Effects of cannabis: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
[[Cannabis]] has both [[psychological]] and [[physiological]] effects on the human body. The '''effects of cannabis''' are caused by [[cannabinoids]], most notably [[tetrahydrocannabinol]] (THC). Five [[Europe]]an countries, [[Canada]], and thirteen [[USA|US]] states have legalized [[medical cannabis]] if prescribed for [[nausea]], [[pain]], and alleviation of symptoms surrounding [[chronic illness]], although it remains banned, but decriminalized under US federal law. |
[[Cannabis]] has both [[psychological]] and [[physiological]] effects on the human body. The '''effects of cannabis''' are caused by [[cannabinoids]], most notably [[tetrahydrocannabinol]] (THC). Five [[Europe]]an countries, [[Canada]], and thirteen [[USA|US]] states have legalized [[medical cannabis]] if prescribed for [[nausea]], [[pain]], and alleviation of symptoms surrounding [[chronic illness]], although it remains banned, but decriminalized under US federal law. |
||
[[Acute toxicity|Acute]] effects while under the influence can include [[euphoria]], [[anxiety]], temporary [[short-term memory]] loss,<ref name=acutememory/> and circulation effects which may increase risks of [[heart attack]]s and [[stroke]]s. However, [[chronic toxicity|chronic]] use is not associated with some cardiovascular risk factors such as blood [[triglyceride]] levels and blood pressure, as indicated in a longitudinal study.<ref name=memorylongterm/> The evidence of long-term effects on memory is preliminary and hindered by [[confounding factor]]s.<ref name=memorylongterm/><ref name=memoryhindered/> Concerns have been raised about the potential for long-term cannabis consumption to increase risk for [[schizophrenia]], bipolar disorders, and major depression,<ref name=mentaldisorders>{{cite journal |author=Leweke FM, Koethe D |title=Cannabis and psychiatric disorders: it is not only addiction |journal=Addict Biol |volume=13 |issue=2 |pages=264–75 |year=2008 |month=June |pmid=18482435 |doi=10.1111/j.1369-1600.2008.00106.x |url=}}</ref><ref name=adolescenceschizo>{{cite journal |author=Rubino T, Parolaro D |title=Long lasting consequences of cannabis exposure in adolescence |journal=Mol. Cell. Endocrinol. |volume=286 |issue=1-2 Suppl 1 |pages=S108–13 |year=2008 |month=April |pmid=18358595 |doi=10.1016/j.mce.2008.02.003 |url=}}</ref> but the ultimate conclusions on these factors are disputed.<ref name=DeLisi2008>{{cite journal |author=DeLisi LE |title=The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? |journal=Curr Opin Psychiatry |volume=21 |issue=2 |pages=140–50 |year=2008 |month=March |pmid=18332661 |doi=10.1097/YCO.0b013e3282f51266 |url=}}</ref><ref name="decreased depression" /> |
[[Acute toxicity|Acute]] effects while under the influence can include [[euphoria]], [[anxiety]], temporary [[short-term memory]] loss,<ref name=acutememory/>,cronic episodes of shitting oneself, and circulation effects which may increase risks of [[heart attack]]s and [[stroke]]s. However, [[chronic toxicity|chronic]] use is not associated with some cardiovascular risk factors such as blood [[triglyceride]] levels and blood pressure, as indicated in a longitudinal study.<ref name=memorylongterm/> The evidence of long-term effects on memory is preliminary and hindered by [[confounding factor]]s.<ref name=memorylongterm/><ref name=memoryhindered/> Concerns have been raised about the potential for long-term cannabis consumption to increase risk for [[schizophrenia]], bipolar disorders, and major depression,<ref name=mentaldisorders>{{cite journal |author=Leweke FM, Koethe D |title=Cannabis and psychiatric disorders: it is not only addiction |journal=Addict Biol |volume=13 |issue=2 |pages=264–75 |year=2008 |month=June |pmid=18482435 |doi=10.1111/j.1369-1600.2008.00106.x |url=}}</ref><ref name=adolescenceschizo>{{cite journal |author=Rubino T, Parolaro D |title=Long lasting consequences of cannabis exposure in adolescence |journal=Mol. Cell. Endocrinol. |volume=286 |issue=1-2 Suppl 1 |pages=S108–13 |year=2008 |month=April |pmid=18358595 |doi=10.1016/j.mce.2008.02.003 |url=}}</ref> but the ultimate conclusions on these factors are disputed.<ref name=DeLisi2008>{{cite journal |author=DeLisi LE |title=The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? |journal=Curr Opin Psychiatry |volume=21 |issue=2 |pages=140–50 |year=2008 |month=March |pmid=18332661 |doi=10.1097/YCO.0b013e3282f51266 |url=}}</ref><ref name="decreased depression" /> |
||
== Biochemical effects == |
== Biochemical effects == |
||
Revision as of 18:04, 11 January 2010
Cannabis has both psychological and physiological effects on the human body. The effects of cannabis are caused by cannabinoids, most notably tetrahydrocannabinol (THC). Five European countries, Canada, and thirteen US states have legalized medical cannabis if prescribed for nausea, pain, and alleviation of symptoms surrounding chronic illness, although it remains banned, but decriminalized under US federal law.
Acute effects while under the influence can include euphoria, anxiety, temporary short-term memory loss,[1],cronic episodes of shitting oneself, and circulation effects which may increase risks of heart attacks and strokes. However, chronic use is not associated with some cardiovascular risk factors such as blood triglyceride levels and blood pressure, as indicated in a longitudinal study.[2] The evidence of long-term effects on memory is preliminary and hindered by confounding factors.[2][3] Concerns have been raised about the potential for long-term cannabis consumption to increase risk for schizophrenia, bipolar disorders, and major depression,[4][5] but the ultimate conclusions on these factors are disputed.[6][7]
Biochemical effects



The most prevalent psychoactive substances in cannabis are cannabinoids, including delta-9-tetrahydrocannabinol (Δ9-THC, commonly called simply THC). In the past two decades, the average content of THC in marijuana sold in North America has increased from about 1% to 3–4% or more.[citation needed] Some varieties, having undergone careful selection and growing techniques, can yield as much as 29% THC.[8] Another psychoactive cannabinoid present in Cannabis sativa is tetrahydrocannabivarin (THCV), but it is only found in small amounts.[9]
In addition, there are also similar compounds contained in cannabis that do not exhibit any psychoactive response but are obligatory for functionality: cannabidiol (CBD), an isomer of THC; cannabinol (CBN), an oxidation product of THC; cannabivarin (CBV), an analog of CBN with a different sidechain, cannabidivarin (CBDV), an analog of CBD with a different side chain, and cannabinolic acid. How these other compounds interact with THC is not fully understood. Some clinical studies have proposed that CBD acts as a balancing force to regulate the strength of the psychoactive agent THC. Marijuana with relatively high ratios of THC:CBD is less likely to induce anxiety than marijuana with low THC:CBD ratios.[10] CBD is also believed to regulate the body’s metabolism of THC by inactivating cytochrome P450, an important class of enzymes that metabolize drugs. Experiments in which mice were treated with CBD followed by THC showed that CBD treatment was associated with a substantial increase in brain concentrations of THC and its major metabolites, most likely because it decreased the rate of clearance of THC from the body.[10] Cannabis cofactor compounds have also been linked to lowering body temperature, modulating immune functioning, and cell protection. The essential oil of cannabis contains many fragrant terpenoids which may synergize with the cannabinoids to produce their unique effects. THC is converted rapidly to 11-hydroxy-THC, which is also pharmacologically active, so the drug effect outlasts measurable THC levels in blood.[8]
THC and cannabidiol are also neuroprotective antioxidants. Research in rats has indicated that THC prevented hydroperoxide-induced oxidative damage as well as or better than other antioxidants in a chemical (Fenton reaction) system and neuronal cultures. Cannabidiol was significantly more protective than either vitamin E or vitamin C.[11]
In 1990, the discovery of cannabinoid receptors located throughout the brain and body, along with endogenous cannabinoid neurotransmitters like anandamide (a lipid material derived ligand from arachidonic acid), suggested that the use of cannabis affects the brain in the same manner as a naturally occurring brain chemical. Cannabinoids usually contain a 1,1'-di-methyl-pyrane ring, a variedly derivatized aromatic ring and a variedly unsaturated cyclohexyl ring and their immediate chemical precursors, constituting a family of about 60 bi-cyclic and tri-cyclic compounds. Like most other neurological processes, the effects of cannabis on the brain follow the standard protocol of signal transduction, the electrochemical system of sending signals through neurons for a biological response. It is now understood that cannabinoid receptors appear in similar forms in most vertebrates and invertebrates and have a long evolutionary history of 500 million years. Cannabinoid receptors decrease adenylyl cyclase activity, inhibit calcium N channels, and disinhibit K+A channels. There are two types of cannabinoid receptors (CB1 and CB2).
The CB1 receptor is found primarily in the brain and mediates the psychological effects of THC. The CB2 receptor is most abundantly found on cells of the immune system. Cannabinoids act as immunomodulators at CB2 receptors, meaning they increase some immune responses and decrease others. For example, nonpsychotropic cannabinoids can be used as a very effective anti-inflammatory.[10] The affinity of cannabinoids to bind to either receptor is about the same, with only a slight increase observed with the plant-derived compound CBD binding to CB2 receptors more frequently. Cannabinoids likely have a role in the brain’s control of movement and memory, as well as natural pain modulation. It is clear that cannabinoids can affect pain transmission and, specifically, that cannabinoids interact with the brain's endogenous opioid system and may affect dopamine transmission.[12] This is an important physiological pathway for the medical treatment of pain.
The cannabinoid receptor is a typical member of the largest known family of receptors called a G protein-coupled receptor. A signature of this type of receptor is the distinct pattern of how the receptor molecule spans the cell membrane seven times. The location of cannabinoid receptors exists on the cell membrane, and both outside (extracellularly) and inside (intracellularly) the cell membrane. CB1 receptors, the bigger of the two, are extraordinarily abundant in the brain: 10 times more plentiful than μ-opioid receptors, the receptors responsible for the effects of morphine. CB2 receptors are structurally different (the homology between the two subtypes of receptors is 44%), found only on cells of the immune system, and seems to function similarly to its CB1 counterpart. CB2 receptors are most commonly prevalent on B-cells, natural killer cells, and monocytes, but can also be found on polymorphonuclear neutrophil cells, T8 cells, and T4 cells. In the tonsils the CB2 receptors appear to be restricted to B-lymphocyte-enriched areas.
THC and endogenous anandamide additionally interact with glycine receptors.
