Sodium oxybate: Difference between revisions
use initial name. update text to match citation; update ref; add ref |
update infobox; ce |
||
| Line 11: | Line 11: | ||
<!-- Clinical data --> |
<!-- Clinical data --> |
||
| pronounce = |
| pronounce = |
||
| tradename = Xyrem, Alcover, Somsanit |
| tradename = Xyrem, Alcover, Somsanit, others<ref name=brands/> |
||
| Drugs.com = {{drugs.com|monograph|sodium-oxybate}} |
| Drugs.com = {{drugs.com|monograph|sodium-oxybate}} |
||
| MedlinePlus = a605032 |
| MedlinePlus = a605032 |
||
| licence_EU = yes |
| licence_EU = yes |
||
| DailyMedID = |
| DailyMedID = Sodium oxybate |
||
| ⚫ | |||
| licence_US = |
|||
| ⚫ | |||
| pregnancy_AU_comment = |
| pregnancy_AU_comment = |
||
| pregnancy_category= |
| pregnancy_category = |
||
| dependency_liability = |
| dependency_liability = |
||
| addiction_liability = |
| addiction_liability = |
||
| Line 103: | Line 102: | ||
}} |
}} |
||
'''Sodium oxybate''', sold under the brand name '''Xyrem''' among others, is a [[medication]] used to treat symptoms of [[narcolepsy]]: [[cataplexy|sudden muscle weakness]] and [[excessive daytime sleepiness]].<ref name="Xyrem FDA label"/><ref name=UKlabel>{{cite web|title=UK label Summary of Product Characteristics|url=https://www.medicines.org.uk/emc/product/178|publisher=Electronic Medicines Compendium|access-date=14 April 2018|date=8 September 2015}}</ref><ref>{{cite web | work = Center for Drug Evaluation and Research | date = 25 January 2017 | title = Xyrem (sodium oxybate) Information. | publisher = U.S. Food and Drug Administration | url = https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/xyrem-sodium-oxybate-information }}</ref> |
'''Sodium oxybate''', sold under the brand name '''Xyrem''' among others, is a [[medication]] used to treat symptoms of [[narcolepsy]]: [[cataplexy|sudden muscle weakness]] and [[excessive daytime sleepiness]].<ref name="Xyrem FDA label"/><ref name=UKlabel>{{cite web|title=UK label Summary of Product Characteristics|url=https://www.medicines.org.uk/emc/product/178|publisher=Electronic Medicines Compendium|access-date=14 April 2018|date=8 September 2015}}</ref><ref>{{cite web | work = Center for Drug Evaluation and Research | date = 25 January 2017 | title = Xyrem (sodium oxybate) Information. | publisher = U.S. Food and Drug Administration | url = https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/xyrem-sodium-oxybate-information }}</ref> It is used sometimes in France and Italy as an [[anesthetic]] given intravenously;<ref name=WHO>{{cite web|title=Critical review of gamma-hydroxybutyric acid (GHB)|url=https://www.who.int/medicines/areas/quality_safety/4.1GHBcritical_review.pdf|date=2012}}</ref>{{rp|15, 27–28}} it is also approved and used in Italy and in Austria to treat alcohol dependence and alcohol withdrawal syndrome.<ref name=AlcoverLabel>{{cite web|title=Alcover: Riassunto delle Caratteristiche del Prodotto|url=https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000223_027751_FI.pdf&retry=0&sys=m0b1l3 |date=31 March 2017|publisher=Agenzia Italiana del Farmaco}} [https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/farmaco?farmaco=027751 Index page]</ref> |
||
Sodium oxybate is the sodium salt of [[gamma-Hydroxybutyric acid|γ-hydroxybutyric acid]] (GHB). |
Sodium oxybate is the sodium salt of [[gamma-Hydroxybutyric acid|γ-hydroxybutyric acid]] (GHB). The clinical trials for narcolepsy were conducted just as abuse of GHB as a [[club drug]] and [[date rape drug]] became a matter of public concern; in 2000 GHB was made a [[Controlled Substances Act#Schedule I controlled substances|Schedule I]] controlled substance, while sodium oxybate, when used under an FDA [[New Drug Application|NDA]] or [[Investigational New Drug|IND]] application, was classified as a [[Controlled Substances Act#Schedule III controlled substances|Schedule III]] controlled substance for medicinal use under the [[Controlled Substances Act]], with illicit use subject to Schedule I penalties.<ref name=GHBfact>{{cite web|title=GHB Fact Sheet|url=https://www.dea.gov/druginfo/drug_data_sheets/GHB.pdf|publisher=DEA|access-date=16 April 2018|archive-url=https://web.archive.org/web/20180416200636/https://www.dea.gov/druginfo/drug_data_sheets/GHB.pdf|archive-date=16 April 2018}}</ref> |
||
Sodium oxybate was approved for use by the US [[Food and Drug Administration]] (FDA) to treat symptoms of narcolepsy in 2002,<ref name="Xyrem FDA label"/> with a strict [[risk evaluation and mitigation strategy]] (REMS) program mandated by the FDA.<ref name="Xyrem FDA label"/> The US label for sodium oxybate also has a [[black box warning]] because it is a [[Central nervous system depression|central nervous system depressant]] and may cause [[respiratory depression]], seizures, coma, or death, especially if used in combination with other central nervous system depressants, such as [[alcohol (drug)|alcohol]] and its use may cause [[Substance dependence|dependence]].<ref name="Xyrem FDA label"/> In Canada and the European Union it was classified as a Schedule III and a [[Convention on Psychotropic Substances#Schedules of Controlled Substances|Schedule IV]] controlled substance, respectively.<ref name="Wang2009">{{cite journal | vauthors = Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL | title = Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion | journal = Journal of Clinical Sleep Medicine | volume = 5 | issue = 4 | pages = 365–371 | date = August 2009 | pmid = 19968016 | pmc = 2725257 | doi = 10.5664/jcsm.27549 }}</ref> |
Sodium oxybate was approved for use by the US [[Food and Drug Administration]] (FDA) to treat symptoms of narcolepsy in 2002,<ref name="Xyrem FDA label"/> with a strict [[risk evaluation and mitigation strategy]] (REMS) program mandated by the FDA.<ref name="Xyrem FDA label"/> The US label for sodium oxybate also has a [[black box warning]] because it is a [[Central nervous system depression|central nervous system depressant]] and may cause [[respiratory depression]], seizures, coma, or death, especially if used in combination with other central nervous system depressants, such as [[alcohol (drug)|alcohol]] and its use may cause [[Substance dependence|dependence]].<ref name="Xyrem FDA label"/> In Canada and the European Union it was classified as a Schedule III and a [[Convention on Psychotropic Substances#Schedules of Controlled Substances|Schedule IV]] controlled substance, respectively.<ref name="Wang2009">{{cite journal | vauthors = Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL | title = Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion | journal = Journal of Clinical Sleep Medicine | volume = 5 | issue = 4 | pages = 365–371 | date = August 2009 | pmid = 19968016 | pmc = 2725257 | doi = 10.5664/jcsm.27549 }}</ref> |
||
| Line 111: | Line 110: | ||
It was approved for treating symptoms of narcolepsy in the European Union in 2005.<ref name=UKlabel/> |
It was approved for treating symptoms of narcolepsy in the European Union in 2005.<ref name=UKlabel/> |
||
Orphan Medical had [[drug development|developed]] it and was acquired by [[Jazz Pharmaceuticals]] in 2005. |
Orphan Medical had [[drug development|developed]] it and was acquired by [[Jazz Pharmaceuticals]] in 2005. The drug is marketed in Europe by [[UCB (company)|UCB]]. Jazz Pharmaceuticals raised the price of the drug dramatically after it acquired Orphan,<ref name="10_big_brands_2014">{{cite web | url=http://www.fiercepharmamarketing.com/story/10-big-brands-keep-pumping-out-big-bucks-little-help-price-hikes/2014-05-07 | title=10 big brands keep pumping out big bucks, with a little help from price hikes | publisher=Fierce Pharma | date=7 May 2014 | access-date=13 November 2015 | vauthors = Staton T }}</ref> and paid a $20M fine for [[off-label marketing]] of the drug in 2007.<ref name=DOJ>{{cite news|title=Press release: US Attorney's Office - Eastern District of New York|url=https://www.justice.gov/archive/usao/nye/pr/2007/2007jul13a.html|work=US Department of Justice|date=13 July 2007}}</ref> |
||
==Medical use== |
==Medical use== |
||
Clinical use of sodium oxybate was introduced in Europe in 1964, as [[anesthetic]] given intravenously but it was not widely used since it sometimes caused seizures; as of 2006, it was still authorized for this use in France and Italy but not widely used.<ref name=WHO/>{{rp|15,27–28}} |
Clinical use of sodium oxybate was introduced in Europe in 1964, as [[anesthetic]] given intravenously but it was not widely used since it sometimes caused seizures; as of 2006, it was still authorized for this use in France and Italy but not widely used.<ref name=WHO/>{{rp|15,27–28}} |
||
The major use of sodium oxybate is in treating two of the symptoms of [[narcolepsy]] – [[cataplexy]] (sudden muscle weakness) and [[excessive daytime sleepiness]].<ref name="Xyrem FDA label"/> |
The major use of sodium oxybate is in treating two of the symptoms of [[narcolepsy]] – [[cataplexy]] (sudden muscle weakness) and [[excessive daytime sleepiness]].<ref name="Xyrem FDA label"/> Reviews of sodium oxybate concluded that it is well tolerated and associated with "significant reductions in cataplexy and daytime sleepiness,"<ref>{{cite journal | vauthors = Alshaikh MK, Tricco AC, Tashkandi M, Mamdani M, Straus SE, BaHammam AS | title = Sodium oxybate for narcolepsy with cataplexy: systematic review and meta-analysis | journal = Journal of Clinical Sleep Medicine | volume = 8 | issue = 4 | pages = 451–458 | date = August 2012 | pmid = 22893778 | pmc = 3407266 | doi = 10.5664/jcsm.2048 | doi-access = free }}</ref> and that its effectiveness "in treating major, clinically relevant narcolepsy symptoms and sleep architecture abnormalities" has been established.<ref>{{cite journal | vauthors = Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, Dauvilliers Y | title = Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials | journal = Sleep Medicine Reviews | volume = 16 | issue = 5 | pages = 431–443 | date = October 2012 | pmid = 22055895 | doi = 10.1016/j.smrv.2011.09.001 }}</ref> However, because of the risks of abuse associated with this medication, it is available in the US only through a [[risk evaluation and mitigation strategy|REMS]] program mandated by the FDA. The program requires that providers who prescribe it are certified to do so, that it is dispensed only from a central pharmacy that is certified to do so, and people to whom it is prescribed must be enrolled in a program for the drug and must document that they are using the drug safely.<ref name="Xyrem FDA label"/> |
||
