Nicotine: Difference between revisions
←Replaced content with '"Nicotine is amazing"' |
m Reverted edits by SammyHass2001 (talk) to last revision by ClueBot NG (HG) |
||
| Line 1: | Line 1: | ||
{{About|the chemical|other uses|Nicotine (disambiguation)}} |
|||
"Nicotine is amazing" |
|||
{{Drugbox| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 420440849 |
|||
| IUPAC_name = 3-[1-Methylpyrrolidin-2-yl]pyridine |
|||
| image = Nicotine.svg |
|||
| width = 200 |
|||
| image2 = Nicotine3Dan2.gif |
|||
| width2 = 200 |
|||
| caption = |
|||
<!--Clinical data--> |
|||
| tradename = Nicorette, Nicotrol |
|||
| Drugs.com = {{drugs.com|monograph|nicotine}} |
|||
| pregnancy_AU = D |
|||
| pregnancy_US = D |
|||
| legal_AU = S4 |
|||
| legal_UK = GSL |
|||
| legal_US = OTC |
|||
| dependency_liability = High |
|||
| routes_of_administration = [[Inhalation]]; [[Insufflation (medicine)|Insufflation]]; [[Oral route|Oral]] – Buccal, Sublingual, and Ingestion; [[Transdermal]]; [[Suppository|Rectal]], |
|||
<!--Pharmacokinetic data--> |
|||
| bioavailability = 20 to 45% (oral), 53% (intranasal), 68% (transdermal) |
|||
| protein_bound = <5% |
|||
| metabolism = [[Hepatic]] |
|||
| elimination_half-life = 1-2 hours; 20 hours active metabolite (cotinine) |
|||
| excretion = Urine (10-20% (gum), pH-dependent; 30% (inhaled); 10-30% (intranasal)) |
|||
<!--Identifiers--> |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CAS_number_Ref = {{cascite|correct|??}} |
|||
| CAS_number = 54-11-5 |
|||
| ATC_prefix = N07 |
|||
| ATC_suffix = BA01 |
|||
| ATC_supplemental = {{ATCvet|P53|AX13}} |
|||
| ChEBI_Ref = {{ebicite|changed|EBI}} |
|||
| ChEBI = 18723 |
|||
| PubChem = 89594 |
|||
| IUPHAR_ligand = 2585 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = DB00184 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 80863 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 6M3C89ZY6R |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = D03365 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 3 |
|||
<!--Chemical data--> |
|||
| C=10 | H=14 | N=2 |
|||
| molecular_weight = 162.12 g/mol |
|||
| smiles = CN(CCC1)[C@@H]1C2=CC=CN=C2 |
|||
| InChI = 1/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = SNICXCGAKADSCV-JTQLQIEISA-N |
|||
| density = 1.01 |
|||
| melting_point = -79 |
|||
| boiling_point = 247 |
|||
}} |
|||
'''Nicotine''' is a [[potency (pharmacology)|potent]] [[parasympathomimetic]] [[alkaloid]] found in the [[nightshade]] family of plants ([[Solanaceae]]) and a [[stimulant]] [[drug]]. It is a [[Nicotinic agonist|nicotinic acetylcholine receptor agonist]]. It [[biosynthesis|is made]] in the roots and accumulates in the leaves of the plants. |
|||
It constitutes approximately 0.6–3.0% of the dry weight of [[tobacco]]<ref>{{cite web|url=http://dccps.nci.nih.gov/tcrb/monographs/9/m9_3.PDF |title=Smoking and Tobacco Control Monograph No. 9 |format=PDF |date= |accessdate=2012-12-19}}</ref> and is present in the range of 2–7 µg/kg of various edible plants.<ref name="acs">{{cite web |url=http://pubs.acs.org/cgi-bin/abstract.cgi/jafcau/1999/47/i08/abs/jf990089w.html |title=Determination of the Nicotine Content of Various Edible Nightshades (Solanaceae) and Their Products and Estimation of the Associated Dietary Nicotine Intake |accessdate=2008-10-05}}</ref> |
|||
It functions as an [[plant defense against herbivory|antiherbivore chemical]]; consequently, nicotine was widely used as an [[insecticide]] in the past<ref>{{Cite book |
|||
| last = Rodgman |
|||
| first = Alan |
|||
| last2 = Perfetti |
|||
| first2 = Thomas A. |
|||
| title = The chemical components of tobacco and tobacco smoke |
|||
| place = Boca Raton, FL |
|||
| publisher = CRC Press |
|||
| year = 2009 |
|||
| lccn=2008018913 |
|||
| isbn = 1-4200-7883-6 }}{{page needed|date=December 2013}} |
|||
</ref><ref name=Ujvary>{{Cite book |
|||
| first = István | last = Ujváry |
|||
| contribution = Nicotine and Other Insecticidal Alkaloids |
|||
| editor-first = Izuru | editor-last = Yamamoto |
|||
| editor2-first = John | editor2-last = Casida |
|||
| title = Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor |
|||
| pages = 29–69 |
|||
| publisher = Springer-Verlag |
|||
| location = Tokyo |
|||
| year = 1999 |
|||
}} |
|||
</ref> |
|||
and nicotine analogs such as [[imidacloprid]] are currently widely used. |
|||
In smaller doses (an average [[cigarette]] yields about 1 mg of absorbed nicotine), the substance acts as a [[stimulant]] in [[mammal]]s, while high amounts (50–100 mg) can be harmful.<ref name=inchem>{{cite web|url=http://www.inchem.org/documents/pims/chemical/nicotine.htm#PartTitle:7.%20TOXICOLOGY |title=Nicotine (PIM) |publisher=Inchem.org |date= |accessdate=2012-12-19}}</ref><ref name=overdose>{{cite web | author = Genetic Science Learning Center | title = How Drugs Can Kill | url = http://learn.genetics.utah.edu/content/addiction/drugskill/ }}</ref><ref name=MayerNewLethalDose2013/> |
|||
This stimulant effect is likely a major contributing factor to the dependence-forming properties of [[tobacco smoking]], nicotine patches, nicotine gum, nicotine inhalers and liquid nicotine vapourizers.{{Citation needed|date=April 2013}} According to the [[American Heart Association]], nicotine [[substance use disorder|addiction]] has historically been one of the hardest addictions to break, while the pharmacological and behavioral characteristics that determine nicotine addiction are similar to those determining addiction to [[heroin]] and [[cocaine]]. The nicotine content of popular American-brand cigarettes has slowly increased over the years, and one study found that there was an average increase of 1.78% per year between the years of 1998 and 2005. This was found for all major market categories of cigarettes.<ref>{{cite journal |
|||
| title = Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005 |
|||
| journal = Tobacco Control |
|||
| volume = 16 |
|||
| issue = 5 |
|||
| pages = e5 |
|||
| year = 2007 |
|||
| pmid = 17897974 |
|||
| doi = 10.1136/tc.2006.019695 |
|||
| author1 = Connolly, G. N |
|||
| author2 = Alpert, H. R |
|||
| author3 = Wayne, G. F |
|||
| author4 = Koh, H |
|||
| pmc = 2598548}}</ref> |
|||
Research in 2011 found that nicotine inhibits chromatin-modifying enzymes (class I and II [[histone deacetylases]]); this inhibition has been shown to increase the ability of [[cocaine]] to cause an [[addiction]].<ref>{{cite journal |author=Volkow ND |title=Epigenetics of nicotine: another nail in the coughing |journal=Sci Transl Med |volume=3 |issue=107 |pages=107ps43 |date=November 2011 |pmid=22049068 |doi=10.1126/scitranslmed.3003278 |pmc=3492949}}</ref> |
|||
==Psychoactive effects== |
|||
{{further|Psychoactive drug}} |
|||
Nicotine's [[mood (psychology)|mood]]-altering effects are different by report: in particular it is both a stimulant and a relaxant.<ref>{{cite journal |url=http://www.ti.ubc.ca/newsletter/effective-clinical-tobacco-intervention |title= Effective Clinical Tobacco Intervention |journal=Therapeutics Letter |issue=21 |date=September–October 1997 |pages=1–4}}</ref> First causing a release of [[glucose]] from the liver and [[epinephrine]](adrenaline) from the [[adrenal medulla]], it causes [[stimulation]]. Users report feelings of [[relaxation (psychology)|relaxation]], sharpness, [[calmness]], and [[alertness]].<ref>{{cite journal |id={{INIST|1081618}} |last1=Lagrue |first1=Gilbert |last2=Cormier |first2=Anne |month=June |year=2001 |title=Des récepteurs nicotiniques à la dépendance tabagique : Perspectives thérapeutiques |trans_title=From nicotinic receptors to smoking dependence: Therapeutic prospects |language=fr |journal=Alcoologie et addictologie |issn=1620-4522 |volume=23 |issue=2 |pages=39S–42S}}</ref> Like any stimulant, it may very rarely cause the often uncomfortable [[neuropsychiatric]] effect of [[akathisia]]. By reducing the [[appetite]] and raising the [[metabolism]], some smokers may [[weight loss|lose weight]] as a consequence.<ref>{{cite journal |id={{INIST|1081638}} |last1=Orsini |first1=Jean-Claude |month=June |year=2001 |title=Dépendance tabagique et contrôle central de la glycémie et de l'appétit |trans_title=Dependence on tobacco smoking and brain systems controlling glycemia and appetite |language=fr |journal=Alcoologie et addictologie |issn=1620-4522 |volume=23 |issue=2 Suppl |pages=28S–36S}}</ref><ref>{{cite journal |doi=10.1038/sj.npp.1300597 |laysummary=http://archive.uninews.unimelb.edu.au/view-49206.html |laysource=The University of Melbourne |laydate=1 November 2004 |title=Effect of Short-Term Cigarette Smoke Exposure on Body Weight, Appetite and Brain Neuropeptide Y in Mice |year=2004 |last1=Chen |first1=Hui |last2=Vlahos |first2=Ross |last3=Bozinovski |first3=Steve |last4=Jones |first4=Jessica |last5=Anderson |first5=Gary P |last6=Morris |first6=Margaret J |journal=Neuropsychopharmacology}}</ref> |
|||
When a [[cigarette]] is smoked, nicotine-rich blood passes from the [[human lung|lung]]s to the [[human brain|brain]] within seven seconds and immediately stimulates the release of many chemical messengers such as [[acetylcholine]], [[norepinephrine]], [[epinephrine]], [[arginine vasopressin]], [[serotonin]], [[dopamine]], and [[beta-endorphin]].<ref>Pomerleau OF, Pomerleau CS (1984). Neuroregulators and the reinforcement of smoking: Towards a biobehavioral explanation. ''Neuroscience and Biobehavioral Reviews'', 8:503-513.</ref> <ref>Pomerleau OF, Rosecrans J (1989). Neuroregulatory effects of nicotine. ''Psychoneuroendocrinology'' 14:407-423.</ref> This release of neurotransmitters and hormones is responsible for most of nicotine's psychoactive effects. Nicotine appears to enhance [[attention|concentration]]<ref name="rusted">{{cite journal |author=Rusted J, Graupner L, O'Connell N, Nicholls C |title=Does nicotine improve cognitive function? |journal=Psychopharmacology (Berl.) |volume=115 |issue=4 |pages=547–9 |date=August 1994 |pmid=7871101 |doi=10.1007/BF02245580}}</ref> and memory due to the increase of [[acetylcholine]]. It also appears to enhance [[alertness]] due to the increases of [[acetylcholine]] and [[norepinephrine]]. [[Arousal]] is increased by the increase of [[norepinephrine]]. [[Pain]] is reduced by the increases of [[acetylcholine]] and beta-endorphin. [[Anxiety]] is reduced by the increase of [[beta-endorphin]]. Nicotine also extends the duration of positive effects of dopamine<ref>{{cite journal |author=Easton, John |title=Nicotine extends duration of pleasant effects of dopamine |journal=The University of Chicago Chronicle|volume=21 |issue=12 |date=March 28, 2002 |url=http://chronicle.uchicago.edu/020328/nicotine.shtml}}</ref> and increases sensitivity in brain reward systems.<ref name=Kenny>{{cite journal |author=Kenny PJ, Markou A |title=Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity |journal=Neuropsychopharmacology |volume=31 |issue=6 |pages=1203–11 |date=Jun 2006 |pmid=16192981 |doi=10.1038/sj.npp.1300905}}</ref> Most cigarettes (in the smoke inhaled) contain 1 to 3 milligrams of nicotine.<ref>{{cite web|author=|url=http://www.erowid.org/chemicals/nicotine/nicotine_dose.shtml|title=Erowid Nicotine Vault : Dosage |publisher=Erowid.org |date=2011-10-14 |accessdate=2012-12-19}}</ref> |
|||
Research suggests that, when smokers wish to achieve a stimulating effect, they take short quick puffs, which produce a low level of blood nicotine.<ref>{{cite book |doi=10.1007/978-1-4899-0888-9_9 |chapter=Factors Governing Recruitment to and Maintenance of Smoking |title=Drug and Alcohol Use |year=1989 |last1=Golding |first1=J. F. |last2=Mangan |first2=G. L. |isbn=978-1-4899-0890-2 |pages=101–17 |chapterurl=http://books.google.com/books?id=s9_EWPshR4QC&pg=PA101 |editor1-first=Stanley |editor1-last=Einstein}}</ref> This stimulates [[action potential|nerve transmission]]. When they wish to relax, they take deep puffs, which produce a high level of blood nicotine, which depresses the passage of [[nerve impulses]], producing a mild sedative effect. At low doses, nicotine potently enhances the actions of [[norepinephrine]] and [[dopamine]] in the brain, causing a drug effect typical of those of [[psychostimulants]]. At higher doses, nicotine enhances the effect of [[serotonin]] and [[opiate]] activity, producing a calming, [[analgesic|pain-killing]] effect. Nicotine is unique in comparison to most [[drug]]s, as its profile changes from [[stimulant]] to [[sedative]]/[[pain killer]] in increasing [[dose (biochemistry)|dosage]]s and use, a phenomenon described by Paul Nesbitt in his doctoral dissertation<ref>Nesbitt P (1969). Smoking, physiological arousal, and emotional response. Unpublished doctoral dissertation, Columbia University.</ref> and subsequently referred to as "Nesbitt's Paradox." |
|||
==Medical uses== |
|||
[[Image:Nicoderm.JPG|thumb|right|A 21 mg patch applied to the left arm. The [[Cochrane Collaboration]] finds that NRT increases a quitter's chance of success by 50 to 70%.<ref name=CD000146>{{cite journal |author=Stead LF, Perera R, Bullen C, Mant D, Lancaster T |title=Nicotine replacement therapy for smoking cessation |journal=Cochrane Database Syst Rev |issue=1 |pages=CD000146 |year=2008 |pmid=18253970 |doi=10.1002/14651858.CD000146.pub3 |editor1-last=Stead |editor1-first=Lindsay F}}</ref> But in 1990, researchers found that 93% of users returned to smoking within six months.<ref>{{cite news|author=Millstone, Ken|title=Nixing the patch: Smokers quit cold turkey|url=http://jscms.jrn.columbia.edu/cns/2007-02-13/millstone-coldturkeyquitters.html|date=February 13, 2007|publisher=Columbia.edu News Service|accessdate=May 23, 2010}}</ref>]] |
|||
