV)". Centers for Disease Control and Prevention. Archived from the original on 14 February 2020. Retrieved 15 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Advice for public". World Health Organization (WHO). Archived from the original on 26 January 2020. Retrieved 15 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention (CDC). 15 February 2020. Archived from the original on 26 February 2020. Retrieved 20 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)". World Health Organization (WHO). Archived from the original on 31 January 2020. Retrieved 11 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Mahtani S, Berger M, O'Grady S, Iati M (6 February 2020). "Hundreds of evacuees to be held on bases in California; Hong Kong and Taiwan restrict travel from mainland China". The Washington Post. Archived from the original on 7 February 2020. Retrieved 11 February 2020.
- ^ World Health Organization (March 2020). "Coronavirus disease 2019 (COVID-19): situation report, 47" (Document). hdl:10665/331444.
{{cite document}}: Cite document requires|publisher=(help); Unknown parameter|website=ignored (help) - ^ a b c Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) (PDF) (Report). World Health Organization (WHO). 16–24 February 2020. Retrieved 21 March 2020.
- ^ "Loss of sense of smell as marker of COVID-19 infection".
{{cite web}}: CS1 maint: url-status (link) - ^ a b Wei, Xiao-Shan; Wang, Xuan; Niu, Yi-Ran; Ye, Lin-Lin; Peng, Wen-Bei; Wang, Zi-Hao; Yang, Wei-Bing; Yang, Bo-Han; Zhang, Jian-Chu; Ma, Wan-Li; Wang, Xiao-Rong (26 February 2020). Clinical Characteristics of SARS-CoV-2 Infected Pneumonia with Diarrhea. Rochester, NY. SSRN 3546120.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. (February 2020). "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study". Lancet. 395 (10223): 507–513. doi:10.1016/S0140-6736(20)30211-7. PMID 32007143.
- ^ Hessen, Margaret Trexler (27 January 2020). "Novel Coronavirus Information Center: Expert guidance and commentary". Elsevier Connect. Archived from the original on 30 January 2020. Retrieved 31 January 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Coronavirus Disease 2019 (COVID-19)—Symptoms". Centers for Disease Control and Prevention. 20 March 2020. Retrieved 21 March 2020.
- ^ Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (February 2020). "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". Lancet. 395 (10223): 497–506. doi:10.1016/S0140-6736(20)30183-5. PMID 31986264.
- ^ Lai, Chih-Cheng; Shih, Tzu-Ping; Ko, Wen-Chien; Tang, Hung-Jen; Hsueh, Po-Ren (1 March 2020). "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges". International Journal of Antimicrobial Agents. 55 (3): 105924. doi:10.1016/j.ijantimicag.2020.105924. ISSN 0924-8579. PMID 32081636.
- ^ a b Zheng YY, Ma YT, Zhang JY, Xie X (March 2020). "COVID-19 and the cardiovascular system". Nature Reviews. Cardiology. doi:10.1038/s41569-020-0360-5. PMID 32139904.
- ^ World Health Organization (19 February 2020). "Coronavirus disease 2019 (COVID-19): situation report, 29" (Document). hdl:10665/331118.
{{cite document}}: Cite document requires|publisher=(help); Unknown parameter|website=ignored (help) - ^ "Q&A on coronaviruses (COVID-19): How long is the incubation period for COVID-19?". World Health Organization (WHO). Archived from the original on 20 January 2020. Retrieved 26 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Lauer, Stephen A.; Grantz, Kyra H.; Bi, Qifang; Jones, Forrest K.; Zheng, Qulu; Meredith, Hannah R.; Azman, Andrew S.; Reich, Nicholas G.; Lessler, Justin (10 March 2020). "The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application". Annals of Internal Medicine. doi:10.7326/M20-0504. ISSN 0003-4819.
- ^ Gorbalenya, Alexander E. (11 February 2020). "Severe acute respiratory syndrome-related coronavirus—The species and its viruses, a statement of the Coronavirus Study Group". bioRxiv (preprint). doi:10.1101/2020.02.07.937862.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "2019 Novel Coronavirus (2019-nCoV)". Centers for Disease Control and Prevention. 11 February 2020. Archived from the original on 7 March 2020. Retrieved 18 February 2020.
The virus is thought to spread mainly from person-to-person ... through respiratory droplets produced when an infected person coughs or sneezes.
- ^ van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. (March 2020). "Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1". The New England Journal of Medicine. Massachusetts Medical Society. doi:10.1056/nejmc2004973. PMID 32182409.
- ^ Water Transmission and COVID-19 (CDC, accessed 19 March 2020)
- ^ Letko M, Marzi A, Munster V (2020). "Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses". Nature Microbiology: 1–8. doi:10.1038/s41564-020-0688-y. PMID 32094589.
- ^ Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (March 2020). "Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target". Intensive Care Medicine. doi:10.1007/s00134-020-05985-9. PMID 32125455.
- ^ a b c Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. (February 2020). "High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa". International Journal of Oral Science. 12 (1): 8. doi:10.1038/s41368-020-0074-x. PMC 7039956. PMID 32094336.
- ^ Gurwitz D (March 2020). "Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics". Drug Development Research. doi:10.1002/ddr.21656. PMID 32129518.