Investigations of its use in dealing with [[alcohol withdrawal syndrome]] and in the maintenance of abstinence have begun in 1989 12bis in Italy where it was then approved in these indications in 1991. It has also been approved for use in Austria.<ref name = Keating>{{cite journal | vauthors = Keating GM | title = Sodium oxybate: a review of its use in alcohol withdrawal syndrome and in the maintenance of abstinence in alcohol dependence | journal = Clinical Drug Investigation | volume = 34 | issue = 1 | pages = 63–80 | date = January 2014 | pmid = 24307430 | doi = 10.1007/s40261-013-0158-x | s2cid = 2056246 }}</ref> Over the years, several studies were conducted to further substantiate sodium oxybate efficacy in these indications. Results of small studies suggest it may be "better than [[naltrexone]] and [[disulfiram]] regarding abstinence maintenance and prevention of craving in the medium term i.e. 3-12 months."<ref>{{cite journal | vauthors = Busardò FP, Kyriakou C, Napoletano S, Marinelli E, Zaami S | title = Clinical applications of sodium oxybate (GHB): from narcolepsy to alcohol withdrawal syndrome | journal = European Review for Medical and Pharmacological Sciences | volume = 19 | issue = 23 | pages = 4654–4663 | date = December 2015 | pmid = 26698265 | url = https://www.europeanreview.org/wp/wp-content/uploads/4654-4663.pdf }}</ref> In a 2014 review, Gillian Keating described sodium oxybate as a "useful option for the treatment of alcohol withdrawal syndrome and for the maintenance of abstinence in alcohol dependence."<ref name = Keating /> However, a 2018 review recognised the evidence for its efficacy but noted safety concerns and concluded that "studies are still limited and investigations including a larger number of patients are needed."<ref>{{cite journal | vauthors = Mannucci C, Pichini S, Spagnolo EV, Calapai F, Gangemi S, Navarra M, Calapai G | title = Sodium Oxybate Therapy for Alcohol Withdrawal Syndrome and Keeping of Alcohol Abstinence | journal = Current Drug Metabolism | volume = 19 | issue = 13 | pages = 1056–1064 | year = 2018 | pmid = 29219048 | doi = 10.2174/1389200219666171207122227 | s2cid = 2166038 |url=https://pubmed.ncbi.nlm.nih.gov/29219048/}}</ref> |
Investigations of its use in dealing with [[alcohol withdrawal syndrome]] and in the maintenance of abstinence have begun in 1989 12bis in Italy where it was then approved in these indications in 1991. It has also been approved for use in Austria.<ref name = Keating>{{cite journal | vauthors = Keating GM | title = Sodium oxybate: a review of its use in alcohol withdrawal syndrome and in the maintenance of abstinence in alcohol dependence | journal = Clinical Drug Investigation | volume = 34 | issue = 1 | pages = 63–80 | date = January 2014 | pmid = 24307430 | doi = 10.1007/s40261-013-0158-x | s2cid = 2056246 }}</ref> Over the years, several studies were conducted to further substantiate sodium oxybate efficacy in these indications. Results of small studies suggest it may be "better than [[naltrexone]] and [[disulfiram]] regarding abstinence maintenance and prevention of craving in the medium term i.e. 3-12 months."<ref>{{cite journal | vauthors = Busardò FP, Kyriakou C, Napoletano S, Marinelli E, Zaami S | title = Clinical applications of sodium oxybate (GHB): from narcolepsy to alcohol withdrawal syndrome | journal = European Review for Medical and Pharmacological Sciences | volume = 19 | issue = 23 | pages = 4654–4663 | date = December 2015 | pmid = 26698265 | url = https://www.europeanreview.org/wp/wp-content/uploads/4654-4663.pdf }}</ref> In a 2014 review, Gillian Keating described sodium oxybate as a "useful option for the treatment of alcohol withdrawal syndrome and for the maintenance of abstinence in alcohol dependence."<ref name = Keating /> However, a 2018 review recognised the evidence for its efficacy but noted safety concerns and concluded that "studies are still limited and investigations including a larger number of patients are needed."<ref>{{cite journal | vauthors = Mannucci C, Pichini S, Spagnolo EV, Calapai F, Gangemi S, Navarra M, Calapai G | title = Sodium Oxybate Therapy for Alcohol Withdrawal Syndrome and Keeping of Alcohol Abstinence | journal = Current Drug Metabolism | volume = 19 | issue = 13 | pages = 1056–1064 | year = 2018 | pmid = 29219048 | doi = 10.2174/1389200219666171207122227 | s2cid = 2166038 |url=https://pubmed.ncbi.nlm.nih.gov/29219048/}}</ref> |
||
| Line 122: | Line 121: | ||
In this context, a study published in 2019 analyzed safety data from 40 clinical trials and from [[pharmacovigilance]] database covering around 260,000 alcohol dependent patients treated with sodium oxybate in Italy and Austria.<ref name=”2019study”>{{cite journal | vauthors = Addolorato G, Lesch OM, Maremmani I, Walter H, Nava F, Raffaillac Q, Caputo F | title = Post-marketing and clinical safety experience with sodium oxybate for the treatment of alcohol withdrawal syndrome and maintenance of abstinence in alcohol-dependent subjects | journal = Expert Opinion on Drug Safety | volume = 19 | issue = 2 | pages = 159–166 | date = February 2020 | doi = 10.1080/14740338.2020.1709821 | pmid = 31876433 | s2cid = 209482660 }}</ref> Results showed that sodium oxybate was well-tolerated, risks were controlled, and no safety concerns were reported.<ref name=”2019study”/> The sodium oxybate approved dose regimen for the treatment of alcohol dependence (i.e. around 3.2g/day) is lower than the one for the treatment of narcolepsy (4.5-9g/night).<ref name=”2019study”/> In 2023, a PhD thesis conducted at the [[University of Amsterdam]], presented results of large clinical trials, including a phase 3 trial, and of meta-analyses which confirmed the efficacy, the good tolerance and the safety of sodium oxybate in the maintenance of abstinence, particularly in severe alcohol-dependent patients.<ref name=”amsterdam”>{{cite book |url=https://hdl.handle.net/11245.1/ad0b0a9e-e28c-432d-81a9-ccdf39b190f8 |title=Sodium oxybate for the treatment of alcohol dependence | vauthors = van den Brink W, Goudriaan A |date=11 May 2023 |publisher=[[University of Amsterdam]] |hdl=11245.1/ad0b0a9e-e28c-432d-81a9-ccdf39b190f8 |isbn=9789464730739 |access-date=11 August 2023}}</ref> A group of international researchers has also considered in 2018 that “sodium oxybate has an excellent risk benefit ratio for this indication” and that it is “a very promising therapeutic option for the most severe alcohol-dependent patients and may provide substantial clinical and public health benefit and costs”.<ref name=”efficacy-safety”>{{cite journal | vauthors = van den Brink W, Addolorato G, Aubin HJ, Benyamina A, Caputo F, Dematteis M, Gual A, Lesch OM, Mann K, Maremmani I, Nutt D, Paille F, Perney P, Rehm J, Reynaud M, Simon N, Söderpalm B, Sommer WH, Walter H, Spanagel R | display-authors = 6 | title = Efficacy and safety of sodium oxybate in alcohol-dependent patients with a very high drinking risk level | journal = Addiction Biology | volume = 23 | issue = 4 | pages = 969–986 | date = July 2018 | pmid = 30043457 | doi = 10.1111/adb.12645 | s2cid = 51716274 }}</ref> |
In this context, a study published in 2019 analyzed safety data from 40 clinical trials and from [[pharmacovigilance]] database covering around 260,000 alcohol dependent patients treated with sodium oxybate in Italy and Austria.<ref name=”2019study”>{{cite journal | vauthors = Addolorato G, Lesch OM, Maremmani I, Walter H, Nava F, Raffaillac Q, Caputo F | title = Post-marketing and clinical safety experience with sodium oxybate for the treatment of alcohol withdrawal syndrome and maintenance of abstinence in alcohol-dependent subjects | journal = Expert Opinion on Drug Safety | volume = 19 | issue = 2 | pages = 159–166 | date = February 2020 | doi = 10.1080/14740338.2020.1709821 | pmid = 31876433 | s2cid = 209482660 }}</ref> Results showed that sodium oxybate was well-tolerated, risks were controlled, and no safety concerns were reported.<ref name=”2019study”/> The sodium oxybate approved dose regimen for the treatment of alcohol dependence (i.e. around 3.2g/day) is lower than the one for the treatment of narcolepsy (4.5-9g/night).<ref name=”2019study”/> In 2023, a PhD thesis conducted at the [[University of Amsterdam]], presented results of large clinical trials, including a phase 3 trial, and of meta-analyses which confirmed the efficacy, the good tolerance and the safety of sodium oxybate in the maintenance of abstinence, particularly in severe alcohol-dependent patients.<ref name=”amsterdam”>{{cite book |url=https://hdl.handle.net/11245.1/ad0b0a9e-e28c-432d-81a9-ccdf39b190f8 |title=Sodium oxybate for the treatment of alcohol dependence | vauthors = van den Brink W, Goudriaan A |date=11 May 2023 |publisher=[[University of Amsterdam]] |hdl=11245.1/ad0b0a9e-e28c-432d-81a9-ccdf39b190f8 |isbn=9789464730739 |access-date=11 August 2023}}</ref> A group of international researchers has also considered in 2018 that “sodium oxybate has an excellent risk benefit ratio for this indication” and that it is “a very promising therapeutic option for the most severe alcohol-dependent patients and may provide substantial clinical and public health benefit and costs”.<ref name=”efficacy-safety”>{{cite journal | vauthors = van den Brink W, Addolorato G, Aubin HJ, Benyamina A, Caputo F, Dematteis M, Gual A, Lesch OM, Mann K, Maremmani I, Nutt D, Paille F, Perney P, Rehm J, Reynaud M, Simon N, Söderpalm B, Sommer WH, Walter H, Spanagel R | display-authors = 6 | title = Efficacy and safety of sodium oxybate in alcohol-dependent patients with a very high drinking risk level | journal = Addiction Biology | volume = 23 | issue = 4 | pages = 969–986 | date = July 2018 | pmid = 30043457 | doi = 10.1111/adb.12645 | s2cid = 51716274 }}</ref> |
||
Multiple trials have shown sodium oxybate to be effective in treating important symptoms of fibromyalgia such as pain and poor sleep structure |
Multiple trials have shown sodium oxybate to be effective in treating important symptoms of fibromyalgia such as pain and poor sleep structure<ref>{{cite journal | vauthors = Swick T| title = Sodium Oxybate: A Potential New Pharmacological Option for the Treatment of Fibromyalgia Syndrome | journal = Ther Adv Musculoskelet Dis | volume = 3 | issue = 4 | pages = 167–178 | year = 2011 | pmid = 29219048 | doi = 10.1177/1759720X11411599 | pmc = 3382678 }}</ref> however in 2010 the FDA voted unanimously against with commenters citing potential for abuse as a street drug. |
||
Pregnant women should not take it, and women should not become pregnant while taking it. |
Pregnant women should not take it, and women should not become pregnant while taking it. It is excreted in breast milk and should not be used by breast feeding mothers.<ref name=UKlabel/> |
||
==Adverse effects== |
==Adverse effects== |
||
The US label for sodium oxybate has a [[black box warning]] because it is a [[Central nervous system depression|central nervous system depressant]] (CNS depressant) and for its potential for [[drug abuse|abuse]]. |