Although population level effectiveness has not been demonstrated,<ref>{{cite journal |doi=10.1016/j.addbeh.2005.05.054 |title=Smoking status of Australian general practice patients and their attempts to quit |year=2006 |last1=Doran |first1=Christopher M. |last2=Valenti |first2=Lisa |last3=Robinson |first3=Maxine |last4=Britt |first4=Helena |last5=Mattick |first5=Richard P. |journal=Addictive Behaviors |volume=31 |issue=5 |pages=758–66 |pmid=16137834}}</ref><ref>{{citation|author=Gallup Poll|title=Most U.S. Smokers Want to Quit, Have Tried Multiple Times|url=http://www.gallup.com/poll/163763/smokers-quit-tried-multiple-times.aspx|date=July 31, 2013|publisher=Gallup|accessdate=December 17, 2013}}</ref><ref>{{cite journal |doi=10.1146/annurev-publhealth-031811-124624 |title=Quitlines and Nicotine Replacement for Smoking Cessation: Do We Need to Change Policy? |year=2012 |last1=Pierce |first1=John P. |last2=Cummins |first2=Sharon E. |last3=White |first3=Martha M. |last4=Humphrey |first4=Aimee |last5=Messer |first5=Karen |journal=Annual Review of Public Health |volume=33 |pages=341–56 |pmid=22224888}}</ref> the primary therapeutic use of nicotine is in treating nicotine dependence in order to eliminate [[smoking]] with the damage it does to health. Controlled levels of nicotine are given to patients through gums, dermal patches, lozenges, electronic/substitute cigarettes or nasal sprays in an effort to wean them off their dependence. |
|||
However, in a few situations, smoking has been observed to be of therapeutic value. |
|||
These are often referred to as "[[Smoker’s Paradoxes]]".<ref name="Cohen_2001">{{cite journal | author = Cohen DJ, Doucet M, Cutlip DE, Ho KK, Popma JJ, Kuntz RE | title = Impact of smoking on clinical and angiographic restenosis after percutaneous coronary intervention: another smoker's paradox? | journal = Circulation | volume = 104 | issue = 7 | pages = 773–8 |date=August 2001 | pmid = 11502701 | doi = 10.1161/hc3201.094225 }}</ref> Although in most cases the actual mechanism is understood only poorly or not at all, it is generally believed that the principal beneficial action is due to the nicotine administered, and that administration of nicotine without smoking may be as beneficial as smoking, without the higher risk to health due to [[tar (tobacco residue)|tar]] and other substances found in tobacco. |
|||
For instance, studies suggest that smokers require less frequent repeated [[revascularization]] after [[percutaneous coronary intervention]](PCI).<ref name="Cohen_2001"/> Risk of [[ulcerative colitis]] has been frequently shown to be reduced by smokers on a dose-dependent basis; the effect is eliminated if the individual stops smoking.<ref name="ohcm">{{cite book |author=Longmore, M., Wilkinson, I., Torok, E. |title=Oxford Handbook of Clinical Medicine |page=232 |edition=5th}}</ref><ref name="pmid11069313">{{cite journal | author = Green JT, Richardson C, Marshall RW, Rhodes J, McKirdy HC, Thomas GA, Williams GT | title = Nitric oxide mediates a therapeutic effect of nicotine in ulcerative colitis | journal = Aliment. Pharmacol. Ther. | volume = 14 | issue = 11 | pages = 1429–34 |date=November 2000 | pmid = 11069313 | doi = 10.1046/j.1365-2036.2000.00847.x }}</ref> Smoking also appears to interfere with development of [[Kaposi's sarcoma]] in patients with HIV.<ref name="pmid12441327">{{cite journal | author = Goedert JJ, Vitale F, Lauria C, Serraino D, Tamburini M, Montella M, Messina A, Brown EE, Rezza G, Gafà L, Romano N | title = Risk factors for classical Kaposi's sarcoma | journal = J. Natl. Cancer Inst. | volume = 94 | issue = 22 | pages = 1712–8 |date=November 2002 | pmid = 12441327 | doi = 10.1093/jnci/94.22.1712 |laysummary=http://www.data-yard.net/10b/kaposi.htm |laysource=United Press International |laydate=March 29, 2001}}</ref> |
|||
Nicotine reduces the chance of [[preeclampsia]],<ref name="pmid10561644">{{cite journal | author = Lain KY, Powers RW, Krohn MA, Ness RB, Crombleholme WR, Roberts JM | title = Urinary cotinine concentration confirms the reduced risk of preeclampsia with tobacco exposure | journal = Am. J. Obstet. Gynecol. | volume = 181 | issue = 5 Pt 1 | pages = 1192–6 |date=November 1999 | pmid = 10561644 | doi = 10.1016/S0002-9378(99)70107-9 }}</ref> and [[atopy|atopic disorder]]s such as [[allergic asthma]].<ref name="pmid11422156">{{cite journal | author = Hjern A, Hedberg A, Haglund B, Rosén M | title = Does tobacco smoke prevent atopic disorders? A study of two generations of Swedish residents |journal = Clin. Exp. Allergy | volume = 31 | issue = 6 | pages = 908–14 |date=June 2001 | pmid = 11422156 | doi = 10.1046/j.1365-2222.2001.01096.x }}</ref>{{dubious|date=March 2013}} A plausible mechanism of action in these cases may be nicotine acting as an [[inflammation|anti-inflammatory agent]], and interfering with the inflammation-related disease process, as nicotine has vasoconstrictive effects.<ref name=sciam>{{cite journal | author = Melton L | title=Body Blazes | journal=Scientific American |date=June 2006 |pmid=16711354|bibcode=2006SciAm.294f..24M |volume=294 |doi=10.1038/scientificamerican0606-24 |issue=6 | pages = 24 }}</ref> |
|||
Tobacco smoke has been shown to contain compounds capable of inhibiting [[monoamine oxidase]], which is responsible for the degradation of dopamine in the human brain. When dopamine is broken down by MAO-B, neurotoxic by-products are formed, possibly contributing to Parkinson's and Alzheimers disease.<ref name="pmid10942038">{{cite journal | author = Fratiglioni L, Wang HX | title = Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies | journal = Behav. Brain Res. | volume = 113 | issue = 1–2 | pages = 117–20 |date=August 2000 | pmid = 10942038 | doi = 10.1016/S0166-4328(00)00206-0 }}</ref> |
|||
While tobacco smoking is associated with an increased risk of Alzheimer's disease,<ref name="pmid19105840">{{cite journal|author=Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C |title=Smoking, dementia and cognitive decline in the elderly, a systematic review |journal=BMC Geriatr |volume=8 |page=36 |year=2008 |pmid=19105840 |pmc=2642819 |doi=10.1186/1471-2318-8-36 }}</ref> there is evidence that nicotine itself has the potential to prevent and treat Alzheimer's disease.<ref name="pmid19184661">{{cite journal |author=Henningfield JE, Zeller M |title=Nicotine psychopharmacology: policy and regulatory |journal=Handb Exp Pharmacol |pages=511–34 |year=2009 |pmid=19184661|doi=10.1007/978-3-540-69248-5_18 |series=Handbook of Experimental Pharmacology |isbn=978-3-540-69246-1 |volume=192 |issue=192 }}</ref> |
|||
Nicotine has been shown to delay the onset of Parkinson's disease in studies involving monkeys and humans.<ref>{{cite journal |doi=10.1111/j.1471-4159.2006.04078.x |laysummary=http://www.webmd.com/parkinsons-disease/news/20060811/nicotine-slows-parkinsons-disease |laysource=WebMD |laydate=August 11, 2006 |title=Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates |year=2006 |last1=Quik |first1=Maryka |last2=Parameswaran |first2=Neeraja |last3=McCallum |first3=Sarah E. |last4=Bordia |first4=Tanuja |last5=Bao |first5=Shanshan |last6=McCormack |first6=Alison |last7=Kim |first7=Amy |last8=Tyndale |first8=Rachel F. |last9=Langston |first9=J. William |last10=Di Monte |first10=Donato A. |journal=Journal of Neurochemistry |volume=98 |issue=6 |pages=1866–75 |pmid=16882311}}</ref><ref>{{cite journal |doi=10.1212/01.wnl.0000225050.57553.6d |laysummary=http://www.nutraingredients.com/Research/More-vitamin-B6-linked-to-lower-Parkinson-s-risk |laysource=NutraIngredients |laydate=August 2, 2006 |title=Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease |year=2006 |last1=De Lau |first1=L.M.L. |last2=Koudstaal |first2=P. J. |last3=Witteman |first3=J. C.M. |last4=Hofman |first4=A. |last5=Breteler |first5=M. M.B. |journal=Neurology |volume=67 |issue=2 |pages=315–8 |pmid=16864826}}</ref><ref>{{cite journal |doi=10.1002/ana.21203 |laysummary=http://www.reuters.com/article/2007/10/24/us-parkinsons-nicotine-idUSN2431402020071024 |laysource=Reuters |laydate=October 24, 2007 |title=Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys |year=2007 |last1=Quik |first1=Maryka |last2=Cox |first2=Heather |last3=Parameswaran |first3=Neeraja |last4=O'Leary |first4=Kathryn |last5=Langston |first5=J. William |last6=Di Monte |first6=Donato |journal=Annals of Neurology |volume=62 |issue=6 |pages=588–96 |pmid=17960553}}</ref> A study has shown a protective effect of nicotine itself on neurons due to nicotine activation of α7-nAChR and the PI3K/Akt pathway which inhibits [[apoptosis-inducing factor]] release and mitochondrial translocation, [[cytochrome c]] release and [[caspase 3]] activation.<ref>{{cite journal |author=Yu W, Mechawar N, Krantic S, Quirion R |title=α7 Nicotinic receptor activation reduces β-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling |journal=J. Neurochem. |volume=119 |issue=4 |pages=848–58 |date=November 2011 |pmid=21884524 |doi=10.1111/j.1471-4159.2011.07466.x }}</ref> |
|||
Studies have indicated that nicotine can be used to help adults suffering from [[autosomal dominant nocturnal frontal lobe epilepsy]]. The same areas that cause seizures in that form of [[epilepsy]] are responsible for processing nicotine in the brain.<ref>{{cite journal |doi=10.1046/j.1528-1157.2003.11903.x |title=Nicotine as an Antiepileptic Agent in ADNFLE: An N-of-One Study |year=2003 |last1=Willoughby |first1=John O. |last2=Pope |first2=Kenneth J. |last3=Eaton |first3=Vaughn |journal=Epilepsia |volume=44 |issue=10 |pages=1363}}</ref> |
|||
Studies suggest a correlation between smoking and [[schizophrenia]], with estimates near 75% for the proportion of schizophrenic patients who smoke. Although the nature of this association remains unclear, it has been argued that the increased level of smoking in schizophrenia may be due to a desire to [[self-medication|self-medicate]] with nicotine.<ref>{{cite journal |author=de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ|title=Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital |journal=Schizophr Res. |volume=56 |issue=1–2|pages=55–65 |date=Jul 2002 |pmid=12084420 |doi=10.1016/S0920-9964(01)00192-X}}</ref><ref>{{cite journal |author=de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM|title=Schizophrenia and smoking: an epidemiological survey in a state hospital |journal=Am J Psychiatry |volume=152 |issue=3 |pages=453–5|date=Mar 1995 |pmid=7864277 |url=http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=7864277}}</ref> Other research found that mildly dependent users got some benefit from nicotine, but not those who were highly dependent.<ref>{{cite journal |author=Aguilar MC, Gurpegui M, Diaz FJ, de Leon J |title=Nicotine dependence and symptoms in schizophrenia: naturalistic study of complex interactions |journal=Br J Psychiatry|volume=186 |issue= 3|pages=215–21 |date=Mar 2005 |pmid=15738502 |doi=10.1192/bjp.186.3.215 }}</ref> |
|||
Research at [[Duke University]] Medical Center found that nicotine may improve the symptoms of depression.<ref>{{cite journal |doi=10.1007/s00213-006-0516-y |laysummary=http://www.dukehealth.org/health_library/news/9863 |laysource=Duke Medicine News and Communications |laydate=September 12, 2006 |title=Transdermal nicotine attenuates depression symptoms in nonsmokers: A double-blind, placebo-controlled trial |year=2006 |last1=McClernon |first1=F. Joseph |last2=Hiott |first2=F. Berry |last3=Westman |first3=Eric C. |last4=Rose |first4=Jed E. |last5=Levin |first5=Edward D. |journal=Psychopharmacology |volume=189 |pages=125–33 |pmid=16977477 |issue=1}}</ref> |
|||
Nicotine appears to improve [[ADHD]] symptoms. Some studies have focused on benefits of nicotine therapy in adults with ADHD.<ref>{{cite web|url=http://adam.about.com/reports/000030_1.htm|title=Attention-Deficit Hyperactivity Disorder|accessdate=21 September 2009}}</ref> |
|||
While acute/initial nicotine intake causes activation of nicotine receptors, chronic low doses of nicotine use leads to desensitisation of nicotine receptors (due to the development of tolerance) and results in an antidepressant effect, with research showing low dose nicotine patches being an effective treatment of [[major depressive disorder]] in non-smokers.<ref name="pmid20965579">{{cite journal |author=Mineur YS, Picciotto MR |title=Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis |journal=Trends Pharmacol. Sci. |volume=31|issue=12 |pages=580–6 |date=December 2010 |pmid=20965579 |pmc=2991594 |doi=10.1016/j.tips.2010.09.004 }}</ref> |
|||
Nicotine (in the form of chewing gum or a transdermal patch) has been explored as an experimental treatment for [[OCD]]. Small studies show some success, even in otherwise treatment-refractory cases.<ref name="pmid15610960">{{cite journal |author=Pasquini M, Garavini A, Biondi M|title=Nicotine augmentation for refractory obsessive-compulsive disorder. A case report |journal=Prog. Neuropsychopharmacol. Biol. Psychiatry|volume=29 |issue=1 |pages=157–9 |date=January 2005 |pmid=15610960 |doi=10.1016/j.pnpbp.2004.08.011 }}</ref><ref name="pmid15610934">{{cite journal |author=Lundberg S, Carlsson A, Norfeldt P, Carlsson ML |title=Nicotine treatment of obsessive-compulsive disorder |journal=Prog. Neuropsychopharmacol. Biol. Psychiatry |volume=28 |issue=7 |pages=1195–9 |date=November 2004 |pmid=15610934|doi=10.1016/j.pnpbp.2004.06.014 }}</ref><ref name="pmid11822995">{{cite journal |author=Tizabi Y, Louis VA, Taylor CT, Waxman D, Culver KE, Szechtman H |title=Effect of nicotine on quinpirole-induced checking behavior in rats: implications for obsessive-compulsive disorder|journal=Biol. Psychiatry |volume=51 |issue=2 |pages=164–71 |date=January 2002 |pmid=11822995 |doi= 10.1016/S0006-3223(01)01207-0}}</ref> |
|||
The relationship between smoking and inflammatory bowel disease has been firmly established, but remains a source of confusion among both patients and doctors. It is negatively associated with ulcerative colitis but positively associated with Crohn's disease. In addition, it has opposite influences on the clinical course of the two conditions with benefit in ulcerative colitis but a detrimental effect in Crohn's disease.<ref>{{cite journal |author=Thomas GA, Rhodes J, Green JT, Richardson C |title=Role of smoking in inflammatory bowel disease: implications for therapy|journal=Postgrad Med J |volume=76 |issue=895 |pages=273–9 |date=May 2000 |pmid=10775279 |pmc=1741576 |doi=10.1136/pmj.76.895.273}}</ref><ref>{{cite journal |author=Rubin DT, Hanauer SB|title=Smoking and inflammatory bowel disease |journal=Eur J Gastroenterol Hepatol |volume=12 |issue=8 |pages=855–62 |date=August 2000|pmid=10958212 |doi=10.1097/00042737-200012080-00004 }}</ref> |
