Cav1.2: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
== Clinical significance == |
== Clinical significance == |
||
Mutation in the CACNA1C gene are associated with a variant of [[Long QT syndrome]] called [[Timothy's syndrome]]<ref name="pmid20301577">{{cite journal | author = Pagon RA, Bird TC, Dolan CR, Stephens K, Splawski I, Timothy KW, Priori SG, Napolitano C, Bloise R | title = Timothy Syndrome| journal = | volume = | issue = | pages = | year = 1993| pmid = 20301577 | doi = | url = | issn = }}</ref> and also with [[Brugada syndrome]].<ref name="pmid19606473">{{cite journal | author = Hedley PL, Jørgensen P, Schlamowitz S, Moolman-Smook J, Kanters JK, Corfield VA, Christiansen M | title = The genetic basis of Brugada syndrome: a mutation update | journal = Hum. Mutat. | volume = 30 | issue = 9 | pages = 1256–66 |date=September 2009 | pmid = 19606473 | doi = 10.1002/humu.21066 | url = | issn = }}</ref> A large-scale genetic analysis conducted in 2008 shows the possibility that CACNA1C is associated with [[bipolar disorder]] <ref name="pmid18711365">{{cite journal | author = Ferreira MA, O'Donovan MC, Meng YA | title = Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder | journal = Nat. Genet. | volume = 40 | issue = 9 | pages = 1056–8 |date=September 2008 | pmid = 18711365 | pmc = 2703780 | doi = 10.1038/ng.209 | laysummary = http://www.schizophreniaforum.org/new/detail.asp?id=1450 | laysource = Schizophrenia Research Forum |display-authors=etal}}</ref> and subsequently also with [[schizophrenia]].<ref name="pmid19621016">{{cite journal | author = Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O'Donovan MC, Craddock N | title = The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia | journal = Mol. Psychiatry | volume = 15 | issue = 10 | pages = 1016–22 |date=October 2010 | pmid = 19621016 | pmc = 3011210 | doi = 10.1038/mp.2009.49 | url = | issn = }}</ref><ref name="pmid21057379">{{cite journal | author = Curtis D, Vine AE, McQuillin A, Bass NJ, Pereira A, Kandaswamy R, Lawrence J, Anjorin A, Choudhury K, Datta SR, Puri V, Krasucki R, Pimm J, Thirumalai S, Quested D, Gurling HM | title = Case-case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes | journal = Psychiatr. Genet. | volume = 21 | issue = 1 | pages = 1–4 |date=February 2011 | pmid = 21057379 | doi = 10.1097/YPG.0b013e3283413382 | url = | issn = | pmc = 3024533 }}</ref> Also, CACNA1C A risk allele has been associated to a disruption in brain connectivity in patients with bipolar disorder, while not or only to a minor degree, in their unaffected relatives or healthy controls.<ref name="pmid22614292">{{cite journal | author = Radua J, Surguladze SA, Marshall N, Walshe M, Bramon E, Collier DA, Prata DP, Murray RM, McDonald C | title = The impact of CACNA1C allelic variation on effective connectivity during emotional processing in bipolar disorder | journal = Mol. Psychiatry. | year = 2012 | pmid = 22614292 | doi = 10.1038/mp.2012.61 | volume=18 | pages=526–527}}</ref> |
Mutation in the CACNA1C gene, the single-nucleotide polymorphism located in the third intron of the Cav1.2 gene<ref>{{Cite journal|title = Major channels involved in neuropsychiatric disorders and therapeutic perspectives|url = http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3646240/|journal = Frontiers in Genetics|date = 2013-05-07|issn = 1664-8021|pmc = 3646240|pmid = 23675382|volume = 4|doi = 10.3389/fgene.2013.00076|first = Paola|last = Imbrici|first2 = Diana Conte|last2 = Camerino|first3 = Domenico|last3 = Tricarico}}</ref> , are associated with a variant of [[Long QT syndrome]] called [[Timothy's syndrome]]<ref name="pmid20301577">{{cite journal | author = Pagon RA, Bird TC, Dolan CR, Stephens K, Splawski I, Timothy KW, Priori SG, Napolitano C, Bloise R | title = Timothy Syndrome| journal = | volume = | issue = | pages = | year = 1993| pmid = 20301577 | doi = | url = | issn = }}</ref> and also with [[Brugada syndrome]].