The US label for sodium oxybate has a [[black box warning]] because it is a [[Central nervous system depression|central nervous system depressant]] (CNS depressant) and for its potential for [[drug abuse|abuse]]. Other potential adverse side effects include [[respiratory depression]], seizures, coma, and death, especially when it is taken in combination with other CNS depressants such as [[alcohol (drug)|alcohol]].<ref name="Xyrem FDA label"/><ref name = EncyclopediaAddictiveDrugs /><ref name = Abadinsky>{{cite book |title = Drug Use and Abuse: A Comprehensive Introduction| vauthors = Abadinsky H |publisher = [[Cengage Learning]]|year = 2010|edition = 7th|isbn = 9780495809913|pages = 197–198|chapter = GHB and GBL|chapter-url = https://books.google.com/books?id=OtC5FjRsE78C&pg=PA169}}</ref> Cases of severe [[Substance dependence|dependence]] and cravings have been reported with excessive and illicit use of this medication.<ref name="Xyrem FDA label"/><ref name = EncyclopediaAddictiveDrugs>{{cite encyclopedia|title = The Encyclopedia of Addictive Drugs | vauthors = Miller RL |chapter = GHB|pages = 182–185|publisher = [[Greenwood Publishing Group]]|year = 2002|isbn = 9780313318078|chapter-url = https://books.google.com/books?id=G7As-qawdzMC&pg=PA182}}</ref><ref>{{cite journal | vauthors = Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup SA, Smith DE | title = Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence | journal = Addiction | volume = 92 | issue = 1 | pages = 89–96 | date = January 1997 | pmid = 9060200 | doi = 10.1111/j.1360-0443.1997.tb03640.x | author-link6 = David E. Smith }}</ref> [[Gamma-Hydroxybutyric acid|GHB]], the [[carboxylic acid|protonated (acidic) form]] of this [[salt (chemistry)|salt]], has been used to commit [[drug-facilitated sexual assault]] and [[date rape]],<ref name = EncyclopediaAddictiveDrugs /><ref name = Abadinsky /><ref>{{cite news|title = FDA Approves 'Date-Rape' Drug to Treat Sleep Disorder|url = https://www.washingtonpost.com/archive/politics/2002/07/18/fda-approves-date-rape-drug-to-treat-sleep-disorder/840e94df-d09d-4eae-b0a0-de27f20b3288|date = July 18, 2002|access-date = June 23, 2018|newspaper = [[The Washington Post]]}}</ref><ref>{{cite journal | vauthors = Wedin GP, Hornfeldt CS, Ylitalo LM | title = The clinical development of gamma-hydroxybutyrate (GHB) | journal = Current Drug Safety | volume = 1 | issue = 1 | pages = 99–106 | date = January 2006 | pmid = 18690919 | doi = 10.2174/157488606775252647 }}</ref> though the illicit form of GHB typically has different characteristics from pharmaceutical-grade sodium oxybate.<ref>{{cite journal | vauthors = Carter LP, Pardi D, Gorsline J, Griffiths RR | title = Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse | journal = Drug and Alcohol Dependence | volume = 104 | issue = 1–2 | pages = 1–10 | date = September 2009 | pmid = 19493637 | pmc = 2713368 | doi = 10.1016/j.drugalcdep.2009.04.012 }}</ref> |
||
It causes dizziness, nausea, and headache in 10% to 20% of people who take it; nausea is more common in women than men.<ref name=UKlabel/><ref>{{cite journal | vauthors = Wise MS, Arand DL, Auger RR, Brooks SN, Watson NF | title = Treatment of narcolepsy and other hypersomnias of central origin | journal = Sleep | volume = 30 | issue = 12 | pages = 1712–1727 | date = December 2007 | pmid = 18246981 | pmc = 2276130 | doi = 10.1093/sleep/30.12.1712 | doi-access = free }}</ref> |
It causes dizziness, nausea, and headache in 10% to 20% of people who take it; nausea is more common in women than men.<ref name=UKlabel/><ref>{{cite journal | vauthors = Wise MS, Arand DL, Auger RR, Brooks SN, Watson NF | title = Treatment of narcolepsy and other hypersomnias of central origin | journal = Sleep | volume = 30 | issue = 12 | pages = 1712–1727 | date = December 2007 | pmid = 18246981 | pmc = 2276130 | doi = 10.1093/sleep/30.12.1712 | doi-access = free }}</ref> |
||
Between 1% and 10% of people experience nasal congestion, runny nose, or sore throat, loss of appetite, distorted sense of taste, cataplexy, weakness, nervousness or anxiety, depressed mood, nightmares or abnormal dreams, sleep paralysis, sleepwalking, or other sleep disturbances including insomnia, sleepiness or sedation, falls, vertigo, tremor, balance disorder, cognitive issues including disturbance in attention, confusion or disorientation, [[Hypoesthesia|numbed sense of touch]], tingling, |
Between 1% and 10% of people experience nasal congestion, runny nose, or sore throat, loss of appetite, distorted sense of taste, cataplexy, weakness, nervousness or anxiety, depressed mood, nightmares or abnormal dreams, sleep paralysis, sleepwalking, or other sleep disturbances including insomnia, sleepiness or sedation, falls, vertigo, tremor, balance disorder, cognitive issues including disturbance in attention, confusion or disorientation, [[Hypoesthesia|numbed sense of touch]], tingling, blurred vision, heart palpitations, high blood pressure, shortness of breath, snoring, vomiting, diarrhea, stomach pain, excessive sweating, rashes, joint pain, muscle pain, back pain, muscle spasms, bedwetting, urinary incontinence, and swelling of the limbs.<ref name=UKlabel/> |
||
==Overdose== |
==Overdose== |
||
Reports of overdose in medical literature are generally from abuse, and often involve other drugs as well. |
Reports of overdose in medical literature are generally from abuse, and often involve other drugs as well. Symptoms include vomiting, excessive sweating, periods of stopped breathing, seizures, agitation, loss of psychomotor skills, and coma. Overdose can lead to death due to respiratory depression. People who overdose may die from asphyxiation resulting from choking on vomit and/or aspiration. People that have overdosed or suspected of overdosing may need to be made to vomit, be intubated, or/and put on a ventilator.<ref name="Xyrem FDA label"/><ref name=UKlabel/> |
||
== Interactions == |
== Interactions == |
||
It should not be used with other drugs that are CNS depressants like alcohol or sedatives.<ref name="Xyrem FDA label"/> |
It should not be used with other drugs that are CNS depressants like alcohol or sedatives.<ref name="Xyrem FDA label"/> Use with [[divalproex]] results in about a 25% increase in the availability of sodium oxybate.<ref name="Xyrem FDA label"/> |
||
==Pharmacology== |
==Pharmacology== |
||
{{Main|gamma-Hydroxybutyric acid}} |
{{Main|gamma-Hydroxybutyric acid}} |
||
The [[mechanism of action]] of sodium oxybate is unknown.<ref name="Xyrem FDA label"/><ref name=UKlabel/> |
The [[mechanism of action]] of sodium oxybate is unknown.<ref name="Xyrem FDA label"/><ref name=UKlabel/> [[gamma-hydroxybutyric acid|GHB]] is a normal metabolite of [[GABA]] that interacts with the [[GABAB receptor|GABA<sub>B</sub> receptor]].<ref name="Xyrem FDA label"/> |
||
Sodium oxybate is rapidly absorbed and is about 88% bioavailable; very little is bound to plasma protein. |
Sodium oxybate is rapidly absorbed and is about 88% bioavailable; very little is bound to plasma protein. The average time to peak plasma concentration ranges from 0.5 to 1.25 hours. Very little of the drug is excreted; instead, it is mostly metabolized through several steps into carbon dioxide and water.<ref name="Xyrem FDA label"/> |
||
==Chemistry== |
==Chemistry== |
||
Sodium oxybate is the sodium [[Salt (chemistry)|salt]] of [[gamma-Hydroxybutyric acid|γ-hydroxybutyric acid]] (GHB). Its systematic chemical name is sodium 4-hydroxybutanoate, though synonyms like sodium γ-hydroxybutyrate are commonly used. |
Sodium oxybate is the sodium [[Salt (chemistry)|salt]] of [[gamma-Hydroxybutyric acid|γ-hydroxybutyric acid]] (GHB). Its systematic chemical name is sodium 4-hydroxybutanoate, though synonyms like sodium γ-hydroxybutyrate are commonly used. Its [[condensed structural formula]] is {{chem|HOCH|2|CH|2|CH|2|CO|2|Na}} ([[molecular formula]]: {{chem|C|4|H|7|Na|O|3}}) and its [[molar mass]] is 126.09 g [[mole (unit)|mol]]<sup>−1</sup>. It is highly hydrophilic.<ref name="Xyrem FDA label"/> Treating the salt with acid allows the [[carboxylic acid]] form of the compound, which is GHB, to be recovered. |
||
==History== |
==History== |
||
| Line 158: | Line 157: | ||
In 2000, the [[Hillory J. Farias and Samantha Reid Date-Rape Prevention Act of 2000]] was signed into law in the US, which put GHB on Schedule I of the [[Controlled Substances Act]], but sodium oxybate, when used under an IND or NDA from the US FDA, was considered a Schedule III substance, but with Schedule I trafficking penalties.<ref>{{cite web|title=2000 - Addition of Gamma-Hydroxybutyric Acid to Schedule I|url=https://www.deadiversion.usdoj.gov/fed_regs/rules/2000/fr0313.htm|publisher=US Department of Justice via the Federal Register|date=March 13, 2000|access-date=April 16, 2018|archive-date=May 1, 2021|archive-url=https://web.archive.org/web/20210501154805/https://www.deadiversion.usdoj.gov/fed_regs/rules/2000/fr0313.htm|url-status=dead}}</ref><ref>{{cite web|title=William J. Clinton: Statement on Signing the Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000|url=http://www.presidency.ucsb.edu/ws/index.php?pid=58098|date=February 18, 2000}}</ref> |
In 2000, the [[Hillory J. Farias and Samantha Reid Date-Rape Prevention Act of 2000]] was signed into law in the US, which put GHB on Schedule I of the [[Controlled Substances Act]], but sodium oxybate, when used under an IND or NDA from the US FDA, was considered a Schedule III substance, but with Schedule I trafficking penalties.<ref>{{cite web|title=2000 - Addition of Gamma-Hydroxybutyric Acid to Schedule I|url=https://www.deadiversion.usdoj.gov/fed_regs/rules/2000/fr0313.htm|publisher=US Department of Justice via the Federal Register|date=March 13, 2000|access-date=April 16, 2018|archive-date=May 1, 2021|archive-url=https://web.archive.org/web/20210501154805/https://www.deadiversion.usdoj.gov/fed_regs/rules/2000/fr0313.htm|url-status=dead}}</ref><ref>{{cite web|title=William J. Clinton: Statement on Signing the Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000|url=http://www.presidency.ucsb.edu/ws/index.php?pid=58098|date=February 18, 2000}}</ref> |
||
It was approved by the FDA in 2002 under the |
It was approved by the FDA, in 2002, under the brand name Xyrem with a strict risk control strategy to prevent [[drug diversion]] and control the risk of abuse by people to whom it was prescribed.<ref>{{cite news|title=FDA Approves 'Date-Rape' Drug to Treat Sleep Disorder|url=https://www.washingtonpost.com/archive/politics/2002/07/18/fda-approves-date-rape-drug-to-treat-sleep-disorder/840e94df-d09d-4eae-b0a0-de27f20b3288/|newspaper=Washington Post|date=18 July 2002}}</ref> |
||
Orphan Medical licensed the right to market the drug in Europe to Celltech in 2003.<ref>{{cite news|title=Celltech acquires rights to Xyrem from Orphan Medical - Pharmaceutical|url=https://www.thepharmaletter.com/article/celltech-acquires-rights-to-xyrem-from-orphan-medical|work=The Pharma Letter|date=3 November 2003}}</ref><ref>{{cite web|title=Form S-1/A EX-10.41 Amended and Restated Xyrem License and Distribution Agreement|url=https://www.sec.gov/Archives/edgar/data/1232524/000119312507126434/dex1041.htm|website=www.sec.gov|publisher=Jazz Pharmaceuticals vis SEC Edgar|date=27 March 2007}} [https://www.sec.gov/Archives/edgar/data/1232524/000119312507065680/0001193125-07-065680-index.htm Form S-1/A Index page]</ref> In 2004, Celltech was acquired by UCB<ref>{{cite news|title=Celltech sold to Belgian firm in £1.5bn deal|url=https://www.theguardian.com/business/2004/may/18/businessofresearch.money|work=the Guardian|date=18 May 2004}}</ref> and in 2005 [[Jazz Pharmaceuticals]] acquired Orphan Medical.<ref>{{cite news|title=Jazz completes Orphan Medical buy - Pharmaceutical industry news|url=https://www.thepharmaletter.com/article/jazz-completes-orphan-medical-buy|work=The Pharma Letter|date=4 July 2005}}</ref> |