|||
==Side effects== |
|||
[[File:Side effects of nicotine.svg|thumb|280px|Possible side effects of nicotine.{{Citation needed|date=September 2013}}]] |
|||
Nicotine increases blood pressure and heart rate in humans.<ref name="pmid10976548">{{cite journal | author = Sabha M, Tanus-Santos JE, Toledo JC, Cittadino M, Rocha JC, Moreno H | title = Transdermal nicotine mimics the smoking-induced endothelial dysfunction | journal = Clin. Pharmacol. Ther. | volume = 68 | issue = 2 | pages = 167–74 |date=August 2000 | pmid = 10976548 | doi = 10.1067/mcp.2000.108851 }}</ref> Nicotine can also induce potentially atherogenic genes in human coronary artery endothelial cells.<ref name="pmid11166759">{{cite journal | author = Zhang S, Day I, Ye S | title = Nicotine induced changes in gene expression by human coronary artery endothelial cells | journal = Atherosclerosis | volume = 154 | issue = 2 | pages = 277–83 |date=February 2001 | pmid = 11166759 | doi =10.1016/S0021-9150(00)00475-5 }}</ref> Microvascular injury can result through its action on nicotinic acetylcholine receptors (nAChRs).<ref name="pmid11830264">{{cite journal | author = Hawkins BT, Brown RC, Davis TP | title = Smoking and ischemic stroke: a role for nicotine? | journal = Trends Pharmacol. Sci. | volume = 23 | issue = 2 | pages = 78–82 |date=February 2002 | pmid = 11830264 | doi = 10.1016/S0165-6147(02)01893-X }}</ref> |
|||
A study on rats showed that nicotine exposure abolishes the beneficial and protective effects of estrogen on the hippocampus,<ref name="pmid19442878">{{cite journal | author = Raval AP, Bhatt A, Saul I | title = Chronic nicotine exposure inhibits 17beta-estradiol-mediated protection of the hippocampal CA1 region against cerebral ischemia in female rats | journal = Neurosci. Lett. | volume = 458 | issue = 2 | pages = 65–9 |date=July 2009 | pmid = 19442878 | doi = 10.1016/j.neulet.2009.04.021 }}</ref> an estrogen-sensitive region of the brain involved in memory formation and retention. |
|||
==Dependence and withdrawal== |
|||
{{See also|Smoking cessation}} |
|||
Modern [[research]] shows that nicotine acts on the brain to produce a number of effects. Specifically, research examining its addictive nature has been found to show that nicotine activates the [[mesolimbic pathway]] ("reward system") – the circuitry within the brain that regulates feelings of pleasure and euphoria.<ref>{{cite book |author=National Institute on Drug Abuse |chapter=Extent, Impact, Delivery, and Addictiveness|chapterurl=http://www.drugabuse.gov/publications/research-reports/tobacco-addiction/what-are-extent-impact-tobacco-use |title=Tobacco Addiction|publisher=National Institutes of Health |location=Bethesda MA |date=June 2009 |series=NIDA Report Research Series |id=09-4342|url=http://www.drugabuse.gov/publications/research-reports/tobacco-addiction}}</ref> |
|||
[[Dopamine]] is one of the key [[neurotransmitters]] actively involved in the brain. Research shows that by increasing the levels of dopamine within the reward circuits in the brain, nicotine acts as a chemical with intense addictive qualities. In many studies it has been shown to be as addictive as [[cocaine]] and [[heroin]] when used in the form of tobacco.<ref>{{cite news|url=http://www.nytimes.com/1994/08/02/science/is-nicotine-addictive-it-depends-on-whose-criteria-you-use.html | work=The New York Times |first=Philip J. | last=Hilts | title=Is Nicotine Addictive? It Depends on Whose Criteria You Use | date=1994-08-02}}</ref><ref>{{cite news|url=http://www.nytimes.com/1987/03/29/magazine/nicotine-harder-to-kickthan-heroin.html | work=The New York Times | first=Sandra | last=Blakeslee |title=Nicotine: Harder to Kick...Than Heroin | date=1987-03-29}}</ref><ref>{{cite web|url=http://www1.umn.edu/perio/tobacco/nicaddct.html|title=Division of Periodontology: Tobacco Use Cessation Program |publisher=.umn.edu |date= |accessdate=2012-12-19}}</ref> Like other physically addictive drugs,{{citation needed|date=November 2013}} [[nicotine withdrawal]] causes [[downregulation]] of the production of dopamine and other stimulatory neurotransmitters as the brain attempts to compensate for artificial stimulation. As dopamine regulates the sensitivity of nicotinic [[acetylcholine]] receptors decreases. To compensate for this compensatory mechanism, the brain in turn upregulates the number of receptors, [[convoluting]] its regulatory effects with compensatory mechanisms meant to counteract other compensatory mechanisms. An example is the increase in [[norepinephrine]], one of the successors to dopamine, which inhibit reuptake of the [[glutamate receptors]],<ref name="pmid6493048">{{cite journal | author = Yoshida T, Nishioka H, Nakamura Y, Kondo M | title = Reduced norepinephrine turnover in mice with monosodium glutamate-induced obesity | journal = Metab. Clin. Exp. |volume = 33 | issue = 11 | pages = 1060–3 |date=November 1984 | pmid = 6493048 | doi = 10.1016/0026-0495(84)90238-5 }}</ref> in charge of memory and cognition. The net effect is an increase in reward pathway sensitivity, the opposite of other addictive drugs such as cocaine and heroin, which reduce reward pathway sensitivity.<ref name=Kenny/> This alteration in neuronal chemistry can persist for months following the last administration. |
|||
===Immunology prevention=== |
|||
[[File:Nicotin 8338.JPG|thumb|A model of a nicotine molecule]] |
|||
Because of the severe addictions and the harmful effects of smoking, vaccination protocols have been developed. The principle operates under the premise that if an antibody is attached to a nicotine molecule, it will be prevented from diffusing through the [[capillaries]], thus making it less likely that it ever affects the brain by binding to [[nicotinic acetylcholine receptors]]. |
|||
These include attaching the nicotine molecule as a [[hapten]] to a protein carrier such as [[keyhole limpet hemocyanin]] or a safe modified bacterial toxin to elicit an active immune response. Often it is added with [[bovine serum albumin]]. |
|||
Additionally, because of concerns with the unique immune systems of individuals being liable to produce antibodies against endogenous hormones and over-the-counter drugs, [[monoclonal antibodies]] have been developed for short term passive immune protection. They have half-lives varying from hours to weeks. Their half-lives depend on their ability to resist degradation from [[pinocytosis]] by [[epithelial cells]].<ref name="pmid19592672">{{cite journal | author = Peterson EC, Owens SM | title = Designing immunotherapies to thwart drug abuse | journal = Mol. Interv. | volume = 9 | issue = 3 | pages = 119–24 |date=June 2009 | pmid = 19592672 | pmc = 2743871 | doi = 10.1124/mi.9.3.5 }}</ref> |
|||
==Toxicology== |
|||
{{See also|Nicotine poisoning}} |
|||
{| class="wikitable" style="float:right; margin:0 0 0 1em;" |
|||
|- |
|||
! style="background:#f90;"|[[NFPA 704]] |
|||
|- |
|||
| style="text-align:left;"|{{NFPA 704|Health = 4|Flammability = 1|Reactivity = 0|}} |
|||
|} |
|||
The {{LD50}} of nicotine is 50 mg/kg for [[rat]]s and 3 mg/kg for [[mouse|mice]]. 30–60 mg (0.5–1.0 mg/kg) can be a lethal dosage for adult humans.<ref name=inchem /><ref>{{cite journal |author=Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T |title=Effects of aging on acute toxicity of nicotine in rats |journal=Pharmacol Toxicol. |volume=75 |issue=1 |pages=1–6 |date=Jul 1994 |pmid=7971729|doi=10.1111/j.1600-0773.1994.tb00316.x}}</ref> However the widely used human LD<sub>50</sub> estimate of 0.5–1.0 mg/kg was questioned in a 2013 review, in light of several documented cases of humans surviving much higher doses; the 2013 review suggests that the lower limit causing fatal outcomes is 500–1000 mg of ingested nicotine, corresponding to an |
|||
oral LD50 of 6.5–13 mg/kg .<ref name=MayerNewLethalDose2013>{{cite journal | author = Mayer B | title = How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century | journal = Arch. Toxicol. | volume = | issue = | pages = |date=October 2013 | pmid = 24091634 | doi = 10.1007/s00204-013-1127-0 }}</ref> Nevertheless nicotine has a relatively high [[toxicity]] in comparison to many other alkaloids such as [[cocaine]], which has an LD<sub>50</sub>of 95.1 mg/kg when administered to mice. It is unlikely that a person would overdose on nicotine through smoking alone, although overdose can occur through combined use of nicotine patches or nicotine gum and cigarettes at the same time.<ref name=overdose />{{verify credibility|date=December 2013}} Spilling a high concentration of nicotine onto the skin can cause intoxication or even death, since nicotine readily passes into the bloodstream following dermal contact.<ref>{{cite journal |author=Lockhart LP |title=Nicotine poisoning |journal=Br Med J |volume=1 |issue= 3762|pages=246–7 |year=1933|doi=10.1136/bmj.1.3762.246-c}}</ref> |
|||
Historically, nicotine has not been regarded as a [[carcinogen]].{{Citation needed|date=February 2014}} The [[International Agency for Research on Cancer|IARC]] has not evaluated nicotine in its standalone form or assigned it to an official carcinogen group. While no epidemiological evidence supports that nicotine alone acts as a carcinogen in the formation of human cancer, research over the last decade has identified nicotine's [[carcinogenic]] potential in animal models and cell culture.<ref>{{cite journal |author=Hecht SS |title=Tobacco smoke carcinogens and lung cancer |journal=J. Natl. Cancer Inst. |volume=91 |issue=14 |pages=1194–210 |date=July 1999 |pmid=10413421 |doi=10.1093/jnci/91.14.1194}}</ref><ref>{{cite journal |author=Wu WK, Cho CH |title=The pharmacological actions of nicotine on the gastrointestinal tract |journal=J. Pharmacol. Sci. |volume=94 |issue=4 |pages=348–58 |date=April 2004 |pmid=15107574 |doi=10.1254/jphs.94.348}}</ref> Nicotine has been noted to directly cause cancer through a number of different mechanisms such as the activation of [[MAP Kinases]].<ref>{{cite journal |author=Chowdhury P, Udupa KB|title=Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation |journal=World J. Gastroenterol. |volume=12 |issue=46|pages=7428–32 |date=December 2006 |pmid=17167829 |url=http://www.wjgnet.com/1007-9327/full/v12/i46/7428.htm}}</ref> Indirectly, nicotine increases [[Nicotinic acetylcholine receptor|cholinergic]] signalling (and [[adrenergic receptor|adrenergic]] signalling in the case of colon cancer<ref>{{cite journal |author=Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH |title=Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation |journal=Toxicol. Sci. |volume=97 |issue=2 |pages=279–87 |date=June 2007 |pmid=17369603 |doi=10.1093/toxsci/kfm060}}</ref>), thereby impeding apoptosis ([[programmed cell death]]), promoting tumor growth, and activating [[growth factors]] and cellular [[mitogenic]] factors such as [[5-Lipoxygenase|5-LOX]], and [[Epidermal growth factor|EGF]]. Nicotine also promotes cancer growth by stimulating [[angiogenesis]] and [[neovascularization]].<ref>{{cite journal |author=Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M |title=Nicotine enhances neovascularization and promotes tumor growth |journal=Mol. Cells|volume=16 |issue=2 |pages=143–6 |date=October 2003 |pmid=14651253 }}</ref><ref>{{cite journal |author=Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH |title=Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway|journal=J. Pharmacol. Exp. Ther. |volume=308 |issue=1 |pages=66–72 |date=January 2004 |pmid=14569062 |doi=10.1124/jpet.103.058321}}</ref> In one study, nicotine administered to mice with tumors caused increases in tumor size (twofold increase), [[metastasis]] (nine-fold increase), and tumor recurrence (threefold increase).<ref name="plosone.org">{{cite journal |author=Davis R, Rizwani W, Banerjee S, ''et al.'' |title=Nicotine promotes tumor growth and metastasis in mouse models of lung cancer |journal=PLoS ONE |volume=4 |issue=10 |pages=e7524 |year=2009 |pmid=19841737 |pmc=2759510 |doi=10.1371/journal.pone.0007524 |editor1-last=Pao |editor1-first=William|bibcode = 2009PLoSO...4.7524D }}</ref> [[N-Nitrosonornicotine|''N''-Nitrosonornicotine]] (NNN), classified by the IARC as a Group 1 carcinogen, is produced endogenously from nitrite in saliva and nicotine. |
|||
The [[teratogenesis|teratogenic]] properties of nicotine have been investigated. According to a study of about 77,000 pregnant women in Denmark,{{citation needed|date=March 2013}} women who used [[nicotine gum]] and patches during the early stages of pregnancy were found to face an increased risk of having babies with birth defects. The study showed that women who used nicotine-replacement therapy in the first 12 weeks of pregnancy had a 60% greater risk of having babies with birth defects compared to women who were non-smokers.{{citation needed|date=December 2013}} |
|||
Tobacco use among pregnant women has also been correlated to increased frequency of ADHD. Children born to mothers who used tobacco were two and a half times more likely to be diagnosed with ADHD.<ref>{{cite journal |author=Rauch, Stephen and Lanphear, Bruce |title=Prevention of Disability in Children:Elevating the Role of Environment |journal= Future of Children |volume=22 |issue=1 |year=2012|url=http://www.futureofchildren.org/futureofchildren/publications/docs/22_01_09.pdf |doi=10.1353/foc.2012.0006 |pages=193–217 |pmid=22550691}}</ref> Froelich estimated that "exposure to higher levels of lead and prenatal tobacco each accounted for 500,000 additional cases of ADHD in U.S. children".<ref>{{cite journal |doi=10.1542/peds.2009-0738 |title=Association of Tobacco and Lead Exposures with Attention-Deficit/Hyperactivity Disorder |year=2009 |last1=Froehlich |first1=T. E. |last2=Lanphear |first2=B. P. |last3=Auinger |first3=P. |last4=Hornung |first4=R. |last5=Epstein |first5=J. N. |last6=Braun |first6=J. |last7=Kahn |first7=R. S. |journal=Pediatrics |volume=124 |issue=6 |pages=e1054–63 |pmid=19933729 |pmc=2853804}}</ref> |