- ^ Hamming, I.; Timens, W.; Bulthuis, M. L. C.; Lely, A. T.; Navis, G. J.; Goor, H. van (2004). "Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis". The Journal of Pathology. 203 (2): 631–637. doi:10.1002/path.1570. ISSN 1096-9896. PMID 15141377.
- ^ a b Zhang, Wei; Du, Rong-Hui; Li, Bei; Zheng, Xiao-Shuang; Yang, Xing-Lou; Hu, Ben; Wang, Yan-Yi; Xiao, Geng-Fu; Yan, Bing; Shi, Zheng-Li; Zhou, Peng (1 January 2020). "Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes". Emerging Microbes & Infections. 9 (1): 386–389. doi:10.1080/22221751.2020.1729071. PMC 7048229. PMID 32065057.
- ^ Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. (23 January 2020). "Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin". bioRxiv (preprint). doi:10.1101/2020.01.22.914952.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (17 March 2020). "The proximal origin of SARS-CoV-2". Nature Medicine: 1–3. doi:10.1038/s41591-020-0820-9. Retrieved 18 March 2020.
- ^ Berger, Kevin (12 March 2020). "The Man Who Saw the Pandemic Coming". Nautilus. Retrieved 16 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d Yanping Z, et al. (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team) (17 February 2020). "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020". China CDC Weekly. 2 (8). Chinese Center for Disease Control and Prevention: 113–122. Retrieved 18 March 2020.
- ^ Heymann DL, Shindo N (February 2020). "COVID-19: what is next for public health?". Lancet. 395 (10224): 542–45. doi:10.1016/S0140-6736(20)30374-3. PMID 32061313.
- ^ Davidson, Helen (13 March 2020). "First Covid-19 case happened in November, China government records show—report". The Guardian. Retrieved 21 March 2020.
- ^ CDC (5 February 2020). "CDC Tests for 2019-nCoV". Centers for Disease Control and Prevention. Archived from the original on 14 February 2020. Retrieved 12 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". World Health Organization (WHO). Retrieved 13 March 2020.
- ^ "2019 Novel Coronavirus (2019-nCoV) Situation Summary". Centers for Disease Control and Prevention. 30 January 2020. Archived from the original on 26 January 2020. Retrieved 30 January 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Real-Time RT-PCR Panel for Detection 2019-nCoV". Centers for Disease Control and Prevention. 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe". GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Brueck, Hilary (30 January 2020). "There's only one way to know if you have the coronavirus, and it involves machines full of spit and mucus". Business Insider. Archived from the original on 1 February 2020. Retrieved 1 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". Archived from the original on 21 February 2020. Retrieved 26 February 2020.
- ^ Cohen J, Normile D (January 2020). "New SARS-like virus in China triggers alarm" (PDF). Science. 367 (6475): 234–35. Bibcode:2020Sci...367..234C. doi:10.1126/science.367.6475.234. PMID 31949058. Archived (PDF) from the original on 11 February 2020. Retrieved 11 February 2020.
- ^ "Severe acute respiratory syndrome coronavirus 2 data hub". NCBI. Retrieved 4 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: url-status (link) - ^ Vogel, Gretchen (2020). "New blood tests for antibodies could show true scale of coronavirus pandemic". Science. doi:10.1126/science.abb8028. ISSN 0036-8075.
- ^ Pang J, Wang MX, Ang IY, Tan SH, Lewis RF, Chen JI, et al. (February 2020). "Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review". Journal of Clinical Medicine. 9 (3): 623. doi:10.3390/jcm9030623. PMID 32110875.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Coronavirus (COVID-19) Update: FDA Issues first Emergency Use Authorization for Point of Care Diagnostic" (Press release). FDA. 21 March 2020. Retrieved 22 March 2020.
- ^ a b c Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. (February 2020). "Clinical Characteristics of Coronavirus Disease 2019 in China". The New England Journal of Medicine. Massachusetts Medical Society. doi:10.1056/nejmoa2002032. PMID 32109013.
- ^ a b c Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. (February 2020). "Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection". Radiology: 200463. doi:10.1148/radiol.2020200463. PMID 32077789.
- ^ a b Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. (18 March 2020). "SARS-CoV-2 Infection in Children". New England Journal of Medicine. Massachusetts Medical Society. doi:10.1056/nejmc2005073. ISSN 0028-4793. PMID 32187458.
- ^ Wiles, Siouxsie (9 March 2020). "The three phases of Covid-19 – and how we can make it manageable". The Spinoff. Retrieved 9 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD (March 2020). "How will country-based mitigation measures influence the course of the COVID-19 epidemic?". Lancet. 395 (10228): 931–934. doi:10.1016/S0140-6736(20)30567-5. PMID 32164834.
A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation—e.g. minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies.
- ^ Barclay, Eliza (10 March 2020). "How canceled events and self-quarantines save lives, in one chart". Vox.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Wiles, Siouxsie (14 March 2020). "After 'Flatten the Curve', we must now 'Stop the Spread'. Here's what that means". The Spinoff. Retrieved 13 March 2020.
- ^ Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD (March 2020). "How will country-based mitigation measures influence the course of the COVID-19 epidemic?". Lancet. 395 (10228): 931–934. doi:10.1016/S0140-6736(20)30567-5. PMID 32164834.