<ref name="pmid19606473">{{cite journal | author = Hedley PL, Jørgensen P, Schlamowitz S, Moolman-Smook J, Kanters JK, Corfield VA, Christiansen M | title = The genetic basis of Brugada syndrome: a mutation update | journal = Hum. Mutat. | volume = 30 | issue = 9 | pages = 1256–66 |date=September 2009 | pmid = 19606473 | doi = 10.1002/humu.21066 | url = | issn = }}</ref> A large-scale genetic analysis conducted in 2008 shows the possibility that CACNA1C is associated with [[bipolar disorder]] <ref name="pmid18711365">{{cite journal | author = Ferreira MA, O'Donovan MC, Meng YA | title = Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder | journal = Nat. Genet. | volume = 40 | issue = 9 | pages = 1056–8 |date=September 2008 | pmid = 18711365 | pmc = 2703780 | doi = 10.1038/ng.209 | laysummary = http://www.schizophreniaforum.org/new/detail.asp?id=1450 | laysource = Schizophrenia Research Forum |display-authors=etal}}</ref> and subsequently also with [[schizophrenia]].<ref name="pmid19621016">{{cite journal | author = Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O'Donovan MC, Craddock N | title = The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia | journal = Mol. Psychiatry | volume = 15 | issue = 10 | pages = 1016–22 |date=October 2010 | pmid = 19621016 | pmc = 3011210 | doi = 10.1038/mp.2009.49 | url = | issn = }}</ref><ref name="pmid21057379">{{cite journal | author = Curtis D, Vine AE, McQuillin A, Bass NJ, Pereira A, Kandaswamy R, Lawrence J, Anjorin A, Choudhury K, Datta SR, Puri V, Krasucki R, Pimm J, Thirumalai S, Quested D, Gurling HM | title = Case-case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes | journal = Psychiatr. Genet. | volume = 21 | issue = 1 | pages = 1–4 |date=February 2011 | pmid = 21057379 | doi = 10.1097/YPG.0b013e3283413382 | url = | issn = | pmc = 3024533 }}</ref> Also, CACNA1C A risk allele has been associated to a disruption in brain connectivity in patients with bipolar disorder, while not or only to a minor degree, in their unaffected relatives or healthy controls.<ref name="pmid22614292">{{cite journal | author = Radua J, Surguladze SA, Marshall N, Walshe M, Bramon E, Collier DA, Prata DP, Murray RM, McDonald C | title = The impact of CACNA1C allelic variation on effective connectivity during emotional processing in bipolar disorder | journal = Mol. Psychiatry. | year = 2012 | pmid = 22614292 | doi = 10.1038/mp.2012.61 | volume=18 | pages=526–527}}</ref> |
||
== Interactive pathway map == |
== Interactive pathway map == |
||
Revision as of 20:37, 24 November 2015
Template:PBB Calcium channel, voltage-dependent, L type, alpha 1C subunit (also known as Cav1.2) is a protein that in humans is encoded by the CACNA1C gene.[1] Cav1.2 is a subunit of L-type voltage-dependent calcium channel.[2]
Structure and function
This gene encodes an alpha-1 subunit of a voltage-dependent calcium channel. Calcium channels mediate the influx of calcium ions into the cell upon membrane polarization. The alpha-1 subunit consists of 24 transmembrane segments and forms the pore through which ions pass into the cell. The calcium channel consists of a complex of alpha-1, alpha-2/delta and beta subunits in a 1:1:1 ratio. The S3-S4 linkers of Cav1.2 determine the gating phenotype and modulated gating kinetics of the channel.[3] Cav1.2 is widely expressed in the smooth muscle, pancreatic cells, fibroblasts, and neurons [4] [5] . However, it is particularly important and well known for its expression in the heart where it mediates L-type currents, which causes calcium-induced calcium release from the ER Stores via ryanodine receptors. It depolarizes at -30mV and helps define the shape of the action potential in cardiac and smooth muscle.[6] The protein encoded by this gene binds to and is inhibited by dihydropyridine.[7] In the arteries of the brain, high levels of calcium in mitochondria elevates activity of nuclear factor kappa B NF-κB and transcription of CACNA1c and functional Cav1.2 expression increases.[8] Cav1.2 also regulates levels of osteoprotegerin.[9]
CaV1.2 is inhibited by the action of STIM1.[10]