|||
Effective April 1, 1990, the Office of Environmental Health Hazard Assessment (OEHHA) of the [[California Environmental Protection Agency]] added nicotine to the list of chemicals known to cause developmental toxicity.<ref>http://oehha.ca.gov/prop65/prop65_list/files/P65single121809.pdf{{full|date=December 2013}}</ref> |
|||
==Chemistry== |
|||
Nicotine is a [[hygroscopy|hygroscopic]], oily liquid that is [[miscible]] with [[water (molecule)|water]] in its [[base (chemistry)|base]] form. As a [[nitrogenous base]], nicotine forms [[salt]]s with [[acid]]s that are usually solid and water soluble. Its [[flash point]] is 95°C and its auto-ignition temperature is 244°C.<ref name=SLMSDS>[http://www.sciencelab.com/msds.php?msdsId=9926222 www.sciencelab.com/msds.php?msdsId=9926222] Material Safety Data Sheet |
|||
L-Nicotine MSDS</ref> |
|||
==Optical activity== |
|||
Nicotine is [[optically active]], having two [[enantiomer]]ic forms. The naturally occurring form of nicotine is [[levorotatory]] with a [[specific rotation]] of [α]<sub>D</sub> = –166.4° ((−)-nicotine). The [[dextrorotatory]] form, (+)-nicotine is physiologically less active than (–)-nicotine. (−)-nicotine is more toxic than (+)-nicotine.<ref>{{cite book|last=Gause|first=G. F.|title=Optical Activity and Living Matter|url=http://www.archive.org/stream/opticalactivityl00gauz/opticalactivityl00gauz_djvu.txt|editor=Luyet, B. J.|publisher=Biodynamica|location= Normandy, Missouri |year=1941|chapter=Chapter V: Analysis of various biological processes by the study of the differential action of optical isomers|volume=2|series= A series of monographs on general physiology}}</ref> The salts of (+)-nicotine are usually dextrorotatory. |
|||
==Biosynthesis== |
|||
[[File:Nicotine biosynthesis june 2012.png|thumb|300px|Nicotine biosynthesis]] |
|||
The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that compose nicotine. Metabolic studies show that the [[pyridine]] ring of nicotine is derived from [[niacin]] (nicotinic acid) while the pyrrolidone is derived from N-methyl-Δ<sup>1</sup>-pyrrollidium cation.<ref>{{cite journal |doi=10.1016/0006-3002(59)90492-5 |title=Ornithine as a precursor for the pyrrolidine ring of nicotine |year=1959 |last1=Lamberts |first1=Burton L. |last2=Dewey |first2=Lovell J. |last3=Byerrum |first3=Richard U. |journal=Biochimica et Biophysica Acta |volume=33 |pages=22–6 |pmid=13651178 |issue=1}}</ref><ref>{{cite journal |doi=10.1021/ja01495a059 |title=The Biosynthesis of Nicotine from Isotopically Labeled Nicotinic Acids1 |year=1960 |last1=Dawson |first1=R. F. |last2=Christman |first2=D. R. |last3=d'Adamo |first3=A. |last4=Solt |first4=M. L. |last5=Wolf |first5=A. P. |journal=Journal of the American Chemical Society |volume=82 |issue=10 |pages=2628}}</ref> Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for niacin and the tropane pathway for N-methyl-Δ<sup>1</sup>-pyrrollidium cation. |
|||
The NAD pathway in the genus ''[[nicotiana]]'' begins with the oxidation of aspartic acid into α-imino succinate by aspartate oxidase (AO). This is followed by a condensation with [[glyceraldehyde-3-phosphate]] and a cyclization catalyzed by quinolinate synthase (QS) to give [[quinolinic acid]]. Quinolinic acid then reacts with phosphoriboxyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form niacin mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce niacin via the conversion of [[nicotinamide]] by the enzyme [[nicotinamidase]].{{citation needed|date=December 2013}} |
|||
The N-methyl-Δ<sup>1</sup>-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with [[decarboxylation]] of [[ornithine]] by ornithine decarboxylase (ODC) to produce [[putrescine]]. Putrescine is then converted into N-methyl putrescine via [[methylation]] by SAM catalyzed by putrescine N-methyltransferase (PMT). N-methylputrescine then undergoes [[deamination]] into 4-methylaminobutanal by the N-methylputrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into N-methyl-Δ<sup>1</sup>-pyrrollidium cation.{{citation needed|date=December 2013}} |
|||
The final step in the synthesis of nicotine is the coupling between N-methyl-Δ<sup>1</sup>-pyrrollidium cation and niacin. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of niacin into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with N-methyl-Δ<sup>1</sup>-pyrrollidium cation to form [[enantiomer]]ically pure (–)-nicotine.<ref name=plant-meta>{{cite book |editor1-first=Hiroshi |editor1-last=Ashihara |editor2-first=Alan |editor2-last=Crozier |editor3-first=Atsushi |editor3-last=Komamine |title=Plant metabolism and biotechnology |publisher=Wiley |location=Cambridge |isbn=978-0-470-74703-2}}{{page needed|date=December 2013}}</ref> |
|||
==Pharmacology== |
|||
===Pharmacokinetics=== |
|||
As nicotine enters the body, it is distributed quickly through the [[blood]]stream and crosses the [[blood–brain barrier]] reaching the [[human brain|brain]] within 10–20 seconds after inhalation.<ref name="pmid12971663">{{cite journal | author = Le Houezec J | title = Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review | journal = Int. J. Tuberc. Lung Dis. | volume = 7 | issue = 9 | pages = 811–9 |date=September 2003 | pmid = 12971663 | doi = }}</ref> The [[elimination half-life]] of nicotine in the body is around two hours.<ref name="pmid7077531">{{cite journal | author = Benowitz NL, Jacob P, Jones RT, Rosenberg J | title = Interindividual variability in the metabolism and cardiovascular effects of nicotine in man | journal = J. Pharmacol. Exp. Ther. | volume = 221 | issue = 2 | pages = 368–72 |date=May 1982 | pmid = 7077531 | doi = }}</ref> |
|||
The amount of nicotine absorbed by the body from smoking can depend on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. However, it has been found that the nicotine yield of individual products has only a small effect (4.4%) on the blood concentration of nicotine,<ref>Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br Med J. 1980 April 5; 280(6219): 972–976.</ref> suggesting "the assumed health advantage of switching to lower-tar and lower-nicotine cigarettes may be largely offset by the tendency of smokers to compensate by increasing inhalation". |
|||
Nicotine is [[metabolized]] in the [[liver]] by [[cytochrome P450]] enzymes (mostly [[CYP2A6]], and also by [[CYP2B6]]). A major metabolite is [[cotinine]]. Other primary metabolites include nicotine ''N'''-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide.<ref name="pmid15734728">{{cite journal | author = Hukkanen J, Jacob P, Benowitz NL | title = Metabolism and disposition kinetics of nicotine | journal = Pharmacol. Rev. | volume = 57 | issue = 1 | pages = 79–115 |date=March 2005 | pmid = 15734728 | doi = 10.1124/pr.57.1.3 }}</ref> Under some conditions, other substances may be formed such as [[myosmine]].<ref>{{cite journal |title=The danger of third-hand smoke |journal=Chromatography Online |volume=7 |issue=3 |date=22 February 2011 |url=http://chromatographyonline.findanalytichem.com/lcgc/News/The-danger-of-third-hand-smoke/ArticleStandard/Article/detail/713385}}</ref> |
|||
[[Glucuronidation]] and oxidative metabolism of nicotine to cotinine are both inhibited by [[menthol]], an additive to [[menthol cigarettes|mentholated cigarettes]], thus increasing the half-life of nicotine ''in vivo''.<ref>{{cite journal |doi=10.1124/jpet.104.066902 |title=Mentholated Cigarette Smoking Inhibits Nicotine Metabolism |year=2004 |last1=Benowitz |first1=N. L. |journal=Journal of Pharmacology and Experimental Therapeutics |volume=310 |issue=3 |pages=1208–15 |pmid=15084646 |last2=Herrera |first2=B |last3=Jacob p |first3=3rd}}</ref> |
|||
===Detection of use=== |
|||
====Medical detection==== |
|||
Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids.<ref>{{cite journal |author=Benowitz NL, Hukkanen J, Jacob P |title=Nicotine Psychopharmacology |pages=29–60 |year=2009 |pmid=19184645 |pmc=2953858 |doi=10.1007/978-3-540-69248-5_2 |series=Handbook of Experimental Pharmacology |isbn=978-3-540-69246-1 |volume=192 |chapter=Nicotine Chemistry, Metabolism, Kinetics and Biomarkers |issue=192 |journal=Handbook of experimental pharmacology }}</ref><ref>{{cite book |first=Randall Clint |last=Baselt |title=Disposition of Toxic Drugs and Chemicals in Man |year=2008 |publisher=Biomedical Publications |isbn=978-0-9626523-7-0 |edition=8th |pages=1103–7}}</ref> Nicotine use is not regulated in competitive sports programs.<ref>{{cite journal |author = Mündel, T. and Jones, D. A.|title = Effect of transdermal nicotine administration on exercise endurance in men|journal = Exp Physiol|year = 2006|volume = 91 |pmid = 16627574 |issue = 4| pages = 705–713 |doi = 10.1113/expphysiol.2006.033373 }}</ref> |
|||
===Pharmacodynamics=== |
|||
Nicotine acts on the [[nicotinic acetylcholine receptor]]s, specifically the [[ganglion type nicotinic receptor]] and one [[Alpha-4 beta-2 nicotinic receptor|CNS nicotinic receptor]]. The former is present in the [[adrenal medulla]] and elsewhere, while the latter is present in the [[central nervous system]] (CNS). In small concentrations, nicotine increases the activity of these receptors. Nicotine also has effects on a variety of other neurotransmitters through less direct mechanisms. |
|||
====In the central nervous system==== |
|||
[[File:NicotineDopaminergic WP1602.png|thumb|right|Effect of nicotine on dopaminergic neurons.]] |
|||
By binding to [[nicotinic acetylcholine receptor]]s, nicotine increases the levels of several [[neurotransmitter]]s – acting as a sort of "volume control". It is thought that increased levels of [[dopamine]] in the [[reward circuit]]s of the [[human brain|brain]] one of the major contributors of the apparent [[euphoria (emotion)|euphoria]] and [[relaxation (psychology)|relaxation]], and addiction caused by nicotine consumption. This release of dopamine induced by nicotine is thought to occur via a cholinergic–dopaminergic link, mediated by a neuropeptide, [[ghrelin]], in the [[ventral tegmentum]].<ref>{{cite journal |doi=10.1016/j.mce.2011.02.017 |title=The role of the central ghrelin system in reward from food and chemical drugs |year=2011 |last1=Dickson |first1=Suzanne L. |last2=Egecioglu |first2=Emil |last3=Landgren |first3=Sara |last4=Skibicka |first4=Karolina P. |last5=Engel |first5=Jörgen A. |last6=Jerlhag |first6=Elisabet |journal=Molecular and Cellular Endocrinology |volume=340 |pages=80–7 |pmid=21354264 |issue=1}}</ref> Nicotine has a higher affinity for [[acetylcholine]] receptors in the brain than those in [[skeletal muscle]], though at toxic doses it can induce contractions and respiratory paralysis.<ref>{{cite book |author=Katzung, Bertram G. |title=Basic and Clinical Pharmacology |publisher=McGraw-Hill Medical |location=New York |year=2006 |pages=99–105 }}</ref> Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.<ref name="pmid19252481">{{cite journal | author = Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA | title = Nicotine binding to brain receptors requires a strong cation-pi interaction | journal = Nature | volume = 458 | issue = 7237 | pages = 534–7 |date=March 2009 | pmid = 19252481 | pmc = 2755585 | doi = 10.1038/nature07768 |bibcode = 2009Natur.458..534X }}</ref> |
|||
Tobacco smoke contains [[anabasine]], [[anatabine]], and [[nornicotine]]. It also contains the [[monoamine oxidase inhibitor]]s [[Harmala alkaloid|harman]] and norharman.<ref name=pmid15582589>{{cite journal |author=Herraiz T, Chaparro C |title=Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors |journal=Biochem. Biophys. Res. Commun. |volume=326 |issue=2 |pages=378–86 |year=2005 |pmid=15582589 |doi=10.1016/j.bbrc.2004.11.033 }}</ref> These [[beta-carboline]] compounds significantly decrease [[MAO]] activity in smokers.<ref name="pmid15582589"/><ref name="pmid9549600">{{cite journal |author=Fowler JS, Volkow ND, Wang GJ, ''et al.'' |title=Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition |journal=J Addict Dis |volume=17 |issue=1 |pages=23–34 |year=1998 |pmid=9549600 |doi= 10.1300/J069v17n01_03 }}</ref> MAO [[enzyme]]s break down [[monoamine|monoaminergic neurotransmitters]] such as [[dopamine]], [[norepinephrine]], and [[serotonin]]. It is thought that the powerful interaction between the MAOIs and the nicotine is responsible for most of the addictive properties of tobacco smoking.<ref name="pmid14592678">{{cite journal |author=Villégier AS, Blanc G, Glowinski J, Tassin JP |title=Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors |journal=Pharmacol. Biochem. Behav. |volume=76 |issue=2 |pages=267–74 |date=September 2003 |pmid=14592678 |doi= 10.1016/S0091-3057(03)00223-5}}</ref> The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats.<ref name="pmid16395299">{{cite journal | author = Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, Tassin JP | title = Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine | journal = Neuropsychopharmacology | volume = 31 | issue = 8 | pages = 1704–13 |date=August 2006 | pmid = 16395299 | doi = 10.1038/sj.npp.1300987 }}</ref> |
|||
