Propofol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Diprivan, others[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Dependence liability | Physical: Very High Psychological: no data |
| Addiction liability | Moderate[2] |
| Routes of administration | Intravenous |
| Drug class | GABA receptor agonist; sedative; hypnotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | 95–99% |
| Metabolism | Liver glucuronidation |
| Onset of action | 15–30 seconds[5] |
| Elimination half-life | 1.5–31 hours[5] |
| Duration of action | ~5–10 minutes[5] |
| Excretion | Liver |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.551 |
| Chemical and physical data | |
| Formula | C12H18O |
| Molar mass | 178.275 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | ΔGsolvH2O = -4.39kcal/mol[6] |
| |
| |
| (verify) | |
Propofol[7] is the active component of an intravenous anesthetic formulation used for induction and maintenance of general anesthesia. It is chemically termed 2,6-diisopropylphenol. The formulation was approved under the brand name Diprivan. Numerous generic versions have since been released. Intravenous administration is used to induce unconsciousness after which anesthesia may be maintained using a combination of medications. It is manufactured as part of a sterile injectable emulsion formulation using soybean oil and lecithin, giving it a white milky coloration.[8]
Recovery from propofol-induced anesthesia is generally rapid and associated with less frequent side effects[9][10] (e.g. drowsiness, nausea, vomiting) compared to other anesthetic agents. Propofol may be used prior to diagnostic procedures requiring anesthesia, in the management of refractory status epilepticus, and for induction and/or maintenance of anesthesia prior to and during surgeries. It may be administered as a bolus or an infusion, or some combination of the two.
First synthesized in 1973, by John B. Glen, a British veterinary anesthesiologist working for Imperial Chemical Industries (ICI, later AstraZeneca),[11] in 1986 propofol was introduced for therapeutic use as a lipid emulsion in the United Kingdom and New Zealand. Propofol (Diprivan) received FDA approval in October 1989. It is on the World Health Organization's List of Essential Medicines.[12]
Uses
[edit]Anesthesia
[edit]To induce general anesthesia, propofol is the drug used almost exclusively, having largely replaced sodium thiopental.[13]
It is often administered as part of an anesthesia maintenance technique called total intravenous anesthesia, using either manually programmed infusion pumps or computer-controlled infusion pumps in a process called target controlled infusion (TCI).[14]
Propofol is also used to sedate individuals who are receiving mechanical ventilation but not undergoing surgery, such as patients in the intensive care unit.[15] In critically ill patients, propofol is superior to lorazepam both in effectiveness and overall cost.[16] Propofol is relatively inexpensive compared to medications of similar use due to shorter ICU stay length.[16] One of the reasons propofol is thought to be more effective (although it has a longer half-life than lorazepam) is that studies have found that benzodiazepines like midazolam and lorazepam tend to accumulate in critically ill patients, prolonging sedation.[16]
Propofol has also been suggested as a sleep aid in critically ill adults in an ICU setting; however, the effectiveness of this medicine in replicating the mental and physical aspects of sleep for people in the ICU is not clear.[15]
Propofol can be administered via a peripheral IV or central line. Propofol is often paired with fentanyl (for pain relief) in intubated and sedated people.[17] The two drugs are molecularly compatible in an IV mixture form.[17]
Propofol is also used for deepening of anesthesia in order to relieve laryngospasm. It may be used alone or followed by succinylcholine. Its use can avoid the need for paralysis and in some instances the potential side-effects of succinylcholine.[18]
Routine procedural sedation
[edit]Propofol is safe and effective for gastrointestinal endoscopy procedures (colonoscopies etc.). Its use in these settings results in a faster recovery compared to midazolam.[19] It can also be combined with opioids or benzodiazepines.[20][21][22] Because of its rapid induction and recovery time, propofol is also widely used for sedation of infants and children undergoing MRI procedures.[23] It is also often used in combination with ketamine with minimal side effects.[24]
COVID-19
[edit]In March 2021, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for Propofol‐Lipuro 1% to maintain sedation via continuous infusion in people older than sixteen with suspected or confirmed COVID-19 who require mechanical ventilation in an intensive care unit ICU setting.[25][26][27][28] During the public health emergency, it was considered unfeasible to limit Fresenius Propoven 2% Emulsion or Propofol-Lipuro 1% to patients with suspected or confirmed COVID-19, so it was made available to all ICU patients under mechanical ventilation.[28] This EUA has since been revoked.[28]
Status epilepticus
[edit]Status epilepticus may be defined as seizure activity lasting beyond five minutes needing anticonvulsant medication. Several guidelines recommend the use of propofol for the treatment of refractory status epilepticus.[29]