- ^ a b c d Centers for Disease Control (3 February 2020). "Coronavirus Disease 2019 (COVID-19): Prevention & Treatment". Archived from the original on 15 December 2019. Retrieved 10 February 2020.
- ^ World Health Organization. "Advice for Public". Archived from the original on 26 January 2020. Retrieved 10 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "My Hand-Washing Song: Readers Offer Lyrics For A 20-Second Scrub". NPR.org. Retrieved 20 March 2020.
- ^ Maragakis, Lisa Lockerd. "Coronavirus, Social Distancing and Self Quarantine". www.hopkinsmedicine.org. John Hopkins University.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Parker-Pope, Tara (19 March 2020). "Deciding How Much Distance You Should Keep". The New York Times. ISSN 0362-4331. Retrieved 20 March 2020.
- ^ Grenfell, Rob; Drew, Trevor (17 February 2020). "Here's Why It's Taking So Long to Develop a Vaccine for the New Coronavirus". Science Alert. Archived from the original on 28 February 2020. Retrieved 26 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "When and how to use masks". World Health Organization (WHO). Retrieved 8 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "For different groups of people: how to choose masks". NHC.gov.cn. National Health Commission of the People's Republic of China. 7 February 2020. Retrieved 22 March 2020.
Disposable medical masks: Recommended for: · People in crowded places · Indoor working environment with a relatively dense population · People going to medical institutions · Children in kindergarten and students at school gathering to study and do other activities
- ^ "Prevention of Coronavirus Disease 2019 (COVID-19)" (PDF). Centre for Health Protection. Retrieved 22 March 2020.
Wear a surgical mask when taking public transport or staying in crowded places.
- ^ Kuhakan, Jiraporn (12 March 2020). "'Better than nothing': Thailand encourages cloth masks amid surgical mask shortage". Reuters.
Thailand's health authorities are encouraging people to make cloth face masks at home to guard against the spread of the coronavirus amid a shortage of surgical masks. ... The droplet from coughing and sneezing is around five microns and we have tested already that cloth masks can protect against droplets bigger than one micron.
- ^ "Shortage of personal protective equipment endangering health workers worldwide" (Press release). WHO. 3 March 2020. Retrieved 24 March 2020.
- ^ "Guidance against wearing masks for the coronavirus is wrong – you should cover your face - The Boston Globe". BostonGlobe.com.
- ^ "Coronavirus Disease 2019 (COVID-19)—Prevention & Treatment". Centers for Disease Control and Prevention. U.S. Department of Health & Human Services. 10 March 2020.
- ^ Centers for Disease Control and Prevention (11 February 2020). "What to do if you are sick with 2019 Novel Coronavirus (2019-nCoV)". Archived from the original on 14 February 2020. Retrieved 13 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "WHO-recommended handrub formulations". WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. World Health Organization. 19 March 2009. Retrieved 19 March 2020.
- ^ "Sequence for Putting On Personal Protective Equipment (PPE)" (PDF). CDC. Retrieved 8 March 2020.
- ^ a b Fisher D, Heymann D (February 2020). "Q&A: The novel coronavirus outbreak causing COVID-19". BMC Medicine. 18 (1): 57. doi:10.1186/s12916-020-01533-w. PMC 7047369. PMID 32106852.
- ^ Kui L, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. (February 2020). "Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province". Chinese Medical Journal: 1. doi:10.1097/CM9.0000000000000744. PMID 32044814.
- ^ Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B (March 2020). "Comorbidities and multi-organ injuries in the treatment of COVID-19". Lancet. 395 (10228). Elsevier BV: e52. doi:10.1016/s0140-6736(20)30558-4. PMID 32171074.
- ^ Vetter P, Eckerle I, Kaiser L (February 2020). "Covid-19: a puzzle with many missing pieces". BMJ. 368: m627. doi:10.1136/bmj.m627. PMID 32075791.
- ^ "Novel Coronavirus—COVID-19: What Emergency Clinicians Need to Know". www.ebmedicine.net. Retrieved 9 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Henry, Brandon Michael (2020). "COVID-19, ECMO, and lymphopenia: a word of caution". The Lancet Respiratory Medicine. Elsevier BV. doi:10.1016/s2213-2600(20)30119-3. ISSN 2213-2600. PMID 32178774.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ AFP (19 March 2020). "Updated: WHO Now Doesn't Recommend Avoiding Ibuprofen For COVID-19 Symptoms". ScienceAlert. Retrieved 19 March 2020.
- ^ Day, Michael (17 March 2020). "Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists". BMJ. 368: m1086. doi:10.1136/bmj.m1086. PMID 32184201. Retrieved 18 March 2020.
- ^ Day, Michael (17 March 2020). "Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists". BMJ. 368. doi:10.1136/bmj.m1086. ISSN 1756-1833. PMID 32184201.
- ^ "Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician". Retrieved 21 March 2020.
- ^ "Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician". American Heart Association (Press release). 17 March 2020. Retrieved 25 March 2020.
- ^ de Simone, Giovanni. "Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers". Council on Hypertension of the European Society of Cardiology. Retrieved 24 March 2020.
- ^ Cheng ZJ, Shan J (February 2020). "2019 Novel coronavirus: where we are and what we know". Infection. doi:10.1007/s15010-020-01401-y. PMID 32072569.