Sustainability in the body
Most cannabinoids are lipophilic (fat soluble) compounds that easily store in fat, thus yielding a long elimination half-life relative to other recreational drugs. The THC molecule, and related compounds, are usually detectable in drug tests from 3 days up to 10 days according to Redwood Laboratories, after using cannabis depending on frequency of use (see drug test). This detection is possible because non-psychoactive THC metabolites are stored for long periods of time in fat cells, and THC has an extremely low water solubility. [citation needed] The rate of elimination of metabolites is slightly greater for more frequent users due to tolerance.[citation needed]
Toxicity
THC has an extremely low toxicity and the amount that can enter the body through the consumption of cannabis plants poses no threat of death. In lab animal tests, scientists have had much difficulty administering a dosage of THC that is high enough to be lethal. It also appears that humans cannot die from ingesting too much THC, unless it were introduced into the body intravenously (See also: Intravenous Marijuana Syndrome).[citation needed] Indeed, a 1988 ruling from the United States Department of Justice concluded that "In practical terms, marijuana cannot induce a lethal response as a result of drug-related toxicity."[13]
According to the Merck Index,[14] the LD50 of THC (the dose which causes the death of 50% of individuals) is 1270 mg/kg for male rats and 730 mg/kg for female rats from oral assumption in sesame oil, and 42 mg/kg for rats from inhalation.[15]
The ratio of cannabis material required to produce a fatal overdose to the amount required to saturate cannabinoid receptors and cause intoxication is 40,000:1;[16][17] consumption of such a large dose is virtually impossible. There had been no reported deaths or permanent injuries sustained as a result of a marijuana overdose. However in 2009 it was reported in the United Kingdom that a man died from the acute affects of cannabis "possibly experiencing one of the toxic effects of cannabis - a fast heart rate and hyperventilating - [which] can lead to heart failure". The coroner recorded a verdict of "death due to misuse of drugs".[18] It is not known if the man had any underlying medical condition. It is generally considered impossible to overdose on marijuana, as the user would certainly either fall asleep or otherwise become incapacitated from the effects of the drug before being able to consume enough THC to be mortally toxic. According to a 2006 United Kingdom government report, using cannabis is much less dangerous than tobacco, prescription drugs, and alcohol in social harms, physical harm, and addiction.[19] It was found in 2007 that while tobacco and cannabis smoke are quite similar, cannabis smoke contained higher amounts of ammonia, hydrogen cyanide, and nitrogen oxides, but lower levels of carcinogenic polycyclic aromatic hydrocarbons (PAHs).[20] This study found that directly inhaled cannabis smoke contained 20 times as much ammonia and 5 times as much hydrogen cyanide as tobacco smoke and compared the properties of both mainstream and sidestream (smoke emitted from a smouldering 'joint' or 'cone') smoke.[20] Sidestream cannabis smoke was found to contain higher concentrations of selected polycyclic aromatic hydrocarbons (PAHs) than sidestream tobacco smoke.[20]
Short-term effects
When smoked, the effects of cannabis manifest within seconds and are fully apparent within a few minutes,[21] typically lasting for 2–3 hours.[22]
Psychoactive effects
The psychoactive effects of cannabis, known as a "high", are subjective and can vary based on the individual and the method of use. Some effects may include an altered state of consciousness, euphoria, feelings of well-being, relaxation or stress reduction, increased appreciation of humor, music or art, joviality, metacognition and introspection, enhanced recollection (episodic memory), increased sensuality, increased awareness of sensation, increased libido, creative or philosophical thinking, disruption of linear memory and paranoia or anxiety.
Cannabis also produces many subjective effects, such as greater enjoyment of food taste and aroma, an enhanced enjoyment of music and comedy, and marked distortions in the perception of time and space (where experiencing an up rush of ideas from the bank of long-term memory can create the subjective impression of long elapsed time, while a clock reveals that only a short time has passed). At higher doses, effects can include altered body image, auditory and/or visual illusions, and ataxia from selective impairment of polysynaptic reflexes. In rare cases, cannabis can lead to depersonalization[23][24] and derealization;[25] such effects are most often considered desirable.
Somatic effects
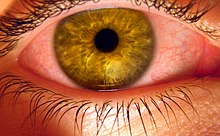
Some of the short-term physical effects of cannabis use include increased heart rate, dry mouth ( cotton mouth or peanut-butter mouth), reddening of the eyes (congestion of the conjunctival blood vessels), a reduction in intra-ocular pressure, muscle relaxation and a sensation of cold or hot hands and feet.[26]
Electroencephalography or EEG shows somewhat more persistent alpha waves of slightly lower frequency than usual.[8] Cannabinoids produce a "marked depression of motor activity" via activation of neuronal cannabinoid receptors belonging to the CB1 subtype.[27]
Duration
The total short-term duration of cannabis intoxication when smoked is based on the potency and how much is smoked. Effects can typically last two to three hours for one gram.[28]
A study of ten healthy, robust, male volunteers who resided in a residential research facility sought to examine both acute and residual subjective, physiologic, and performance effects of smoking marijuana cigarettes. On three separate days, subjects smoked one NIDA marijuana cigarette containing either 0%, 1.8%, or 3.6% THC, documenting subjective, physiologic, and performance measures prior to smoking, five times following smoking on that day, and three times on the following morning. Subjects reported robust subjective effects following both active doses of marijuana, which returned to baseline levels within 3.5 hours. Heart rate increased and the pupillary light reflex decreased following active dose administration with return to baseline on that day. Additionally, marijuana smoking acutely produced decrements in smooth pursuit eye tracking. Although robust acute effects of marijuana were found on subjective and physiological measures, no effects were evident the day following administration, indicating that the residual effects of smoking a single marijuana cigarette are minimal.[29]
A Dutch double blind, randomized, placebo-controlled, cross-over study examining male volunteers aged 18–45 years with a self-reported history of regular cannabis use concluded that smoking of cannabis with very high THC levels (marijuana with 9–23% THC), as currently sold in coffee shops in the Netherlands, may lead to higher THC blood-serum concentrations. This is reflected by an increase of the occurrence of impaired psychomotor skills, particularly among younger or inexperienced cannabis smokers, who do not adapt their smoking-style to the higher THC content.[30] High THC concentrations in cannabis were associated with a dose-related increase of physical effects (such as increase of heart rate, and decrease of blood pressure) and psychomotor effects (such as reacting more slowly, being less concentrated, making more mistakes during performance testing, having less motor control, and experiencing drowsiness). It was also observed during the study that the effects from a single joint lasted for more than eight hours. Reaction times remained impaired five hours after smoking, when the THC serum concentrations were significantly reduced, but still present. When subjects smoke on several occasions per day, accumulation of THC in blood-serum may occur.
Neurological effects
The areas of the brain where cannabinoid receptors are most prevalently located are consistent with the behavioral effects produced by cannabinoids. Brain regions in which cannabinoid receptors are very abundant are the basal ganglia, associated with movement control; the cerebellum, associated with body movement coordination; the hippocampus, associated with learning, memory, and stress control; the cerebral cortex, associated with higher cognitive functions; and the nucleus accumbens, regarded as the reward center of the brain. Other regions where cannabinoid receptors are moderately concentrated are the hypothalamus, which regulates homeostatic functions; the amygdala, associated with emotional responses and fears; the spinal cord, associated with peripheral sensations like pain; the brain stem, associated with sleep, arousal, and motor control; and the nucleus of the solitary tract, associated with visceral sensations like nausea and vomiting.[31]
Most notably, the two areas of motor control and memory are where the effects of cannabis are directly and irrefutably evident. Cannabinoids, depending on the dose, inhibit the transmission of neural signals through the basal ganglia and cerebellum. At lower doses, cannabinoids seem to stimulate locomotion while greater doses inhibit it, most commonly manifested by lack of steadiness (body sway and hand steadiness) in motor tasks that require a lot of attention. Other brain regions, like the cortex, the cerebellum, and the neural pathway from cortex to striatum, are also involved in the control of movement and contain abundant cannabinoid receptors, indicating their possible involvement as well.
Experiments on animal and human tissue have demonstrated disruption of short-term memory,[10] which is consistent with the abundance of CB1 receptors on the hippocampus, the region of the brain most closely associated with memory. Cannabinoids inhibit the release of several neurotransmitters in the hippocampus, like acetylcholine, norepinephrine, and glutamate, resulting in a major decrease in neuronal activity in that region. This decrease in activity resembles a "temporary hippocampal lesion."[10] In the end, this procedure could lead to the blocking of cellular processes that are associated with memory formation.
In in-vitro experiments THC at extremely high concentrations, which could not be reached with commonly consumed doses, caused competitive inhibition of the AChE enzyme and inhibition of β-amyloid peptide aggregation, the cause of Alzheimer's disease. Compared to currently approved drugs prescribed for the treatment of Alzheimer's disease, THC is a considerably superior inhibitor of A aggregation, and this study provides a previously unrecognized molecular mechanism through which cannabinoid molecules may directly impact the progression of this debilitating disease.[32]
Effects on driving
This section needs additional citations for verification. (October 2008) |
It is known that cannabis consumption affects motor skills, reflexes, and attention.
A 2001 study by the United Kingdom Transit Research Laboratory (TRL) specifically focuses on the effects of cannabis use on driving,[33] and is one of the most recent and commonly quoted studies on the subject. The report summarizes current knowledge about the effects of cannabis on driving and accident risk based on a review of available literature published since 1994 and the effects of cannabis on laboratory based tasks.
The study identified young males, amongst whom cannabis consumption is frequent and increasing, and in whom alcohol consumption is also common, as a risk group for traffic accidents. This is due to driving inexperience and factors associated with youth relating to risk taking, delinquency and motivation. These demographic and psychosocial variables may relate to both drug use and accident risk, thereby presenting an artificial relationship between use of drugs and accident involvement.
The effects of cannabis on laboratory-based tasks show clear impairment with respect to tracking ability, attention, and other tasks depending on the dose administered. These effects however, are not as pronounced on real world tasks, like driving or simulator tasks. Both simulation and road trials generally find that driving behavior shortly after consumption of larger doses of cannabis results in:
- increased variability in lane position (such as taking a curve too tightly or too loosely).
- longer decision times, leading to slower responses to driving situations; and
- a more cautious driving style, including slower average speed and greater following distance.
Kelly, Darke and Ross [34] show similar results, with laboratory studies examining the effects of cannabis on skills utilised while driving showing impairments in tracking, attention, reaction time, short-term memory, hand-eye coordination, vigilance, time and distance perception, and decision making and concentration. An EMCDDA[35] review concluded that "the acute effect of moderate of higher doses of cannabis impairs the skills related to safe driving and injury risk", specifically "attention, tracking and psychomotor skills"[35]. In their review of driving simulator studies, Kelly et al.[34] conclude that there is evidence of dose-dependent impairments in cannabis-affected drivers' ability to control a vehicle in the areas of steering, headway control, speed variability, car following, reaction time and lane positioning. The researchers note that "even in those who learn to compensate for a drug's impairing effects, substantial impairment in performance can still be observed under conditions of general task performance (ie. when no contingencies are present to maintain compensated performance."[35]
Whereas these results indicate a 'change' from normal conditions, they do not necessarily reflect 'impairment' in terms of performance effectiveness, since few studies report increased accident risk. However, the results do suggest 'impairment' in terms of performance efficiency given that some of these behaviors may limit the available resources to cope with any additional, unexpected or high demand, events. Indeed, compensatory effort may be invoked to offset impairment in the driving task. Subjects under cannabis treatment may perceive that they are impaired and may strategically compensate, for example, by not overtaking, by slowing down and by focusing their attention when they know a response will be required. This compensatory effort may be one reason for the failure to implicate cannabis consumption as an accident risk factor, particularly at lower doses or with more than about one hour after consumption. [citation needed] According to the TRL study, the same compensatory behavior could also be an unconscious adaptation, similar to reduced driving speeds used by a sleepy driver.
Specifically, 4-12% of accident fatalities have detected levels of cannabis.[citation needed]However, most studies report that the majority of fatal cases with detected levels of cannabis are compounded by alcohol. The study estimates 11 ng/ml THC as the equivalent dose to the legal limit of alcohol (0.08% BAC in the UK). Complicating this assessment is the fact that cannabis effects on driving fade after a short period of time, while some THC may be present in the body for weeks.
Grotenherman et al.[36] conclude that after reviewing the limited epidemiological and laboratory evidence, driving under the influence of cannabis appears to increase the risk of motor vehicle crashes by a factor of two to three times. In their review of recent studies featuring blood samples that detect active THC metabolites, Ramaekers et al.[37] concluded that although crash culpability was not elevated for low concentrations of THC, risk of involvement in a traffic crash increased as drivers' THC levels increased, and became up to 6.6 times greater than that for drug-free drivers, at higher concentrations of THC.