Other uses
[edit]Assisted death in Canada
[edit]A lethal dose of propofol is used for medical assistance in dying in Canada to quickly induce deep coma and death, but rocuronium is always given as a paralytic ensuring death, even when the patient has died as a result of initial propofol overdose.[30]
Capital punishment
[edit]Use of propofol as part of an execution protocol has been considered, although no individual has been executed using this agent. This is largely due to European manufacturers and governments banning the export of this propofol for such use.[31][32]
Recreational use
[edit]Recreational use of the drug via self-administration has been reported[33][34] but is relatively rare due to its potency and the level of monitoring required for safe use. Critically, a steep dose-response curve makes recreational use of propofol very dangerous, and deaths from self-administration continue to be reported.[35][36] The short-term effects sought via recreational use include mild euphoria, hallucinations, and disinhibition.[37][38]
Recreational use of the drug has been described among medical staff, such as anesthetists who have access to the drug.[39][40] It is reportedly more common among anesthetists on rotations with short rest periods, as usage generally produces a well-rested feeling.[41] Long-term use has been reported to result in addiction.[39][42]
Attention to the risks of off-label use of propofol increased in August 2009 due to the Los Angeles County coroner's conclusion that musician Michael Jackson died from a mixture of propofol and the benzodiazepine drugs lorazepam, midazolam, and diazepam on 25 June 2009.[43][44][45][46] According to a 22 July 2009 search warrant affidavit unsealed by the district court of Harris County, Texas, Jackson's physician, Conrad Murray, administered 25 milligrams of propofol diluted with lidocaine shortly before Jackson's death.[44][45][47]
Manufacturing
[edit]Propofol as a commercial sterile emulsified formulation is considered difficult to manufacture.[48][49][50]
It was initially formulated in Cremophor for human use, but this original formulation was implicated in an unacceptable number of anaphylactic events. It was eventually manufactured as a 1% emulsion in soybean oil.[51] Sterile emulsions represent complex formulation, the stability of which is dependent on the interplay of many factors such as micelle size and distribution.[52] [53][54]
Side effects
[edit]One of propofol's most common side effects is pain on injection, especially in smaller veins. This pain arises from activation of the pain receptor, TRPA1,[55] found on sensory nerves and can be mitigated by pretreatment with lidocaine.[56] Less pain is experienced when infused at a slower rate in a large vein (antecubital fossa). Patients show considerable variability in their response to propofol, at times showing profound sedation with small doses.
Additional side effects include low blood pressure related to vasodilation, transient apnea following induction doses, and cerebrovascular effects. Propofol has more pronounced hemodynamic effects relative to many intravenous anesthetic agents.[57] Reports of blood pressure drops of 30% or more are thought to be at least partially due to inhibition of sympathetic nerve activity.[58] This effect is related to the dose and rate of propofol administration. It may also be potentiated by opioid analgesics.[59]
Propofol can also cause decreased systemic vascular resistance, myocardial blood flow, and oxygen consumption, possibly through direct vasodilation.[60] There are also reports that it may cause green discoloration of the urine.[61]
Although propofol is widely used in the adult ICU setting, the side effects associated with medication seem to be more concerning in children. In the 1990s, multiple reported deaths of children in ICUs associated with propofol sedation prompted the FDA to issue a warning.[62]
As a respiratory depressant, propofol frequently produces apnea. The persistence of apnea can depend on factors such as premedication, dose administered, and rate of administration, and may sometimes persist for longer than 60 seconds.[63] Possibly as the result of depression of the central inspiratory drive, propofol may produce significant decreases in respiratory rate, minute volume, tidal volume, mean inspiratory flow rate, and functional residual capacity.[57]
Propofol administration also results in decreased cerebral blood flow, cerebral metabolic oxygen consumption, and intracranial pressure.[64] In addition, propofol may decrease intraocular pressure by as much as 50% in patients with normal intraocular pressure.[65]
A more serious but rare side effect is dystonia.[66] Mild myoclonic movements are common, as with other intravenous hypnotic agents. Propofol appears to be safe for use in porphyria, and has not been known to trigger malignant hyperpyrexia.[citation needed]
Propofol is also reported to induce priapism in some individuals,[67][68] and has been observed to suppress REM sleep stage and to worsen the poor sleep quality in some patients.[69]
Rare side effects include:[70]
- anxiety
- changes in vision
- cloudy urine