- ^ "Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected". World Health Organization (WHO). Archived from the original on 31 January 2020. Retrieved 13 February 2020.
- ^ Farkas, Josh (March 2020). COVID-19—The Internet Book of Critical Care (digital) (Reference manual). USA: EMCrit. Archived from the original on 11 March 2020. Retrieved 13 March 2020.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "COVID19—Resources for Health Care Professionals". Penn Libraries. 11 March 2020. Retrieved 13 March 2020.
- ^ Cheung JC, Ho LT, Cheng JV, Cham EY, Lam KN (February 2020). "Staff safety during emergency airway management for COVID-19 in Hong Kong". Lancet Respiratory Medicine. doi:10.1016/s2213-2600(20)30084-9. PMID 32105633.
- ^ "Hospital workers battling coronavirus turn to bandannas, sports goggles and homemade face shields amid shortages". The Washington Post. 19 March 2020.
- ^ Filtering out Confusion: Frequently Asked Questions about Respiratory Protection, User Seal Check. The National Institute for Occupational Safety and Health (April 2018). Retrieved 16 March 2020.
- ^ Proper N95 Respirator Use for Respiratory Protection Preparedness. NIOSH Science Blog (16 March 2020). Retrieved 16 March 2020.
- ^ "Strategies for Optimizing the Supply of Eye Protection". CDC.
- ^ "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020. Retrieved 8 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020. Retrieved 11 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Coronavirus Disease 2019 (COVID-19) Frequently Asked Questions". Food and Drug Administration.
- ^ "Strategies for Optimizing the Supply of Facemasks". CDC.
- ^ a b c Murthy S, Gomersall CD, Fowler RA (11 March 2020). "Care for Critically Ill Patients With COVID-19". JAMA. doi:10.1001/jama.2020.3633. PMID 32159735.
- ^ World Health Organization (28 January 2020). "Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected" (PDF).
- ^ a b Scott, Dylan (16 March 2020). "Coronavirus is exposing all of the weaknesses in the US health system High health care costs and low medical capacity made the US uniquely vulnerable to the coronavirus". Vox. Retrieved 18 March 2020.
- ^ a b c d Li G, De Clercq E (March 2020). "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)". Nature Reviews. Drug Discovery. 19 (3): 149–150. doi:10.1038/d41573-020-00016-0. PMID 32127666.
- ^ "Chinese doctors using plasma therapy on coronavirus, WHO says 'very valid' approach". Reuters. 17 February 2020 – via www.reuters.com.
- ^ Steenhuysen, Julie; Kelland, Kate (24 January 2020). "With Wuhan virus genetic code in hand, scientists begin work on a vaccine". Reuters. Archived from the original on 25 January 2020. Retrieved 25 January 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Duddu, Praveen (19 February 2020). "Coronavirus outbreak: Vaccines/drugs in the pipeline for Covid-19". clinicaltrialsarena.com. Archived from the original on 19 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Lu H (28 January 2020). "Drug treatment options for the 2019-new coronavirus (2019-nCoV)". Biosci Trends. 14 (1): 69–71. doi:10.5582/bst.2020.01020. PMID 31996494.
- ^ Nebehay, Stephanie; Kelland, Kate; Liu, Roxanne (5 February 2020). "WHO: 'no known effective' treatments for new coronavirus". Thomson Reuters. Archived from the original on 5 February 2020. Retrieved 5 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "China launches coronavirus 'close contact' app". BBC News. 11 February 2020. Retrieved 7 March 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Chen, Angela. "China's coronavirus app could have unintended consequences". MIT Technology Review. Retrieved 7 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Gov in the Time of Corona". GovInsider. 19 March 2020. Retrieved 20 March 2020.
- ^ Manancourt, Vincent (10 March 2020). "Coronavirus tests Europe's resolve on privacy". POLITICO. Retrieved 20 March 2020.
- ^ Tidy, Joe (17 March 2020). "Coronavirus: Israel enables emergency spy powers". BBC News. Retrieved 18 March 2020.
- ^ Paksoy, Yunus. "German telecom giant shares private data with government amid privacy fears". trtworld. Retrieved 20 March 2020.
- ^ "Moscow deploys facial recognition technology for coronavirus quarantine". Reuters. 21 February 2020. Retrieved 20 March 2020.
- ^ "Italians scolded for flouting lockdown as death toll nears 3,000". Pittsburgh Post-Gazette. Retrieved 20 March 2020.
- ^ "Kreative Lösungen gesucht". Startseite (in German).
- ^ Dannewitz, Juliane (23 March 2020). "Hackathon Germany: #WirvsVirus". Datenschutzbeauftragter (in German).
- ^ ERR, BNS (21 March 2020). "President makes global call to combat coronavirus via hackathon". ERR.
{{cite news}}: Cite has empty unknown parameter:|1=(help) - ^ Xiang YT, Yang Y, Li W, Zhang L, Zhang Q, Cheung T, et al. (March 2020). "Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed". The Lancet. Psychiatry. 7 (3): 228–29. doi:10.1016/S2215-0366(20)30046-8. PMID 32032543.