Similar conclusions have been reached by studies maintained by the federal governments of Australia, United Kingdom, New Zealand and the United States (see here for a list of studies). Those studies that have concluded that cannabis has a significant negative effect on driving ability generally involve the use of roadside sobriety tests as an indicator of reduced ability (for example, see this NIDA report). Driver culpability studies in Australia have suggested that drivers testing positive to cannabis are significantly more likely to be responsible for fatal car crashes than drug-free drivers.[38] However, studies that employ this methodology show that a majority of subjects who tested positive for THC also tested positive for alcohol, already described as a limiting factor of validity. Kelly et al.[34] conclude that the combined effects of cannabis and alcohol on laboratory performance measures are typically greater than the effects of cannabis alone, and act in either an additive or multiplicative manner. Increasing recognition of limitations on driving skill when the driver tests positive to cannabis has resulted in the development of guidelines for research on drugged driving[39]
Vascular effects
Cannabis arteritis is a very rare peripheral vascular disease similar to Buerger's disease. There were about 50 confirmed cases from 1960 to 2008.[40]
A 2008 study by the National Institutes of Health Biomedical Research Centre in Baltimore found that heavy, chronic smoking of marijuana changed blood proteins associated with heart disease and stroke.[41] A 2005 study by the U.S. National Institute on Drug Abuse found that moderate to heavy marijuana use has an effect on blood flow to the brain, potentially increasing the risk of memory damage and stroke.[42]
A 2005 article in the Journal of Neurology, Neurosurgery and Psychiatry reported on a 36-year-old man who suffered a stroke on three separate occasions after smoking a large amount of marijuana, despite having no known risk factors for the disorder, suggesting that a rare side effect of marijuana use may be an increase in the incidence of strokes among young smokers.[43] A 2000 study by researchers at Boston's Beth Israel Deaconess Medical Center, Massachusetts General Hospital and Harvard School of Public Health also found that a middle-age person's risk of heart attack rises nearly fivefold in the first hour after smoking marijuana.[44]
Adulterated cannabis
Contaminants may be found in hashish obtained from "soap bar"-type sources.[45] The dried flowers of the plant may be contaminated by the plant taking up heavy metals and other toxins from its growing environment,[46] or by the addition of lead or glass beads, used to increase the weight or to make the cannabis appear as if it has more crystal-looking trichomes indicating a higher THC content.[47] Users who burn hot or mix cannabis with tobacco are at risk of failing to detect deviations from appropriate cannabis taste.
Despite cannabis being generally perceived as a natural or "chemical-free" product,[48] in a recent Australian survey[49] one in four Australians consider cannabis grown indoors under hydroponic conditions to be a greater health risk due to increased contamination, added to the plant during cultivation to enhance the actual or perceived plant growth and quality.
Combination with other drugs
The most obvious confounding factor in cannabis research is the prevalent usage of other recreational drugs, especially alcohol and tobacco.[50] Such complications demonstrate the need for studies on cannabis that have stronger controls, and investigations into alleged symptoms of cannabis use that may also be caused by tobacco. Some critics question whether agencies doing the research make an honest effort to present an accurate, unbiased summary of the evidence, or whether they "cherry-pick" their data to please funding sources which may include the tobacco industry or governments dependent on cigarette tax revenue; others caution that the raw data, and not the final conclusions, are what should be examined.[51]
Cannabis also has been shown to have a synergistic cytotoxic effect on lung cancer cell cultures in vitro with the food additive butylated hydroxyanisole (BHA) and possibly the related compound butylated hydroxytoluene (BHT). The study concluded, "Exposure to marijuana smoke in conjunction with BHA, a common food additive, may promote deleterious health effects in the lung." BHA & BHT are human-made fat preservatives, and are found in many packaged foods including: plastics in boxed cereal, Jello, Slim Jims, and more.[52]
The Australian National Household Survey[5] of 2001 showed that cannabis use in Australia is rarely used without other drugs. 95% of cannabis users also drank alcohol; 26% took amphetamines; 19% took ecstasy and only 2.7% reported not having used any other drug with cannabis.[53] While research has been undertaken on the combined effects of alcohol and cannabis on performing certain tasks, little research has been conducted on the reasons why this combination is so popular. Evidence from a controlled experimental study undertaken by Lukas and Orozco [54] suggests that alcohol causes THC to be absorbed more rapidly into the blood plasma of the user. Data from the Australian National Survey of Mental Health and Wellbeing [6]found that three-quarters of recent cannabis users reported using alcohol when cannabis was not available.[55]
Memory and learning
Studies on cannabis and memory are hindered by small sample sizes, confounding drug abuse, and other factors.[3] The strongest evidence regarding cannabis and memory focuses on its short-term negative effects on short-term and working memory.[3]
A 2008 review of the evidence surrounding the acute impact on memory concluded that cannabinoids impair all aspects of short-term memory, especially short-term episodic and working memory.[1] One small study found that no learning occurred during the 2 hour period in which the subjects (infrequent users) were "stoned".[56]
The feeling of increased appetite following the use of cannabis has been documented for hundreds of years,[57] and is commonly known as 'the munchies' in popular culture. Clinical studies and survey data have found that cannabis increases food enjoyment and interest in food.[58][59] Scientists have claimed to be able to explain what causes the increase in appetite, concluding that "endocannabinoids in the hypothalamus activate cannabinoid receptors that are responsible for maintaining food intake".[59] Endogenous cannabinoids have recently been discovered in foods such as chocolate and human and bovine milk.[60][61]
It is widely accepted that the neonatal survival of many species "is largely dependent upon their suckling behavior, or appetite for breast milk"[62] and recent research has identified the endogenous cannabinoid system to be the first neural system to display complete control over milk ingestion and neonatal survival.[63] It is possible that "cannabinoid receptors in our body interact with the cannabinoids in milk to stimulate a suckling response in newborns so as to prevent growth failure".[62]
Long-term effects
Reproductive effects
Cannabis has been reported both to enhance and lessen the subjective enjoyment of sex.[64] It has been shown that administration of high doses [clarification needed] of THC to animals lowers serum testosterone levels, impairs sperm production, motility, and viability, disrupts the ovulation cycle, and decreases output of gonadotropic hormones.[8][65] According to the 1997 Merck Manual of Diagnosis and Therapy, fertility effects related to cannabis use are uncertain.
Research has demonstrated that human sperm contains receptors which are stimulated by substances like THC and other cannabis-related chemicals. Tests have implied that smoking of marijuana could impact the sperm's functions, though this impact is unknown.[66]. There is some evidence that cannabis may compromise female fertility with a modest association reported between cannabis use and infertility in Mueller et al.'s[67] case controlled study of 150 women with primary anovulatory infertility. While Wenger et al.[68] indicate evidence that THC and anandamide increase the duration of pregnancy and increase the frequency of stillbirth in rats. In a report prepared for the Australian National Council on Drugs, Copeland, Gerber and Swift conclude that current understanding suggests cannabis-related substances are contraindicated in pregnancy, as are compounds that interact with endocannabinoid synthesis and metabolism.[69][70]
Pregnancy
A 1989 study of 1226 mothers published in the New England Journal of Medicine concluded that "the use of marijuana or cocaine during pregnancy is associated with impaired fetal growth".[71] Compared to fetal alcohol syndrome, similar types of facial features and related symptoms are not associated with prenatal marijuana exposure.[72] THC passes into the breast milk and might affect a breastfed infant.[73] Many studies about drug use during pregnancy are self-administered by the applicants and not always anonymous. The stigma of using illicit drugs while pregnant discourages honest reporting and can invalidate the results. Studies show that women who consume cannabis while they are pregnant may also be likely to consume alcohol, tobacco, caffeine, or other illicit drugs, which makes it difficult to deduce scientific facts about just marijuana use from statistical results. Very few large, well-controlled epidemiological studies have taken place to understand the connection of marijuana use and pregnancy.[citation needed]
A study of the development of 59 Jamaican children was conducted with the children being monitored from child birth to age 5 years. One-half of the sample's mothers used marijuana during pregnancy; they were paired with non-using mothers who matched age, parity, and socioeconomic status. Testing was done at 1, 3, and 30 days of age with the Brazelton Neonatal Behavioral Assessment Scales, and at ages 4 and 5 years with the McCarthy Scales of Children's Abilities test. Data was also collected from the child's home environment and temperament, as well as standardized tests. The results over the entire research period showed no significant differences in development testing outcomes between using and non-using mothers. At 30 days of age, however, the children of marijuana-using mothers had higher scores on autonomic stability and reflexes.[74] The absence of any differences between the exposed and non-exposed groups in the early neonatal period suggest that the better scores of exposed neonates at 1 month are traceable to the cultural positioning and social and economic characteristics of mothers using marijuana that select for the use of marijuana but also promote neonatal development.[75]
Some studies have found that children of tobacco and marijuana-smoking mothers more frequently suffer from permanent cognitive deficits, concentration disorders, hyperactivity, and impaired social interactions than non-exposed children of the same age and social background.[76][77] A recent study, with participation of scientists from Europe and the United States, has now identified that naturally occurring endocannabinoid molecules play a role in establishing how certain nerve cells connect to each other in the fetal brain.[78][79][80] Another study examining cannabinoid receptor proteins (CBRs) expressed in brain cells of mice determined that endogenous endocannabinoids assist in directing brain cell directional development while in the womb.[81] The researchers suggest that elevated blood THC levels due to cannabis consumption would affect brain development of human fetuses. In contrast, other studies in Jamaica have suggested that cannabis use by expectant mothers does not appear to cause birth defects or developmental delays in their newborn children.[74][82]
Addiction potential
Research has shown the overall addiction potential for cannabis to be much less than for tobacco, alcohol, cocaine or heroin.[83] There is some evidence that dependence on cannabis can exist in some heavy users. One study with 500 heavy users of cannabis showed that when trying to cease consumption, some experience one or more symptoms such as insomnia, restlessness, loss of appetite, depression, irritability, and anger.[84] Prolonged marijuana use produces both pharmacokinetic changes (how the drug is absorbed, distributed, metabolized, and excreted) and pharmacodynamic changes (how the drug interacts with target cells) to the body. These changes require the user to consume higher doses of the drug to achieve a common desirable effect (known as a higher tolerance), and reinforce the body’s metabolic systems for synthesizing and eliminating the drug more efficiently.[10]
Preliminary research, published in the April 2006 issue of the Journal of Consulting and Clinical Psychology, indicates that cannabis addiction can be offset by a combination of cognitive-behavioral therapy and motivational incentives. Participants in the study (previously diagnosed with marijuana dependence) received either vouchers as incentives to stay drug free, cognitive-behavioral therapy, or both over a 14-week period. At the end of 3 months, 43 percent of those who received both treatments were no longer using marijuana, compared with 40 percent of the voucher group, and 30 percent of the therapy group. At the end of a 12-month follow-up, 37 percent of those who got both treatments remained abstinent, compared with 17 percent of the voucher group, and 23 percent of the therapy group.[85]
A 1998 French governmental report commissioned by Health Secretary of State Bernard Kouchner, and directed by Dr. Pierre-Bernard Roques, classed drugs according to addictiveness and neurotoxicity. It placed heroin, cocaine and alcohol in the most addictive and lethal categories; benzodiazepine, hallucinogens and tobacco in the medium category, and cannabis in the last category. The report stated that "Addiction to cannabis does not involve neurotoxicity such as it was defined in chapter 3 by neuroanatomical, neurochemical and behavioral criteria. Thus, former results suggesting anatomic changes in the brain of chronic cannabis users, measured by tomography, were not confirmed by the accurate modern neuro-imaging techniques. Moreover, morphological impairment of the hippocampus [which plays a part in memory and navigation] of rats after administration of very high doses of THC (Langfield et al., 1988) was not shown (Slikker et al., 1992)." Health Secretary Bernard Kouchner concluded that : "Scientific facts show that, for cannabis, no neurotoxicity is demonstrated, to the contrary of alcohol and cocaine."[86]
In treating marijuana use, Dr. David McDowell of Columbia University found that there is a need for the clinician to differentiate in the spectrum between a casual user who still has difficulty with drug screens, and a daily, heavy user.[87] McDowell found that the sedating and anxiolytic properties of THC in some users might make the use of cannabis an attempt to self-medicate personality or psychiatric disorders.[87]
Mental health
Cannabis use has been assessed by several studies[88] to be correlated with the development of anxiety, psychosis, and depression.[89][90] Some studies assess that the causality is more likely to involve a path from cannabis use to psychotic symptoms rather than a path from psychotic symptoms to cannabis use,[91] while others assess the opposite direction of the causality, or hold cannabis to only form parts of the "causal constellation", while not inflicting mental health problems that would have occurred in the absence of the cannabis use.[92][93] A common interpretation of the correlation and theorized direction of the causality is the self-medication hypothesis, which is based on partially or fully attributing the correlation between psychiatric diseases and cannabis to the extensive substance abuse among sufferers of certain mental disorders, before diagnosis in many cases, which increases the likeliness of cannabis use among the mentally ill and the undiagnosed, thus accounting for correlation and debunking some claims of causality with the opposite direction.[94] As much as 60% of the mentally ill are suspected to be substance abusers, and many seem to prefer cannabis and alcohol.[95] Dr Stanley Zammit of Bristol and Cardiff universities (in the Daily Express newspaper of the 27th of July 2007) reported, "Even if cannabis did increase the risk of psychosis, most people using the drug would not get ill" But he added: "Nevertheless, we would still advise people to avoid or limit their use of this drug, especially if they start to develop any mental health symptoms or if they have relatives with psychotic illnesses." A 2007 study of studies published in the Lancet concluded that cannabis users are 40% more likely to be sufferers of a psychotic illness than non-users.[96]
A large, unselected population-based study, published in British Journal of Psychiatry (2008), examined cannabis use and prodromal symptoms of psychosis at age 15–16 years and conclude that cannabis use is associated with prodromal symptoms of psychosis in adolescence.[97]
The direction of causation was more directly examined in a study by Dr. Mikkel Arendt of Aarhus University in Risskov, Denmark, and colleagues, which found that individuals treated for psychotic episodes following cannabis use had the same likelihood of having a mother, sister or other "first-degree" relative with schizophrenia as did the individuals who had actually been treated for schizophrenia themselves. This suggests that the psychosis blamed on cannabis use is in fact the result of a genetic predisposition towards schizophrenia. "These people would have developed schizophrenia whether or not they used cannabis"[98]
In a recent study at the Institute of Psychiatry at King's College London, scientists have confirmed a link between potent cannabis use ("skunk" cannabis, which accounts for 80 per cent of street seizures of the drug in the UK[99]) and transient psychotic symptoms in healthy people. After testing 22 healthy males in their late 20s by injecting them with THC, with a control dummy injection administered to a percentage of the sample group, a link was found between the chemical and psychosis, "in which hallucinations leave sufferers unable to know what is real and what is imagined". Dr Paul Morrison, who lead the team, concluded, "these findings confirm that THC can induce a transient acute psychological reaction in psychiatrically well individuals"[99]. In addition, it was found that the extent of the psychotic reaction was not related to "the degree of anxiety or cognitive impairment" in the sample group. Further research is needed into the chemical makeup of skunk cannabis as it is believed stronger strains have virtually no traces of CBD (cannabidiol), which appears to counteract the damaging effects of THC.[99] However, there is likely to be wide variation in the THC and CBD levels (and ratios) since numerous (perhaps even hundreds) of different strains of cannabis have been marketed by dealers as "skunk," some of which are descended from the original 1980s Amsterdam variety.