- coughing up blood
- delirium or hallucinations
- difficult urination
- difficulty swallowing
- dry eyes, mouth, nose, or throat
As with any other general anesthetic agent, propofol should be administered only where appropriately trained staff and facilities for monitoring are available, as well as proper airway management, a supply of supplemental oxygen, artificial ventilation, and cardiovascular resuscitation.[71]
Because of propofol's formulation (using lecithin and soybean oil) it is prone to bacterial contamination, despite the presence of the bacterial inhibitor benzyl alcohol; consequently, some hospital facilities require the IV tubing (of continuous propofol infusions) to be changed after 12 hours. This is a preventive measure against microbial growth and potential infection.[72]
Propofol infusion syndrome
[edit]A rare, but serious, side effect is propofol infusion syndrome. This potentially lethal metabolic derangement has been reported in critically ill patients after a prolonged infusion of high-dose propofol, sometimes in combination with catecholamines and/or corticosteroids.[73]
Interactions
[edit]The respiratory effects of propofol are increased if given with other respiratory depressants, including benzodiazepines.[74]
Pharmacology
[edit]Pharmacodynamics
[edit]Propofol has been proposed to have several mechanisms of action,[75][76][77] both through potentiation of GABAA receptor activity and therefore acting as a GABAA receptor positive allosteric modulator, thereby slowing the channel-closing time. At high doses, propofol may be able to activate GABAA receptors in the absence of GABA, behaving as a GABAA receptor agonist as well.[78][79][80] Propofol analogs have been shown to also act as sodium channel blockers.[81][82] Some research has also suggested that the endocannabinoid system may contribute significantly to propofol's anesthetic action and to its unique properties, as endocannabinoids also play an important role in the physiologic control of sleep, pain processing and emesis.[83][84] An EEG study on patients undergoing general anesthesia with propofol found that it causes a prominent reduction in the brain's information integration capacity.[85]
Propofol is an inhibitor of the enzyme fatty acid amide hydrolase, which metabolizes the endocannabinoid anandamide (AEA). Activation of the endocannabinoid system by propofol, possibly via inhibition of AEA catabolism, generates a significant increase in the whole-brain content of AEA, contributing to the sedative properties of propofol via CB1 receptor activation.[86] This may explain the psychotomimetic and antiemetic properties of propofol. By contrast, there is a high incidence of postoperative nausea and vomiting after administration of volatile anesthetics, which contribute to a significant decrease in the whole-brain content of AEA that can last up to forty minutes after induction.[84]
Pharmacokinetics
[edit]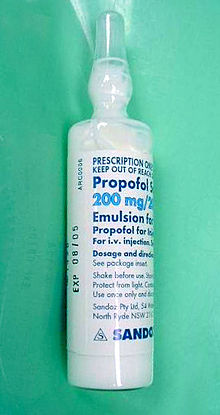
Propofol is highly protein-bound in vivo and is metabolized by conjugation in the liver.[87] The half-life of elimination of propofol has been estimated to be between 2 and 24 hours. However, its duration of clinical effect is much shorter, because propofol is rapidly distributed into peripheral tissues. When used for IV sedation, a single dose of propofol typically wears off within minutes. Onset is rapid, in as little as 15–30 seconds.[5] Propofol is versatile; the drug can be given for short or prolonged sedation, as well as for general anesthesia. Its use is not associated with nausea as is often seen with opioid medications. These characteristics of rapid onset and recovery along with its amnestic effects[88] have led to its widespread use for sedation and anesthesia.
History
[edit]John B. Glen, a veterinarian and researcher at Imperial Chemical Industries (ICI), spent thirteen years developing propofol, an effort for which he was awarded the 2018 Lasker Award for clinical research.
Originally developed as ICI 35868, propofol was chosen after extensive evaluation and structure–activity relationship studies of the anesthetic potencies and pharmacokinetic profiles of a series of ortho-alkylated phenols.[89]
First identified as a drug candidate in 1973, propofol entered clinical trials in 1977, using a form solubilized in cremophor EL.[90] However, due to anaphylactic reactions to cremophor, this formulation was withdrawn from the market and subsequently reformulated as an emulsion of a soya oil and propofol mixture in water. The emulsified formulation was relaunched in 1986 by ICI (whose pharmaceutical division later became a constituent of AstraZeneca) under the brand name Diprivan. The preparation contains 1% propofol, 10% soybean oil, and 1.2% purified egg phospholipid as an emulsifier, with 2.25% glycerol as a tonicity-adjusting agent, and sodium hydroxide to adjust the pH. Diprivan contains EDTA, a common chelation agent, that also acts alone (bacteriostatically against some bacteria) and synergistically with some other antimicrobial agents. Newer generic formulations contain sodium metabisulfite as an antioxidant and benzyl alcohol as antimicrobial agent. Propofol emulsion is an opaque white fluid due to the scattering of light from the emulsified micelle formulation.
