Desoxypipradrol: Difference between revisions
PMIDs for citation bot to work with |
Citation bot (talk | contribs) m Citations: [239]+: last1, first1, last2, first2, title, journal, volume, issue, pages, year, doi. Tweaked: pmid. User-activated. |
||
| Line 38: | Line 38: | ||
}} |
}} |
||
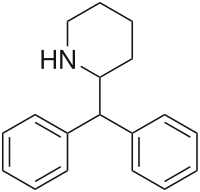
'''Desoxypipradrol''', also known as '''2-diphenylmethylpiperidine''' ('''2-DPMP''', '''12-Hour Caffeine''') acts as a [[norepinephrine-dopamine reuptake inhibitor]] (NDRI).<ref name="Ferris RM 1979">{{cite journal | pmid = 39160}}</ref> |
'''Desoxypipradrol''', also known as '''2-diphenylmethylpiperidine''' ('''2-DPMP''', '''12-Hour Caffeine''') acts as a [[norepinephrine-dopamine reuptake inhibitor]] (NDRI).<ref name="Ferris RM 1979">{{cite journal | last1 = Ferris | first1 = RM | last2 = Tang | first2 = FL | title = Comparison of the effects of the isomers of amphetamine, methylphenidate and deoxypipradrol on the uptake of l-3Hnorepinephrine and 3Hdopamine by synaptic vesicles from rat whole brain, striatum and hypothalamus | journal = The Journal of pharmacology and experimental therapeutics | volume = 210 | issue = 3 | pages = 422–8 | year = 1979 | pmid = 39160}}</ref> |
||
Desoxypipradrol is closely related on a [[chemical structure|structural]] level to the compounds [[methylphenidate]] and [[pipradrol]], all three of which share a similar [[pharmacological]] [[mechanism of action|action]].<ref name="Ferris RM 1979"/> Of these three [[piperidine]]s, desoxypipradrol has the longest elimination [[half-life]], as it is a highly [[lipophilic]] [[molecule]] lacking [[Chemical polarity|polar]] [[functional group]]s that are typically targeted by [[metabolic]] [[enzyme]]s. Methylphenidate, on the other hand, is a short-acting compound, as it possesses a [[methyl]]-[[ester]] [[Moiety (chemistry)|moiety]] that is easily cleaved, forming a highly polar [[acid]] group, while pipradrol is intermediate in duration, possessing a [[hydroxyl]] group which can be [[Xenobiotic conjugation|conjugated]] (e.g. with [[glucuronide]]) to increase its [[hydrophilicity]] and facilitate [[excretion]], but no easily metabolized groups. |
Desoxypipradrol is closely related on a [[chemical structure|structural]] level to the compounds [[methylphenidate]] and [[pipradrol]], all three of which share a similar [[pharmacological]] [[mechanism of action|action]].<ref name="Ferris RM 1979"/> Of these three [[piperidine]]s, desoxypipradrol has the longest elimination [[half-life]], as it is a highly [[lipophilic]] [[molecule]] lacking [[Chemical polarity|polar]] [[functional group]]s that are typically targeted by [[metabolic]] [[enzyme]]s. Methylphenidate, on the other hand, is a short-acting compound, as it possesses a [[methyl]]-[[ester]] [[Moiety (chemistry)|moiety]] that is easily cleaved, forming a highly polar [[acid]] group, while pipradrol is intermediate in duration, possessing a [[hydroxyl]] group which can be [[Xenobiotic conjugation|conjugated]] (e.g. with [[glucuronide]]) to increase its [[hydrophilicity]] and facilitate [[excretion]], but no easily metabolized groups. |
||
Desoxypipradrol was developed by the pharmaceutical company CIBA (now called [[Novartis]]) in the 1950s,<ref>{{cite journal | author = Tripod J, Sury E, Hoffmann K. | title = Zentralerregende Wirkung eines neuen Piperidinderivates. (German) | journal = Experientia | year = 1954 | volume = 10 | pages = |
Desoxypipradrol was developed by the pharmaceutical company CIBA (now called [[Novartis]]) in the 1950s,<ref>{{cite journal | doi = 10.1007/BF02157398 | author = Tripod J, Sury E, Hoffmann K. | title = Zentralerregende Wirkung eines neuen Piperidinderivates. (German) | journal = Experientia | year = 1954 | volume = 10 | issue = 6 | pages = 261–262 | pmid = 13183068}}</ref> and researched for applications such as the treatment of [[narcolepsy]] and [[ADHD]]; however, it was dropped from development after the related drug methylphenidate was developed by the same company. Methylphenidate was felt to be the superior drug for treating ADHD due to its shorter duration of action and more predictable [[pharmacokinetics]], and while desoxypipradrol was researched for other applications (such as facilitation of rapid recovery from [[anaesthesia]]<ref>{{cite journal|last1=Bellucci|first1=G|title=(2-Diphenylmethyl-piperidine hydrochloride and the methyl ester of 2-chloro-2-phenyl-2-(2-piperidyl)-acetic acid), drugs with waking effect in anesthesia.|journal=Minerva anestesiologica|volume=21|issue=6|pages=125–8|year=1955|pmid=13244387}}</ref>), its development was not continued. The hydroxylated derivative [[pipradrol]] was, however, introduced as a clinical drug indicated for [[Major depressive disorder|depression]], [[narcolepsy]] and cognitive enhancement in organic [[dementia]]. |
||