- ^ Kang L, Li Y, Hu S, Chen M, Yang C, Yang BX, et al. (March 2020). "The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus". The Lancet. Psychiatry. 7 (3): e14. doi:10.1016/S2215-0366(20)30047-X. PMID 32035030.
- ^ Roser, Max; Ritchie, Hannah; Ortiz-Ospina, Esteban (4 March 2020). "Coronavirus Disease (COVID-19)". Our World in Data. Retrieved 12 March 2020.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S (2020). "Epidemiological Characteristics of 2143 Pediatric Patients With 2019 Coronavirus Disease in China" (PDF). Pediatrics: e20200702. doi:10.1542/peds.2020-0702. PMID 32179660.
- ^ Fang L, Karakiulakis G, Roth M (March 2020). "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?". The Lancet Respiratory Medicine. 395 (10224): e40. doi:10.1016/S0140-6736(20)30311-1. PMID 32171062.
- ^ "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020. Retrieved 2 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Heymann DL, Shindo N, et al. (WHO Scientific and Technical Advisory Group for Infectious Hazards) (February 2020). "COVID-19: what is next for public health?". Lancet. 395 (10224). Elsevier BV: 542–545. doi:10.1016/s0140-6736(20)30374-3. PMID 32061313.
- ^ Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020). "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32150360. Retrieved 18 March 2020.
- ^ Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. (2020). "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study". The Lancet. Elsevier BV. doi:10.1016/s0140-6736(20)30566-3. ISSN 0140-6736. PMID 32171076.
- ^ Xu L, Liu J, Lu M, Yang D, Zheng X (March 2020). "Liver injury during highly pathogenic human coronavirus infections". Liver International. doi:10.1111/liv.14435. PMID 32170806.
- ^ Tian, Dai-Shi; Wang, Wei; Shang, Ke; Ma, Ke; Xie, Cuihong; Tao, Yu; Yang, Sheng; Zhang, Shuoqi; Hu, Ziwei; Zhou, Luoqi; Qin, Chuan (12 March 2020). "Dysregulation of immune response in patients with COVID-19 in Wuhan, China". Clinical Infectious Diseases. doi:10.1093/cid/ciaa248. PMID 32161940.
- ^ "WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus". World Health Organization (WHO).
- ^ a b Epidemia COVID-19. Aggiornamento nazionale 19 marzo 2020 (PDF) (Report) (in Italian). Rome: Istituto Superiore di Sanità. 19 March 2020. Retrieved 22 March 2020.
- ^ a b Report sulle caratteristiche dei pazienti deceduti positivi a COVID-19 in Italia (PDF) (Report) (in Italian). Rome: Istituto Superiore di Sanità. 21 March 2020. Retrieved 23 March 2020.
- ^ Wang W, Tang J, Wei F (April 2020). "Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China". Journal of Medical Virology. 92 (4): 441–47. doi:10.1002/jmv.25689. PMID 31994742.
- ^ "Coronavirus Age, Sex, Demographics (COVID-19)". www.worldometers.info. Archived from the original on 27 February 2020. Retrieved 26 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Ji Y, Ma Z, Peppelenbosch MP, Pan Q (February 2020). "Potential association between COVID-19 mortality and health-care resource availability". Lancet Global Health. 8 (4): e480. doi:10.1016/S2214-109X(20)30068-1. PMID 32109372.
- ^ Li XQ, Cai WF, Huang LF, Chen C, Liu YF, Zhang ZB, et al. (March 2020). "[Comparison of epidemic characteristics between SARS in2003 and COVID-19 in 2020 in Guangzhou]". Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi (in Chinese). 41 (5): 634–637. doi:10.3760/cma.j.cn112338-20200228-00209. PMID 32159317.
- ^ Jung SM, Akhmetzhanov AR, Hayashi K, Linton NM, Yang Y, Yuan B, et al. (February 2020). "Real-Time Estimation of the Risk of Death from Novel Coronavirus (COVID-19) Infection: Inference Using Exported Cases". Journal of Clinical Medicine. 9 (2): 523. doi:10.3390/jcm9020523. PMC 7074479. PMID 32075152.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Chughtai A, Malik A (March 2020). "Is Coronavirus disease (COVID-19) case fatality ratio underestimated?". Global Biosecurity. 1 (3). doi:10.31646/gbio.56 (inactive 19 March 2020).
{{cite journal}}: CS1 maint: DOI inactive as of March 2020 (link) - ^ Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G (March 2020). "Real estimates of mortality following COVID-19 infection". The Lancet Infectious Diseases. doi:10.1016/S1473-3099(20)30195-X. PMID 32171390.
- ^ "BSI open letter to Government on SARS-CoV-2 outbreak response | British Society for Immunology". www.immunology.org. Retrieved 15 March 2020.
- ^ "Can you get coronavirus twice or does it cause immunity?". The Independent. 13 March 2020. Retrieved 15 March 2020.
- ^ "They survived the coronavirus. Then they tested positive again. Why?". Los Angeles Times. 13 March 2020. Retrieved 15 March 2020.
- ^ "14% of Recovered Covid-19 Patients in Guangdong Tested Positive Again—Caixin Global". www.caixinglobal.com. Retrieved 15 March 2020.