Developments
[edit]A water-soluble prodrug form, fospropofol, has been developed and tested with positive results. Fospropofol is rapidly broken down by the enzyme alkaline phosphatase to form propofol. Marketed as Lusedra, this formulation may not produce the pain at injection site that often occurs with the conventional form of the drug. The U.S. Food and Drug Administration (FDA) approved the product in 2008.[91]
By incorporation of an azobenzene unit, a photoswitchable version of propofol (AP2) was developed in 2012 that allows for optical control of GABAA receptors with light.[92] In 2013, a propofol binding site on mammalian GABAA receptors has been identified by photolabeling using a diazirine derivative.[93] Additionally, it was shown that the hyaluronan polymer present in the synovia can be protected from free-radical depolymerization by propofol.[94]
Ciprofol is another derivative of propofol that is 4–6 times more potent than propofol. As of 2022[update] it is undergoing Phase III trials. Ciprofol appears to have a lower incidence of injection site pain and respiratory depression than propofol.[95]
Propofol has also been studied for treatment resistant depression.[96]
References
[edit]- ^ "Propofol". Drugs.com. Retrieved 2 January 2019.
- ^ Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–437. doi:10.3109/00952990.2014.933840. PMID 25083822. S2CID 19157711.
Propofol is a general anesthetic, however its abuse for recreational purpose has been documented (120). Using control drugs implicated in both ΔFosB induction and addiction (ethanol and nicotine), similar ΔFosB expression was apparent when propofol was given to rats. Moreover, this cascade was shown to act via the dopamine D1 receptor in the NAc, suggesting that propofol has abuse potential (119)
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Diprivan- propofol injection, emulsion". DailyMed. Retrieved 17 April 2021.
- ^ a b c d "Propofol". The American Society of Health-System Pharmacists. Archived from the original on 9 October 2016. Retrieved 21 January 2017.
- ^ Arcario MJ, Mayne CG, Tajkhorshid E (October 2014). "Atomistic models of general anesthetics for use in in silico biological studies". The Journal of Physical Chemistry B. 118 (42). American Chemical Society (ACS): 12075–12086. doi:10.1021/jp502716m. PMC 4207551. PMID 25303275.
- ^ "Propofol". PubChem. U.S. National Library of Medicine. Retrieved 25 October 2023.
- ^ "Making Stable, Sterile Propofol". www.microfluidics-mpt.com. Retrieved 25 October 2023.
- ^ "Propofol". go.drugbank.com. Retrieved 25 October 2023.
- ^ Glen JB (25 September 2018). "The Discovery and Development of Propofol Anesthesia: The 2018 Lasker-DeBakey Clinical Medical Research Award". JAMA. 320 (12): 1235–1236. doi:10.1001/jama.2018.12756. ISSN 0098-7484. PMID 30208399.
- ^ Glen JB (July 2019). "Try, try, and try again: personal reflections on the development of propofol". British Journal of Anaesthesia. 123 (1): 3–9. doi:10.1016/j.bja.2019.02.031. PMID 30982566. S2CID 115198733.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Discovery and development of propofol, a widely used anesthetic". The Lasker Foundation. Retrieved 8 September 2020.
Propofol is used today to initiate anesthesia in nearly 100% of general anesthesia cases worldwide.
- ^ Gale T, Leslie K, Kluger M (December 2001). "Propofol anaesthesia via target controlled infusion or manually controlled infusion: effects on the bispectral index as a measure of anaesthetic depth". Anaesthesia and Intensive Care. 29 (6): 579–584. doi:10.1177/0310057X0102900602. ISSN 0310-057X. PMID 11771598. S2CID 27253877.
- ^ a b Lewis SR, Schofield-Robinson OJ, Alderson P, Smith AF (January 2018). "Propofol for the promotion of sleep in adults in the intensive care unit". The Cochrane Database of Systematic Reviews. 1 (1): CD012454. doi:10.1002/14651858.CD012454.pub2. PMC 6353271. PMID 29308828.
- ^ a b c Cox CE, Reed SD, Govert JA, Rodgers JE, Campbell-Bright S, Kress JP, Carson SS (March 2008). "Economic evaluation of propofol and lorazepam for critically ill patients undergoing mechanical ventilation". Critical Care Medicine. 36 (3): 706–714. doi:10.1097/CCM.0B013E3181544248. PMC 2763279. PMID 18176312.
- ^ a b Isert PR, Lee D, Naidoo D, Carasso ML, Kennedy RA (June 1996). "Compatibility of propofol, fentanyl, and vecuronium mixtures designed for potential use in anesthesia and patient transport". Journal of Clinical Anesthesia. 8 (4): 329–336. doi:10.1016/0952-8180(96)00043-8. PMID 8695138.
- ^ Gavel G, Walker RW (April 2014). "Laryngospasm in anaesthesia". Continuing Education in Anaesthesia Critical Care & Pain. 14 (2): 47–51. doi:10.1093/bjaceaccp/mkt031.
- ^ McQuaid KR, Laine L (May 2008). "A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures". Gastrointestinal Endoscopy. 67 (6): 910–923. doi:10.1016/j.gie.2007.12.046. PMID 18440381.
- ^ Canadian National Formulary 2010
- ^ Shannon MT, Wilson BA, Stang CL (1999). Appleton & Lange's 1999 drug guide. Stamford, CT: Appleton & Lange. ISBN 978-0-8385-0371-3.