The BEACH[100] study (Bettering the Evaluation and Care of Health) conducted by the Australian General Practice Statistics and Classification Centre, based at the University of Sydney, found that "cannabis smokers are more likely to suffer depression, anxiety and psychosis". The report continues that of the number of patients who mentioned cannabis use to their GP, 48% had a psychological problem, including 19% with depression, 9% with psychosis and 6% had anxiety.[101] However, it was also noted that few cannabis users actually tell their doctors that they use it, which could potentially bias the results of the study. Much of the evidence for a short-lived cannabis psychosis is largely based on case reports where heavy cannabis use has preceded the onset of a psychotic episode, which then remits on abstinence.[102] Depictions of a toxic or acute cannabis psychosis have been reported in a number of countries such as New Zealand[103], South Africa[104], Sweden[105] and the UK.[106]
The largest longitudinal study examining the link between cannabis and psychosis was undertaken by Andreasson and colleagues [107] and followed 45,570 male Swedish Army conscripts for 15 years. After controlling for other factors such as parental mental illness or a pre-existing psychotic illnes at conscription, the study found that the odds of developing schizophrenia later in life were "1.5 times higher for those who had used cannabis 1-10 times and 2.3 times more likely for those who had used cannabis 10 times or more".[108] Further to criticism that the study did not control for the use of other potentially psychotogenic substances such as amphetamines, a follow-up study re-analysed the data and ruled out this argument, finding that cannabis use remained predictive of schizophrenia in a dose-dependent manner even after accounting for other substance use and pre-morbid social integration.[109]
Research findings from the University of Melbourne and the [Orygen Research Centre], reported in New Scientist[110] reveal links between heavy cannabis use and brain size. In this study to determine whether long-term and heavy cannabis use is associated with gross anatomical abnormalities in two regions of the brain that are particularly rich in cannabinoid receptors, researchers found that the brain scans of 15 heavy users, who had smoked at least five joints a day for over 10 years, showed that on average the hippocampus and amygdales of the test group were 12% and 7.1% smaller than non-users, respectively. According to commentary provided by the National Cannabis Prevention and Information Centre, these brain regions are intricately involved in learning and memory processes and are considered core components of the emotional brain and the research found that in addition left hippocampal and amygdala volume was inversely associated with cumulative doses of cannabis over the previous 10 years, as well as subthreshold positive psychotic symptoms. In their commentary, NCPIC state: "While modest use may not lead to significant neurotoxicity, these results corroborate similar findings within the animal literature and indicate that heavy daily cannabis use over protracted periods exerts harmful effects on brain tissue and mental health".[111]
Less attention has been given to the association between cannabis use and depression, though it is possible this is because cannabis users who suffer from depression are less likely to access treatment than those suffering from psychosis.[112] Chen and colleagues (2002) re-analyzed the US National Comorbidity Survey (NCS) to examine the relationship between cannabis use and a major depressive episode and discovered that some degree of cannabis dependence was associated with a 3.4 time greater risk of major depression.[113] Similarly, data retrieved from the US Longitudinal Alcohol Epidemiologic Survey showed that cannabis dependency within the past year was associated with a 6.4 fold chance of also receiving a diagnosis for major depression in that time.[114] The issue of suicide and cannabis use is considered by Borges, Walters, and Kessler who examined whether cannabis use heightens the risk of suicide or attempted suicide. Cross-sectional data from the US National Comorbidity Survey indicated that cannabis-dependent individuals were 2.4 times more likely to report a suicide attempt than non-cannabis-dependent individuals, after controlling for socio-demographic factors, psychiatric disorders and other drug use.[115] Beautrais et al. (1999) examined 302 hospitalized cases of suicide attempts and found that 16% screened positive for cannabis abuse or dependence, compared with 2% of a random community sample. After controlling for depression and social disadvantage the study found this translated to a two-fold suicide attempt risk for those who had a cannabis use disorder.[116]
Co-occurrence of mental illness
A 2005 meta analysis of available data which evaluated several hypotheses regarding the correlation of cannabis and psychosis found that there is no support for the hypothesis that cannabis can cause cases of psychosis which would not have occurred otherwise, however further study is needed to explore the correlation between cannabis and other types of psychosis patients.[117] Studies have shown that a risk does exist in some individuals with a predisposition to mental illness to develop symptoms of psychosis.[89] The risk was found to be directly related to high dosage and frequency of use, early age of introduction to the drug, and was especially pronounced for those with a predisposition for mental illness. These results have been questioned as being biased by failing to account for medicinal versus recreational usage[7] — critics contend it could be a causal relationship, or it could be that people who are susceptible to mental problems tend to smoke cannabis, or it could be connected to the criminalization of cannabis. Another important question is whether the observed symptoms of mental illness are actually connected to development of a permanent mental disorder; cannabis may trigger latent conditions, or be part of a complex coordination of causes of mental illness, referred to in psychology as the diathesis-stress model. People with developed psychological disorders are known to self-medicate their symptoms with cannabis as well, although one study has claimed that those with a predisposition for psychosis did not show a statistically significant increase in likelihood of cannabis use four years later.[89]
A 2005 literature review of the use of cannabis in mental health patients found that the drug can have very different effects on different patients. Although "no controlled trials of THC have been done in bipolar disorder," there is anecdotal evidence that "for some people marijuana is beneficial" as a treatment for bipolar disorder. The reviewers suggested that randomized studies and standardized administration techniques would be required to create conclusive evidence.[118]
Correlation versus causation
Some studies conclude that there is a correlation of cannabis use and some symptoms of psychosis, but do not necessarily support the notion that cannabis use is a sufficient or necessary cause for psychosis. It might be a component cause, part of a complex constellation of factors leading to psychosis, or it might be a correlation without forward causality at all.
For example, a review of the evidence by Louise Arsenault, et al., in 2004 reports that on an individual level, cannabis use confers an overall twofold increase in the relative risk of later schizophrenia, assuming a causal relationship. This same research also states that "There is little dispute that cannabis intoxication can lead to acute transient psychotic episodes in some individuals". The study synthesizes the results of several studies into a statistical model. The study does not correct for the use of other illicit drugs, and relies on self-reporting of cannabis dosage. The study also does not determine if the cannabis use preceded or followed the mental health problem.[93]
Similarly, the landmark study, in 1987, of 50,000 Swedish Army conscripts, mentioned earlier, found that those who admitted at age 18 to having taken cannabis on more than 50 occasions, were six times more likely to develop schizophrenia in the following 15 years. In fact, psychosis cases were restricted to patients requiring a hospital admission. These findings have not been replicated in another population based sample. As the study did not control for symptoms preexisting onset of cannabis use, the use of other illicit drugs, the study does not resolve the correlation versus causality question but has fueled a major debate within the scientific community. This study also used self reporting for cannabis dosage.[119]
A 2005 study found that "the onset of schizotypal symptoms generally precedes the onset of cannabis use. The findings do not support a causal link between cannabis use and schizotypal traits".[120] It should be noted that a schizotypal personality disorder is a personality disorder different from schizophrenia, though there is some evidence that the former may predispose to the latter. A 2007 British study concluded, "We found few appreciable differences in symptomatology between schizophrenic patients who were or were not cannabis users. There were no differences in the proportion of people with a positive family history of schizophrenia between cannabis users and non-users. This argues against a distinct schizophrenia-like psychosis caused by cannabis."[121]
Research based on the Dunedin Multidisciplinary Health and Development Study has found that those who begin regular use of cannabis in early adolescence (from age 15, median 25 days per year by age 18) and also fit a certain genetic profile (specifically, the Val/Val variant of the COMT gene) are five times more likely to develop psychotic illnesses than individuals with differing genotypes, or those who do not use cannabis.[122][123] The study was noted for having controlled for preexisting symptoms, but is open to the criticism that it cannot control for late adolescent onset of psychotic illness. Also, the study was on a cohort population, so there is no way to correlate a change in the rate of adolescent use with a change in the rate of incidence of schizophrenia in the study population. These points undermine its value in resolving the correlation versus causality question.