Orphan Medical licensed the right to market the drug in Europe to Celltech in 2003.<ref>{{cite news|title=Celltech acquires rights to Xyrem from Orphan Medical - Pharmaceutical|url=https://www.thepharmaletter.com/article/celltech-acquires-rights-to-xyrem-from-orphan-medical|work=The Pharma Letter|date=3 November 2003}}</ref><ref>{{cite web|title=Form S-1/A EX-10.41 Amended and Restated Xyrem License and Distribution Agreement|url=https://www.sec.gov/Archives/edgar/data/1232524/000119312507126434/dex1041.htm|website=www.sec.gov|publisher=Jazz Pharmaceuticals vis SEC Edgar|date=27 March 2007}} [https://www.sec.gov/Archives/edgar/data/1232524/000119312507065680/0001193125-07-065680-index.htm Form S-1/A Index page]</ref> In 2004, Celltech was acquired by UCB<ref>{{cite news|title=Celltech sold to Belgian firm in £1.5bn deal|url=https://www.theguardian.com/business/2004/may/18/businessofresearch.money|work=the Guardian|date=18 May 2004}}</ref> and in 2005 [[Jazz Pharmaceuticals]] acquired Orphan Medical.<ref>{{cite news|title=Jazz completes Orphan Medical buy - Pharmaceutical industry news|url=https://www.thepharmaletter.com/article/jazz-completes-orphan-medical-buy|work=The Pharma Letter|date=4 July 2005}}</ref> |
||
In January 2007, Valeant announced that Jazz had licensed the rights to market Xyrem in Canada to Valeant.<ref>{{cite news|title=Press release: Valeant Pharmaceuticals Signs Licensing Agreement for Canadian Rights to (C)Xyrem(R) (Sodium Oxybate) from Jazz Pharmaceuticals. - Free Online Library|url=https://www.thefreelibrary.com/Valeant+Pharmaceuticals+Signs+Licensing+Agreement+for+Canadian+Rights...-a0157349646|work=Valeant via Business Wire|date=January 12, 2007|access-date=April 16, 2018|archive-date=June 18, 2018|archive-url=https://web.archive.org/web/20180618075346/https://www.thefreelibrary.com/Valeant+Pharmaceuticals+Signs+Licensing+Agreement+for+Canadian+Rights...-a0157349646|url-status=dead}}</ref> Jazz and Valeant terminated the agreement in 2017.<ref>{{cite web|title=10-K For the fiscal year ended December 31, 2017|url=https://www.sec.gov/Archives/edgar/data/1232524/000123252418000018/jazz1231201710k.htm|publisher=Jazz via SEC Edgar|access-date=16 April 2018}}</ref> |
In January 2007, Valeant announced that Jazz Pharmaceuticals had licensed the rights to market Xyrem in Canada to Valeant.<ref>{{cite news|title=Press release: Valeant Pharmaceuticals Signs Licensing Agreement for Canadian Rights to (C)Xyrem(R) (Sodium Oxybate) from Jazz Pharmaceuticals. - Free Online Library|url=https://www.thefreelibrary.com/Valeant+Pharmaceuticals+Signs+Licensing+Agreement+for+Canadian+Rights...-a0157349646|work=Valeant via Business Wire|date=January 12, 2007|access-date=April 16, 2018|archive-date=June 18, 2018|archive-url=https://web.archive.org/web/20180618075346/https://www.thefreelibrary.com/Valeant+Pharmaceuticals+Signs+Licensing+Agreement+for+Canadian+Rights...-a0157349646|url-status=dead}}</ref> Jazz Pharmaceuticals and Valeant terminated the agreement in 2017.<ref>{{cite web|title=10-K For the fiscal year ended December 31, 2017|url=https://www.sec.gov/Archives/edgar/data/1232524/000123252418000018/jazz1231201710k.htm|publisher=Jazz via SEC Edgar|access-date=16 April 2018}}</ref> |
||
In July 2007, Jazz and their subsidiary, Orphan Medical, pleaded guilty to a criminal charge of felony misbranding in their marketing of sodium oxybate; they also settled a civil suit at the same time. Jazz paid $20 million in total and agreed to a [[corporate integrity agreement]] and to implement internal reforms.<ref name=DOJ/><ref>{{cite news| vauthors = Berenson A |title=Indictment of Doctor Tests Drug Marketing Rules|url=https://www.nytimes.com/2006/07/22/business/22drugdoc.html |work=The New York Times|date=22 July 2006}}</ref><ref>{{cite news| vauthors = Berenson A |title=Maker of Narcolepsy Drug Pleads Guilty in U.S. Case |url= https://www.nytimes.com/2007/07/14/business/14jazz.html |work=The New York Times |date=14 July 2007}}</ref> |
In July 2007, Jazz Pharmaceuticals and their subsidiary, Orphan Medical, pleaded guilty to a criminal charge of felony misbranding in their marketing of sodium oxybate; they also settled a civil suit at the same time. Jazz Pharmaceuticals paid $20 million in total and agreed to a [[corporate integrity agreement]] and to implement internal reforms.<ref name=DOJ/><ref>{{cite news| vauthors = Berenson A |title=Indictment of Doctor Tests Drug Marketing Rules|url=https://www.nytimes.com/2006/07/22/business/22drugdoc.html |work=The New York Times|date=22 July 2006}}</ref><ref>{{cite news| vauthors = Berenson A |title=Maker of Narcolepsy Drug Pleads Guilty in U.S. Case |url= https://www.nytimes.com/2007/07/14/business/14jazz.html |work=The New York Times |date=14 July 2007}}</ref> The FDA sent Jazz Pharmaceuticals an [[FDA warning letter]] about safety violations in September 2007.<ref name="FDAWarning"/> |
||
| ⚫ | In 2010, the FDA rejected Jazz Pharmaceuticals' [[New Drug Application]] for use of sodium oxybate in [[fibromyalgia]].<ref>{{Cite news |url=https://www.nytimes.com/aponline/2010/10/12/business/AP-APFN-US-Jazz-Pharma-Fibromyalgia.html |title=FDA Says No to Jazz Pharma Fibromyalgia Drug |agency=Associated Press |date=2010-10-12 |access-date=2010-10-12 |work=[[The New York Times]]}}{{dead link}}</ref> |
||
The FDA sent Jazz an [[FDA warning letter]] about safety violations in September 2007.<ref name="FDAWarning"/> |
|||
| ⚫ | In October 2011, the FDA sent Jazz Pharmaceuticals another [[FDA warning letter]] for failing to collect, evaluate, and promptly report adverse effects to the FDA after it started marketing the drug.<ref name="FDAWarning">{{cite web |url=https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm275565.htm | title = FDA Warning Letter, Jazz Pharmaceuticals, Inc 10/11/11| website = [[Food and Drug Administration]]}}</ref> It sent another letter in 2013 saying that the problems described in the 2011 letter appeared to be resolved.<ref>{{cite web|title=2013 - Jazz Pharmaceuticals, Inc.- Close Out Letter |url=https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm371211.htm|publisher=FDA|date=2 August 2013}}</ref> |
||
| ⚫ | In 2010, the FDA rejected Jazz' [[New Drug Application]] for use of sodium oxybate in [[fibromyalgia]].<ref>{{Cite news |url=https://www.nytimes.com/aponline/2010/10/12/business/AP-APFN-US-Jazz-Pharma-Fibromyalgia.html |title=FDA Says No to Jazz Pharma Fibromyalgia Drug | |
||
| ⚫ | In January 2017, the FDA approved the first generic sodium oxybate product for narcolepsy symptoms, which is also subject to the same REMS program conditions as the original.<ref name=generic>{{cite news|title=Press release: FDA approves a generic of Xyrem with a REMS Program|url=https://www.fda.gov/Drugs/DrugSafety/ucm537281.htm|work=FDA Center for Drug Evaluation and Research|date=17 January 2017}}</ref> By April 2017, seven companies had filed [[Abbreviated New Drug Application]]s (ANDAs) with the FDA to market generic versions of Xyrem, which resulted in Jazz Pharmaceuticals filing patent infringement cases against them. [[Hikma Pharmaceuticals]] had been the first company to file an ANDA and Jazz Pharmaceuticals settled with them in April 2017; under the agreement Hikma could begin selling an [[authorized generic]] in 2023 under Jazz Pharmaceuticals' REMS, and would have five years of exclusivity, however, those conditions could change if Jazz Pharmaceuticals' patents were invalidated.<ref>{{cite news|title=This Rival Might Swipe 20% Of Jazz's Sleep Business, But Stock Perks Up {{!}} Investor's Business Daily|url=https://www.investors.com/news/technology/this-rival-might-swipe-20-of-jazzs-sleep-business-but-stock-perks-up/|work=Investor's Business Daily|date=6 April 2017}}</ref><ref>{{cite web|title=8-K|url=https://www.sec.gov/Archives/edgar/data/1232524/000119312517111882/d369719d8k.htm|website=www.sec.gov|publisher=Jazz via SEC Edgar|date=April 5, 2017}}</ref> In 2023, Jazz Pharmaceuticals licensed the right to produce an authorized generic of Xyrem to [[Hikma Pharmaceuticals]], marketed as "Sodium Oxybate Oral Solution".<ref name=":0">{{Cite web |title=Hikma launches authorized generic of Xyrem® (sodium oxybate) in the US |url=https://www.hikma.com/newsroom/article-i6081-hikma-launches-authorized-generic-of-xyrem-sodium-oxybate-in-the-us/ |access-date=2023-02-03 |website=Hikma }}</ref><ref name=":1">{{Cite web |title=Authorized Generic Sodium Oxybate Oral Solution {{!}} For Patients |url=https://sodiumoxybateoralsolution.com/ |access-date=2023-02-03 |website=Sodium Oxybate Oral Solution }}</ref> |
||
| ⚫ | In October 2011, the FDA sent Jazz another [[FDA warning letter]] for failing to collect, evaluate, and promptly report adverse effects to the FDA after it started marketing the drug.<ref name="FDAWarning">{{cite web |url=https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm275565.htm | title = FDA Warning Letter, Jazz Pharmaceuticals, Inc 10/11/11| website = [[Food and Drug Administration]]}}</ref> It sent another letter in 2013 saying that the problems described in the 2011 letter appeared to be resolved.<ref>{{cite web|title=2013 - Jazz Pharmaceuticals, Inc.- Close Out Letter |url=https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm371211.htm|publisher=FDA|date=2 August 2013}}</ref> |
||
| ⚫ | In May 2023, the FDA approved Lumryz, an extended-release oral suspension of sodium oxybate. Lumryz replaces the traditional twice-nightly dosage of instant-release sodium oxybate formulations (like Xyrem) with a single dose at bedtime.<ref name="Brooks 2023">{{cite web | vauthors = Brooks M |title= FDA OKs Once-Nightly Sodium Oxybate for Narcolepsy |website=Medscape |url=https://www.medscape.com/viewarticle/991464 |access-date=14 July 2023}}</ref> |
||
| ⚫ | In January 2017, the FDA approved the first generic sodium oxybate product for narcolepsy symptoms, which is also subject to the same REMS program conditions as the original.<ref name=generic>{{cite news|title=Press release: FDA approves a generic of Xyrem with a REMS Program|url=https://www.fda.gov/Drugs/DrugSafety/ucm537281.htm|work=FDA Center for Drug Evaluation and Research|date=17 January 2017}}</ref> By April 2017, seven companies had filed [[Abbreviated New Drug Application]]s (ANDAs) with the FDA to market generic versions of Xyrem, which resulted in Jazz filing patent infringement cases against them. [[Hikma Pharmaceuticals]] had been the first company to file an ANDA and Jazz settled with them in April 2017; under the agreement Hikma could begin selling an [[authorized generic]] in 2023 under Jazz' REMS, and would have five years of exclusivity, however, those conditions could change if Jazz' patents were invalidated.<ref>{{cite news|title=This Rival Might Swipe 20% Of Jazz's Sleep Business, But Stock Perks Up {{!}} Investor's Business Daily|url=https://www.investors.com/news/technology/this-rival-might-swipe-20-of-jazzs-sleep-business-but-stock-perks-up/|work=Investor's Business Daily|date=6 April 2017}}</ref><ref>{{cite web|title=8-K|url=https://www.sec.gov/Archives/edgar/data/1232524/000119312517111882/d369719d8k.htm|website=www.sec.gov|publisher=Jazz via SEC Edgar|date=April 5, 2017}}</ref> In 2023, Jazz licensed the right to produce an authorized generic of Xyrem to [[Hikma Pharmaceuticals]], marketed as "Sodium Oxybate Oral Solution".<ref name=":0">{{Cite web |title=Hikma launches authorized generic of Xyrem® (sodium oxybate) in the US |url=https://www.hikma.com/newsroom/article-i6081-hikma-launches-authorized-generic-of-xyrem-sodium-oxybate-in-the-us/ |access-date=2023-02-03 |website=Hikma |