- ^ Numorphan® (oxymorphone) package insert (English), Endo 2009
- ^ Machata AM, Willschke H, Kabon B, Kettner SC, Marhofer P (August 2008). "Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging". British Journal of Anaesthesia. 101 (2): 239–243. doi:10.1093/bja/aen153. PMID 18534971.
- ^ Yan JW, McLeod SL, Iansavitchene A (September 2015). "Ketamine-Propofol Versus Propofol Alone for Procedural Sedation in the Emergency Department: A Systematic Review and Meta-analysis". Academic Emergency Medicine. 22 (9): 1003–1013. doi:10.1111/acem.12737. PMID 26292077.
- ^ "Propofol-Lipuro 1% (propofol) Injectable emulsion for infusion – 1,000 mg in 100 ml (10 mg /ml) : Fact Sheet for health Care Providers" (PDF). Bbraunusa.com. Archived from the original (PDF) on 14 May 2021. Retrieved 5 March 2022.
- ^ "Letter RE: Emergency Use Authorization 096". Fda.gov. Retrieved 5 March 2022.
- ^ "Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Propofol-Lipuro 1% Injectable Emulsion for Infusion". Fda.gov. Retrieved 5 March 2022.
- ^ a b c "Emergency Use Authorization". U.S. Food and Drug Administration (FDA). Retrieved 17 April 2021.
- ^ Rao VR, Lowenstein DH (2022). "Seizures and epilepsy.". In Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson J (eds.). Harrison's Principles of Internal Medicine (21st ed.). McGraw Hill. ISBN 978-1-264-26851-1.
- ^ Reggler J, Daws T (May 2017). "Medical Assistance in Dying (MAiD): Protocols and Procedures Handbook" (PDF). Divisions of Family Practice (2nd ed.). Comox Valley, British Columbia.
- ^ Kim E, Levy RJ (February 2020). "The role of anaesthesiologists in lethal injection: a call to action". Lancet. 395 (10225): 749–754. doi:10.1016/S0140-6736(19)32986-1. PMC 7416913. PMID 32014115.
- ^ "Lethal injection: Secretive US states resort to untested drugs". BBC News. 15 November 2013. Retrieved 8 November 2023.
- ^ Riezzo I, Centini F, Neri M, Rossi G, Spanoudaki E, Turillazzi E, Fineschi V (April 2009). "Brugada-like EKG pattern and myocardial effects in a chronic propofol abuser". Clinical Toxicology. 47 (4): 358–363. doi:10.1080/15563650902887842. hdl:11392/2357145. PMID 19514884. S2CID 22531823.
- ^ Belluck P (6 August 2009). "With High-Profile Death, Focus on High-Risk Drug". The New York Times. Archived from the original on 11 November 2011. Retrieved 7 August 2009.
- ^ Iwersen-Bergmann S, Rösner P, Kühnau HC, Junge M, Schmoldt A (2001). "Death after excessive propofol abuse". International Journal of Legal Medicine. 114 (4–5): 248–251. CiteSeerX 10.1.1.528.7395. doi:10.1007/s004149900129. PMID 11355404. S2CID 25963187.
- ^ Kranioti EF, Mavroforou A, Mylonakis P, Michalodimitrakis M (March 2007). "Lethal self administration of propofol (Diprivan). A case report and review of the literature". Forensic Science International. 167 (1): 56–58. doi:10.1016/j.forsciint.2005.12.027. PMID 16431058.
- ^ Sweetman SC, ed. (2005). Martindale: The Complete Drug Reference (34th ed.). London: Pharmaceutical Press. pp. 1305–1307. ISBN 978-0-85369-550-9.
- ^ Baudoin Z (2000). "General anesthetics and anesthetic gases.". In Dukes MN, Aronson JK (eds.). Meyler's Side Effects of Drugs (14th ed.). Amsterdam: Elsevier Science. p. 330. ISBN 978-0-444-50093-9.
- ^ a b Roussin A, Montastruc JL, Lapeyre-Mestre M (October 2007). "Pharmacological and clinical evidences on the potential for abuse and dependence of propofol: a review of the literature". Fundamental & Clinical Pharmacology. 21 (5): 459–466. doi:10.1111/j.1472-8206.2007.00497.x. PMID 17868199. S2CID 22477291.
- ^ Ward CF (2008). "Propofol: dancing with a "White Rabbit."" (PDF). California Society Anesthesiology Bulletin. 57 (Spring): 61–63. Archived from the original (PDF) on 8 September 2017. Retrieved 24 November 2014.
- ^ Charatan F (September 2009). "Concerns mount over misuse of anaesthetic propofol among US health professionals". BMJ. 339: b3673. doi:10.1136/bmj.b3673. PMID 19737827. S2CID 9877560.