Chronic nicotine exposure via tobacco smoking [[up-regulation|up-regulates]] [[alpha-4 beta-2 nicotinic receptor|alpha4beta2]]* nAChR in [[cerebellum]] and [[brainstem]] regions<ref name="pmid17997038">{{cite journal | author = Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schütz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J | title = Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain | journal = Neurosci. Lett. | volume = 430 | issue = 1 | pages = 34–7 |date=January 2008 | pmid = 17997038 | doi = 10.1016/j.neulet.2007.10.011 }}</ref><ref name="pmid18174175">{{cite journal |author=Walsh H, Govind AP, Mastro R, ''et al.'' |title=Up-regulation of nicotinic receptors by nicotine varies with receptor subtype |journal=J. Biol. Chem. |volume=283 |issue=10 |pages=6022–32 |year=2008 |pmid=18174175 |doi=10.1074/jbc.M703432200 }}</ref> but not [[habenula|habenulopeduncular]] structures.<ref name="pmid14560040">{{cite journal |author=Nguyen HN, Rasmussen BA, Perry DC |title=Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography |journal=J. Pharmacol. Exp. Ther. |volume=307 |issue=3 |pages=1090–7 |year=2003 |pmid=14560040 |doi=10.1124/jpet.103.056408 }}</ref> Alpha4beta2 and alpha6beta2 receptors, present in the [[ventral tegmental area]], play a crucial role in mediating the reinforcement effects of nicotine.<ref name="pmid19020025">{{cite journal |author=Pons S, Fattore L, Cossu G, ''et al.'' |title=Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration |journal=J. Neurosci. |volume=28 |issue=47 |pages=12318–27 |date=November 2008 |pmid=19020025 |doi=10.1523/JNEUROSCI.3918-08.2008 |pmc=2819191}}</ref> |
|||
====In the sympathetic nervous system==== |
|||
Nicotine also activates the [[sympathetic nervous system]],<ref>{{cite journal |author=Yoshida T, Sakane N, Umekawa T, Kondo M |title=Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress |journal=Physiol. Behav. |volume=55 |issue=1 |pages=53–7 |date=Jan 1994 |pmid=8140174 |doi=10.1016/0031-9384(94)90009-4}}</ref> acting via [[splanchnic nerves]] to the adrenal medulla, stimulates the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and noradrenaline) into the [[bloodstream]]. Nicotine also has an affinity for [[melanin]]-containing tissues due to its precursor function in melanin synthesis or due to the irreversible binding of melanin and nicotine. This has been suggested to underlie the increased [[nicotine dependence]] and lower [[smoking cessation]] rates in darker pigmented individuals. However, further research is warranted before a definite conclusive link can be inferred.<ref>{{cite journal |author=King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET |title=Link between facultative melanin and tobacco use among African Americans |journal=Pharmacol. Biochem. Behav. |volume=92 |issue=4 |pages=589–96 |date=June 2009 |pmid=19268687 |doi=10.1016/j.pbb.2009.02.011}}</ref> |
|||
====In adrenal medulla==== |
|||
[[File:NicotineChromaffinCells WP1603.png|thumb|300px|Effect of nicotine on chromaffin cells.]] |
|||
By binding to [[ganglion type nicotinic receptor]]s in the adrenal medulla nicotine increases flow of [[adrenaline]] (epinephrine), a stimulating [[hormone]] and neurotransmitter. By binding to the receptors, it causes cell depolarization and an influx of [[calcium]] through voltage-gated calcium channels. Calcium triggers the [[exocytosis]] of [[Chromaffin cell|chromaffin granules]] and thus the release of [[epinephrine]] (and norepinephrine) into the [[bloodstream]]. The release of [[epinephrine]] (adrenaline) causes an increase in [[heart rate]], [[blood pressure]] and [[breathing|respiration]], as well as higher [[blood glucose]] levels.<ref name="Marieb" >{{cite book | author = Elaine N. Marieb and Katja Hoehn | title = Human Anatomy & Physiology (7th Ed.) | publisher = Pearson | pages = ? | year = 2007 | isbn = 0-8053-5909-5}}{{page needed|date=December 2013}}</ref> |
|||
Nicotine is the natural product of tobacco, having a half-life of 1 to 2 hours. [[Cotinine]] is an active metabolite of nicotine that remains in the blood for 18 to 20 hours, making it easier to analyze due to its longer half-life.<ref>{{cite journal |last=Bhalala |first=Oneil |title=Detection of Cotinine in Blood Plasma by HPLC MS/MS |journal=MIT Undergraduate Research Journal |volume=8 |date=Spring 2003 |pages=45–50 |url=http://www.docstoc.com/docs/89426297/Detection-of-Cotinine-in-Blood-Plasma-by-HPLC-MS-MS}}</ref> |
|||
==History and name== |
|||
===Name=== |
|||
Nicotine is named after the tobacco plant ''[[Nicotiana tabacum]],'' which in turn is named after the [[France|French]] ambassador in [[Portugal]], [[Jean Nicot|Jean Nicot de Villemain]], who sent tobacco and seeds to [[Paris]] in 1560, and who promoted their medicinal use. The tobacco and its seeds were brought to Ambassador Nicot from [[Brazil]] by [[Luis de Gois]], a Portuguese colonist in [[São Paulo]].{{citation needed|date=December 2013}} |
|||
===Chemical identification=== |
|||
Nicotine was first isolated from the tobacco plant in 1828 by physician Wilhelm Heinrich Posselt and chemist Karl Ludwig Reimann of [[Germany]], who considered it a poison.<ref>{{cite journal |author=Posselt, W.; Reimann, L. |title=Chemische Untersuchung des Tabaks und Darstellung eines eigenthümlich wirksamen Prinzips dieser Pflanze |trans_title=Chemical investigation of tobacco and preparation of a characteristically active constituent of this plant|language=German |journal=Magazin für Pharmacie |volume=6 |issue=24 |pages=138–161 |year=1828 |url=http://books.google.com/books?id=cgkCAAAAYAAJ&pg=RA1-PA138}}</ref><ref name="pmid16463054">{{cite journal | author = Henningfield JE, Zeller M | title = Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward | journal = Psychopharmacology (Berl.) | volume = 184 | issue = 3–4 | pages = 286–91 |date=March 2006 | pmid = 16463054 | doi = 10.1007/s00213-006-0308-4 }}</ref> Its chemical [[empirical formula]] was described by [[Louis Melsens|Melsens]] in 1843,<ref>Melsens, Louis-Henri-Frédéric (1843) [http://books.google.com/books?id=j-E3AAAAMAAJ&pg=PA465#v=onepage&q&f=false "Note sur la nicotine,"] ''Annales de chimie et de physique'', third series, vol. 9, pages 465-479; see especially page 470. [Note: The empirical formula that Melsens provides is incorrect because at that time, chemists used the wrong atomic mass for carbon (6 instead of 12).]</ref> its structure was |
|||
discovered by [[Adolf Pinner]] and [[Richard Wolffenstein (chemist)|Richard Wolffenstein]] in 1893,<ref>{{cite journal |doi=10.1002/cber.189102401242 |title=Ueber Nicotin |year=1891 |last1=Pinner |first1=A. |last2=Wolffenstein |first2=R. |journal=Berichte der deutschen chemischen Gesellschaft |volume=24 |pages=1373}}</ref><ref>{{cite journal |doi=10.1002/cber.18930260165 |title=Ueber Nicotin. Die Constitution des Alkaloïds |year=1893 |last1=Pinner |first1=A. |journal=Berichte der deutschen chemischen Gesellschaft |volume=26 |pages=292}}</ref><ref>{{cite journal |doi=10.1002/ardp.18932310508 |title=Ueber Nicotin. I. Mitteilung |year=1893 |last1=Pinner |first1=A. |journal=Archiv der Pharmazie |volume=231 |issue=5–6 |pages=378}}</ref>{{Clarify|reason=It's not clear that Wolffenstein should be attributed credit for identifying the structure of nicotine. Please see the talk page.|date=March 2013}} and it was first synthesized by Amé Pictet and A. Rotschy in 1904.<ref>{{cite journal |doi=10.1002/cber.19040370206 |title=Synthese des Nicotins |year=1904 |last1=Pictet |first1=Amé |last2=Rotschy |first2=A. |journal=Berichte der deutschen chemischen Gesellschaft |volume=37 |issue=2 |pages=1225}}</ref> |
|||
===As an insecticide=== |
|||
[[Tobacco]] was introduced to [[Europe]] in 1559, and by the late 17th century, it was used not only for [[smoking]] but also as an [[insecticide]]. After [[World War II]], over 2,500 tons of nicotine insecticide (waste from the tobacco industry) were used worldwide, but by the 1980s the use of nicotine insecticide had declined below 200 tons. This was due to the availability of other insecticides that are cheaper and less harmful to [[mammal]]s.<ref name=Ujvary /> |
|||
Currently, nicotine, even in the form of tobacco dust, is prohibited as a [[pesticide]] for [[organic farming]] in the United States.<ref>US Code of Federal Regulations. [http://www.law.cornell.edu/cfr/text/7/205.602 7 CFR 205.602 - Nonsynthetic substances prohibited for use in organic crop production]</ref><ref>Staff, IFOAM. [http://classic.ifoam.org/growing_organic/1_arguments_for_oa/criticisms_misconceptions/misconceptions_no7.html Criticisms and Frequent Misconceptions about Organic Agriculture: The Counter-Arguments: Misconception Number 7]</ref>{{dead link|date=February 2014}} |
|||
In 2008, the [[United States Environmental Protection Agency|EPA]] received a request, from the registrant, to cancel the registration of the last nicotine pesticide registered in the United States.<ref name=epacancel1>{{Cite journal | author = USEPA | title = Nicotine; Notice of Receipt of Request to Voluntarily Cancel a Pesticide Registration | journal = Federal Register | pages = 64320–64322 | date = 29 October 2008 | url = https://federalregister.gov/a/E8-25831| accessdate = 8 April 2012 }}</ref> This request was granted, and since 1 January 2014, this pesticide has not been available for sale.<ref name=epacancel2>{{Cite journal | author = USEPA | title = Nicotine; Product Cancellation Order | journal = Federal Register | pages = 26695–26696 | url =https://federalregister.gov/a/E9-12561 | date = 3 June 2009 | accessdate = 8 April 2012 }}</ref> |
|||
==See also== |
|||
*''[[Nicotiana rustica]]'' |
|||
*''[[Nicotiana tabacum]]'' |
|||
*[[Substance dependence]] |
|||
*[[Tobacco products]] |
|||
==References== |
|||
{{Reflist|35em}} |
|||
==Further reading== |
|||
{{refbegin|30em}} |
|||
*{{Cite journal |author=Bilkei-Gorzo A, Rácz I, Michel K, Darvas M, Rafael Maldonado López, Zimmer A.|title=A common genetic predisposition to stress sensitivity and stress-induced nicotine craving|journal=Biol. Psychiatry |year=2008 |volume=63 |pages= 164–71 |pmid=17570348 |doi=10.1016/j.biopsych.2007.02.010 |issue=2}} |
|||
*{{cite book |
|||
| editor1-last =Gorrod |
|||
| editor1-first =John W. |
|||
| editor2-last =Peyton |
|||
| editor2-first =Jacob,III |
|||
| title =Analytical Determination of Nicotine and Related Compounds and their Metabolites |
|||
| date =November 16, 1999 |
|||
| publisher =Elsevier |
|||
| location =Amsterdam |
|||
| isbn =978-0-08-052551-8 |
|||
}} |
|||
*{{Cite journal |author=Willoughby JO, Pope KJ, Eaton V |title=Nicotine as an antiepileptic agent in ADNFLE: an N-of-one study |journal=Epilepsia |volume=44 |issue=9 |pages=1238–40 |date=Sep 2003 |pmid=12919397 |doi=10.1046/j.1528-1157.2003.11903.x}} |
|||
*{{Cite journal |author=Minna JD |title=Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer |journal=J Clin Invest. |volume=111 |issue=1 |pages=31–3 |date=Jan 2003 |pmid=12511585 |pmc=151841 |doi=10.1172/JCI17492 }} |
|||
*{{Cite journal |author=[[James Fallon|Fallon JH]], Keator DB, Mbogori J, Taylor D, Potkin SG |title=Gender: a major determinant of brain response to nicotine |journal=Int J Neuropsychopharmacol. |volume=8 |issue=1 |pages=17–26 |date=Mar 2005 |pmid=15579215 |doi=10.1017/S1461145704004730}} |
|||
*{{Cite journal |author=West KA, Brognard J, Clark AS, ''et al.'' |title=Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells |journal=J Clin Invest. |volume=111 |issue=1 |pages=81–90 |date=Jan 2003 |pmid=12511591 |pmc=151834 |doi=10.1172/JCI16147 }} |
|||
*[http://www.nida.nih.gov/researchreports/nicotine/nicotine.html National Institute on Drug Abuse] |
|||
*[http://www.erowid.org/plants/tobacco/tobacco.shtml Erowid information on tobacco] |
|||
{{refend}} |
|||
==External links== |
|||
{{commons category|Nicotine}} |
|||
*[http://www.ebi.ac.uk/pdbe-srv/PDBeXplore/ligand/?ligand=NCT Nicotine bound to proteins] in the [[Protein Data Bank|PDB]] |
|||
*[http://www.howstuffworks.com/nicotine.htm Description of nicotine mechanisms] |
|||
*[http://www.erowid.org/chemicals/nicotine/nicotine_data_sheet1.shtml Erowid Nicotine Vault : Nicotine Material Safety Data Sheet] |
|||
*{{cite journal |doi=10.1038/ncpgasthep0316 |title=Mechanisms of Disease: Nicotine—a review of its actions in the context of gastrointestinal disease |year=2005 |last1=Thomas |first1=Gareth AO |last2=Rhodes |first2=John |last3=Ingram |first3=John R |journal=Nature Clinical Practice Gastroenterology & Hepatology |volume=2 |issue=11 |pages=536}} |
|||
*[http://www.cdc.gov/niosh/npg/npgd0446.html CDC - NIOSH Pocket Guide to Chemical Hazards] |
|||
{{Addiction}} |
|||
{{Drug use}} |
|||
{{Stimulants}} |
|||
{{Nootropics}} |
|||
{{Euphoriants}} |
|||
{{Antiaddictives}} |
|||
{{Cholinergics}} |
|||
{{Cigarettes}} |
|||
[[Category:Pyrrolidine alkaloids]] |
|||
[[Category:Neurotoxins]] |
|||
[[Category:Nicotinic agonists]] |
|||
[[Category:Plant toxin insecticides]] |
|||
[[Category:Pyridines]] |
|||
[[Category:Smoking]] |
|||
[[Category:Stimulants]] |
|||
[[Category:Pollinator decline pesticides]] |
|||
[[Category:Alkaloids found in Nicotiana]] |
|||
[[Category:Alkaloids found in Erythroxylum coca]] |
|||
{{Link FA|bs}} |
|||
Revision as of 09:24, 5 March 2014
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nicorette, Nicotrol |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Dependence liability | High |
| Routes of administration | Inhalation; Insufflation; Oral – Buccal, Sublingual, and Ingestion; Transdermal; Rectal, |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20 to 45% (oral), 53% (intranasal), 68% (transdermal) |
| Protein binding | <5% |
| Metabolism | Hepatic |
| Elimination half-life | 1-2 hours; 20 hours active metabolite (cotinine) |
| Excretion | Urine (10-20% (gum), pH-dependent; 30% (inhaled); 10-30% (intranasal)) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.177 |
| Chemical and physical data | |
| Formula | C10H14N2 |
| Molar mass | 162.12 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.01 g/cm3 |
| Melting point | −79 °C (−110 °F) |
| Boiling point | 247 °C (477 °F) |
| |
| |
| | |
Nicotine is a potent parasympathomimetic alkaloid found in the nightshade family of plants (Solanaceae) and a stimulant drug. It is a nicotinic acetylcholine receptor agonist. It is made in the roots and accumulates in the leaves of the plants. It constitutes approximately 0.6–3.0% of the dry weight of tobacco[2] and is present in the range of 2–7 µg/kg of various edible plants.[3] It functions as an antiherbivore chemical; consequently, nicotine was widely used as an insecticide in the past[4][5] and nicotine analogs such as imidacloprid are currently widely used.