||
| ⚫ | In May 2023, the FDA approved Lumryz, an extended-release oral suspension of sodium oxybate. Lumryz replaces the traditional twice-nightly dosage of instant-release sodium oxybate formulations (like Xyrem) with a single dose at bedtime.<ref name="Brooks 2023">{{cite web | vauthors = Brooks M |
||
==Society and culture== |
==Society and culture== |
||
| Line 185: | Line 182: | ||
In the US, the cost (as of Q3 2015) of Xyrem is $5,468.09 per 180 mL bottle (500 mg/mL)(a 10 to 15-day supply){{citation needed|date=April 2018}} As of 2017 the cost of sodium oxybate in the UK was £540.00 to £1,080.00 for a thirty-day supply,<ref>{{cite web|title=Narcolepsy with or without cataplexy in adults: pitolisant {{!}} Guidance and guidelines: Other Treatments|url=https://www.nice.org.uk/advice/es8/chapter/estimated-impact-for-the-nhs#other-treatments|publisher=NICE|access-date=14 April 2018|date=March 2017}}</ref> which at typical doses is £6,500 to £13,100 per year.<ref name=NTAG2017>{{cite web|vauthors=Kane N|title=Sodium oxybate for the treatment of narcolepsy with cataplexy in adults|url=http://ntag.nhs.uk/docs/app/Sodium-oxybate-NTAG-Appraisal.pdf|publisher=NHS Regional Drug & Therapeutics Centre (Newcastle)|date=May 2017|access-date=2018-04-14|archive-date=2020-10-21|archive-url=https://web.archive.org/web/20201021094717/http://ntag.nhs.uk/docs/app/Sodium-oxybate-NTAG-Appraisal.pdf|url-status=dead}}</ref> |
In the US, the cost (as of Q3 2015) of Xyrem is $5,468.09 per 180 mL bottle (500 mg/mL)(a 10 to 15-day supply){{citation needed|date=April 2018}} As of 2017 the cost of sodium oxybate in the UK was £540.00 to £1,080.00 for a thirty-day supply,<ref>{{cite web|title=Narcolepsy with or without cataplexy in adults: pitolisant {{!}} Guidance and guidelines: Other Treatments|url=https://www.nice.org.uk/advice/es8/chapter/estimated-impact-for-the-nhs#other-treatments|publisher=NICE|access-date=14 April 2018|date=March 2017}}</ref> which at typical doses is £6,500 to £13,100 per year.<ref name=NTAG2017>{{cite web|vauthors=Kane N|title=Sodium oxybate for the treatment of narcolepsy with cataplexy in adults|url=http://ntag.nhs.uk/docs/app/Sodium-oxybate-NTAG-Appraisal.pdf|publisher=NHS Regional Drug & Therapeutics Centre (Newcastle)|date=May 2017|access-date=2018-04-14|archive-date=2020-10-21|archive-url=https://web.archive.org/web/20201021094717/http://ntag.nhs.uk/docs/app/Sodium-oxybate-NTAG-Appraisal.pdf|url-status=dead}}</ref> |
||
[[Jazz Pharmaceuticals]] raised the price of Xyrem 841% earning a total of $569 million in 2013 and representing more than 50% of Jazz Pharmaceutical's revenues.<ref name="10_big_brands_2014"/> |
[[Jazz Pharmaceuticals]] raised the price of Xyrem 841% earning a total of $569 million in 2013 and representing more than 50% of Jazz Pharmaceutical's revenues.<ref name="10_big_brands_2014"/> In 2007 it cost $2.04; by 2014 it cost $19.40 per 1-milliliter dose.<ref name="10_big_brands_2014" /> Jazz offers copay assistance to help patients access the expensive drug.<ref name="10_big_brands_2014" /> According to DRX, a drug-data report published by Bloomberg, Jazz Pharmaceuticals price increase on Xyrem topped the list of price hikes in 2014.<ref name="10_big_brands_2014" /> |
||
Historically, [[orphan drugs]] cost more than other drugs and have received special treatment since the enactment of the US [[Orphan Drug Act of 1983]]. However, these steep price increases of orphan and other [[specialty drugs]] has come under scrutiny.<ref name="10_big_brands_2014" /> The average cost of a specialty drug in the US was $65,000 annually in June 2013 (about $5,416 a month). The price of Xyrem in the US has inflated by an average of 40% annually since it became available as a prescription.<ref name="NYT_jackpot">{{cite news| url=http://opinionator.blogs.nytimes.com/2013/06/30/the-orphan-jackpot/ | work=The New York Times | vauthors = Rattner S | title=An Orphan Jackpot | date=2013-06-30}}</ref> |
Historically, [[orphan drugs]] cost more than other drugs and have received special treatment since the enactment of the US [[Orphan Drug Act of 1983]]. However, these steep price increases of orphan and other [[specialty drugs]] has come under scrutiny.<ref name="10_big_brands_2014" /> The average cost of a specialty drug in the US was $65,000 annually in June 2013 (about $5,416 a month). The price of Xyrem in the US has inflated by an average of 40% annually since it became available as a prescription.<ref name="NYT_jackpot">{{cite news| url=http://opinionator.blogs.nytimes.com/2013/06/30/the-orphan-jackpot/ | work=The New York Times | vauthors = Rattner S | title=An Orphan Jackpot | date=2013-06-30}}</ref> |
||
| Line 193: | Line 190: | ||
In European Union countries, the government either provides national health insurance (as in the [[UK]] and [[Italy]]) or strictly regulates quasi-private social insurance funds (as in [[Germany]], [[France]], and the [[Netherlands]]). These government agencies are the sole purchaser (or regulator) of medical goods and services and have the power to set prices.<ref name="Danzon_2000">{{cite journal |vauthors=Danzon PM |title=Making sense of drug prices |journal=Regulation |date=Spring 2000 |volume=23 |issue=1 |pages=56–63 |url=http://www.cato.org/pubs/regulation/regv23n1/danzon.pdf |access-date=2010-10-05 |archive-date=2012-10-11 |archive-url=https://web.archive.org/web/20121011175143/http://www.cato.org/pubs/regulation/regv23n1/danzon.pdf |url-status=dead }}</ref> The cost of pharmaceuticals, including sodium oxybate, tends to be lower in these countries.<ref name="Danzon_2000"/> |
In European Union countries, the government either provides national health insurance (as in the [[UK]] and [[Italy]]) or strictly regulates quasi-private social insurance funds (as in [[Germany]], [[France]], and the [[Netherlands]]). These government agencies are the sole purchaser (or regulator) of medical goods and services and have the power to set prices.<ref name="Danzon_2000">{{cite journal |vauthors=Danzon PM |title=Making sense of drug prices |journal=Regulation |date=Spring 2000 |volume=23 |issue=1 |pages=56–63 |url=http://www.cato.org/pubs/regulation/regv23n1/danzon.pdf |access-date=2010-10-05 |archive-date=2012-10-11 |archive-url=https://web.archive.org/web/20121011175143/http://www.cato.org/pubs/regulation/regv23n1/danzon.pdf |url-status=dead }}</ref> The cost of pharmaceuticals, including sodium oxybate, tends to be lower in these countries.<ref name="Danzon_2000"/> |
||
[[NHS England]] authorises and pays for sodium oxybate by means of individual funding requests on the basis of exceptional circumstances. The British [[Department of Health (United Kingdom)|Department of Health]] pays for the medication for 80 patients who are taking legal action over problems linked to the use of the swine flu vaccine [[Pandemrix]] at a cost of £12,000 a year. As of 2016 there were many areas in the UK where NHS did not pay for it.<ref>{{cite web|title=Narcolepsy|url=https://www.nhs.uk/conditions/narcolepsy/treatment/|publisher=NHS Choices|access-date=14 April 2018|date=29 May 2016}}</ref><ref>{{cite news|title=DH funds private prescriptions for drug denied to NHS patients|url=http://www.hsj.co.uk/5087926.article?WT.tsrc=email&WT.mc_id=Newsletter2|access-date=20 July 2015|publisher=Health Service Journal|date=20 July 2015}}</ref> |
[[NHS England]] authorises and pays for sodium oxybate by means of individual funding requests on the basis of exceptional circumstances. The British [[Department of Health (United Kingdom)|Department of Health]] pays for the medication for 80 patients who are taking legal action over problems linked to the use of the swine flu vaccine [[Pandemrix]] at a cost of £12,000 a year. As of 2016 there were many areas in the UK where NHS did not pay for it.<ref>{{cite web|title=Narcolepsy|url=https://www.nhs.uk/conditions/narcolepsy/treatment/|publisher=NHS Choices|access-date=14 April 2018|date=29 May 2016}}</ref><ref>{{cite news|title=DH funds private prescriptions for drug denied to NHS patients|url=http://www.hsj.co.uk/5087926.article?WT.tsrc=email&WT.mc_id=Newsletter2|access-date=20 July 2015|publisher=Health Service Journal|date=20 July 2015}}</ref> In May 2016 they were ordered by the High Court to provide funding to treat a teenager with severe narcolepsy. The judge criticised their "thoroughly bad decision" and "absurd" policy discriminating against the girl when hundreds of other NHS patients already receive the drug.<ref>{{cite news|title=Judge criticises NHS England for 'totally irrational' drug decision|url=http://www.hsj.co.uk/newsletter/sectors/commissioning/judge-criticises-nhs-england-for-totally-irrational-drug-decision/7004512.article?WT.tsrc=email&WT.mc_id=Newsletter308|access-date=4 May 2016|publisher=Health Service Journal|date=4 May 2016}}</ref> |
||
==Names== |
==Names== |
||
Sodium oxybate is the common name for the chemical; it has no [[international nonproprietary name]] (INN).<ref>{{cite web|title=Sodium oxybate: CHMP Scientific Discussion|url=http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000593/WC500057101.pdf|publisher=EMA|date=9 August 2006}} Linked from [http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000593/human_med_001163.jsp&mid=WC0b01ac058001d124 EMA index page for EMEA 000593]</ref> |
Sodium oxybate is the common name for the chemical; it has no [[international nonproprietary name]] (INN).<ref>{{cite web|title=Sodium oxybate: CHMP Scientific Discussion|url=http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000593/WC500057101.pdf|publisher=EMA|date=9 August 2006}} Linked from [http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000593/human_med_001163.jsp&mid=WC0b01ac058001d124 EMA index page for EMEA 000593]</ref> |
||
As of April 2018, sodium oxybate is sold under the following brands: |
As of April 2018, sodium oxybate is sold under the following brands: Alcover (Italy), Gamma-OH (France), Natrii oxybutyras Kalceks (Latvia), Somsanit (Germany), Xyrem (many countries by Jazz Pharmaceuticals and UCB).<ref name=brands>{{cite web|title=International brands for Sodium Oxybate -|url=https://www.drugs.com/international/sodium-oxybate.html|publisher=Drugs.com|access-date=16 April 2018}}</ref> |