A study that inversely correlated cerebrospinal anandamide (an endogenous cannabinoid) levels with severity of schizophrenia (i.e., that anandamide was released in order to suppress psychosis) suggests that cannabis use may be an effect of schizophrenia or its predisposition, as opposed to a cause.[124]
The fact that the prevalence of cannabis use has increased substantially during the last decades whereas the prevalence of psychotic illness has not suggests no causal relationship.[117]
Cannabidiol and schizophrenia
A recent study has shown that cannabidiol (a major constituent of cannabis) may be as effective as atypical antipsychotics in treating schizophrenia,[125] Further research has verified these results. Leweke et al., (2009) performed a double blind, 4 week, explorative study controlled clinical trial, to compare the effects of purified cannabidiol and the atypical antipsychotic amisulpride on improving the symptoms of schizophrenia in 42 patients with acute paranoid schizophrenia. 'Both treatments were associated with a significant decrease of psychotic symptoms after 2 and 4 weeks as assessed by BPRS and PANSS. However, there was no statistical difference between both treatment groups. In contrast, cannabidiol induced significantly less side effects (EPS, increase in prolactin, weight gain) when compared to amisulpride'.[126] The authors conclude cannabidiol revealed substantial antipsychotic properties in acute paranoid schizophirenia (Leweke et al., 2009). This led the authors to suggest the endocannabinoid system plays an adaptive role in the development of paranoid schizophirenia and that this research provides evidence that this mechanism may be a valuable target for 'antipsychotic treament strategies' .[126]
Smoking
The process most popularly used to ingest cannabis is smoking, and for this reason most research has evaluated health effects from this method of ingestion. Other methods of ingestion may have lower or higher health risks. See section on harm reduction below for more information on other methods of ingestion. Tobacco smoking has well-established risks such as bronchitis, coughing, overproduction of mucus, wheezing, and addiction. Similar risks for smoking cannabis related to airway inflammation have been suggested in a study of healthy cannabis users who exhibited similar early characteristics to tobacco smoking.[127]
The effects of tobacco and cannabis smoking differ, however, as they affect different parts of the respiratory tract: whereas tobacco tends to penetrate to the smaller, peripheral passageways of the lungs, cannabis tends to concentrate on the larger, central passageways. One consequence of this is that cannabis, unlike tobacco, does not appear to cause emphysema, though this claim is disputed. A 2002 report by the British Lung Foundation estimated that three to four cannabis cigarettes a day were associated with the same amount of damage to the lungs as 20 or more tobacco cigarettes a day.[128] Unlike tobacco, regular cannabis use does not appear to cause chronic obstructive pulmonary disease.[129]
It is important to note that, in some cases, cannabis users mix commercial tobacco in joints, called "Spliff" (popular in Europe), tobacco mixed with hash in a chillum (India), or cannabis rolled in tobacco leaves (a blunt), which would expose the user to the additional risks of tobacco, such as rapid physical addiction to nicotine.[130]
Cancer risk
Cannabis smoke contains numerous carcinogens.[131][132][133] Surprisingly, an extensive study published in 2006 by Donald Tashkin of the University of California, Los Angeles found that there is no significant link between smoking cannabis and lung cancer.[134] The study, which involved a large population sample (1,200 people with lung, neck, or head cancer, and a matching group of 1,040 without cancer) found no correlation between marijuana smoking and increased lung cancer risk, with the same being true for head and neck cancers as well. The results indicated no correlation between long and short-term cannabis use and cancer, indicating a possible therapeutic effect. Extensive cellular studies and some studies in animal models suggest that THC or cannabidiol has antitumor properties, either by encouraging programmed cell death of genetically damaged cells that can become cancerous, or by restricting the development of the blood supply that feeds tumors, or both.[135] Unlike most other studies, this one had a very large sample size and was controlled for tobacco, alcohol, and several socio-demographic factors, which likely confounded the other studies.
In 2008 a smaller study was released by the Medical Research Institute of New Zealand suggested that smoking cannabis increased the risk of lung cancer by 5.7 times over non-smokers.[136] The small 79 person study noted that "In the near future we may see an 'epidemic' of lung cancers connected with this new carcinogen. And the future risk probably applies to many other countries, where increasing use of cannabis among young adults and adolescents is becoming a major public health problem."
Prior, a 1997 study examining the records of 64,855 Kaiser patients (14,033 of whom identified themselves as current smokers), also found no positive correlation between cannabis use and cancer.[137]
Conversely, the 2008 case-control study from New Zealand mentioned earlier by the Cannabis and Respiratory Disease Research Group examining adults under age 55 with lung cancer concluded that smoking cannabis was significantly associated with risk of developing lung cancer, after controlling for smoking tobacco; the highest tertile of marijuana smokers were estimated to have a 5.7-fold higher risk of lung cancer compared to nonusers.[138]
A Research Triangle Institute study concluded that THC, a dilative agent (bronchodilator), may help cleanse the lungs by dilating the bronchi, and could actively reduce the instance of tumors.[139] Additionally, a study by Rosenblatt et al. found no association between marijuana use and the development of head and neck squamous cell carcinoma.[140] However, a contrasting study conducted in 2000 linked the smoking of cannabis to the growth of cancerous tumors through the impairment of anti-tumor defenses in mice.[141]
A preliminary 2009 study found that cannabis use may increase the risk of testicular cancer. In particular, the risk of developing nonseminoma testicular cancer, a more aggressive form of the disease, was increased in current cannabis users and even greater in long-term chronic users. This however is overshadowed by the fact that researchers are unable to determine what about marijuana causes the increased risk, or for that matter if a positive correlation can be established. A statement was released by the researchers that performed the study as seen below: "This is the first study to look at this question, and by itself is not definitive. And there's a lot more research that would have to be done in order to prove that marijuana use really increases a man's risk of developing testicular cancer," - Stephen Schwartz.[142]
Cannabis smoke (but not the plant itself) has recently been added to a "list of substances California regulators say cause cancer"[143]. California's Office of Environmental Health Hazard assessment has added cannabis smoke to the list after it found that it "contains 33 of the same harmful chemicals as tobacco smoke."[144]
UCLA study
On 23 May 2006, Donald Tashkin, M.D., Professor of Medicine at the David Geffen School of Medicine at UCLA in Los Angeles announced that the use of cannabis does not appear to increase the risk of developing lung cancer, or increase the risk of head and neck cancers, such as cancer of the tongue, mouth, throat, or esophagus.[145] The study involved 2252 participants, with some of the most chronic marijuana smokers having smoked over 22,000 marijuana cigarettes.[145][146][147][148] The finding of Donald Tashkin, M.D., and his team of researchers in 2006 refined their earlier studies published in a Dec. 17th 2000 edition of the peer-reviewed journal Cancer Epidemiology Biomarker and Prevention.[149] Many opponents of marijuana incorrectly cite the original finding of UCLA Medical Center from 2000 as "proof" that marijuana leaves the users at higher risk for cancer of the lung, and cancerous tumors,[141] even though the researchers at the UCLA Medical Center have revised their finding with a more in-depth study on the effects of the use of marijuana. This seemed to contradict assumptions made after some studies, like those from Dale Geirringer et al., which found that 118 carcinogens were produced when marijuana underwent combustion, and two carcinogens {2-Methyl-2, 4(2H-1-benzopyran-5-ol) & 5-[Acetyl benz[e]azulene-3,8-dione} formed when marijuana underwent vaporization with the Volcano Vaporizer.[52] To help explain this seemingly chemical proof of carcinogenity inherent in the process of combustion, Tashkin noted that "one possible explanation for the new findings, he said, is that THC, a chemical in marijuana smoke, may encourage aging cells to die earlier and therefore be less likely to undergo cancerous transformation."[145]
In a study of ten smokers with mild respiratory issues Hii et al. found evidence of lung disease in the form of severe bullae (fluid-filled, thin-walled blisters) of different shapes and sizes. Despite such lung disease, the patients chest x-rays were normal and lung function was only mildly reduced in nearly half of the patients. The cannabis-smoking patient group was, on average, 41 years old—considerably younger than previously research tobacco-smoking patients with lung disease, who had an average age of between 62–67 years. The researchers conclude that the younger age of lung disease and poorer lung function may be due to different smoking patterns demonstrated in cannabis smokers[150], who have been found to inhale larger amounts of smoke, which is held in the lungs for longer periods of time.[151]
Behavioral effects
Government studies often point to statistical data accumulated by methods like the National Household Survey on Drug Abuse (NHSDA), the Monitoring the Future study (MTF), and the Arrestee Drug Abuse Monitoring (ADAM) program, which claim lower school averages and higher dropout rates among users than nonusers. However, the major contributor to a lack of credibility in these studies, is that in many cases, like with NHSDA and MTF, these surveys are usually self-administered and may be anonymous. The likeliness of over or under representing data definitely undermines the effectiveness of these instruments.[12] The ADAM study is conducted anonymously, but only seeks information from a sample of people who have been arrested for drug-related offenses. Socially deviant behavior may be found more frequently in individuals of the criminal justice system compared to those in the general population, including non users. In response, independent studies of college students have shown that there was no difference in grade point average, and achievement, between marijuana users and nonusers, but the users had a little more difficulty deciding on career goals, and a smaller number were seeking advanced professional degrees.[152] Laboratory studies of the relationship between motivation and marijuana outside of the classroom, where volunteers worked on operant tasks for a wage representing a working world model, also fail to distinguish a noticeable difference between users and non users.[153]
A USC study of 4,400 Internet users found that "adults apparently do not increase their risk for depression by using marijuana", and in fact show less self-reported symptoms of depression.[7]
Gateway drug hypothesis
The gateway drug hypothesis asserts that the use of cannabis may ultimately lead to the use of harder drugs. For the most part, it was commonly thought that cannabis gateways to other drugs because of social factors. For example, the criminalization of cannabis in many countries associates its users with organized crime promoting the illegal drug trade.
A July 2006 study by Ellgren et al.[154] strictly tested lab rats for the biological mechanism of the gateway drug effect. The study administered 6 "teenage" (28 and 49 days old) rats delta-9-tetrahydrocannabinol, and 6 were the control. One week after the first part was completed, catheters were inserted in the jugular vein of all of the adult rats and they were able to self-administer themselves heroin by pushing a lever. The study found that initially both groups behaved the same and began to self-administer heroin frequently, but then stabilized at different levels. The rats that had previously been administered THC consumed about 1.5 times more heroin than those that had not. Because many THC receptors interact with the opioid system, the study surmised that adolescent cannabis use overstimulates and alters the pleasure and reward structures of the brain, thus increasing the already high risk of addiction for people who start to use heroin. However, the rats took up self-administration at the same rate regardless of adolescent THC exposure, and observed levels of "drug-seeking behavior" were also the same.[154] Psychopharmacologist Ian Stolerman, from King's College London, finds the biological cannabis gateway drug effect "somewhat preliminary", and states "it's too early to say there's a consensus, but a small number of studies like this suggest that there is a physiological basis for this effect." Other drugs, he notes, such as cocaine and amphetamines are involved in another brain pathway called the dopaminergic system. Cells in that system also interact with THC receptors and could be modified by cannabis exposure.[155] Cannabinoid receptors are 10 times more prevalent in the brain than opioid receptors. According to Dr. Hurd, one of the study leaders, two other drugs that also stimulate opioid cells, and could therefore also feasibly cause a gateway effect, are nicotine and alcohol.
However, a December 2006 study by the American Psychiatric Association[156][157] challenges these findings. A 12 year study on 214 boys from ages 10–12 showed that adolescents who used marijuana prior to using other drugs, including alcohol and tobacco, were no more likely to develop a substance abuse disorder than other subjects in the study. "This evidence supports what's known as the common liability model… states the likelihood that someone will transition to the use of illegal drugs is determined not by the preceding use of a particular drug, but instead by the user's individual tendencies and environmental circumstances," investigators stated in a press release. They added, "The emphasis on the drugs themselves, rather than other, more important factors that shape a person's behavior, has been detrimental to drug policy and prevention programs."
Models used in a 2002 study[158] by RAND cast doubt on the gateway effect and show "that the marijuana gateway effect is not the best explanation for the link between marijuana use and the use of harder drugs," as noted by Andrew Morral, associate director of RAND's Public Safety and Justice unit and lead author of the study.
Memory and intelligence
A 2002 longitudinal study published in the Canadian Medical Association Journal concluded that "marijuana does not have a long-term negative impact on global intelligence", and that "current marijuana use had a negative effect on global IQ score only in subjects who smoked 5 or more joints per week." In fact, current light users and former users showed larger IQ gains than those who had never used cannabis.[159]
A 2008 study suggests that long-term, heavy cannabis use (over five joints daily for more than ten years) are associated with structural abnormalities in the hippocampus and amygdala areas of the brain. The hippocampus, thought to regulate emotion and memory, and the amygdala, involved with fear and aggression, tended to be smaller in heavy and long term cannabis users than in controls (volume was reduced by an average of 12 percent in the hippocampus and 7.1 percent in the amygdala). The study concluded that "heavy daily cannabis use across protracted periods exerts harmful effects on brain tissue and mental health."[160]
A 2002 study published in Neurology concluded that "very heavy use of marijuana is associated with persistent decrements in neurocognitive performance even after 28 days of abstinence."[161]
The strongest evidence regarding cannabis and memory focuses on its short-term negative effects on short-term and working memory.[3] Evidence also suggests that long-term effects exist, but these appear to be reversible except possibly in very heavy users.[2]
A 1998 Journal of Neuroscience in vitro research, which was carried out on hippocampal cells excised from decapitated rats, using THC carried in ethanol to saturate the neurons, suggests that THC is toxic for cultured hippocampal neurons.[162]
A 1998 report by INSERM and CNRS, which was directed by Dr. Pierre-Bernard Roques, determined that, "former results suggesting anatomic changes in the brain of chronic cannabis users, measured by tomography, were not confirmed by the accurate modern neuro-imaging techniques (such as MRI) ... Moreover, morphological impairment of the hippocampus [which plays a part in memory and navigation] of rat after administration of very high doses of THC was not shown."[163][164][165]
Legal and political constraints on open research

In many countries, experimental science regarding cannabis is restricted due to its illegality. Thus, cannabis as a drug is often hard to fit into the structural confines of medical research because appropriate, research-grade samples are difficult to obtain for research purposes, unless granted under authority of national governments.