Regulation
The activity of CaV1.2 channels is tightly regulated by the Ca2+ signals they produce. An increase in intracellular Ca2+ concentration implicated in Cav1.2 facilitation, a form of positive feedback called Ca2+-dependent facilitation, that amplifies Ca2+ influx. In addition, increasing influx intracellular Ca2+ concentration has implicated to exert the opposite effect Ca2+ dependent inactivation.[11] These activation and inactivation mechanisms both involve Ca2+ binding to calmodulin (CaM) in the IQ domain in the C-terminal tail of these channels.[12] Cav1.2 channels are arranged in cluster of eight, on average, in the cell membrane. When calcium ions bind to calmodulin, which in turn binds to a Cav1.2 channel, it allows the Cav1.2 channels within a cluster to interact with each other.[13] This results in channels working cooperatively when they open at the same time to allow more calcium ions to enter and then close together to allow the cell to relax.[13]

Clinical significance
Mutation in the CACNA1C gene, the single-nucleotide polymorphism located in the third intron of the Cav1.2 gene[14] , are associated with a variant of Long QT syndrome called Timothy's syndrome[15] and also with Brugada syndrome.[16] A large-scale genetic analysis conducted in 2008 shows the possibility that CACNA1C is associated with bipolar disorder [17] and subsequently also with schizophrenia.[18][19] Also, CACNA1C A risk allele has been associated to a disruption in brain connectivity in patients with bipolar disorder, while not or only to a minor degree, in their unaffected relatives or healthy controls.[20]
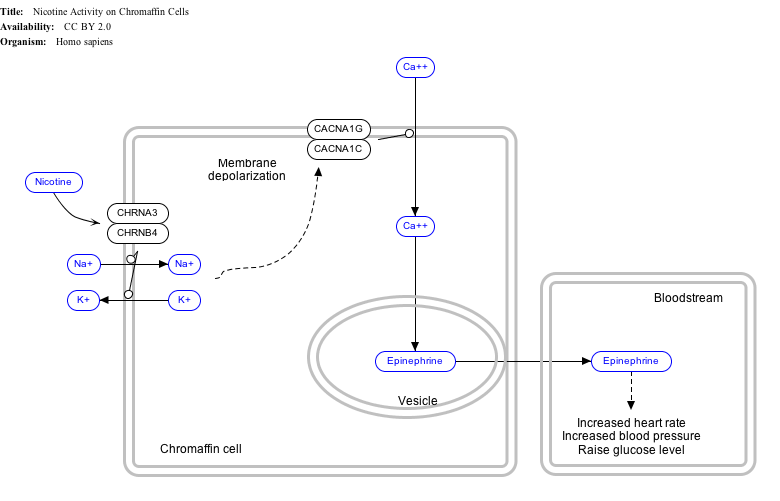
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective Wikipedia articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "NicotineActivityonChromaffinCells_WP1603".
See also
References
- ^ Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM (August 1991). "Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel". Nature. 352 (6335): 527–30. doi:10.1038/352527a0. PMID 1650913.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (December 2005). "International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels". Pharmacol. Rev. 57 (4): 411–25. doi:10.1124/pr.57.4.5. PMID 16382099.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lipscombe, Diane; Helton, Thomas D.; Xu, Weifeng (2004-11-01). "L-type calcium channels: the low down". Journal of Neurophysiology. 92 (5): 2633–2641. doi:10.1152/jn.00486.2004. ISSN 0022-3077. PMID 15486420.
- ^ Christel, Carl; Lee, Amy (2012-08-01). "Ca2+-dependent modulation of voltage-gated Ca2+ channels: analysis in native and heterologous expression systems". Biochimica et Biophysica Acta. 1820 (8): 1243–1252. doi:10.1016/j.bbagen.2011.12.012. ISSN 0006-3002. PMC 3345169. PMID 22223119.
- ^ Berger, Stefan M.; Bartsch, Dusan (2014-08-01). "The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function". Cell and Tissue Research. 357 (2): 463–476. doi:10.1007/s00441-014-1936-3. ISSN 1432-0878. PMID 24996399.
- ^ Lipscombe, Diane; Helton, Thomas D.; Xu, Weifeng (2004-11-01). "L-type calcium channels: the low down". Journal of Neurophysiology. 92 (5): 2633–2641. doi:10.1152/jn.00486.2004. ISSN 0022-3077. PMID 15486420.
- ^ "Entrez Gene: , voltage-dependent, L type, alpha 1C subunit".
- ^ Narayanan D, Xi Q, Pfeffer LM, Jaggar JH (September 2010). "Mitochondria control functional CaV1.2 expression in smooth muscle cells of cerebral arteries". Circ. Res. 107 (5): 631–41. doi:10.1161/CIRCRESAHA.110.224345. PMC 3050675. PMID 20616314.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bergh JJ, Xu Y, Farach-Carson MC (January 2004). "Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel". Endocrinology. 145 (1): 426–36. doi:10.1210/en.2003-0319. PMID 14525906.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Calahan MD (October 2010). "Cell biology. How to STIMulate calcium channels". Science. 330 (1): 43–4. doi:10.1126/science.1196348. PMID 20929798.