- ^ Bonnet U, Harkener J, Scherbaum N (June 2008). "A case report of propofol dependence in a physician". Journal of Psychoactive Drugs. 40 (2): 215–217. doi:10.1080/02791072.2008.10400634. PMID 18720673. S2CID 15779389.
- ^ Moore S (28 August 2009). "Jackson's Death Ruled a Homicide". The New York Times. Archived from the original on 14 November 2013.
- ^ a b Surdin A (25 August 2009). "Coroner Attributes Michael Jackson's Death to Propofol". The Washington Post. Archived from the original on 9 November 2012. Retrieved 22 May 2010.
- ^ a b Itzkoff D (24 August 2009). "Coroner's Findings in Jackson Death Revealed". The New York Times. Archived from the original on 11 June 2010. Retrieved 22 May 2010.
- ^ "Jackson's Death: How Dangerous Is Propofol?". Time. 25 August 2009. Archived from the original on 25 July 2010. Retrieved 22 May 2010.
- ^ "Michael Jackson search warrant". Scribd. Archived from the original on 5 March 2016. Retrieved 12 August 2015.
- ^ Rooimans T, Damen M, Markesteijn CM, Schuurmans CC, de Zoete NH, van Hasselt PM, et al. (June 2023). "Development of a compounded propofol nanoemulsion using multiple non-invasive process analytical technologies". International Journal of Pharmaceutics. 640: 122960. doi:10.1016/j.ijpharm.2023.122960. PMC 10101488. PMID 37061210.
- ^ Zorrilla-Vaca A, Arevalo JJ, Escandón-Vargas K, Soltanifar D, Mirski MA (June 2016). "Infectious Disease Risk Associated with Contaminated Propofol Anesthesia, 1989-2014(1)". Emerging Infectious Diseases. 22 (6): 981–992. doi:10.3201/eid2206.150376. PMC 4880094. PMID 27192163.
- ^ WO 2014033751A2, Pramanick S, Gurjar S, Mehta SS, "Pharmaceutical composition of propofol", issued 2014-03-06, assigned to Emcure Pharmaceuticals Limited
- ^ Glen JB (July 2019). "Try, try, and try again: personal reflections on the development of propofol". British Journal of Anaesthesia. 123 (1): 3–9. doi:10.1016/j.bja.2019.02.031. PMID 30982566. S2CID 115198733.
- ^ "Hospira recalls lot of Propofol Injectable Emulsion". European Pharmaceutical Review. Retrieved 8 November 2023.
- ^ "Understanding Emulsion Formulation | Ascendia Pharmaceuticals". ascendiapharma.com. Retrieved 8 November 2023.
- ^ "Stability of Emulsion - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 9 November 2023.
- ^ Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP (June 2008). "General anesthetics activate a nociceptive ion channel to enhance pain and inflammation". Proceedings of the National Academy of Sciences of the United States of America. 105 (25): 8784–8789. doi:10.1073/pnas.0711038105. PMC 2438393. PMID 18574153.
- ^ "Propofol Drug Information, Professional". m drugs.com. Archived from the original on 23 January 2007. Retrieved 2 January 2007.
- ^ a b Sebel PS, Lowdon JD (August 1989). "Propofol: a new intravenous anesthetic". Anesthesiology. 71 (2): 260–277. doi:10.1097/00000542-198908000-00015. PMID 2667401. S2CID 34331379.
- ^ Robinson BJ, Ebert TJ, O'Brien TJ, Colinco MD, Muzi M (January 1997). "Mechanisms whereby propofol mediates peripheral vasodilation in humans. Sympathoinhibition or direct vascular relaxation?". Anesthesiology. 86 (1): 64–72. doi:10.1097/00000542-199701000-00010. PMID 9009941. S2CID 31288656.
- ^ "New awakening in anaesthesia—at a price". Lancet. 329 (8548): 1469–70. 1987. doi:10.1016/s0140-6736(87)92214-8. S2CID 28545161.
- ^ Larijani GE, Gratz I, Afshar M, Jacobi AG (October 1989). "Clinical pharmacology of propofol: an intravenous anesthetic agent". DICP. 23 (10): 743–749. doi:10.1177/106002808902301001. PMID 2683416. S2CID 43010280.
- ^ Lee JS, Jang HS, Park BJ (August 2013). "Green discoloration of urine after propofol infusion". Korean Journal of Anesthesiology. 65 (2): 177–179. doi:10.4097/kjae.2013.65.2.177. PMC 3766788. PMID 24024005.
- ^ Parke TJ, Stevens JE, Rice AS, Greenaway CL, Bray RJ, Smith PJ, et al. (September 1992). "Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports". BMJ. 305 (6854): 613–616. doi:10.1136/bmj.305.6854.613. PMC 1883365. PMID 1393073.