United States
This issue was recently highlighted in the United States by the clash between Multidisciplinary Association for Psychedelic Studies (MAPS), an independent research group, and the National Institute on Drug Abuse (NIDA), a federal agency charged with the application of science to the study of drug abuse. The NIDA largely operates under the general control of the Office of National Drug Control Policy (ONDCP), a White House office responsible for the direct coordination of all legal, legislative, scientific, social and political aspects of federal drug control policy.
The cannabis that is available for research studies in the United States is grown at the University of Mississippi and solely controlled by the NIDA, which has veto power over the Food and Drug Administration (FDA) to define accepted protocols. Since 1942, when cannabis was removed from the U.S. Pharmacopoeia and its medical use was prohibited, there have been no legal (under federal law) privately funded cannabis production projects. This has resulted in a limited amount of research being done and possibly in NIDA producing cannabis which has been alleged to be of very low potency and inferior quality.[166]
MAPS, in conjunction with Professor Lyle Craker, PhD, the director of the Medicinal Plant Program of the University of Massachusetts at Amherst, sought to provide independently grown cannabis of more appropriate research quality for FDA-approved research studies, and encountered opposition by NIDA, the ONDCP, and the U.S. Drug Enforcement Administration (DEA).
United Kingdom
In countries such as the United Kingdom a license for growing cannabis is required if it is to be used for botanical or scientific reasons. It is referred to as a "controlled drug". In such countries a greater depth and variety of scientific research has been performed. Recently several habitual smokers were invited to partake in various tests by British medical companies in order for the UK government to ascertain the influence of cannabis on operating a motor vehicle.
Pathogens and microtoxins
Most microorganisms found in cannabis only affect plants and not humans, but some microorganisms, especially those that proliferate when the herb is not correctly dried and stored, can be harmful to humans. Some users may store marijuana in an airtight bag or jar in a refrigerator to prevent fungi and bacterial growth.[167]
Fungi
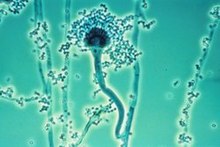
The fungi Aspergillus flavus,[168] Aspergillus fumigatus,[168] Aspergillus niger,[168] Aspergillus parasiticus, Aspergillus tamarii, Aspergillus sulphureus, Aspergillus repens, Mucor hiemalis (not a human pathogen), Penicillin chrysogenum, Penicillin italicum and Rhizopus nigrans have been found in moldy cannabis.[167] Aspergillus mold species can infect the lungs via smoking or handling of infected cannabis and cause opportunistic and sometimes deadly Aspergillosis.[citation needed] Some of the microorganisms found create aflatoxins, which are toxic and carcinogenic. Researchers suggest that moldy cannabis thus be discarded.[citation needed]
Mold is also found in smoke from mold infected cannabis,[167][168] and the lungs and nasal passages are a major means of contracting fungal infections. "Levitz and Diamond (1991) suggested baking marijuana in home ovens at 150 °C [302 °F], for five minutes before smoking. Oven treatment killed conidia of A. fumigatus, A. flavus and A. niger, and did not degrade the active component of marijuana, tetrahydrocannabinol (THC)."[167]
Bacteria
Cannabis contaminated with Salmonella muenchen was positively correlated with dozens of cases of salmonellosis in 1981.[169] "Thermophilic actinomycetes" were also found in cannabis.[168]
See also
- Contact high
- Harm reduction
- Health effects of tobacco smoking
- Medical cannabis
- National Cannabis Prevention and Information Centre
- Psychoactive drug
- Responsible drug use
References
- ^ a b Ranganathan M, D'Souza DC (2006). "The acute effects of cannabinoids on memory in humans: a review". Psychopharmacology (Berl.). 188 (4): 425–44. doi:10.1007/s00213-006-0508-y. PMID 17019571.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Grotenhermen F (2007). "The toxicology of cannabis and cannabis prohibition". Chemistry & Biodiversity. 4 (8): 1744–69. doi:10.1002/cbdv.200790151. PMID 17712818.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d Riedel G, Davies SN (2005). "Cannabinoid function in learning, memory and plasticity". Handb Exp Pharmacol. 168 (168): 445–77. doi:10.1007/3-540-26573-2_15. PMID 16596784.
- ^ Leweke FM, Koethe D (2008). "Cannabis and psychiatric disorders: it is not only addiction". Addict Biol. 13 (2): 264–75. doi:10.1111/j.1369-1600.2008.00106.x. PMID 18482435.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rubino T, Parolaro D (2008). "Long lasting consequences of cannabis exposure in adolescence". Mol. Cell. Endocrinol. 286 (1-2 Suppl 1): S108–13. doi:10.1016/j.mce.2008.02.003. PMID 18358595.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ DeLisi LE (2008). "The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia?". Curr Opin Psychiatry. 21 (2): 140–50. doi:10.1097/YCO.0b013e3282f51266. PMID 18332661.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c T.F. Denson, M. Earleywine (June 20, 2005). "Decreased depression in marijuana users". Addictive Behaviors.
- ^ a b c d H.K. Kalant & W.H.E. Roschlau (1998). Principles of Medical Pharmacology (6th ed.). pp. 373–375.
- ^ Turner CE, Bouwsma OJ, Billets S, Elsohly MA (1980). "Constituents of Cannabis sativa L. XVIII--Electron voltage selected ion monitoring study of cannabinoids". Biomedical Mass Spectrometry. 7 (6): 247–56. doi:10.1002/bms.1200070605. PMID 7426688.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f J.E. Joy, S. J. Watson, Jr., and J.A. Benson, Jr, (1999). Marijuana and Medicine: Assessing The Science Base. Washington D.C: National Academy of Sciences Press.
{{cite book}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Hampson AJ, Grimaldi M, Axelrod J, Wink D (1998). "Cannabidiol and (-)Δ9-tetrahydrocannabinol are neuroprotective antioxidants". Proc. Natl. Acad. Sci. U.S.A. 95 (14): 8268–73. doi:10.1073/pnas.95.14.8268. PMC 20965. PMID 9653176.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b H. Abadinsky (2004). Drugs: An Introduction (5th ed.). pp. 62–77, 160–166.
- ^ Judge Young - Part 4
- ^ 1996. The Merck Index, 12th ed., Merck & Co., Rahway, New Jersey
- ^ Erowid. "Cannabis Chemistry". Retrieved 2006-03-20.
- ^ http://www.druglibrary.org/schaffer/library/mjfaq1.htm [unreliable source?]
- ^ http://www.askmen.com/sports/health/20_mens_health.html [unreliable source?]
- ^ http://www.mirror.co.uk/news/top-stories/2009/05/21/rock-star-killed-by-smoking-pot-115875-21376212/[unreliable source?]
- ^ "UK government report" (PDF). House of Commons Science and Technology Committee. 2006-07-18. Retrieved 2006-08-29.]
- ^ a b c Moir D, Rickert WS, Levasseur G; et al. (2008). "A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions". Chemical Research in Toxicology. 21 (2): 494–502. doi:10.1021/tx700275p. PMID 18062674.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ashton CH (2001). "Pharmacology and effects of cannabis: a brief review". Br J Psychiatry. 178: 101–6. doi:10.1192/bjp.178.2.101. PMID 11157422.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ http://www.dasc.sa.gov.au/site/page.cfm?u=128
- ^ "Medication-Associated Depersonalization Symptoms".
- ^ Shufman, E. (2005). "Depersonalization after withdrawal from cannabis usage". Harefuah (in Hebrew). 144 (4): 249–51 and 303. PMID 15889607.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ http://www.ncbi.nlm.nih.gov/pubmed/2178712
- ^ Web4Health: Physical effects of cannabis/haschish/marijuana Written by: Wendy Moelker, Psychologist in charge, tutor, Emergis center for mental health care, Goes, the Netherlands. Latest revision: 19 Sep 2008.
- ^ Andersson M, Usiello A, Borgkvist A; et al. (2005). "Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons". J. Neurosci. 25 (37): 8432–8. doi:10.1523/JNEUROSCI.1289-05.2005. PMID 16162925.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://www.erowid.org/plants/cannabis/cannabis_effects.shtml
- ^ Fant R, Heishman S, Bunker E, Pickworth W (1998). "Acute and residual effects of marijuana in humans". Pharmacol. Biochem. Behav. 60 (4): 777–84. doi:10.1016/S0091-3057(97)00386-9. PMID 9700958.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tj. T. Mensinga; et al. A double-blind, randomized, placebo-controlled, cross-over study on the pharmacokinetics and effects of cannabis (PDF). RIVM. Retrieved 2007-09-21.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Pertwee R (1997). "Pharmacology of cannabinoid CB1 and CB2 receptors". Pharmacol. Ther. 74 (2): 129–80. doi:10.1016/S0163-7258(97)82001-3. PMID 9336020.
- ^ Eubanks L, Rogers C, Beuscher A, Koob G, Olson A, Dickerson T, Janda K (2006). "A molecular link between the active component of marijuana and Alzheimer's disease pathology". Mol. Pharm. 3 (6): 773–7. doi:10.1021/mp060066m. PMC 2562334. PMID 17140265.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.dft.gov.uk/pgr/roadsafety/research/rsrr/theme3/cannabisanddrivingareviewoft4764
- ^ a b c Kelly E, Darke S, Ross J (2004). "A review of drug use and driving: epidemiology, impairment, risk factors and risk perceptions". Drug and Alcohol Review. 23 (3): 319–44. doi:10.1080/09595230412331289482. PMID 15370012.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c EMCDDA(2008a). A cannabis reader: Global issues and local experiences,Monograph series 8, Volume 2. Lisbon: European Monitoring Centre for Drugs and Addiction
- ^ Grotenhermen F, Leson G, Berghaus G; et al. (2007). "Developing limits for driving under cannabis". Addiction. 102 (12): 1910–7. doi:10.1111/j.1360-0443.2007.02009.x. PMID 17916224.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ramaekers JG, Berghaus G, van Laar M, Drummer OH (2004). "Dose related risk of motor vehicle crashes after cannabis use". Drug and Alcohol Dependence. 73 (2): 109–19. doi:10.1016/j.drugalcdep.2003.10.008. PMID 14725950.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Drummer OH, Gerostamoulos J, Batziris H; et al. (2004). "The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes". Accident Analysis & Prevention. 36 (2): 239–48. doi:10.1016/S0001-4575(02)00153-7. PMID 14642878.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Walsh JM, Verstraete AG, Huestis MA, Mørland J (2008). "Guidelines for research on drugged driving". Addiction. 103 (8): 1258–68. doi:10.1111/j.1360-0443.2008.02277.x. PMC 2690607. PMID 18855814.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Peyrot I, Garsaud AM, Saint-Cyr I, Quitman O, Sanchez B, Quist D (2007). "Cannabis arteritis: a new case report and a review of literature". Journal of the European Academy of Dermatology and Venereology. 21 (3): 388–91. doi:10.1111/j.1468-3083.2006.01947.x. PMID 17309465.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Heavy pot smoking could raise risk of heart attack, stroke". CBC. Retrieved 2009-04-17.
- ^ "Marijuana affects blood vessels". BBC News. 2005-02-08. Retrieved 2009-04-26.
- ^ Norton, Amy (2005-02-22). "More Evidence Ties Marijuana to Stroke Risk". Reuters Health. Retrieved 2009-04-26.
- ^ Noble, Holcomb B. (2000-03-03). "Report Links Heart Attacks To Marijuana". New York Times. Retrieved 2009-04-26.