- ^ Isaev, Dmytro; Solt, Karisa; Gurtovaya, Oksana; Reeves, John P.; Shirokov, Roman (2004-05-01). "Modulation of the Voltage Sensor of L-type Ca2+ Channels by Intracellular Ca2+". The Journal of General Physiology. 123 (5): 555–571. doi:10.1085/jgp.200308876. ISSN 0022-1295. PMC 2234499. PMID 15111645.
- ^ Kim, Eun Young; Rumpf, Christine H; Van Petegem, Filip; Arant, Ryan J; Findeisen, Felix; Cooley, Elizabeth S; Isacoff, Ehud Y; Minor, Daniel L (2010-12-01). "Multiple C-terminal tail Ca2+/CaMs regulate CaV1.2 function but do not mediate channel dimerization". The EMBO Journal. 29 (23): 3924–3938. doi:10.1038/emboj.2010.260. ISSN 0261-4189. PMC 3020648. PMID 20953164.
- ^ a b [1], Graded Ca2+/calmodulin-dependent coupling of voltage-gated CaV1.2 channels
- ^ Imbrici, Paola; Camerino, Diana Conte; Tricarico, Domenico (2013-05-07). "Major channels involved in neuropsychiatric disorders and therapeutic perspectives". Frontiers in Genetics. 4. doi:10.3389/fgene.2013.00076. ISSN 1664-8021. PMC 3646240. PMID 23675382.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pagon RA, Bird TC, Dolan CR, Stephens K, Splawski I, Timothy KW, Priori SG, Napolitano C, Bloise R (1993). "Timothy Syndrome". PMID 20301577.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Hedley PL, Jørgensen P, Schlamowitz S, Moolman-Smook J, Kanters JK, Corfield VA, Christiansen M (September 2009). "The genetic basis of Brugada syndrome: a mutation update". Hum. Mutat. 30 (9): 1256–66. doi:10.1002/humu.21066. PMID 19606473.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ferreira MA, O'Donovan MC, Meng YA; et al. (September 2008). "Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder". Nat. Genet. 40 (9): 1056–8. doi:10.1038/ng.209. PMC 2703780. PMID 18711365.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O'Donovan MC, Craddock N (October 2010). "The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia". Mol. Psychiatry. 15 (10): 1016–22. doi:10.1038/mp.2009.49. PMC 3011210. PMID 19621016.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Curtis D, Vine AE, McQuillin A, Bass NJ, Pereira A, Kandaswamy R, Lawrence J, Anjorin A, Choudhury K, Datta SR, Puri V, Krasucki R, Pimm J, Thirumalai S, Quested D, Gurling HM (February 2011). "Case-case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes". Psychiatr. Genet. 21 (1): 1–4. doi:10.1097/YPG.0b013e3283413382. PMC 3024533. PMID 21057379.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Radua J, Surguladze SA, Marshall N, Walshe M, Bramon E, Collier DA, Prata DP, Murray RM, McDonald C (2012). "The impact of CACNA1C allelic variation on effective connectivity during emotional processing in bipolar disorder". Mol. Psychiatry. 18: 526–527. doi:10.1038/mp.2012.61. PMID 22614292.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Kempton MJ, Ruberto G, Vassos E, Tatarelli R, Girardi P, Collier D, Frangou S (December 2009). "Effects of the CACNA1C risk allele for bipolar disorder on cerebral gray matter volume in healthy individuals". Am J Psychiatry. 166 (12): 1413–4. doi:10.1176/appi.ajp.2009.09050680. PMID 19952088.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Soldatov NM (1992). "Molecular diversity of L-type Ca2+ channel transcripts in human fibroblasts". Proc. Natl. Acad. Sci. U.S.A. 89 (10): 4628–32. doi:10.1073/pnas.89.10.4628. PMC 49136. PMID 1316612.