- ^ Cheung, Elizabeth (13 March 2020). "Some recovered Covid-19 patients may have lung damage, doctors say". South China Morning Post.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b 코로나바이러스감염증-19 국내 발생 현황 (3월 23일, 정례브리핑) (Report). Korea Centers for Disease Control and Prevention. 23 March 2020. Retrieved 24 March 2020.
- ^ Actualización nº53. Enfermedad por el coronavirus (COVID-19) (PDF) (Report) (in Spanish). Ministerio de Sanidad, Consumo y Bienestar Social. 23 March 2020. Retrieved 24 March 2020.
- ^ "Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States, February 12–March 16, 2020". Centers for Disease Control. 16 March 2020. Retrieved 22 March 2020.
- ^ "Limited data on coronavirus may be skewing assumptions about severity". STAT. 30 January 2020. Archived from the original on 1 February 2020. Retrieved 1 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sparrow, Annie. "How China's Coronavirus Is Spreading—and How to Stop It". Foreign Policy. Archived from the original on 31 January 2020. Retrieved 2 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "WHOが"致死率3%程度" 専門家「今後 注意が必要」". NHK. 24 January 2020. Archived from the original on 26 January 2020. Retrieved 3 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Boseley, Sarah (17 February 2020). "Coronavirus causes mild disease in four in five patients, says WHO". The Guardian. Archived from the original on 18 February 2020. Retrieved 18 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Diao, Ying; Liu, Xiaoyun; Wang, Tao; Zeng, Xiaofei; Dong, Chen; Zhou, Changlong; Zhang, Yuanming; She, Xuan; Liu, Dingfu; Hu, Zhongli (20 February 2020). "Estimating the cure rate and case fatality rate of the ongoing epidemic COVID-19". MedRxiv (preprint). doi:10.1101/2020.02.18.20024513.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "2019-nCoV: preliminary estimates of the confirmed-case-fatality-ratio and infection-fatality-ratio, and initial pandemic risk assessment". institutefordiseasemodeling.github.io. Retrieved 1 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Report 4: Severity of 2019-novel coronavirus (nCoV)" (PDF). Archived (PDF) from the original on 10 February 2020. Retrieved 10 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Wu, Joseph T.; Leung, Kathy; Bushman, Mary; Kishore, Nishant; Niehus, Rene; de Salazar, Pablo M.; Cowling, Benjamin J.; Lipsitch, Marc; Leung, Gabriel M. (19 March 2020). "Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China". Nature Medicine: 1–5. doi:10.1038/s41591-020-0822-7 – via www.nature.com.
- ^ Kucharski, Adam J; Russell, Timothy W; Diamond, Charlie; Liu, Yang; Edmunds, John; Funk, Sebastian; Eggo, Rosalind M; Sun, Fiona; Jit, Mark; Munday, James D; Davies, Nicholas; Gimma, Amy; van Zandvoort, Kevin; Gibbs, Hamish; Hellewell, Joel; Jarvis, Christopher I; Clifford, Sam; Quilty, Billy J; Bosse, Nikos I; Abbott, Sam; Klepac, Petra; Flasche, Stefan (March 2020). "Early dynamics of transmission and control of COVID-19: a mathematical modelling study". The Lancet Infectious Diseases. doi:10.1016/S1473-3099(20)30144-4. PMID 32171059.
- ^ Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. (February 2020). "Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records". Lancet. 395 (10226): 809–15. doi:10.1016/S0140-6736(20)30360-3. PMID 32151335.
- ^ Cui, Pengfei; Chen, Zhe; Wang, Tian; Dai, Jun; Zhang, Jinjin; Ding, Ting; Jiang, Jingjing; Liu, Jia; Zhang, Cong; Shan, Wanying; Wang, Sheng; Rong, Yueguang; Chang, Jiang; Miao, Xiaoping; Ma, Xiangyi; Wang, Shixuan (27 February 2020). "Clinical features and sexual transmission potential of SARS-CoV-2 infected female patients: a descriptive study in Wuhan, China". MedRxiv (preprint). doi:10.1101/2020.02.26.20028225.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Total confirmed cases of COVID-19 per million people". Our World in Data. Retrieved 20 March 2020.
- ^ "Total confirmed deaths due to COVID-19 per million people". Our World in Data. Retrieved 24 March 2020.
- ^ "Novel coronavirus named 'Covid-19': WHO". TODAYonline. Retrieved 11 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "The coronavirus spreads racism against—and among—ethnic Chinese". The Economist. 17 February 2020. Archived from the original on 17 February 2020. Retrieved 17 February 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b "Naming the coronavirus disease (COVID-19) and the virus that causes it". World Health Organization (WHO). Retrieved 13 March 2020.
- ^ "Novel Coronavirus(2019-nCoV) Situation Report—10" (PDF). World Health Organization (WHO). 30 January 2020. Retrieved 15 March 2020.
- ^ "Virology: Coronaviruses". Nature. 220 (5168): 650–650. 1968. doi:10.1038/220650b0. ISSN 0028-0836.
- ^ Definition of Coronavirus by Merriam-Webster, Merriam-Webster, retrieved 24 March 2020
- ^ Definition of Corona by Merriam-Webster, Merriam-Webster, retrieved 24 March 2020
- ^ Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (March 2020). "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls [Internet]. PMID 32150360. Bookshelf ID: NBK554776.