In smaller doses (an average cigarette yields about 1 mg of absorbed nicotine), the substance acts as a stimulant in mammals, while high amounts (50–100 mg) can be harmful.[6][7][8] This stimulant effect is likely a major contributing factor to the dependence-forming properties of tobacco smoking, nicotine patches, nicotine gum, nicotine inhalers and liquid nicotine vapourizers.[citation needed] According to the American Heart Association, nicotine addiction has historically been one of the hardest addictions to break, while the pharmacological and behavioral characteristics that determine nicotine addiction are similar to those determining addiction to heroin and cocaine. The nicotine content of popular American-brand cigarettes has slowly increased over the years, and one study found that there was an average increase of 1.78% per year between the years of 1998 and 2005. This was found for all major market categories of cigarettes.[9]
Research in 2011 found that nicotine inhibits chromatin-modifying enzymes (class I and II histone deacetylases); this inhibition has been shown to increase the ability of cocaine to cause an addiction.[10]
Psychoactive effects
Nicotine's mood-altering effects are different by report: in particular it is both a stimulant and a relaxant.[11] First causing a release of glucose from the liver and epinephrine(adrenaline) from the adrenal medulla, it causes stimulation. Users report feelings of relaxation, sharpness, calmness, and alertness.[12] Like any stimulant, it may very rarely cause the often uncomfortable neuropsychiatric effect of akathisia. By reducing the appetite and raising the metabolism, some smokers may lose weight as a consequence.[13][14]
When a cigarette is smoked, nicotine-rich blood passes from the lungs to the brain within seven seconds and immediately stimulates the release of many chemical messengers such as acetylcholine, norepinephrine, epinephrine, arginine vasopressin, serotonin, dopamine, and beta-endorphin.[15] [16] This release of neurotransmitters and hormones is responsible for most of nicotine's psychoactive effects. Nicotine appears to enhance concentration[17] and memory due to the increase of acetylcholine. It also appears to enhance alertness due to the increases of acetylcholine and norepinephrine. Arousal is increased by the increase of norepinephrine. Pain is reduced by the increases of acetylcholine and beta-endorphin. Anxiety is reduced by the increase of beta-endorphin. Nicotine also extends the duration of positive effects of dopamine[18] and increases sensitivity in brain reward systems.[19] Most cigarettes (in the smoke inhaled) contain 1 to 3 milligrams of nicotine.[20]
Research suggests that, when smokers wish to achieve a stimulating effect, they take short quick puffs, which produce a low level of blood nicotine.[21] This stimulates nerve transmission. When they wish to relax, they take deep puffs, which produce a high level of blood nicotine, which depresses the passage of nerve impulses, producing a mild sedative effect. At low doses, nicotine potently enhances the actions of norepinephrine and dopamine in the brain, causing a drug effect typical of those of psychostimulants. At higher doses, nicotine enhances the effect of serotonin and opiate activity, producing a calming, pain-killing effect. Nicotine is unique in comparison to most drugs, as its profile changes from stimulant to sedative/pain killer in increasing dosages and use, a phenomenon described by Paul Nesbitt in his doctoral dissertation[22] and subsequently referred to as "Nesbitt's Paradox."
Medical uses
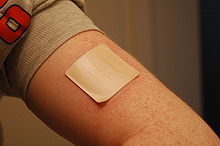
Although population level effectiveness has not been demonstrated,[25][26][27] the primary therapeutic use of nicotine is in treating nicotine dependence in order to eliminate smoking with the damage it does to health. Controlled levels of nicotine are given to patients through gums, dermal patches, lozenges, electronic/substitute cigarettes or nasal sprays in an effort to wean them off their dependence.
However, in a few situations, smoking has been observed to be of therapeutic value. These are often referred to as "Smoker’s Paradoxes".[28] Although in most cases the actual mechanism is understood only poorly or not at all, it is generally believed that the principal beneficial action is due to the nicotine administered, and that administration of nicotine without smoking may be as beneficial as smoking, without the higher risk to health due to tar and other substances found in tobacco.
For instance, studies suggest that smokers require less frequent repeated revascularization after percutaneous coronary intervention(PCI).[28] Risk of ulcerative colitis has been frequently shown to be reduced by smokers on a dose-dependent basis; the effect is eliminated if the individual stops smoking.[29][30] Smoking also appears to interfere with development of Kaposi's sarcoma in patients with HIV.[31]
Nicotine reduces the chance of preeclampsia,[32] and atopic disorders such as allergic asthma.[33][dubious – discuss] A plausible mechanism of action in these cases may be nicotine acting as an anti-inflammatory agent, and interfering with the inflammation-related disease process, as nicotine has vasoconstrictive effects.[34]
Tobacco smoke has been shown to contain compounds capable of inhibiting monoamine oxidase, which is responsible for the degradation of dopamine in the human brain. When dopamine is broken down by MAO-B, neurotoxic by-products are formed, possibly contributing to Parkinson's and Alzheimers disease.[35]
While tobacco smoking is associated with an increased risk of Alzheimer's disease,[36] there is evidence that nicotine itself has the potential to prevent and treat Alzheimer's disease.[37] Nicotine has been shown to delay the onset of Parkinson's disease in studies involving monkeys and humans.[38][39][40] A study has shown a protective effect of nicotine itself on neurons due to nicotine activation of α7-nAChR and the PI3K/Akt pathway which inhibits apoptosis-inducing factor release and mitochondrial translocation, cytochrome c release and caspase 3 activation.[41]
Studies have indicated that nicotine can be used to help adults suffering from autosomal dominant nocturnal frontal lobe epilepsy. The same areas that cause seizures in that form of epilepsy are responsible for processing nicotine in the brain.[42]
Studies suggest a correlation between smoking and schizophrenia, with estimates near 75% for the proportion of schizophrenic patients who smoke. Although the nature of this association remains unclear, it has been argued that the increased level of smoking in schizophrenia may be due to a desire to self-medicate with nicotine.[43][44] Other research found that mildly dependent users got some benefit from nicotine, but not those who were highly dependent.[45]
Research at Duke University Medical Center found that nicotine may improve the symptoms of depression.[46] Nicotine appears to improve ADHD symptoms. Some studies have focused on benefits of nicotine therapy in adults with ADHD.[47]
While acute/initial nicotine intake causes activation of nicotine receptors, chronic low doses of nicotine use leads to desensitisation of nicotine receptors (due to the development of tolerance) and results in an antidepressant effect, with research showing low dose nicotine patches being an effective treatment of major depressive disorder in non-smokers.[48]
Nicotine (in the form of chewing gum or a transdermal patch) has been explored as an experimental treatment for OCD. Small studies show some success, even in otherwise treatment-refractory cases.[49][50][51]
The relationship between smoking and inflammatory bowel disease has been firmly established, but remains a source of confusion among both patients and doctors. It is negatively associated with ulcerative colitis but positively associated with Crohn's disease. In addition, it has opposite influences on the clinical course of the two conditions with benefit in ulcerative colitis but a detrimental effect in Crohn's disease.[52][53]
Side effects
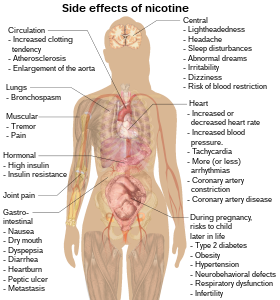
Nicotine increases blood pressure and heart rate in humans.[54] Nicotine can also induce potentially atherogenic genes in human coronary artery endothelial cells.[55] Microvascular injury can result through its action on nicotinic acetylcholine receptors (nAChRs).[56]
A study on rats showed that nicotine exposure abolishes the beneficial and protective effects of estrogen on the hippocampus,[57] an estrogen-sensitive region of the brain involved in memory formation and retention.
Dependence and withdrawal
Modern research shows that nicotine acts on the brain to produce a number of effects. Specifically, research examining its addictive nature has been found to show that nicotine activates the mesolimbic pathway ("reward system") – the circuitry within the brain that regulates feelings of pleasure and euphoria.[58]
Dopamine is one of the key neurotransmitters actively involved in the brain. Research shows that by increasing the levels of dopamine within the reward circuits in the brain, nicotine acts as a chemical with intense addictive qualities. In many studies it has been shown to be as addictive as cocaine and heroin when used in the form of tobacco.[59][60][61] Like other physically addictive drugs,[citation needed] nicotine withdrawal causes downregulation of the production of dopamine and other stimulatory neurotransmitters as the brain attempts to compensate for artificial stimulation. As dopamine regulates the sensitivity of nicotinic acetylcholine receptors decreases. To compensate for this compensatory mechanism, the brain in turn upregulates the number of receptors, convoluting its regulatory effects with compensatory mechanisms meant to counteract other compensatory mechanisms. An example is the increase in norepinephrine, one of the successors to dopamine, which inhibit reuptake of the glutamate receptors,[62] in charge of memory and cognition. The net effect is an increase in reward pathway sensitivity, the opposite of other addictive drugs such as cocaine and heroin, which reduce reward pathway sensitivity.[19] This alteration in neuronal chemistry can persist for months following the last administration.
Immunology prevention

Because of the severe addictions and the harmful effects of smoking, vaccination protocols have been developed. The principle operates under the premise that if an antibody is attached to a nicotine molecule, it will be prevented from diffusing through the capillaries, thus making it less likely that it ever affects the brain by binding to nicotinic acetylcholine receptors.
These include attaching the nicotine molecule as a hapten to a protein carrier such as keyhole limpet hemocyanin or a safe modified bacterial toxin to elicit an active immune response. Often it is added with bovine serum albumin.
Additionally, because of concerns with the unique immune systems of individuals being liable to produce antibodies against endogenous hormones and over-the-counter drugs, monoclonal antibodies have been developed for short term passive immune protection. They have half-lives varying from hours to weeks. Their half-lives depend on their ability to resist degradation from pinocytosis by epithelial cells.[63]
Toxicology
| NFPA 704 | ||||
|---|---|---|---|---|
| ||||
The LD50 of nicotine is 50 mg/kg for rats and 3 mg/kg for mice. 30–60 mg (0.5–1.0 mg/kg) can be a lethal dosage for adult humans.[6][64] However the widely used human LD50 estimate of 0.5–1.0 mg/kg was questioned in a 2013 review, in light of several documented cases of humans surviving much higher doses; the 2013 review suggests that the lower limit causing fatal outcomes is 500–1000 mg of ingested nicotine, corresponding to an oral LD50 of 6.5–13 mg/kg .[8] Nevertheless nicotine has a relatively high toxicity in comparison to many other alkaloids such as cocaine, which has an LD50of 95.1 mg/kg when administered to mice. It is unlikely that a person would overdose on nicotine through smoking alone, although overdose can occur through combined use of nicotine patches or nicotine gum and cigarettes at the same time.[7][unreliable source?] Spilling a high concentration of nicotine onto the skin can cause intoxication or even death, since nicotine readily passes into the bloodstream following dermal contact.[65]
Historically, nicotine has not been regarded as a carcinogen.[citation needed] The IARC has not evaluated nicotine in its standalone form or assigned it to an official carcinogen group. While no epidemiological evidence supports that nicotine alone acts as a carcinogen in the formation of human cancer, research over the last decade has identified nicotine's carcinogenic potential in animal models and cell culture.[66][67] Nicotine has been noted to directly cause cancer through a number of different mechanisms such as the activation of MAP Kinases.[68] Indirectly, nicotine increases cholinergic signalling (and adrenergic signalling in the case of colon cancer[69]), thereby impeding apoptosis (programmed cell death), promoting tumor growth, and activating growth factors and cellular mitogenic factors such as 5-LOX, and EGF. Nicotine also promotes cancer growth by stimulating angiogenesis and neovascularization.[70][71] In one study, nicotine administered to mice with tumors caused increases in tumor size (twofold increase), metastasis (nine-fold increase), and tumor recurrence (threefold increase).[72] N-Nitrosonornicotine (NNN), classified by the IARC as a Group 1 carcinogen, is produced endogenously from nitrite in saliva and nicotine.