||
In 2023, the first authorized generic of Xyrem was made available in the US.<ref name=":1" /> |
In 2023, the first authorized generic of Xyrem was made available in the US.<ref name=":1" /> |
||
==Research== |
==Research== |
||
Jazz has been developing JZP-386, a [[Deuterated drug|deuterated]] analog of sodium oxybate. The company presented Phase I results in 2015, stating that deuterium-related effects made it necessary to do further formulation work as part of the drug's development.<ref>{{cite journal | vauthors = de Biase S, Nilo A, Gigli GL, Valente M | title = Investigational therapies for the treatment of narcolepsy | journal = Expert Opinion on Investigational Drugs | volume = 26 | issue = 8 | pages = 953–963 | date = August 2017 | pmid = 28726523 | doi = 10.1080/13543784.2017.1356819 | s2cid = 25638377 }}</ref> |
Jazz Pharmaceuticals has been developing JZP-386, a [[Deuterated drug|deuterated]] analog of sodium oxybate. The company presented Phase I results in 2015, stating that deuterium-related effects made it necessary to do further formulation work as part of the drug's development.<ref>{{cite journal | vauthors = de Biase S, Nilo A, Gigli GL, Valente M | title = Investigational therapies for the treatment of narcolepsy | journal = Expert Opinion on Investigational Drugs | volume = 26 | issue = 8 | pages = 953–963 | date = August 2017 | pmid = 28726523 | doi = 10.1080/13543784.2017.1356819 | s2cid = 25638377 }}</ref> |
||
== References == |
== References == |
||
Revision as of 20:18, 3 September 2023
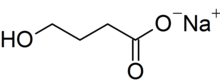 | |
| Clinical data | |
|---|---|
| Trade names | Xyrem, Alcover, Somsanit, others[1] |
| Other names | NSC-84223, WY-3478 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605032 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 88%[3] |
| Protein binding | <1%[3] |
| Elimination half-life | 0.5 to 1 hour. |
| Excretion | Almost entirely by biotransformation to carbon dioxide, which is then eliminated by expiration |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.231 |
| Chemical and physical data | |
| Formula | C4H7NaO3 |
| Molar mass | 126.087 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sodium oxybate, sold under the brand name Xyrem among others, is a medication used to treat symptoms of narcolepsy: sudden muscle weakness and excessive daytime sleepiness.[3][4][5] It is used sometimes in France and Italy as an anesthetic given intravenously;[6]: 15, 27–28 it is also approved and used in Italy and in Austria to treat alcohol dependence and alcohol withdrawal syndrome.[7]
Sodium oxybate is the sodium salt of γ-hydroxybutyric acid (GHB). The clinical trials for narcolepsy were conducted just as abuse of GHB as a club drug and date rape drug became a matter of public concern; in 2000 GHB was made a Schedule I controlled substance, while sodium oxybate, when used under an FDA NDA or IND application, was classified as a Schedule III controlled substance for medicinal use under the Controlled Substances Act, with illicit use subject to Schedule I penalties.[8]
Sodium oxybate was approved for use by the US Food and Drug Administration (FDA) to treat symptoms of narcolepsy in 2002,[3] with a strict risk evaluation and mitigation strategy (REMS) program mandated by the FDA.[3] The US label for sodium oxybate also has a black box warning because it is a central nervous system depressant and may cause respiratory depression, seizures, coma, or death, especially if used in combination with other central nervous system depressants, such as alcohol and its use may cause dependence.[3] In Canada and the European Union it was classified as a Schedule III and a Schedule IV controlled substance, respectively.[9]
It was approved for treating symptoms of narcolepsy in the European Union in 2005.[4]
Orphan Medical had developed it and was acquired by Jazz Pharmaceuticals in 2005. The drug is marketed in Europe by UCB. Jazz Pharmaceuticals raised the price of the drug dramatically after it acquired Orphan,[10] and paid a $20M fine for off-label marketing of the drug in 2007.[11]
Medical use
Clinical use of sodium oxybate was introduced in Europe in 1964, as anesthetic given intravenously but it was not widely used since it sometimes caused seizures; as of 2006, it was still authorized for this use in France and Italy but not widely used.[6]: 15, 27–28
The major use of sodium oxybate is in treating two of the symptoms of narcolepsy – cataplexy (sudden muscle weakness) and excessive daytime sleepiness.[3] Reviews of sodium oxybate concluded that it is well tolerated and associated with "significant reductions in cataplexy and daytime sleepiness,"[12] and that its effectiveness "in treating major, clinically relevant narcolepsy symptoms and sleep architecture abnormalities" has been established.[13] However, because of the risks of abuse associated with this medication, it is available in the US only through a REMS program mandated by the FDA. The program requires that providers who prescribe it are certified to do so, that it is dispensed only from a central pharmacy that is certified to do so, and people to whom it is prescribed must be enrolled in a program for the drug and must document that they are using the drug safely.[3]
Investigations of its use in dealing with alcohol withdrawal syndrome and in the maintenance of abstinence have begun in 1989 12bis in Italy where it was then approved in these indications in 1991. It has also been approved for use in Austria.[14] Over the years, several studies were conducted to further substantiate sodium oxybate efficacy in these indications. Results of small studies suggest it may be "better than naltrexone and disulfiram regarding abstinence maintenance and prevention of craving in the medium term i.e. 3-12 months."[15] In a 2014 review, Gillian Keating described sodium oxybate as a "useful option for the treatment of alcohol withdrawal syndrome and for the maintenance of abstinence in alcohol dependence."[14] However, a 2018 review recognised the evidence for its efficacy but noted safety concerns and concluded that "studies are still limited and investigations including a larger number of patients are needed."[16]
In this context, a study published in 2019 analyzed safety data from 40 clinical trials and from pharmacovigilance database covering around 260,000 alcohol dependent patients treated with sodium oxybate in Italy and Austria.[17] Results showed that sodium oxybate was well-tolerated, risks were controlled, and no safety concerns were reported.[17] The sodium oxybate approved dose regimen for the treatment of alcohol dependence (i.e. around 3.2g/day) is lower than the one for the treatment of narcolepsy (4.5-9g/night).[17] In 2023, a PhD thesis conducted at the University of Amsterdam, presented results of large clinical trials, including a phase 3 trial, and of meta-analyses which confirmed the efficacy, the good tolerance and the safety of sodium oxybate in the maintenance of abstinence, particularly in severe alcohol-dependent patients.[18] A group of international researchers has also considered in 2018 that “sodium oxybate has an excellent risk benefit ratio for this indication” and that it is “a very promising therapeutic option for the most severe alcohol-dependent patients and may provide substantial clinical and public health benefit and costs”.[19]
Multiple trials have shown sodium oxybate to be effective in treating important symptoms of fibromyalgia such as pain and poor sleep structure[20] however in 2010 the FDA voted unanimously against with commenters citing potential for abuse as a street drug.
Pregnant women should not take it, and women should not become pregnant while taking it. It is excreted in breast milk and should not be used by breast feeding mothers.[4]
Adverse effects
The US label for sodium oxybate has a black box warning because it is a central nervous system depressant (CNS depressant) and for its potential for abuse. Other potential adverse side effects include respiratory depression, seizures, coma, and death, especially when it is taken in combination with other CNS depressants such as alcohol.[3][21][22] Cases of severe dependence and cravings have been reported with excessive and illicit use of this medication.[3][21][23] GHB, the protonated (acidic) form of this salt, has been used to commit drug-facilitated sexual assault and date rape,[21][22][24][25] though the illicit form of GHB typically has different characteristics from pharmaceutical-grade sodium oxybate.[26]
It causes dizziness, nausea, and headache in 10% to 20% of people who take it; nausea is more common in women than men.[4][27]
Between 1% and 10% of people experience nasal congestion, runny nose, or sore throat, loss of appetite, distorted sense of taste, cataplexy, weakness, nervousness or anxiety, depressed mood, nightmares or abnormal dreams, sleep paralysis, sleepwalking, or other sleep disturbances including insomnia, sleepiness or sedation, falls, vertigo, tremor, balance disorder, cognitive issues including disturbance in attention, confusion or disorientation, numbed sense of touch, tingling, blurred vision, heart palpitations, high blood pressure, shortness of breath, snoring, vomiting, diarrhea, stomach pain, excessive sweating, rashes, joint pain, muscle pain, back pain, muscle spasms, bedwetting, urinary incontinence, and swelling of the limbs.[4]
Overdose
Reports of overdose in medical literature are generally from abuse, and often involve other drugs as well. Symptoms include vomiting, excessive sweating, periods of stopped breathing, seizures, agitation, loss of psychomotor skills, and coma. Overdose can lead to death due to respiratory depression. People who overdose may die from asphyxiation resulting from choking on vomit and/or aspiration. People that have overdosed or suspected of overdosing may need to be made to vomit, be intubated, or/and put on a ventilator.[3][4]
Interactions
It should not be used with other drugs that are CNS depressants like alcohol or sedatives.[3] Use with divalproex results in about a 25% increase in the availability of sodium oxybate.[3]
Pharmacology
The mechanism of action of sodium oxybate is unknown.[3][4] GHB is a normal metabolite of GABA that interacts with the GABAB receptor.[3]
Sodium oxybate is rapidly absorbed and is about 88% bioavailable; very little is bound to plasma protein. The average time to peak plasma concentration ranges from 0.5 to 1.25 hours. Very little of the drug is excreted; instead, it is mostly metabolized through several steps into carbon dioxide and water.[3]
Chemistry
Sodium oxybate is the sodium salt of γ-hydroxybutyric acid (GHB). Its systematic chemical name is sodium 4-hydroxybutanoate, though synonyms like sodium γ-hydroxybutyrate are commonly used. Its condensed structural formula is HOCH
2CH
2CH
2CO
2Na (molecular formula: C
4H
7NaO
3) and its molar mass is 126.09 g mol−1. It is highly hydrophilic.[3] Treating the salt with acid allows the carboxylic acid form of the compound, which is GHB, to be recovered.