- ^ Langley MS, Heel RC (April 1988). "Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic". Drugs. 35 (4): 334–372. doi:10.2165/00003495-198835040-00002. PMID 3292208.
- ^ Bailey JM, Mora CT, Shafer SL (June 1996). "Pharmacokinetics of propofol in adult patients undergoing coronary revascularization. The Multicenter Study of Perioperative Ischemia Research Group". Anesthesiology. 84 (6): 1288–1297. doi:10.1097/00000542-199606000-00003. PMID 8669668. S2CID 26019589.
- ^ Reilly CS, Nimmo WS (July 1987). "New intravenous anaesthetics and neuromuscular blocking drugs. A review of their properties and clinical use". Drugs. 34 (1): 98–135. doi:10.2165/00003495-198734010-00004. PMID 3308413. S2CID 46973781.
- ^ Schramm BM, Orser BA (May 2002). "Dystonic reaction to propofol attenuated by benztropine (cogentin)". Anesthesia and Analgesia. 94 (5): 1237–40, table of contents. doi:10.1097/00000539-200205000-00034. PMID 11973196.
- ^ Vesta KS, Martina SD, Kozlowski EA (May 2006). "Propofol-induced priapism, a case confirmed with rechallenge". The Annals of Pharmacotherapy. 40 (5): 980–982. doi:10.1345/aph.1G555. PMID 16638914. S2CID 36563320.
- ^ Fuentes EJ, Garcia S, Garrido M, Lorenzo C, Iglesias JM, Sola JE (July 2009). "Successful treatment of propofol-induced priapism with distal glans to corporal cavernosal shunt". Urology. 74 (1): 113–115. doi:10.1016/j.urology.2008.12.066. PMID 19371930.
- ^ Kondili E, Alexopoulou C, Xirouchaki N, Georgopoulos D (October 2012). "Effects of propofol on sleep quality in mechanically ventilated critically ill patients: a physiological study". Intensive Care Medicine. 38 (10): 1640–1646. doi:10.1007/s00134-012-2623-z. PMID 22752356. S2CID 21206446.
- ^ "Propofol (Intravenous Route) Side Effects - Mayo Clinic". Mayoclinic.org. Retrieved 24 January 2022.
- ^ "AstraZeneca – United States Home Page" (PDF). .astrazeneca-us.com. Archived from the original (PDF) on 4 October 2011. Retrieved 8 June 2013.
- ^ Kim TE, Shankel T, Reibling ET, Paik J, Wright D, Buckman M, et al. (1 January 2017). "Healthcare students interprofessional critical event/disaster response course". American Journal of Disaster Medicine. 12 (1): 11–26. doi:10.5055/ajdm.2017.0254. PMID 28822211.
- ^ Vasile B, Rasulo F, Candiani A, Latronico N (September 2003). "The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome". Intensive Care Medicine. 29 (9): 1417–1425. doi:10.1007/s00134-003-1905-x. PMID 12904852. S2CID 23932736.
- ^ Doheny K, Chang L, Vila Jr H (24 August 2009). "Propofol Linked to Michael Jackson's Death". WebMD. Archived from the original on 28 August 2009. Retrieved 26 August 2009.
- ^ Trapani G, Altomare C, Liso G, Sanna E, Biggio G (February 2000). "Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery". Current Medicinal Chemistry. 7 (2): 249–271. doi:10.2174/0929867003375335. PMID 10637364.
- ^ Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H (Summer 2008). "The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties". CNS Neuroscience & Therapeutics. 14 (2): 95–106. doi:10.1111/j.1527-3458.2008.00043.x. PMC 6494023. PMID 18482023.
- ^ Vanlersberghe C, Camu F (2008). "Propofol". Modern Anesthetics. Handbook of Experimental Pharmacology. Vol. 182. pp. 227–52. doi:10.1007/978-3-540-74806-9_11. ISBN 978-3-540-72813-9. PMID 18175094.
- ^ Trapani G, Latrofa A, Franco M, Altomare C, Sanna E, Usala M, et al. (May 1998). "Propofol analogues. Synthesis, relationships between structure and affinity at GABAA receptor in rat brain, and differential electrophysiological profile at recombinant human GABAA receptors". Journal of Medicinal Chemistry. 41 (11): 1846–1854. doi:10.1021/jm970681h. PMID 9599235.
- ^ Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL (April 2001). "General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility". The Journal of Pharmacology and Experimental Therapeutics. 297 (1): 338–351. PMID 11259561.
- ^ Krasowski MD, Hong X, Hopfinger AJ, Harrison NL (July 2002). "4D-QSAR analysis of a set of propofol analogues: mapping binding sites for an anesthetic phenol on the GABA(A) receptor". Journal of Medicinal Chemistry. 45 (15): 3210–3221. doi:10.1021/jm010461a. PMC 2864546. PMID 12109905.