- ^ Soapbar - Just Say No to polluted hash
- ^ Flin Flon Mine Area Marijuana Contamination
- ^ BBC NEWS | England | Devon | Warnings over glass in cannabis
- ^ Hall,W. & Nelson,J. (1995). Public perceptions of health and psychological consequences of cannabis use. Canberra: Australian Government Publishing Service
- ^ StollzNow. (2006). Market research report: Australians on cannabis. Report prepared for NDARC and Pfizer Australia. Sydney: StollzNow Research and Advisory
- ^ Zhang ZF, Morgenstern H, Spitz MR; et al. (1999). "Marijuana use and increased risk of squamous cell carcinoma of the head and neck". Cancer Epidemiology, Biomarkers & Prevention. 8 (12): 1071–8. PMID 10613339.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Public opinion on drugs and drug policy. Transform Drug Policy Foundation: Fact Research Guide. "Data is notoriously easy to cherry pick or spin to support a particular agenda or position. Often the raw data will conceal all sorts of interesting facts that the headlines have missed." Transform Drug Policy Foundation, Easton Business Centre, Felix Rd., Bristol, UK. Retrieved on 24 March 2007.
- ^ a b Sarafian TA, Kouyoumjian S, Tashkin D, Roth MD (2002). "Synergistic cytotoxicity of Delta(9)-tetrahydrocannabinol and butylated hydroxyanisole". Toxicol. Lett. 133 (2–3): 171–9. doi:10.1016/S0378-4274(02)00134-0. PMID 12119125.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Australian Institute of Health and Welfare (2002). 2001 National Drug Stategy Household Survey: first results. (Drug Statistics Series, no. 9). AIHW cat no. PHE 35. Canberra: AIHW.
- ^ Lukas, S.E. and Orozco, S. (2001). Ethanol increases plasma Delta-9 tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug and Alcohol Dependence 64(2): 143–149
- ^ Degenhardt, L. and Hall, W. (2001). The relationship between tobacco use, substance-use disorders and mental health: results from the National Survey of Mental Health and Wellbeing. Nicotine & Tobacco Research 3(3): 225–234.
- ^ Curran HV, Brignell C, Fletcher S, Middleton P, Henry J (2002). "Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users". Psychopharmacology (Berl.). 164 (1): 61–70. doi:10.1007/s00213-002-1169-0. PMID 12373420.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mechoulam,R. (1984). Cannabinoids as therapeutic agents. Boca Raton, FL: CRC Press
- ^ Ad Hoc Group of Experts. "Report to the Director, National Institutes of Health" (Workshop on the Medical Utility of Marijauana). Institute of Medicine.
- ^ a b Bonsor, Kevin. "How Marijauan Works: Other Physiological Effects". HowStuffWorks. Retrieved on 2007-11-03
- ^ Di Marzo, V., Sepe, N., De Petrocellis, L., Berger, A.,Crozier, G., Fride, E. & Mechoulam, R.(1998) Trick or treat from food endocannabinoids?Nature 396, 636.
- ^ Di Tomaso, E., Beltramo, M. & Piomelli, D(1996). Brain cannabinoids in chocolate.Nature 382, 677-678
- ^ a b http://ncpic.org.au/ncpic/publications/research-briefs/?page=1
- ^ Fride, E. (2004). The endocannabinoid-CB receptor system in pre- and postnatal life.European Journal of Pharmacology 500, 289-297
- ^ UCSB's SexInfo - Sex Under the Influence
- ^ W. Hall, N. Solowij (1998). "Adverse Effects of Cannabis". The Lancet. 352: 1611–6. doi:10.1016/S0140-6736(98)05021-1.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Schuel H, Burkman LJ, Lippes J; et al. (2002). "Evidence that anandamide-signaling regulates human sperm functions required for fertilization". Molecular Reproduction and Development. 63 (3): 376–87. doi:10.1002/mrd.90021. PMID 12237954.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mueller BA, Daling JR, Weiss NS, Moore DE (1990). "Recreational drug use and the risk of primary infertility". Epidemiology. 1 (3): 195–200. PMID 2081252.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wenger T, Tóth BE, Juanéda C, Leonardelli J, Tramu G (1999). "The effects of cannabinoids on the regulation of reproduction". Life Sciences. 65 (6–7): 695–701. doi:10.1016/S0024-3205(99)00292-1. PMID 10462070.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Coperland, J., Gerber, S., Swift, W., Evidence-based Answers to Cannabis Questions: a review of the literatureA report prepared for the Australian National Council on Drugs, December 2004
- ^ http://ncpic.org.au/ncpic/links/information/
- ^ Zuckerman B, Frank DA, Hingson R; et al. (1989). "Effects of maternal marijuana and cocaine use on fetal growth". The New England Journal of Medicine. 320 (12): 762–8. PMID 2784193.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Astley SJ, Clarren SK, Little RE, Sampson PD, Daling JR (1992). "Analysis of facial shape in children gestationally exposed to marijuana, alcohol, and/or cocaine". Pediatrics. 89 (1): 67–77. PMID 1728025.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ R. Berkow MD; et al. (1997). The Merck Manual of Medical Information (Home Edition). p. 449.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ a b Hayes JS, Lampart R, Dreher MC, Morgan L (1991). "Five-year follow-up of rural Jamaican children whose mothers used marijuana during pregnancy". The West Indian Medical Journal. 40 (3): 120–3. doi:10.1590/S1516-31802004000300007. PMID 1957518.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ M.C. Dreher, K. Nugent, R. Hudgins (1994). "Prenatal Marijuana Exposure and Neonatal Outcomes in Jamaica: An Ethnographic Study". Pediatrics. 93 (3): 254–260. doi:10.1590/S1516-31802004000300007.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Huizink AC, Mulder EJ (2006). "Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring". Neurosci Biobehav Rev. 30 (1): 24–41. doi:10.1016/j.neubiorev.2005.04.005. PMID 16095697.
- ^ Fried PA, Watkinson B, Gray R (2003). "Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana". Neurotoxicol Teratol. 25 (4): 427–36. doi:10.1016/S0892-0362(03)00029-1. PMID 12798960.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hardwiring the Brain: Endocannabinoids Shape Neuronal Connectivity Science, May 25, 2007
- ^ Paul Berghuis,...:Endocannabinoids regulate interneuron migration and morphogenesis... 2007
- ^ Harkany et al.: The emerging functions of endocannabinoid signaling during CNS development
- ^ JR Minkel: Marijuana-Like Chemicals Guide Fetal Brain Cells, Scientific American, May24, 2007
- ^ Dreher MC, Nugent K, Hudgins R (1994). "Prenatal marijuana exposure and neonatal outcomes in Jamaica: an ethnographic study". Pediatrics. 93 (2): 254–60. PMID 8121737.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://www.antiproibizionisti.it/public/docs/thelancet_20070323.pdf
- ^ Laino, Charlene (7 May 2008). "Withdrawal Symptoms From Smoking Pot?". CBS News. Retrieved 2009-09-05.
- ^ "Combination of Cognitive-Behavioral Therapy and Motivational Incentives Enhance Treatment for Marijuana Addiction" (Press release). National Institutes of Health. April 1, 2006.
- ^ 1998 INSERM-CNRS report, directed by Pr. Bernard Roques and commissioned by Health Secretary of State Bernard Kouchner [1] [2] [3] [4]
- ^ a b Clinical Textbook of Addictive Disorders, Marijuana, David McDowell, page 169, Published by Guilford Press, 2005 ISBN 159385174X.
- ^ McLaren, Jennifer; Lemon, Jim; Robins, Lisa; Mattick, Richard P. (2008). Cannabis and Mental Health: Put into Context. National Drug Strategy Monograph Series. Australian Government Department of Health and Ageing. Retrieved 17 October 2009.
{{cite book}}: Unknown parameter|month=ignored (help) - ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1136/bmj.38267.664086.63, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1136/bmj.38267.664086.63instead. - ^ Patton, G. C., Coffey, C., Carlin, J. B., Degenhardt, L., Lynskey, M., and Hall, W. 2002. Cannabis use and mental health in young people: cohort study. BMJ 325(7374): 1195-1198. Retrieved 45 March 2007
- ^ Fergusson, David M.; Horwood, John L.; Ridder, Elizabeth M. "Research Report: Tests of causal linkages between cannabis use and psychotic symptoms. University of Otago Christchurch School of Medicine published in the Society for the Study of Addiction (2004-11-05). Retrieved on 2007-11-03.
- ^ Hall, Wayne; Degenhardt, Lousia; Teesson, Maree. "Cannabis use and psychotic disorders: an update". Office of Public Policy and Ethics, Institute for Molecular Bioscience University of Queensland Australia, and National Drug and Alcohol Research Centre University of New South Wales Australia published in Drug and Alcohol Review (December 2004). Vol 23 Issue 4. Pg 433-443
- ^ a b L. Arseneault; et al. (2004). "Causal association between cannabis and psychosis: examination of the evidence". The British Journal of Psychiatry. 184: 110–117. doi:10.1192/bjp.184.2.110. PMID 14754822.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Earth Erowid (2005). "Cannabis & Psychosis - A guide to current research about cannabis and mental health".
- ^ Drake RE, Wallach MA (1989). "Substance abuse among the chronic mentally ill". Hospital & Community Psychiatry. 40 (10): 1041–6. PMID 2807205.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Cannabis 'raises psychosis risk'". BBC News (2007-07-27). Retrieved on 2007-11-03.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1192/bjp.bp.107.045740, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1192/bjp.bp.107.045740instead. - ^ Harding, Anne (3 November 2008). "Pot-induced psychosis may signal schizophrenia". Reuters. Retrieved 17 October 2009.
- ^ a b c Hope, C., Skunk cannabis can make well users psychotic: study, The Telegraph, 28 July 2009
- ^ "The BEACH Project". Retrieved 17 October 2009.
- ^ "Depression, psychosis strike dope smokers". The Australian. Australian Associated Press. 29 September 2009. Retrieved 17 October 2009.
- ^ Chopra, G.S. and Smith, J.W. (1974). Psychotic reactions following cannabis use in East Indians. Archives of General Psychiatry 30: 24–27.
- ^ Eva, J. (1992). Cannabis psychosis. Psychiatric Bulletin 16: 310–311
- ^ Solomons, K., Neppe, V.M. and Kuyl, J.M. (1990). Toxic cannabis psychosis is a valid entity. South African Medical Journal 78(8): 476–481.
- ^ Bernhardson, G. and Gunne, L.M. (1972). Forty-six cases of psychosis in cannabis abusers. International Journal of the Addictions 7(1): 9–16
- ^ Carney, M., Bacelle, L. and Robinson, B. (1984). Psychosis after cannabis use. British Medical Journal 288: 1047.
- ^ Andreasson, S., Allebeck, P. and Rydberg, U. (1989). Schizophrenia in users and nonusers of cannabis: a longitudinal study in Stockholm County. Acta Psychiatrica Scandinavica 79(5): 505–510
- ^ Copeland, J.; Gerber, Saul; Swift, Wendy (2006). Evidence-based answers to cannabis questions: a review of the literature. ANCD Research Paper. Canberra: Australian National Council on Drugs. ISBN 1877018120.
{{cite book}}: More than one of|author=and|last1=specified (help) - ^ Zammit, S., et al., Self-reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: Historical cohort study. British Medical Journal, 2002. 325: p. 1199–1201.
- ^ "Heavy pot smoking linked to smaller brains". New Scientist (2659). 4 June 2008. Retrieved 17 October 2009.
- ^ Lubman, Dan (2008). "Long-term cannabis use and regional brain abnormalities". NCPIC e-Zine. Retrieved 17 October 2009.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Copeland, J., Gerber, S., Swift, W. (2004), evidence-based answers to cannabis questions a review of the literature. National Drug and Alcohol Research Centre University of New South Wales A report prepared for the Australian National Council on Drugs. Available at http://ncpic.org.au/ncpic/links/information
- ^ Chen, C.-Y., Wagner, F.A. and Anthony, J.C. (2002). Marijuana use and the risk of major depressive episode: epidemiological evidence from the United States National Comorbidity Survey. Social Psychiatry and Psychiatric Epidemiology 37(5): 199–206
- ^ Grant, B.F. (1995). Comorbidity between DSM-IV drug use disorders and major depression: results of a national survey of adults. Journal of Substance Abuse 7: 481–497
- ^ Borges, G., Walters, E.E. and Kessler, R.C. (2000). Associations of substance use, abuse and dependence with subsequent suicidal behavior. American Journal of Epidemiology 151: 781–789
- ^ Beautrais, A.L., Joyce, P.R. and Mulder, R.T. (1999). Cannabis abuse and serious suicide attempts. Addiction 94(8): 1155–1164
- ^ a b Degenhardt L, Hall W, Lynskey M (2001). "Comorbidity between cannabis use and psychosis: Modelling some possible relationships. Technical Report No. 121" (PDF). Sydney: National Drug and Alcohol Research Centre. Retrieved 19.18.2006.