History
Alexander Zaytsev worked on this chemical family and published work on it in 1874.[28]: 79 [29] The first extended research into sodium oxybate and its use in humans was conducted in the early 1960s by Henri Laborit to study the neurotransmitter GABA.[6]: 11–12 [30] It was studied for a range of uses, including obstetric surgery, during childbirth, and as an anxiolytic; there were anecdotal reports of it having antidepressant and aphrodisiac effects as well.[6]: 27 It was also studied as an intravenous anesthetic agent and was marketed for that purpose starting in 1964 in Europe, but it was not widely adopted as it caused seizures; as of 2006, that use was still authorized in France and Italy but not widely used.[6]: 27–28 sodium oxybate was also studied to treat alcohol addiction[6]: 28–29 and for use in narcolepsy from the 1960s onwards.[6]: 28
In May 1990, GHB was introduced as a dietary supplement and was marketed to bodybuilders for help with weight control, as a sleep aid, and as a "replacement" for L-tryptophan, which was removed from the market in November 1989 when batches of it were found to cause eosinophilia-myalgia syndrome. By November of that year, 57 cases of illness caused by the GHB supplements had been reported to the Centers for Disease Control and Prevention, with people having taken up to three teaspoons of GHB; there were no deaths, but nine people needed care in an intensive care unit.[31][32] The FDA issued a warning in November 1990 that the sale of GHB was illegal.[31] GHB continued to be manufactured and sold illegally, and it and its analogs were adopted as a club drug and came to be used as a date rape drug. The DEA made seizures and the FDA reissued warnings several times throughout the 1990s.[33][34][35]
At the same time, research on the use of sodium oxybate had formalized, as a company called Orphan Medical Inc. had filed an Investigational New Drug application and was running clinical trials with the intention of gaining regulatory approval for use to treat narcolepsy.[6]: 18–25, 28 [36]: 10 In 1996, Orphan contracted with Lonza Group, a contract manufacturer for supply of the drug.[37]
In 2000, the Hillory J. Farias and Samantha Reid Date-Rape Prevention Act of 2000 was signed into law in the US, which put GHB on Schedule I of the Controlled Substances Act, but sodium oxybate, when used under an IND or NDA from the US FDA, was considered a Schedule III substance, but with Schedule I trafficking penalties.[38][39]
It was approved by the FDA, in 2002, under the brand name Xyrem with a strict risk control strategy to prevent drug diversion and control the risk of abuse by people to whom it was prescribed.[40]
Orphan Medical licensed the right to market the drug in Europe to Celltech in 2003.[41][42] In 2004, Celltech was acquired by UCB[43] and in 2005 Jazz Pharmaceuticals acquired Orphan Medical.[44]
In January 2007, Valeant announced that Jazz Pharmaceuticals had licensed the rights to market Xyrem in Canada to Valeant.[45] Jazz Pharmaceuticals and Valeant terminated the agreement in 2017.[46]
In July 2007, Jazz Pharmaceuticals and their subsidiary, Orphan Medical, pleaded guilty to a criminal charge of felony misbranding in their marketing of sodium oxybate; they also settled a civil suit at the same time. Jazz Pharmaceuticals paid $20 million in total and agreed to a corporate integrity agreement and to implement internal reforms.[11][47][48] The FDA sent Jazz Pharmaceuticals an FDA warning letter about safety violations in September 2007.[49]
In 2010, the FDA rejected Jazz Pharmaceuticals' New Drug Application for use of sodium oxybate in fibromyalgia.[50]
In October 2011, the FDA sent Jazz Pharmaceuticals another FDA warning letter for failing to collect, evaluate, and promptly report adverse effects to the FDA after it started marketing the drug.[49] It sent another letter in 2013 saying that the problems described in the 2011 letter appeared to be resolved.[51]
In January 2017, the FDA approved the first generic sodium oxybate product for narcolepsy symptoms, which is also subject to the same REMS program conditions as the original.[52] By April 2017, seven companies had filed Abbreviated New Drug Applications (ANDAs) with the FDA to market generic versions of Xyrem, which resulted in Jazz Pharmaceuticals filing patent infringement cases against them. Hikma Pharmaceuticals had been the first company to file an ANDA and Jazz Pharmaceuticals settled with them in April 2017; under the agreement Hikma could begin selling an authorized generic in 2023 under Jazz Pharmaceuticals' REMS, and would have five years of exclusivity, however, those conditions could change if Jazz Pharmaceuticals' patents were invalidated.[53][54] In 2023, Jazz Pharmaceuticals licensed the right to produce an authorized generic of Xyrem to Hikma Pharmaceuticals, marketed as "Sodium Oxybate Oral Solution".[55][56]
In May 2023, the FDA approved Lumryz, an extended-release oral suspension of sodium oxybate. Lumryz replaces the traditional twice-nightly dosage of instant-release sodium oxybate formulations (like Xyrem) with a single dose at bedtime.[57]
Society and culture
Regulation
In the United States, GHB is a Schedule I controlled substance, while sodium oxybate, when used under an FDA NDA or IND application, is classified as a Schedule III controlled substance for medicinal use under the Controlled Substances Act, with illicit use subject to Schedule I penalties.[8][dubious ]
In Canada and the European Union, as of 2009, it is classified as a Schedule III and a Schedule IV controlled substance, respectively.[9]
Cost
In the US, the cost (as of Q3 2015) of Xyrem is $5,468.09 per 180 mL bottle (500 mg/mL)(a 10 to 15-day supply)[citation needed] As of 2017 the cost of sodium oxybate in the UK was £540.00 to £1,080.00 for a thirty-day supply,[58] which at typical doses is £6,500 to £13,100 per year.[59]
Jazz Pharmaceuticals raised the price of Xyrem 841% earning a total of $569 million in 2013 and representing more than 50% of Jazz Pharmaceutical's revenues.[10] In 2007 it cost $2.04; by 2014 it cost $19.40 per 1-milliliter dose.[10] Jazz offers copay assistance to help patients access the expensive drug.[10] According to DRX, a drug-data report published by Bloomberg, Jazz Pharmaceuticals price increase on Xyrem topped the list of price hikes in 2014.[10]
Historically, orphan drugs cost more than other drugs and have received special treatment since the enactment of the US Orphan Drug Act of 1983. However, these steep price increases of orphan and other specialty drugs has come under scrutiny.[10] The average cost of a specialty drug in the US was $65,000 annually in June 2013 (about $5,416 a month). The price of Xyrem in the US has inflated by an average of 40% annually since it became available as a prescription.[60]
The first authorized generic sodium oxybate, produced by Hikma Pharmaceuticals, was made available in January of 2023.[55]
In European Union countries, the government either provides national health insurance (as in the UK and Italy) or strictly regulates quasi-private social insurance funds (as in Germany, France, and the Netherlands). These government agencies are the sole purchaser (or regulator) of medical goods and services and have the power to set prices.[61] The cost of pharmaceuticals, including sodium oxybate, tends to be lower in these countries.[61]
NHS England authorises and pays for sodium oxybate by means of individual funding requests on the basis of exceptional circumstances. The British Department of Health pays for the medication for 80 patients who are taking legal action over problems linked to the use of the swine flu vaccine Pandemrix at a cost of £12,000 a year. As of 2016 there were many areas in the UK where NHS did not pay for it.[62][63] In May 2016 they were ordered by the High Court to provide funding to treat a teenager with severe narcolepsy. The judge criticised their "thoroughly bad decision" and "absurd" policy discriminating against the girl when hundreds of other NHS patients already receive the drug.[64]
Names
Sodium oxybate is the common name for the chemical; it has no international nonproprietary name (INN).[65]
As of April 2018, sodium oxybate is sold under the following brands: Alcover (Italy), Gamma-OH (France), Natrii oxybutyras Kalceks (Latvia), Somsanit (Germany), Xyrem (many countries by Jazz Pharmaceuticals and UCB).[1]
In 2023, the first authorized generic of Xyrem was made available in the US.[56]
Research
Jazz Pharmaceuticals has been developing JZP-386, a deuterated analog of sodium oxybate. The company presented Phase I results in 2015, stating that deuterium-related effects made it necessary to do further formulation work as part of the drug's development.[66]
References
- ^ a b "International brands for Sodium Oxybate -". Drugs.com. Retrieved 16 April 2018.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f g h i j k l m n o p q r "Xyrem- sodium oxybate solution". DailyMed. Retrieved 14 November 2020.
- ^ a b c d e f g "UK label Summary of Product Characteristics". Electronic Medicines Compendium. 8 September 2015. Retrieved 14 April 2018.
- ^ "Xyrem (sodium oxybate) Information". Center for Drug Evaluation and Research. U.S. Food and Drug Administration. 25 January 2017.
- ^ a b c d e f g h "Critical review of gamma-hydroxybutyric acid (GHB)" (PDF). 2012.
- ^ "Alcover: Riassunto delle Caratteristiche del Prodotto". Agenzia Italiana del Farmaco. 31 March 2017. Index page
- ^ a b "GHB Fact Sheet" (PDF). DEA. Archived from the original (PDF) on 16 April 2018. Retrieved 16 April 2018.
- ^ a b Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL (August 2009). "Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion". Journal of Clinical Sleep Medicine. 5 (4): 365–371. doi:10.5664/jcsm.27549. PMC 2725257. PMID 19968016.
- ^ a b c d e f Staton T (7 May 2014). "10 big brands keep pumping out big bucks, with a little help from price hikes". Fierce Pharma. Retrieved 13 November 2015.
- ^ a b "Press release: US Attorney's Office - Eastern District of New York". US Department of Justice. 13 July 2007.
- ^ Alshaikh MK, Tricco AC, Tashkandi M, Mamdani M, Straus SE, BaHammam AS (August 2012). "Sodium oxybate for narcolepsy with cataplexy: systematic review and meta-analysis". Journal of Clinical Sleep Medicine. 8 (4): 451–458. doi:10.5664/jcsm.2048. PMC 3407266. PMID 22893778.