- ^ Haeseler G, Leuwer M (March 2003). "High-affinity block of voltage-operated rat IIA neuronal sodium channels by 2,6 di-tert-butylphenol, a propofol analogue". European Journal of Anaesthesiology. 20 (3): 220–224. doi:10.1017/s0265021503000371 (inactive 1 November 2024). PMID 12650493. S2CID 25072723.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Haeseler G, Karst M, Foadi N, Gudehus S, Roeder A, Hecker H, et al. (September 2008). "High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues". British Journal of Pharmacology. 155 (2): 265–275. doi:10.1038/bjp.2008.255. PMC 2538694. PMID 18574460.
- ^ Fowler CJ (February 2004). "Possible involvement of the endocannabinoid system in the actions of three clinically used drugs". Trends in Pharmacological Sciences. 25 (2): 59–61. doi:10.1016/j.tips.2003.12.001. PMID 15106622.
- ^ a b Schelling G (2006). "Effects of General Anesthesia on Anandamide Blood Levels in Humans". Anesthesiology. 104 (2): 273–277. doi:10.1097/00000542-200602000-00012. PMID 16436846. S2CID 27303365. Retrieved 11 December 2022.
- ^ Lee U, Mashour GA, Kim S, Noh GJ, Choi BM (March 2009). "Propofol induction reduces the capacity for neural information integration: implications for the mechanism of consciousness and general anesthesia". Consciousness and Cognition. 18 (1): 56–64. doi:10.1016/j.concog.2008.10.005. PMID 19054696. S2CID 14699319.
- ^ Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, et al. (July 2003). "The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase". British Journal of Pharmacology. 139 (5): 1005–1013. doi:10.1038/sj.bjp.0705334. PMC 1573928. PMID 12839875.
- ^ Favetta P, Degoute CS, Perdrix JP, Dufresne C, Boulieu R, Guitton J (May 2002). "Propofol metabolites in man following propofol induction and maintenance". British Journal of Anaesthesia. 88 (5): 653–658. doi:10.1093/bja/88.5.653. PMID 12067002.
- ^ Veselis RA, Reinsel RA, Feshchenko VA, Wroński M (October 1997). "The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations". Anesthesiology. 87 (4): 749–764. doi:10.1097/00000542-199710000-00007. PMID 9357875. S2CID 30185553.
- ^ James R, Glen JB (December 1980). "Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents". Journal of Medicinal Chemistry. 23 (12): 1350–1357. doi:10.1021/jm00186a013. PMID 7452689.
- ^ Lasker Foundation. "Discovery and development of propofol, a widely used anesthetic". The Lasker Foundation. Retrieved 25 July 2020.
- ^ "Drugs@FDA: FDA Approved Drug Products". U.S. Food and Drug Administration (FDA). Archived from the original on 13 August 2014. Retrieved 8 June 2013.
- ^ Stein M, Middendorp SJ, Carta V, Pejo E, Raines DE, Forman SA, et al. (October 2012). "Azo-propofols: photochromic potentiators of GABA(A) receptors". Angewandte Chemie. 51 (42): 10500–10504. doi:10.1002/anie.201205475. PMC 3606271. PMID 22968919.
- ^ Yip GM, Chen ZW, Edge CJ, Smith EH, Dickinson R, Hohenester E, et al. (November 2013). "A propofol binding site on mammalian GABAA receptors identified by photolabeling". Nature Chemical Biology. 9 (11): 715–720. doi:10.1038/nchembio.1340. PMC 3951778. PMID 24056400.
- ^ Kvam C, Granese D, Flaibani A, Pollesello P, Paoletti S (June 1993). "Hyaluronan can be protected from free-radical depolymerisation by 2,6-diisopropylphenol, a novel radical scavenger". Biochemical and Biophysical Research Communications. 193 (3): 927–933. doi:10.1006/bbrc.1993.1714. PMID 8391811.
- ^ Chen BZ, Yin XY, Jiang LH, Liu JH, Shi YY, Yuan BY (August 2022). "The efficacy and safety of ciprofol use for the induction of general anesthesia in patients undergoing gynecological surgery: a prospective randomized controlled study". BMC Anesthesiology. 22 (1): 245. doi:10.1186/s12871-022-01782-7. PMC 9347095. PMID 35922771.
- ^ Wu G, Xu H (October 2023). "A synopsis of multitarget therapeutic effects of anesthetics on depression". European Journal of Pharmacology. 957: 176032. doi:10.1016/j.ejphar.2023.176032. PMID 37660970. S2CID 261479363.
External links
[edit]- "Propofol". Drug Information Portal. U.S. National Library of Medicine.
- GB patent 1472793, John B Glen & Roger James, "Pharmaceutical Compositions", published 1977-05-04, assigned to Imperial Chemical Industries Ltd