Desoxypipradrol might prove quite useful for its original application of treating [[attention-deficit hyperactivity disorder]] (ADHD) and depression, considering that the short half-life of common medications such as methylphenidate and [[dextroamphetamine]] has led to the development of long-acting, delayed-release formulations of these drugs. Some individuals with ADHD prefer long-acting stimulant formulations, which allow for once-daily dosing. |
Desoxypipradrol might prove quite useful for its original application of treating [[attention-deficit hyperactivity disorder]] (ADHD) and depression, considering that the short half-life of common medications such as methylphenidate and [[dextroamphetamine]] has led to the development of long-acting, delayed-release formulations of these drugs. Some individuals with ADHD prefer long-acting stimulant formulations, which allow for once-daily dosing. |
||
Revision as of 01:39, 8 January 2011
This article possibly contains original research. (January 2010) |
This article needs additional citations for verification. (January 2010) |
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, nasal and sublingual |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | >90% |
| Metabolism | Hepatic |
| Elimination half-life | 16-20 hours |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.525 |
| Chemical and physical data | |
| Formula | C18H21N |
| Molar mass | 251.366 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Desoxypipradrol, also known as 2-diphenylmethylpiperidine (2-DPMP, 12-Hour Caffeine) acts as a norepinephrine-dopamine reuptake inhibitor (NDRI).[1]
Desoxypipradrol is closely related on a structural level to the compounds methylphenidate and pipradrol, all three of which share a similar pharmacological action.[1] Of these three piperidines, desoxypipradrol has the longest elimination half-life, as it is a highly lipophilic molecule lacking polar functional groups that are typically targeted by metabolic enzymes. Methylphenidate, on the other hand, is a short-acting compound, as it possesses a methyl-ester moiety that is easily cleaved, forming a highly polar acid group, while pipradrol is intermediate in duration, possessing a hydroxyl group which can be conjugated (e.g. with glucuronide) to increase its hydrophilicity and facilitate excretion, but no easily metabolized groups.
Desoxypipradrol was developed by the pharmaceutical company CIBA (now called Novartis) in the 1950s,[2] and researched for applications such as the treatment of narcolepsy and ADHD; however, it was dropped from development after the related drug methylphenidate was developed by the same company. Methylphenidate was felt to be the superior drug for treating ADHD due to its shorter duration of action and more predictable pharmacokinetics, and while desoxypipradrol was researched for other applications (such as facilitation of rapid recovery from anaesthesia[3]), its development was not continued. The hydroxylated derivative pipradrol was, however, introduced as a clinical drug indicated for depression, narcolepsy and cognitive enhancement in organic dementia.
Desoxypipradrol might prove quite useful for its original application of treating attention-deficit hyperactivity disorder (ADHD) and depression, considering that the short half-life of common medications such as methylphenidate and dextroamphetamine has led to the development of long-acting, delayed-release formulations of these drugs. Some individuals with ADHD prefer long-acting stimulant formulations, which allow for once-daily dosing.
Desoxypipradrol is not specifically listed as a controlled drug in any country at the present time, but its structural similarity to pipradrol makes it possible that it would be considered a controlled substance analogue in several countries such as Australia and New Zealand. As of the 4th November 2010, the UK Home Office announced a ban on the importation of 2-DPMP, following a recommendation from the ACMD.[4]
Prior to the import ban, desoxypipradrol was sold as a 'legal high' in several products, most notably "Ivory wave". Its use lead to several ER visits which prompted the government to commission a review from the ACMD
See also
References
- ^ a b Ferris, RM; Tang, FL (1979). "Comparison of the effects of the isomers of amphetamine, methylphenidate and deoxypipradrol on the uptake of l-3Hnorepinephrine and 3Hdopamine by synaptic vesicles from rat whole brain, striatum and hypothalamus". The Journal of pharmacology and experimental therapeutics. 210 (3): 422–8. PMID 39160.
- ^ Tripod J, Sury E, Hoffmann K. (1954). "Zentralerregende Wirkung eines neuen Piperidinderivates. (German)". Experientia. 10 (6): 261–262. doi:10.1007/BF02157398. PMID 13183068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bellucci, G (1955). "(2-Diphenylmethyl-piperidine hydrochloride and the methyl ester of 2-chloro-2-phenyl-2-(2-piperidyl)-acetic acid), drugs with waking effect in anesthesia". Minerva anestesiologica. 21 (6): 125–8. PMID 13244387.
- ^ Import ban on psychoactive drug, UK Home Office