- ^ Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, Dauvilliers Y (October 2012). "Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials". Sleep Medicine Reviews. 16 (5): 431–443. doi:10.1016/j.smrv.2011.09.001. PMID 22055895.
- ^ a b Keating GM (January 2014). "Sodium oxybate: a review of its use in alcohol withdrawal syndrome and in the maintenance of abstinence in alcohol dependence". Clinical Drug Investigation. 34 (1): 63–80. doi:10.1007/s40261-013-0158-x. PMID 24307430. S2CID 2056246.
- ^ Busardò FP, Kyriakou C, Napoletano S, Marinelli E, Zaami S (December 2015). "Clinical applications of sodium oxybate (GHB): from narcolepsy to alcohol withdrawal syndrome" (PDF). European Review for Medical and Pharmacological Sciences. 19 (23): 4654–4663. PMID 26698265.
- ^ Mannucci C, Pichini S, Spagnolo EV, Calapai F, Gangemi S, Navarra M, Calapai G (2018). "Sodium Oxybate Therapy for Alcohol Withdrawal Syndrome and Keeping of Alcohol Abstinence". Current Drug Metabolism. 19 (13): 1056–1064. doi:10.2174/1389200219666171207122227. PMID 29219048. S2CID 2166038.
- ^ a b c Addolorato G, Lesch OM, Maremmani I, Walter H, Nava F, Raffaillac Q, Caputo F (February 2020). "Post-marketing and clinical safety experience with sodium oxybate for the treatment of alcohol withdrawal syndrome and maintenance of abstinence in alcohol-dependent subjects". Expert Opinion on Drug Safety. 19 (2): 159–166. doi:10.1080/14740338.2020.1709821. PMID 31876433. S2CID 209482660.
- ^ van den Brink W, Goudriaan A (11 May 2023). Sodium oxybate for the treatment of alcohol dependence. University of Amsterdam. hdl:11245.1/ad0b0a9e-e28c-432d-81a9-ccdf39b190f8. ISBN 9789464730739. Retrieved 11 August 2023.
- ^ van den Brink W, Addolorato G, Aubin HJ, Benyamina A, Caputo F, Dematteis M, et al. (July 2018). "Efficacy and safety of sodium oxybate in alcohol-dependent patients with a very high drinking risk level". Addiction Biology. 23 (4): 969–986. doi:10.1111/adb.12645. PMID 30043457. S2CID 51716274.
- ^ Swick T (2011). "Sodium Oxybate: A Potential New Pharmacological Option for the Treatment of Fibromyalgia Syndrome". Ther Adv Musculoskelet Dis. 3 (4): 167–178. doi:10.1177/1759720X11411599. PMC 3382678. PMID 29219048.
- ^ a b c Miller RL (2002). "GHB". The Encyclopedia of Addictive Drugs. Greenwood Publishing Group. pp. 182–185. ISBN 9780313318078.
- ^ a b Abadinsky H (2010). "GHB and GBL". Drug Use and Abuse: A Comprehensive Introduction (7th ed.). Cengage Learning. pp. 197–198. ISBN 9780495809913.
- ^ Galloway GP, Frederick SL, Staggers FE, Gonzales M, Stalcup SA, Smith DE (January 1997). "Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence". Addiction. 92 (1): 89–96. doi:10.1111/j.1360-0443.1997.tb03640.x. PMID 9060200.
- ^ "FDA Approves 'Date-Rape' Drug to Treat Sleep Disorder". The Washington Post. July 18, 2002. Retrieved June 23, 2018.
- ^ Wedin GP, Hornfeldt CS, Ylitalo LM (January 2006). "The clinical development of gamma-hydroxybutyrate (GHB)". Current Drug Safety. 1 (1): 99–106. doi:10.2174/157488606775252647. PMID 18690919.
- ^ Carter LP, Pardi D, Gorsline J, Griffiths RR (September 2009). "Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse". Drug and Alcohol Dependence. 104 (1–2): 1–10. doi:10.1016/j.drugalcdep.2009.04.012. PMC 2713368. PMID 19493637.
- ^ Wise MS, Arand DL, Auger RR, Brooks SN, Watson NF (December 2007). "Treatment of narcolepsy and other hypersomnias of central origin". Sleep. 30 (12): 1712–1727. doi:10.1093/sleep/30.12.1712. PMC 2276130. PMID 18246981.
- ^ Lewis DE (2012). "Section 4.4.3 Aleksandr Mikhailovich Zaitsev". Early Russian organic chemists and their legacy. Springer. ISBN 9783642282195.
- ^ Saytzeff A (1874). "Über die Reduction des Succinylchlorids". Liebigs Annalen der Chemie (in German). 171 (2): 258–290. doi:10.1002/jlac.18741710216.
- ^ Laborit H, Jouany JM, Gerard J, Fabiani F (October 1960). "[Generalities concerning the experimental study and clinical use of gamma hydroxybutyrate of Na]". Agressologie (in French). 1: 397–406. PMID 13758011.
- ^ a b Centers for Disease Control (CDC) (November 1990). "Multistate outbreak of poisonings associated with illicit use of gamma hydroxy butyrate". MMWR. Morbidity and Mortality Weekly Report. 39 (47): 861–863. PMID 2122223.
- ^ Dyer JE (July 1991). "gamma-Hydroxybutyrate: a health-food product producing coma and seizurelike activity". The American Journal of Emergency Medicine. 9 (4): 321–324. doi:10.1016/0735-6757(91)90050-T. PMID 2054002.
- ^ Institute of Medicine; National Research Council (US) Committee on the Framework for Evaluating the Safety of Dietary Supplements (2002). "Appendix D: Table of Food and Drug Administration Actions on Dietary Supplements". Proposed Framework for Evaluating the Safety of Dietary Supplements: For Comment. National Academies Press (US).
- ^ "GHB: A Club Drug To Watch" (PDF). Substance Abuse Treatment Advisory. 2 (1). November 2002. Archived from the original (PDF) on 2017-08-01. Retrieved 2018-04-16.
- ^ Mason PE, Kerns WP (July 2002). "Gamma hydroxybutyric acid (GHB) intoxication". Academic Emergency Medicine. 9 (7): 730–739. doi:10.1197/aemj.9.7.730. PMID 12093716.
- ^ "Transcript: FDA Peripheral and Central Nervous System Drugs Advisory Committee Meeting". FDA. 6 June 2001.
- ^ "Jazz Pharma (JAZZ) Announces that Lonza has Terminated Their Sodium Oxybate Supply Agreement". Street Insider. March 25, 2010.
- ^ "2000 - Addition of Gamma-Hydroxybutyric Acid to Schedule I". US Department of Justice via the Federal Register. March 13, 2000. Archived from the original on May 1, 2021. Retrieved April 16, 2018.
- ^ "William J. Clinton: Statement on Signing the Hillory J. Farias and Samantha Reid Date-Rape Drug Prohibition Act of 2000". February 18, 2000.
- ^ "FDA Approves 'Date-Rape' Drug to Treat Sleep Disorder". Washington Post. 18 July 2002.
- ^ "Celltech acquires rights to Xyrem from Orphan Medical - Pharmaceutical". The Pharma Letter. 3 November 2003.
- ^ "Form S-1/A EX-10.41 Amended and Restated Xyrem License and Distribution Agreement". www.sec.gov. Jazz Pharmaceuticals vis SEC Edgar. 27 March 2007. Form S-1/A Index page
- ^ "Celltech sold to Belgian firm in £1.5bn deal". the Guardian. 18 May 2004.
- ^ "Jazz completes Orphan Medical buy - Pharmaceutical industry news". The Pharma Letter. 4 July 2005.
- ^ "Press release: Valeant Pharmaceuticals Signs Licensing Agreement for Canadian Rights to (C)Xyrem(R) (Sodium Oxybate) from Jazz Pharmaceuticals. - Free Online Library". Valeant via Business Wire. January 12, 2007. Archived from the original on June 18, 2018. Retrieved April 16, 2018.
- ^ "10-K For the fiscal year ended December 31, 2017". Jazz via SEC Edgar. Retrieved 16 April 2018.
- ^ Berenson A (22 July 2006). "Indictment of Doctor Tests Drug Marketing Rules". The New York Times.
- ^ Berenson A (14 July 2007). "Maker of Narcolepsy Drug Pleads Guilty in U.S. Case". The New York Times.
- ^ a b "FDA Warning Letter, Jazz Pharmaceuticals, Inc 10/11/11". Food and Drug Administration.
- ^ "FDA Says No to Jazz Pharma Fibromyalgia Drug". The New York Times. Associated Press. 2010-10-12. Retrieved 2010-10-12.[dead link]
- ^ "2013 - Jazz Pharmaceuticals, Inc.- Close Out Letter". FDA. 2 August 2013.
- ^ "Press release: FDA approves a generic of Xyrem with a REMS Program". FDA Center for Drug Evaluation and Research. 17 January 2017.
- ^ "This Rival Might Swipe 20% Of Jazz's Sleep Business, But Stock Perks Up | Investor's Business Daily". Investor's Business Daily. 6 April 2017.
- ^ "8-K". www.sec.gov. Jazz via SEC Edgar. April 5, 2017.
- ^ a b "Hikma launches authorized generic of Xyrem® (sodium oxybate) in the US". Hikma. Retrieved 2023-02-03.
- ^ a b "Authorized Generic Sodium Oxybate Oral Solution | For Patients". Sodium Oxybate Oral Solution. Retrieved 2023-02-03.
- ^ Brooks M. "FDA OKs Once-Nightly Sodium Oxybate for Narcolepsy". Medscape. Retrieved 14 July 2023.
- ^ "Narcolepsy with or without cataplexy in adults: pitolisant | Guidance and guidelines: Other Treatments". NICE. March 2017. Retrieved 14 April 2018.
- ^ Kane N (May 2017). "Sodium oxybate for the treatment of narcolepsy with cataplexy in adults" (PDF). NHS Regional Drug & Therapeutics Centre (Newcastle). Archived from the original (PDF) on 2020-10-21. Retrieved 2018-04-14.
- ^ Rattner S (2013-06-30). "An Orphan Jackpot". The New York Times.
- ^ a b Danzon PM (Spring 2000). "Making sense of drug prices" (PDF). Regulation. 23 (1): 56–63. Archived from the original (PDF) on 2012-10-11. Retrieved 2010-10-05.
- ^ "Narcolepsy". NHS Choices. 29 May 2016. Retrieved 14 April 2018.
- ^ "DH funds private prescriptions for drug denied to NHS patients". Health Service Journal. 20 July 2015. Retrieved 20 July 2015.
- ^ "Judge criticises NHS England for 'totally irrational' drug decision". Health Service Journal. 4 May 2016. Retrieved 4 May 2016.
- ^ "Sodium oxybate: CHMP Scientific Discussion" (PDF). EMA. 9 August 2006. Linked from EMA index page for EMEA 000593
- ^ de Biase S, Nilo A, Gigli GL, Valente M (August 2017). "Investigational therapies for the treatment of narcolepsy". Expert Opinion on Investigational Drugs. 26 (8): 953–963. doi:10.1080/13543784.2017.1356819. PMID 28726523. S2CID 25638377.