The teratogenic properties of nicotine have been investigated. According to a study of about 77,000 pregnant women in Denmark,[citation needed] women who used nicotine gum and patches during the early stages of pregnancy were found to face an increased risk of having babies with birth defects. The study showed that women who used nicotine-replacement therapy in the first 12 weeks of pregnancy had a 60% greater risk of having babies with birth defects compared to women who were non-smokers.[citation needed]
Tobacco use among pregnant women has also been correlated to increased frequency of ADHD. Children born to mothers who used tobacco were two and a half times more likely to be diagnosed with ADHD.[73] Froelich estimated that "exposure to higher levels of lead and prenatal tobacco each accounted for 500,000 additional cases of ADHD in U.S. children".[74]
Effective April 1, 1990, the Office of Environmental Health Hazard Assessment (OEHHA) of the California Environmental Protection Agency added nicotine to the list of chemicals known to cause developmental toxicity.[75]
Chemistry
Nicotine is a hygroscopic, oily liquid that is miscible with water in its base form. As a nitrogenous base, nicotine forms salts with acids that are usually solid and water soluble. Its flash point is 95°C and its auto-ignition temperature is 244°C.[76]
Optical activity
Nicotine is optically active, having two enantiomeric forms. The naturally occurring form of nicotine is levorotatory with a specific rotation of [α]D = –166.4° ((−)-nicotine). The dextrorotatory form, (+)-nicotine is physiologically less active than (–)-nicotine. (−)-nicotine is more toxic than (+)-nicotine.[77] The salts of (+)-nicotine are usually dextrorotatory.
Biosynthesis

The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that compose nicotine. Metabolic studies show that the pyridine ring of nicotine is derived from niacin (nicotinic acid) while the pyrrolidone is derived from N-methyl-Δ1-pyrrollidium cation.[78][79] Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for niacin and the tropane pathway for N-methyl-Δ1-pyrrollidium cation.
The NAD pathway in the genus nicotiana begins with the oxidation of aspartic acid into α-imino succinate by aspartate oxidase (AO). This is followed by a condensation with glyceraldehyde-3-phosphate and a cyclization catalyzed by quinolinate synthase (QS) to give quinolinic acid. Quinolinic acid then reacts with phosphoriboxyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form niacin mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce niacin via the conversion of nicotinamide by the enzyme nicotinamidase.[citation needed]
The N-methyl-Δ1-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine. Putrescine is then converted into N-methyl putrescine via methylation by SAM catalyzed by putrescine N-methyltransferase (PMT). N-methylputrescine then undergoes deamination into 4-methylaminobutanal by the N-methylputrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into N-methyl-Δ1-pyrrollidium cation.[citation needed]
The final step in the synthesis of nicotine is the coupling between N-methyl-Δ1-pyrrollidium cation and niacin. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of niacin into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with N-methyl-Δ1-pyrrollidium cation to form enantiomerically pure (–)-nicotine.[80]
Pharmacology
Pharmacokinetics
As nicotine enters the body, it is distributed quickly through the bloodstream and crosses the blood–brain barrier reaching the brain within 10–20 seconds after inhalation.[81] The elimination half-life of nicotine in the body is around two hours.[82]
The amount of nicotine absorbed by the body from smoking can depend on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. However, it has been found that the nicotine yield of individual products has only a small effect (4.4%) on the blood concentration of nicotine,[83] suggesting "the assumed health advantage of switching to lower-tar and lower-nicotine cigarettes may be largely offset by the tendency of smokers to compensate by increasing inhalation".
Nicotine is metabolized in the liver by cytochrome P450 enzymes (mostly CYP2A6, and also by CYP2B6). A major metabolite is cotinine. Other primary metabolites include nicotine N'-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide.[84] Under some conditions, other substances may be formed such as myosmine.[85]
Glucuronidation and oxidative metabolism of nicotine to cotinine are both inhibited by menthol, an additive to mentholated cigarettes, thus increasing the half-life of nicotine in vivo.[86]
Detection of use
Medical detection
Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids.[87][88] Nicotine use is not regulated in competitive sports programs.[89]
Pharmacodynamics
Nicotine acts on the nicotinic acetylcholine receptors, specifically the ganglion type nicotinic receptor and one CNS nicotinic receptor. The former is present in the adrenal medulla and elsewhere, while the latter is present in the central nervous system (CNS). In small concentrations, nicotine increases the activity of these receptors. Nicotine also has effects on a variety of other neurotransmitters through less direct mechanisms.
In the central nervous system

By binding to nicotinic acetylcholine receptors, nicotine increases the levels of several neurotransmitters – acting as a sort of "volume control". It is thought that increased levels of dopamine in the reward circuits of the brain one of the major contributors of the apparent euphoria and relaxation, and addiction caused by nicotine consumption. This release of dopamine induced by nicotine is thought to occur via a cholinergic–dopaminergic link, mediated by a neuropeptide, ghrelin, in the ventral tegmentum.[90] Nicotine has a higher affinity for acetylcholine receptors in the brain than those in skeletal muscle, though at toxic doses it can induce contractions and respiratory paralysis.[91] Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.[92]
Tobacco smoke contains anabasine, anatabine, and nornicotine. It also contains the monoamine oxidase inhibitors harman and norharman.[93] These beta-carboline compounds significantly decrease MAO activity in smokers.[93][94] MAO enzymes break down monoaminergic neurotransmitters such as dopamine, norepinephrine, and serotonin. It is thought that the powerful interaction between the MAOIs and the nicotine is responsible for most of the addictive properties of tobacco smoking.[95] The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats.[96]
Chronic nicotine exposure via tobacco smoking up-regulates alpha4beta2* nAChR in cerebellum and brainstem regions[97][98] but not habenulopeduncular structures.[99] Alpha4beta2 and alpha6beta2 receptors, present in the ventral tegmental area, play a crucial role in mediating the reinforcement effects of nicotine.[100]
In the sympathetic nervous system
Nicotine also activates the sympathetic nervous system,[101] acting via splanchnic nerves to the adrenal medulla, stimulates the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and noradrenaline) into the bloodstream. Nicotine also has an affinity for melanin-containing tissues due to its precursor function in melanin synthesis or due to the irreversible binding of melanin and nicotine. This has been suggested to underlie the increased nicotine dependence and lower smoking cessation rates in darker pigmented individuals. However, further research is warranted before a definite conclusive link can be inferred.[102]
In adrenal medulla
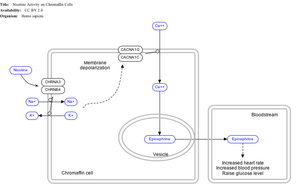
By binding to ganglion type nicotinic receptors in the adrenal medulla nicotine increases flow of adrenaline (epinephrine), a stimulating hormone and neurotransmitter. By binding to the receptors, it causes cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of epinephrine (and norepinephrine) into the bloodstream. The release of epinephrine (adrenaline) causes an increase in heart rate, blood pressure and respiration, as well as higher blood glucose levels.[103]
Nicotine is the natural product of tobacco, having a half-life of 1 to 2 hours. Cotinine is an active metabolite of nicotine that remains in the blood for 18 to 20 hours, making it easier to analyze due to its longer half-life.[104]
History and name
Name
Nicotine is named after the tobacco plant Nicotiana tabacum, which in turn is named after the French ambassador in Portugal, Jean Nicot de Villemain, who sent tobacco and seeds to Paris in 1560, and who promoted their medicinal use. The tobacco and its seeds were brought to Ambassador Nicot from Brazil by Luis de Gois, a Portuguese colonist in São Paulo.[citation needed]
Chemical identification
Nicotine was first isolated from the tobacco plant in 1828 by physician Wilhelm Heinrich Posselt and chemist Karl Ludwig Reimann of Germany, who considered it a poison.[105][106] Its chemical empirical formula was described by Melsens in 1843,[107] its structure was discovered by Adolf Pinner and Richard Wolffenstein in 1893,[108][109][110][clarification needed] and it was first synthesized by Amé Pictet and A. Rotschy in 1904.[111]
As an insecticide
Tobacco was introduced to Europe in 1559, and by the late 17th century, it was used not only for smoking but also as an insecticide. After World War II, over 2,500 tons of nicotine insecticide (waste from the tobacco industry) were used worldwide, but by the 1980s the use of nicotine insecticide had declined below 200 tons. This was due to the availability of other insecticides that are cheaper and less harmful to mammals.[5]
Currently, nicotine, even in the form of tobacco dust, is prohibited as a pesticide for organic farming in the United States.[112][113][dead link]
In 2008, the EPA received a request, from the registrant, to cancel the registration of the last nicotine pesticide registered in the United States.[114] This request was granted, and since 1 January 2014, this pesticide has not been available for sale.[115]
See also
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Smoking and Tobacco Control Monograph No. 9" (PDF). Retrieved 2012-12-19.
- ^ "Determination of the Nicotine Content of Various Edible Nightshades (Solanaceae) and Their Products and Estimation of the Associated Dietary Nicotine Intake". Retrieved 2008-10-05.
- ^ Rodgman, Alan; Perfetti, Thomas A. (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 1-4200-7883-6. LCCN 2008018913.[page needed]
- ^ a b Ujváry, István (1999). "Nicotine and Other Insecticidal Alkaloids". In Yamamoto, Izuru; Casida, John (eds.). Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 29–69.
- ^ a b "Nicotine (PIM)". Inchem.org. Retrieved 2012-12-19.
- ^ a b Genetic Science Learning Center. "How Drugs Can Kill".
- ^ a b Mayer B (October 2013). "How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century". Arch. Toxicol. doi:10.1007/s00204-013-1127-0. PMID 24091634.
- ^ Connolly, G. N; Alpert, H. R; Wayne, G. F; Koh, H (2007). "Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005". Tobacco Control. 16 (5): e5. doi:10.1136/tc.2006.019695. PMC 2598548. PMID 17897974.
- ^ Volkow ND (November 2011). "Epigenetics of nicotine: another nail in the coughing". Sci Transl Med. 3 (107): 107ps43. doi:10.1126/scitranslmed.3003278. PMC 3492949. PMID 22049068.
- ^ "Effective Clinical Tobacco Intervention". Therapeutics Letter (21): 1–4. September–October 1997.
- ^ Lagrue, Gilbert; Cormier, Anne (2001). "Des récepteurs nicotiniques à la dépendance tabagique : Perspectives thérapeutiques". Alcoologie et addictologie (in French). 23 (2): 39S–42S. ISSN 1620-4522. INIST 1081618.
{{cite journal}}: Unknown parameter|month=ignored (help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ Orsini, Jean-Claude (2001). "Dépendance tabagique et contrôle central de la glycémie et de l'appétit". Alcoologie et addictologie (in French). 23 (2 Suppl): 28S–36S. ISSN 1620-4522. INIST 1081638.
{{cite journal}}: Unknown parameter|month=ignored (help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ Chen, Hui; Vlahos, Ross; Bozinovski, Steve; Jones, Jessica; Anderson, Gary P; Morris, Margaret J (2004). "Effect of Short-Term Cigarette Smoke Exposure on Body Weight, Appetite and Brain Neuropeptide Y in Mice". Neuropsychopharmacology. doi:10.1038/sj.npp.1300597.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Pomerleau OF, Pomerleau CS (1984). Neuroregulators and the reinforcement of smoking: Towards a biobehavioral explanation. Neuroscience and Biobehavioral Reviews, 8:503-513.
- ^ Pomerleau OF, Rosecrans J (1989). Neuroregulatory effects of nicotine. Psychoneuroendocrinology 14:407-423.
- ^ Rusted J, Graupner L, O'Connell N, Nicholls C (August 1994). "Does nicotine improve cognitive function?". Psychopharmacology (Berl.). 115 (4): 547–9. doi:10.1007/BF02245580. PMID 7871101.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Easton, John (March 28, 2002). "Nicotine extends duration of pleasant effects of dopamine". The University of Chicago Chronicle. 21 (12).
- ^ a b Kenny PJ, Markou A (Jun 2006). "Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity". Neuropsychopharmacology. 31 (6): 1203–11. doi:10.1038/sj.npp.1300905. PMID 16192981.
- ^ "Erowid Nicotine Vault : Dosage". Erowid.org. 2011-10-14. Retrieved 2012-12-19.
- ^ Golding, J. F.; Mangan, G. L. (1989). "Factors Governing Recruitment to and Maintenance of Smoking". In Einstein, Stanley (ed.). Drug and Alcohol Use. pp. 101–17. doi:10.1007/978-1-4899-0888-9_9. ISBN 978-1-4899-0890-2.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Nesbitt P (1969). Smoking, physiological arousal, and emotional response. Unpublished doctoral dissertation, Columbia University.
- ^ Stead LF, Perera R, Bullen C, Mant D, Lancaster T (2008). Stead, Lindsay F (ed.). "Nicotine replacement therapy for smoking cessation". Cochrane Database Syst Rev (1): CD000146. doi:10.1002/14651858.CD000146.pub3. PMID 18253970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Millstone, Ken (February 13, 2007). "Nixing the patch: Smokers quit cold turkey". Columbia.edu News Service. Retrieved May 23, 2010.
- ^ Doran, Christopher M.; Valenti, Lisa; Robinson, Maxine; Britt, Helena; Mattick, Richard P. (2006). "Smoking status of Australian general practice patients and their attempts to quit". Addictive Behaviors. 31 (5): 758–66. doi:10.1016/j.addbeh.2005.05.054. PMID 16137834.
- ^ Gallup Poll (July 31, 2013), Most U.S. Smokers Want to Quit, Have Tried Multiple Times, Gallup, retrieved December 17, 2013
- ^ Pierce, John P.; Cummins, Sharon E.; White, Martha M.; Humphrey, Aimee; Messer, Karen (2012). "Quitlines and Nicotine Replacement for Smoking Cessation: Do We Need to Change Policy?". Annual Review of Public Health. 33: 341–56. doi:10.1146/annurev-publhealth-031811-124624. PMID 22224888.
- ^ a b Cohen DJ, Doucet M, Cutlip DE, Ho KK, Popma JJ, Kuntz RE (August 2001). "Impact of smoking on clinical and angiographic restenosis after percutaneous coronary intervention: another smoker's paradox?". Circulation. 104 (7): 773–8. doi:10.1161/hc3201.094225. PMID 11502701.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Longmore, M., Wilkinson, I., Torok, E. Oxford Handbook of Clinical Medicine (5th ed.). p. 232.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Green JT, Richardson C, Marshall RW, Rhodes J, McKirdy HC, Thomas GA, Williams GT (November 2000). "Nitric oxide mediates a therapeutic effect of nicotine in ulcerative colitis". Aliment. Pharmacol. Ther. 14 (11): 1429–34. doi:10.1046/j.1365-2036.2000.00847.x. PMID 11069313.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goedert JJ, Vitale F, Lauria C, Serraino D, Tamburini M, Montella M, Messina A, Brown EE, Rezza G, Gafà L, Romano N (November 2002). "Risk factors for classical Kaposi's sarcoma". J. Natl. Cancer Inst. 94 (22): 1712–8. doi:10.1093/jnci/94.22.1712. PMID 12441327.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lain KY, Powers RW, Krohn MA, Ness RB, Crombleholme WR, Roberts JM (November 1999). "Urinary cotinine concentration confirms the reduced risk of preeclampsia with tobacco exposure". Am. J. Obstet. Gynecol. 181 (5 Pt 1): 1192–6. doi:10.1016/S0002-9378(99)70107-9. PMID 10561644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hjern A, Hedberg A, Haglund B, Rosén M (June 2001). "Does tobacco smoke prevent atopic disorders? A study of two generations of Swedish residents". Clin. Exp. Allergy. 31 (6): 908–14. doi:10.1046/j.1365-2222.2001.01096.x. PMID 11422156.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Melton L (June 2006). "Body Blazes". Scientific American. 294 (6): 24. Bibcode:2006SciAm.294f..24M. doi:10.1038/scientificamerican0606-24. PMID 16711354.