- ^ Chen WH, Strych U, Hotez PJ, Bottazzi ME (3 March 2020). "The SARS-CoV-2 Vaccine Pipeline: an Overview". Current Tropical Medicine Reports. doi:10.1007/s40475-020-00201-6.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Roberts, Michelle (17 March 2020). "Coronavirus: US volunteers test first vaccine". BBC News. Retrieved 17 March 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Aspinall, R., & Lang, P. O. (2018). Interventions to restore appropriate immune function in the elderly. Immunity & Ageing, 15(1), 5. doi:10.1186/s12979-017-0111-6 PMC 5785902
- ^ Aspinall, R., & Lang, P. O. (2018). Interleukin-7 and Immunorejuvenation In: Fulop T., Franceschi C., Hirokawa K., Pawelec G. (eds) Handbook of Immunosenescence. Springer, Cham. https://doi.org/10.1007/978-3-319-64597-1_72-1
- ^ Kupferschmidt, Kai; CohenMar. 22, Jon; 2020; Pm, 3:28 (22 March 2020). "WHO launches global megatrial of the four most promising coronavirus treatments". Science | AAAS. Retrieved 23 March 2020.
{{cite web}}:|last3=has numeric name (help)CS1 maint: numeric names: authors list (link) - ^ "UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment". UN News. 18 March 2020. Retrieved 23 March 2020.
- ^ Ko WC, Rolain JM, Lee NY, Chen PL, Huang CT, Lee PI, Hsueh PR (March 2020). "Arguments in favor of remdesivir for treating SARS-CoV-2 infections". International Journal of Antimicrobial Agents: 105933. doi:10.1016/j.ijantimicag.2020.105933. PMID 32147516.
- ^ a b c Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. (February 2020). "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro". Cell Research. 30 (3): 269–71. doi:10.1038/s41422-020-0282-0. PMC 7054408. PMID 32020029.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Beeching, Nicholas J.; Fletcher, Tom E.; Fowler, Robert (2020). "BMJ Best Practices: COVID-19" (PDF). BMJ.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: url-status (link) - ^ "AIFA e Gilead annunciano che l'Italia è tra i Paesi che testeranno l'antivirale remdesivir per il trattamento del COVID-19". aifa.gov.it (in Italian). Retrieved 19 March 2020.
- ^ a b Gao J, Tian Z, Yang X (February 2020). "Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies". Bioscience Trends. 14 (1): 72–73. doi:10.5582/bst.2020.01047. PMID 32074550.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. (March 2020). "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)". Clinical Infectious Diseases. doi:10.1093/cid/ciaa237. PMID 32150618.
- ^ Touret F, de Lamballerie X (March 2020). "Of chloroquine and COVID-19". Antiviral Research. 177: 104762. doi:10.1016/j.antiviral.2020.104762. PMID 32147496.
- ^ multicenter collaboration group of Department of Science Technology of Guangdong Province Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia (February 2020). "[Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]". Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua Jiehe He Huxi Zazhi = Chinese Journal of Tuberculosis and Respiratory Diseases. 43: E019. doi:10.3760/cma.j.issn.1001-0939.2020.0019. PMID 32075365.
- ^ "Azioni intraprese per favorire la ricerca e l'accesso ai nuovi farmaci per il trattamento del COVID-19". aifa.gov.it (in Italian). Retrieved 18 March 2020.
- ^ "Physicians work out treatment guidelines for coronavirus". m.koreabiomed.com (in Korean). 13 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b "Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Provisional 7th Edition)". China Law Translate. 4 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Malaria drug touted as coronavirus treatment by Trump and Elon Musk can be deadly, China finds". Fortune. Retrieved 23 March 2020.
- ^ Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. (March 2020). "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19". The New England Journal of Medicine. doi:10.1056/NEJMoa2001282. PMID 32187464.
- ^ a b Hoffmann, Markus; Kleine-Weber, Hannah; Krüger, Nadine; Müller, Marcel; Drosten, Christian; Pöhlmann, Stefan (31 January 2020). "The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells". bioRxiv (preprint). doi:10.1101/2020.01.31.929042.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N (March 2019). "TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection". Journal of Virology. 93 (6). doi:10.1128/JVI.01815-18. PMC 6401451. PMID 30626688.
- ^ Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nature Reviews Drug Discovery 2020 Feb doi:10.1038/d41573-020-00016-0
- ^ BRIEF-Corrected-Zhejiang Hisun Pharma gets approval for clinical trial to test flu drug Favipiravir for pneumonia caused by new coronavirus. Reuters Healthcare, 16 February 2020.
- ^ Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D (March 2020). "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)". Clin. Infect. Dis. doi:10.1093/cid/ciaa237. PMID 32150618.
- ^ Liu, Roxanne; Miller, Josh (3 March 2020). "China approves use of Roche drug in battle against coronavirus complications". Reuters. Retrieved 14 March 2020.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Effective Treatment of Severe COVID-19 Patients with Tocilizumab". ChinaXiv.org. 5 March 2020. doi:10.12074/202003.00026 (inactive 16 March 2020). Retrieved 14 March 2020.
{{cite web}}: CS1 maint: DOI inactive as of March 2020 (link) - ^ "3 patients get better on arthritis drug". 5 March 2020. Retrieved 14 March 2020.