- Powers PA, Gregg RG, Hogan K (1992). "Linkage mapping of the human gene for the alpha 1 subunit of the cardiac DHP-sensitive Ca2+ channel (CACNL1A1) to chromosome 12p13.2-pter using a dinucleotide repeat". Genomics. 14 (1): 206–7. doi:10.1016/S0888-7543(05)80312-X. PMID 1330882.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Sun W, McPherson JD, Hoang DQ; et al. (1993). "Mapping of a human brain voltage-gated calcium channel to human chromosome 12p13-pter". Genomics. 14 (4): 1092–4. doi:10.1016/S0888-7543(05)80135-1. PMID 1335957.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Powers PA, Gregg RG, Lalley PA; et al. (1991). "Assignment of the human gene for the alpha 1 subunit of the cardiac DHP-sensitive Ca2+ channel (CCHL1A1) to chromosome 12p12-pter". Genomics. 10 (3): 835–9. doi:10.1016/0888-7543(91)90471-P. PMID 1653763.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Perez-Reyes E, Wei XY, Castellano A, Birnbaumer L (1990). "Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes". J. Biol. Chem. 265 (33): 20430–6. PMID 2173707.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Soldatov NM, Bouron A, Reuter H (1995). "Different voltage-dependent inhibition by dihydropyridines of human Ca2+ channel splice variants". J. Biol. Chem. 270 (18): 10540–3. doi:10.1074/jbc.270.18.10540. PMID 7737988.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Soldatov NM (1994). "Genomic structure of human L-type Ca2+ channel". Genomics. 22 (1): 77–87. doi:10.1006/geno.1994.1347. PMID 7959794.
- Tang S, Mikala G, Bahinski A; et al. (1993). "Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel". J. Biol. Chem. 268 (18): 13026–9. PMID 8099908.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Schultz D, Mikala G, Yatani A; et al. (1993). "Cloning, chromosomal localization, and functional expression of the alpha 1 subunit of the L-type voltage-dependent calcium channel from normal human heart". Proc. Natl. Acad. Sci. U.S.A. 90 (13): 6228–32. doi:10.1073/pnas.90.13.6228. PMC 46901. PMID 8392192.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Perets T, Blumenstein Y, Shistik E; et al. (1996). "A potential site of functional modulation by protein kinase A in the cardiac Ca2+ channel alpha 1C subunit". FEBS Lett. 384 (2): 189–92. doi:10.1016/0014-5793(96)00303-1. PMID 8612821.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Andersson B, Wentland MA, Ricafrente JY; et al. (1996). "A "double adaptor" method for improved shotgun library construction". Anal. Biochem. 236 (1): 107–13. doi:10.1006/abio.1996.0138. PMID 8619474.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Soldatov NM, Zühlke RD, Bouron A, Reuter H (1997). "Molecular structures involved in L-type calcium channel inactivation. Role of the carboxyl-terminal region encoded by exons 40-42 in alpha1C subunit in the kinetics and Ca2+ dependence of inactivation". J. Biol. Chem. 272 (6): 3560–6. doi:10.1074/jbc.272.6.3560. PMID 9013606.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Klöckner U, Mikala G, Eisfeld J; et al. (1997). "Properties of three COOH-terminal splice variants of a human cardiac L-type Ca2+-channel alpha1-subunit". Am. J. Physiol. 272 (3 Pt 2): H1372–81. PMID 9087614.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Yu W, Andersson B, Worley KC; et al. (1997). "Large-scale concatenation cDNA sequencing". Genome Res. 7 (4): 353–8. doi:10.1101/gr.7.4.353. PMC 139146. PMID 9110174.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gao T, Yatani A, Dell'Acqua ML; et al. (1997). "cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits". Neuron. 19 (1): 185–96. doi:10.1016/S0896-6273(00)80358-X. PMID 9247274.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Zühlke RD, Bouron A, Soldatov NM, Reuter H (1998). "Ca2+ channel sensitivity towards the blocker isradipine is affected by alternative splicing of the human alpha1C subunit gene". FEBS Lett. 427 (2): 220–4. doi:10.1016/S0014-5793(98)00425-6. PMID 9607315.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Meyers MB, Puri TS, Chien AJ; et al. (1998). "Sorcin associates with the pore-forming subunit of voltage-dependent L-type Ca2+ channels". J. Biol. Chem. 273 (30): 18930–5. doi:10.1074/jbc.273.30.18930. PMID 9668070.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Liu WS, Soldatov NM, Gustavsson I, Chowdhary BP (1999). "Fiber-FISH analysis of the 3'-terminal region of the human L-type Ca2+ channel alpha 1C subunit gene". Hereditas. 129 (2): 169–75. doi:10.1111/j.1601-5223.1998.00169.x. PMID 10022083.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- GeneReviews/NIH/NCBI/UW entry on Brugada syndrome
- CACNA1C+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- GeneReviews/NIH/NCBI/UW entry on Timothy Syndrome
This article incorporates text from the United States National Library of Medicine, which is in the public domain.