{{cite journal}}: Check date values in:|accessdate=(help); Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ http://www.pendulum.org/bpnews/archive/001628.html
- ^ S. Andreasson; et al. (1987). "Cannabis and Schizophrenia: A Longitudinal Study of Swedish Conscripts". The Lancet. 2: 1483–1486. doi:10.1016/S0140-6736(87)92620-1.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Schiffman J, Nakamura B, Earleywine M, LaBrie J (2005). "Symptoms of schizotypy precede cannabis use". Psychiatry Research. 134 (1): 37–42. doi:10.1016/j.psychres.2005.01.004. PMID 15808288.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Boydell J, Dean K, Dutta R, Giouroukou E, Fearon P, Murray R (2007). "A comparison of symptoms and family history in schizophrenia with and without prior cannabis use: implications for the concept of cannabis psychosis". Schizophr. Res. 93 (1–3): 203–10. doi:10.1016/j.schres.2007.03.014. PMID 17462864.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1136/bmj.325.7374.1212, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1136/bmj.325.7374.1212instead. - ^ Avshalom Caspi, Terrie E. Moffitt, Mary Cannon, Joseph McClay, Robin Murray, HonaLee Harrington, Alan Taylor, Louise Arseneault, Ben Williams, Antony Braithwaite, Richie Poulton, and Ian W. Craig (18 January 2005). "Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction" (PDF). Society of Biological Psychiatry. 122. doi:10.1590/S1516-31802004000300007.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Giuffrida A, Leweke FM, Gerth CW; et al. (2004). "Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms". Neuropsychopharmacology. 29 (11): 2108–14. doi:10.1038/sj.npp.1300558. PMID 15354183.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link), New Scientist - ^ Zuardi, A.W (2006). "Cannabidiol as an antipsychotic drug" (PDF). Brazilian Journal of Medical and Biological Research. 39 (4): 421–429. doi:/S0100-879X2006000400001. ISSN 0100-879X ISSN 0100-879X. PMID 16612464.
{{cite journal}}: Check|doi=value (help); Check|issn=value (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0924-9338(09)70440-7, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0924-9338(09)70440-7instead. - ^ M.D. Roth; et al. (1 March 1998 pages= 928–937). "Airway Inflammation in Young Marijuana and Tobacco Smokers". American Journal of Respiratory and Critical Care Medicine. 157 (3): 928. PMID 9517614.
{{cite journal}}: Check date values in:|date=(help); Explicit use of et al. in:|author=(help); Missing pipe in:|date=(help) - ^ http://www.lunguk.org/Resources/British%20Lung%20Foundation/Migrated%20Resources/Documents/A/A_Smoking_Gun.pdf
- ^ Tashkin DP, Simmons MS, Sherrill DL, Coulson AH (1997). "Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age". American Journal of Respiratory and Critical Care Medicine. 155 (1): 141–8. doi:10.1136/thx.2006.077081. PMID 9001303.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Australian Government Department of Health: National Cannabis Strategy Consultation Paper, page 4. "Cannabis has been described as a 'Trojan Horse' for nicotine addiction, given the usual method of mixing cannabis with tobacco when preparing marijuana for administration."
- ^ Hashibe M, Straif K, Tashkin DP, Morgenstern H, Greenland S, Zhang ZF (2005). "Epidemiologic review of marijuana use and cancer risk". Alcohol. 35 (3): 265–75. doi:10.1016/j.alcohol.2005.04.008. PMID 16054989.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Novotny M, Lee ML, Bartle KD (1976). "A possible chemical basis for the higher mutagenicity of marijuana smoke as compared to tobacco smoke". Experientia. 32 (3): 280–2. doi:10.1007/BF01940790. PMID 1253890.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://www.norml.org/pdf_files/NORML_Cannabis_Smoke_Cancer.pdf
- ^ "Study Finds No Cancer-Marijuana Connection". Washington Post. 2006-05-26. Retrieved 2007-02-23.
{{cite news}}: External link in|publisher= - ^ WebMD (23 May 2006). "Pot Smoking Not Linked to Lung Cancer". ScienceNOW, Abstract
- ^ Cannabis bigger cancer risk than cigarettes: study | Health | Reuters
- ^ S. Sidney; Quesenberry Jr, CP; Friedman, GD; Tekawa, IS (1997). "Marijuana use and cancer incidence (California, United States)". Cancer Causes and Control. 8 (5): 722–728. doi:10.1023/A:1018427320658. PMID 9328194.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Aldington S, Harwood M, Cox B; et al. (2008). "Cannabis use and risk of lung cancer: a case-control study". The European Respiratory Journal. 31 (2): 280–6. doi:10.1183/09031936.00065707. PMC 2516340. PMID 18238947.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Huff J, Chan P (2000). "Antitumor effects of THC". Environmental Health Perspectives. 108 (10): A442–3. doi:10.2307/3435034. PMC 1240145. PMID 11097557.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ K.A. Rosenblatt; et al. (1 June 2004). "Marijuana Use and Risk of Oral Squamous Cell Carcinoma". Cancer Research. 64 (11): 4049–4054. doi:10.1158/0008-5472.CAN-03-3425. PMID 15173020.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Dr. Steven M. Dubinett (July 2006). "Study Finds Marijuana Ingredient Promotes Tumor Growth, Impairs Anti-Tumor Defenses".
{{cite news}}: Unknown parameter|org=ignored (help) - ^ "Marijuana may raise testicular cancer risk", MSNBC, Reuters, 9 February 2009
- ^ Sunday Examiner, June 21, 2009
- ^ http://search.ca.gov/search?access=p&entqr=0&output=xml_no_dtd&sort=date%3AD%3AL%3Ad1&ud=1&site=ca_oehha&ie=UTF-8&client=ca_oehha&oe=UTF-8&proxystylesheet=ca_oehha&q=cannabis+smoke&ip=59.101.63.116&filter=0// Office of Environmental Health Hazard Assessment
- ^ a b c "Study Finds No Link Between Marijuana Use And Lung Cancer". 26 May 2006.
{{cite news}}: Unknown parameter|org=ignored (help) Cite error: The named reference "Study Finds No Link Between Marijuana Use And Lung Cancer" was defined multiple times with different content (see the help page). - ^ Fred Gardner (2006-07-06). "Marijuana Smoking Does Not Cause Lung Cancer".
{{cite news}}: Unknown parameter|org=ignored (help) - ^ Tashkin, D. P., Simmons, M. S., Sherrill, D. L., and Coulson, A. H. 1997. Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age. American Journal of Respiratory and Critical Care Medicine 155(1): 141-148. Retrieved on 5 March 2007
- ^ "Study finds no marijuana-lung cancer link". Washington Post. 2006-05-26. Retrieved 2006-07-13.
- ^ http://www.sciencedaily.com/releases/1999/12/991220082058.htm
- ^ Hii, S.W., Tam, J.D.C., Thompson, B.R. & Naughton, M.T.(2008). Bullous lung disease due to marijuana. Respirology 13, 122-127
- ^ http://ncpic.org.au/ncpic/publications/factsheets/article/cannabis-and-tobacco-use// NCPIC Cannabis and tobacco factsheet
- ^ Brill NQ, Christie RL (1974). "Marihuana use and psychosocial adaptation". Archives of General Psychiatry. 31 (5): 713–9. PMID 4441242.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ H.H. Mendelson, J.C. Kuehnle, I. Greenberg; N.K. Mello (1976). "The Effects of Marihuana Use on Human Operant Behavior: Individual Data…". Pharmacology of Marihuana. Vol. 2. New York: Academic Press. pp. 643–653.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Ellgren M, Spano SM, Hurd YL (2007). "Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats". Neuropsychopharmacology. 32 (3): 607–15. doi:10.1038/sj.npp.1301127. PMID 16823391.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hopkin, Michael (2006). "Rats taking cannabis get taste for heroin". News@nature. doi:10.1038/news060703-9.
- ^ Marijuana Use Per Se Not a 'Gateway' To Illicit Drug Use, Study Says - NORML
- ^ Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB (2006). "Predictors of marijuana use in adolescents before and after licit drug use: examination of the gateway hypothesis". The American Journal of Psychiatry. 163 (12): 2134–40. doi:10.1176/appi.ajp.163.12.2134. PMID 17151165.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ RAND | News Release | RAND Study Casts Doubt on Claims That Marijuana Acts as "Gateway" to the Use of Cocaine and Heroin
- ^ Fried P, Watkinson B, James D, Gray R (2002). "Current and former marijuana use: preliminary findings of a longitudinal study of effects on IQ in young adults". CMAJ. 166 (7): 887–91. PMC 100921. PMID 11949984.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yücel M, Solowij N, Respondek C; et al. (2008). "Regional brain abnormalities associated with long-term heavy cannabis use". Archives of General Psychiatry. 65 (6): 694–701. doi:10.1001/archpsyc.65.6.694. PMID 18519827.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002). "Dose-related neurocognitive effects of marijuana use". Neurology. 59 (9): 1337–43. PMID 12427880.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chan GC, Hinds TR, Impey S, Storm DR (1998). "Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol". The Journal of Neuroscience. 18 (14): 5322–32. PMID 9651215.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ INSERM-CNRS. Released June 1998. Excerpts from the Roques report. Hemp Info. Retrieved 5 March 2007
- ^ Rapport Roques sur la dangerosité des drogues. (in French). Retrieved on 5 March 2007
- ^ L'alcool aussi dangereux que l'héroïne. (in French) Retrieved on 5 March 2007
- ^ Lyle E. Craker, Ph. D. v. U.S. Drug Enforcement Administration, Docket No. 05-16, May 8, 2006, 8-27 PDF
- ^ a b c d "Microbiological contaminants of marijuana". www.hempfood.com. Retrieved 2008-06-22.
- ^ a b c d e Kagen SL, Kurup VP, Sohnle PG, Fink JN (1983). "Marijuana smoking and fungal sensitization". The Journal of Allergy and Clinical Immunology. 71 (4): 389–93. doi:10.1016/0091-6749(83)90067-2. PMID 6833678.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ lib.bioinfo.pl
External links
- How marijuana works from HowStuffWorks
- Microbiological contaminants of marijuana
- Cannabis Use and Psychosis from National Drug and Alcohol Research Centre, Australia
- The key research on cannabis use and mental illness at BBC News
- Provision of Marijuana and Other Compounds For Scientific Research recommendations of The National Institute on Drug Abuse National Advisory Council
- Scientific American Magazine (December 2004 Issue) The Brain's Own Marijuana
- Ramström, J. (2003), Adverse Health Consequences of Cannabis Use, A Survey of Scientific Studies Published up to and including the Autumn of 2003, National institute of public health, Sweden, Stockholm.
- Hall, W., Solowij, N., Lemon, J., The Health and Psychological Consequences of Cannabis Use. Canberra: Australian Government Publishing Service; 1994.
- World Health Organisation, PROGRAMME ON SUBSTANCE ABUSE, Cannabis: a health perspective and research agenda;1997.
- Cannabis and mental health factsheet
- Bibliography of scholarly histories on cannabis and hashish. (Updated to include article abstracts.)
- Marijuana and Immunity
- The National Cannabis Prevention and Information Centre (Australia)
- EU Research paper on the potency of Cannabis (2004)(.pdf)
- Driving under the influence of cannabis: a brief review of the literature
- Cannabis Contamination Research Brief
- Cannabinoids and appetite
- NCPIC e-zine December 2007/ January 2008 Commentary on cannabis toxicity research and Moir report
- Cannabis use and reproduction
- Evidence-based answers to cannabis questions
- NCPIC Cannabis and alcohol factsheet
- Fast Facts on Mental Health + Cannabis National Cannabis Prevention and Information Centre (Australia)