- ^ "Coronavirus, via libera dell'Aifa al farmaco anti-artrite efficace su 3 pazienti e a un antivirale: test in 5 centri" [Coronavirus, Aifa gives go-ahead to effective anti-arthritis drug on 3 patients and an antiviral: test in 5 centers]. Il Messaggero (in Italian). Retrieved 14 March 2020.
- ^ "How doctors can potentially significantly reduce the number of deaths from Covid-19". Vox. 12 March 2020. Retrieved 14 March 2020.
- ^ Ruan Q, Yang K, Wang W, Jiang L, Song J (March 2020). "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China". Intensive Care Medicine. doi:10.1007/s00134-020-05991-x. PMID 32125452.
- ^ Mehta P, McAuley DF, Brown M, et al. (16 March 2020). "COVID-19: consider cytokine storm syndromes and immunosuppression". The Lancet. doi:10.1016/S0140-6736(20)30628-0. PMID 32192578. Retrieved 19 March 2020.
- ^ "China turns Roche arthritis drug Actemra against COVID-19 in new treatment guidelines". FiercePharma. Retrieved 14 March 2020.
- ^ "Northwell Health Initiates Clinical Trials of 2 COVID-19 Drugs". 21 March 2020.
- ^ Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA (November 1989). "Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability". J. Clin. Invest. 84 (5): 1609–19. doi:10.1172/JCI114338. PMC 304027. PMID 2553777.
- ^ Gaudreault E, Gosselin J (May 2008). "Leukotriene B4 induces release of antimicrobial peptides in lungs of virally infected mice". J. Immunol. 180 (9): 6211–21. doi:10.4049/jimmunol.180.9.6211. PMID 18424743.
- ^ Gaudreault E, Gosselin J (August 2009). "Leukotriene B4 potentiates CpG signaling for enhanced cytokine secretion by human leukocytes". J. Immunol. 183 (4): 2650–8. doi:10.4049/jimmunol.0804135. PMID 19620296.
- ^ Pernet E, Downey J, Vinh DC, Powell WS, Divangahi M (August 2019). "Leukotriene B4-type I interferon axis regulates macrophage-mediated disease tolerance to influenza infection". Nat Microbiol. 4 (8): 1389–1400. doi:10.1038/s41564-019-0444-3. PMID 31110361.
- ^ Widegren H, Andersson M, Borgeat P, Flamand L, Johnston S, Greiff L (July 2011). "LTB4 increases nasal neutrophil activity and conditions neutrophils to exert antiviral effects". Respir Med. 105 (7): 997–1006. doi:10.1016/j.rmed.2010.12.021. PMID 21251805.
- ^ a b c d Casadevall A, Pirofski LA (March 2020). "The convalescent sera option for containing COVID-19". The Journal of Clinical Investigation. doi:10.1172/JCI138003. PMID 32167489.
- ^ Pearce, Katie (13 March 2020). "Antibodies from COVID-19 survivors could be used to treat patients, protect those at risk: Infusions of antibody-laden blood have been used with reported success in prior outbreaks, including the SARS epidemic and the 1918 flu pandemic". The Hub at Johns Hopkins University. Retrieved 14 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
Further reading
- Guo YR, Cao QD, Hong ZS, et al. (March 2020). "The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status". Mil Med Res. 7 (1): 11. doi:10.1186/s40779-020-00240-0. PMC 7068984. PMID 32169119.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- Coronavirus disease (COVID-19) outbreak by the World Health Organization
- Coronavirus 2019 (COVID-19) by the U.S. Centers for Disease Control and Prevention
- Coronavirus Disease 2019 (COVID-19) by The Journal of the American Medical Association
- Coronavirus: Latest news and resources by the BMJ Publishing Group
- Novel Coronavirus Information Center by Elsevier
- COVID-19 Resource Centre by The Lancet
- SARS-CoV-2 and COVID-19 by Nature
- Coronavirus (Covid-19) by New England Journal of Medicine
- Covid-19: Novel Coronavirus by Wiley Publishing
- "SARS-CoV-2 related protein structures". Protein Data Bank.


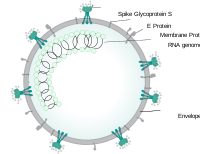


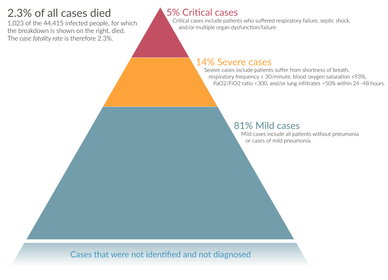




![Total confirmed cases of COVID-19 per million people, 20 March 2020[181]](http://upload.wikimedia.org/wikipedia/commons/thumb/1/17/World_map_of_total_confirmed_COVID-19_cases_per_million_people.png/446px-World_map_of_total_confirmed_COVID-19_cases_per_million_people.png)
![Total confirmed deaths due to COVID-19 per million people, 24 March 2020[182]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/26/World_map_of_total_confirmed_COVID-19_deaths_per_million_people_by_country.png/446px-World_map_of_total_confirmed_COVID-19_deaths_per_million_people_by_country.png)