- ^ Fratiglioni L, Wang HX (August 2000). "Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies". Behav. Brain Res. 113 (1–2): 117–20. doi:10.1016/S0166-4328(00)00206-0. PMID 10942038.
- ^ Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C (2008). "Smoking, dementia and cognitive decline in the elderly, a systematic review". BMC Geriatr. 8: 36. doi:10.1186/1471-2318-8-36. PMC 2642819. PMID 19105840.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Henningfield JE, Zeller M (2009). "Nicotine psychopharmacology: policy and regulatory". Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 192 (192): 511–34. doi:10.1007/978-3-540-69248-5_18. ISBN 978-3-540-69246-1. PMID 19184661.
- ^ Quik, Maryka; Parameswaran, Neeraja; McCallum, Sarah E.; Bordia, Tanuja; Bao, Shanshan; McCormack, Alison; Kim, Amy; Tyndale, Rachel F.; Langston, J. William; Di Monte, Donato A. (2006). "Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates". Journal of Neurochemistry. 98 (6): 1866–75. doi:10.1111/j.1471-4159.2006.04078.x. PMID 16882311.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ De Lau, L.M.L.; Koudstaal, P. J.; Witteman, J. C.M.; Hofman, A.; Breteler, M. M.B. (2006). "Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease". Neurology. 67 (2): 315–8. doi:10.1212/01.wnl.0000225050.57553.6d. PMID 16864826.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Quik, Maryka; Cox, Heather; Parameswaran, Neeraja; O'Leary, Kathryn; Langston, J. William; Di Monte, Donato (2007). "Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys". Annals of Neurology. 62 (6): 588–96. doi:10.1002/ana.21203. PMID 17960553.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Yu W, Mechawar N, Krantic S, Quirion R (November 2011). "α7 Nicotinic receptor activation reduces β-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling". J. Neurochem. 119 (4): 848–58. doi:10.1111/j.1471-4159.2011.07466.x. PMID 21884524.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Willoughby, John O.; Pope, Kenneth J.; Eaton, Vaughn (2003). "Nicotine as an Antiepileptic Agent in ADNFLE: An N-of-One Study". Epilepsia. 44 (10): 1363. doi:10.1046/j.1528-1157.2003.11903.x.
- ^ de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ (Jul 2002). "Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital". Schizophr Res. 56 (1–2): 55–65. doi:10.1016/S0920-9964(01)00192-X. PMID 12084420.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (Mar 1995). "Schizophrenia and smoking: an epidemiological survey in a state hospital". Am J Psychiatry. 152 (3): 453–5. PMID 7864277.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Aguilar MC, Gurpegui M, Diaz FJ, de Leon J (Mar 2005). "Nicotine dependence and symptoms in schizophrenia: naturalistic study of complex interactions". Br J Psychiatry. 186 (3): 215–21. doi:10.1192/bjp.186.3.215. PMID 15738502.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McClernon, F. Joseph; Hiott, F. Berry; Westman, Eric C.; Rose, Jed E.; Levin, Edward D. (2006). "Transdermal nicotine attenuates depression symptoms in nonsmokers: A double-blind, placebo-controlled trial". Psychopharmacology. 189 (1): 125–33. doi:10.1007/s00213-006-0516-y. PMID 16977477.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ "Attention-Deficit Hyperactivity Disorder". Retrieved 21 September 2009.
- ^ Mineur YS, Picciotto MR (December 2010). "Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis". Trends Pharmacol. Sci. 31 (12): 580–6. doi:10.1016/j.tips.2010.09.004. PMC 2991594. PMID 20965579.
- ^ Pasquini M, Garavini A, Biondi M (January 2005). "Nicotine augmentation for refractory obsessive-compulsive disorder. A case report". Prog. Neuropsychopharmacol. Biol. Psychiatry. 29 (1): 157–9. doi:10.1016/j.pnpbp.2004.08.011. PMID 15610960.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lundberg S, Carlsson A, Norfeldt P, Carlsson ML (November 2004). "Nicotine treatment of obsessive-compulsive disorder". Prog. Neuropsychopharmacol. Biol. Psychiatry. 28 (7): 1195–9. doi:10.1016/j.pnpbp.2004.06.014. PMID 15610934.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tizabi Y, Louis VA, Taylor CT, Waxman D, Culver KE, Szechtman H (January 2002). "Effect of nicotine on quinpirole-induced checking behavior in rats: implications for obsessive-compulsive disorder". Biol. Psychiatry. 51 (2): 164–71. doi:10.1016/S0006-3223(01)01207-0. PMID 11822995.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thomas GA, Rhodes J, Green JT, Richardson C (May 2000). "Role of smoking in inflammatory bowel disease: implications for therapy". Postgrad Med J. 76 (895): 273–9. doi:10.1136/pmj.76.895.273. PMC 1741576. PMID 10775279.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rubin DT, Hanauer SB (August 2000). "Smoking and inflammatory bowel disease". Eur J Gastroenterol Hepatol. 12 (8): 855–62. doi:10.1097/00042737-200012080-00004. PMID 10958212.
- ^ Sabha M, Tanus-Santos JE, Toledo JC, Cittadino M, Rocha JC, Moreno H (August 2000). "Transdermal nicotine mimics the smoking-induced endothelial dysfunction". Clin. Pharmacol. Ther. 68 (2): 167–74. doi:10.1067/mcp.2000.108851. PMID 10976548.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zhang S, Day I, Ye S (February 2001). "Nicotine induced changes in gene expression by human coronary artery endothelial cells". Atherosclerosis. 154 (2): 277–83. doi:10.1016/S0021-9150(00)00475-5. PMID 11166759.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hawkins BT, Brown RC, Davis TP (February 2002). "Smoking and ischemic stroke: a role for nicotine?". Trends Pharmacol. Sci. 23 (2): 78–82. doi:10.1016/S0165-6147(02)01893-X. PMID 11830264.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Raval AP, Bhatt A, Saul I (July 2009). "Chronic nicotine exposure inhibits 17beta-estradiol-mediated protection of the hippocampal CA1 region against cerebral ischemia in female rats". Neurosci. Lett. 458 (2): 65–9. doi:10.1016/j.neulet.2009.04.021. PMID 19442878.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ National Institute on Drug Abuse (June 2009). "Extent, Impact, Delivery, and Addictiveness". Tobacco Addiction. NIDA Report Research Series. Bethesda MA: National Institutes of Health. 09-4342.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Hilts, Philip J. (1994-08-02). "Is Nicotine Addictive? It Depends on Whose Criteria You Use". The New York Times.
- ^ Blakeslee, Sandra (1987-03-29). "Nicotine: Harder to Kick...Than Heroin". The New York Times.
- ^ "Division of Periodontology: Tobacco Use Cessation Program". .umn.edu. Retrieved 2012-12-19.
- ^ Yoshida T, Nishioka H, Nakamura Y, Kondo M (November 1984). "Reduced norepinephrine turnover in mice with monosodium glutamate-induced obesity". Metab. Clin. Exp. 33 (11): 1060–3. doi:10.1016/0026-0495(84)90238-5. PMID 6493048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peterson EC, Owens SM (June 2009). "Designing immunotherapies to thwart drug abuse". Mol. Interv. 9 (3): 119–24. doi:10.1124/mi.9.3.5. PMC 2743871. PMID 19592672.
- ^ Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T (Jul 1994). "Effects of aging on acute toxicity of nicotine in rats". Pharmacol Toxicol. 75 (1): 1–6. doi:10.1111/j.1600-0773.1994.tb00316.x. PMID 7971729.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lockhart LP (1933). "Nicotine poisoning". Br Med J. 1 (3762): 246–7. doi:10.1136/bmj.1.3762.246-c.
- ^ Hecht SS (July 1999). "Tobacco smoke carcinogens and lung cancer". J. Natl. Cancer Inst. 91 (14): 1194–210. doi:10.1093/jnci/91.14.1194. PMID 10413421.
- ^ Wu WK, Cho CH (April 2004). "The pharmacological actions of nicotine on the gastrointestinal tract". J. Pharmacol. Sci. 94 (4): 348–58. doi:10.1254/jphs.94.348. PMID 15107574.
- ^ Chowdhury P, Udupa KB (December 2006). "Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation". World J. Gastroenterol. 12 (46): 7428–32. PMID 17167829.
- ^ Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH (June 2007). "Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation". Toxicol. Sci. 97 (2): 279–87. doi:10.1093/toxsci/kfm060. PMID 17369603.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M (October 2003). "Nicotine enhances neovascularization and promotes tumor growth". Mol. Cells. 16 (2): 143–6. PMID 14651253.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH (January 2004). "Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway". J. Pharmacol. Exp. Ther. 308 (1): 66–72. doi:10.1124/jpet.103.058321. PMID 14569062.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Davis R, Rizwani W, Banerjee S; et al. (2009). Pao, William (ed.). "Nicotine promotes tumor growth and metastasis in mouse models of lung cancer". PLoS ONE. 4 (10): e7524. Bibcode:2009PLoSO...4.7524D. doi:10.1371/journal.pone.0007524. PMC 2759510. PMID 19841737.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Rauch, Stephen and Lanphear, Bruce (2012). "Prevention of Disability in Children:Elevating the Role of Environment" (PDF). Future of Children. 22 (1): 193–217. doi:10.1353/foc.2012.0006. PMID 22550691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Froehlich, T. E.; Lanphear, B. P.; Auinger, P.; Hornung, R.; Epstein, J. N.; Braun, J.; Kahn, R. S. (2009). "Association of Tobacco and Lead Exposures with Attention-Deficit/Hyperactivity Disorder". Pediatrics. 124 (6): e1054–63. doi:10.1542/peds.2009-0738. PMC 2853804. PMID 19933729.
- ^ http://oehha.ca.gov/prop65/prop65_list/files/P65single121809.pdf[full citation needed]
- ^ www.sciencelab.com/msds.php?msdsId=9926222 Material Safety Data Sheet L-Nicotine MSDS
- ^ Gause, G. F. (1941). "Chapter V: Analysis of various biological processes by the study of the differential action of optical isomers". In Luyet, B. J. (ed.). Optical Activity and Living Matter. A series of monographs on general physiology. Vol. 2. Normandy, Missouri: Biodynamica.
- ^ Lamberts, Burton L.; Dewey, Lovell J.; Byerrum, Richard U. (1959). "Ornithine as a precursor for the pyrrolidine ring of nicotine". Biochimica et Biophysica Acta. 33 (1): 22–6. doi:10.1016/0006-3002(59)90492-5. PMID 13651178.
- ^ Dawson, R. F.; Christman, D. R.; d'Adamo, A.; Solt, M. L.; Wolf, A. P. (1960). "The Biosynthesis of Nicotine from Isotopically Labeled Nicotinic Acids1". Journal of the American Chemical Society. 82 (10): 2628. doi:10.1021/ja01495a059.
- ^ Ashihara, Hiroshi; Crozier, Alan; Komamine, Atsushi (eds.). Plant metabolism and biotechnology. Cambridge: Wiley. ISBN 978-0-470-74703-2.[page needed]
- ^ Le Houezec J (September 2003). "Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review". Int. J. Tuberc. Lung Dis. 7 (9): 811–9. PMID 12971663.
- ^ Benowitz NL, Jacob P, Jones RT, Rosenberg J (May 1982). "Interindividual variability in the metabolism and cardiovascular effects of nicotine in man". J. Pharmacol. Exp. Ther. 221 (2): 368–72. PMID 7077531.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br Med J. 1980 April 5; 280(6219): 972–976.
- ^ Hukkanen J, Jacob P, Benowitz NL (March 2005). "Metabolism and disposition kinetics of nicotine". Pharmacol. Rev. 57 (1): 79–115. doi:10.1124/pr.57.1.3. PMID 15734728.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "The danger of third-hand smoke". Chromatography Online. 7 (3). 22 February 2011.
- ^ Benowitz, N. L.; Herrera, B; Jacob p, 3rd (2004). "Mentholated Cigarette Smoking Inhibits Nicotine Metabolism". Journal of Pharmacology and Experimental Therapeutics. 310 (3): 1208–15. doi:10.1124/jpet.104.066902. PMID 15084646.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Benowitz NL, Hukkanen J, Jacob P (2009). "Nicotine Psychopharmacology". Handbook of experimental pharmacology. Handbook of Experimental Pharmacology. 192 (192): 29–60. doi:10.1007/978-3-540-69248-5_2. ISBN 978-3-540-69246-1. PMC 2953858. PMID 19184645.
{{cite journal}}:|chapter=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Baselt, Randall Clint (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Biomedical Publications. pp. 1103–7. ISBN 978-0-9626523-7-0.
- ^ Mündel, T. and Jones, D. A. (2006). "Effect of transdermal nicotine administration on exercise endurance in men". Exp Physiol. 91 (4): 705–713. doi:10.1113/expphysiol.2006.033373. PMID 16627574.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dickson, Suzanne L.; Egecioglu, Emil; Landgren, Sara; Skibicka, Karolina P.; Engel, Jörgen A.; Jerlhag, Elisabet (2011). "The role of the central ghrelin system in reward from food and chemical drugs". Molecular and Cellular Endocrinology. 340 (1): 80–7. doi:10.1016/j.mce.2011.02.017. PMID 21354264.
- ^ Katzung, Bertram G. (2006). Basic and Clinical Pharmacology. New York: McGraw-Hill Medical. pp. 99–105.
- ^ Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA (March 2009). "Nicotine binding to brain receptors requires a strong cation-pi interaction". Nature. 458 (7237): 534–7. Bibcode:2009Natur.458..534X. doi:10.1038/nature07768. PMC 2755585. PMID 19252481.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Herraiz T, Chaparro C (2005). "Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors". Biochem. Biophys. Res. Commun. 326 (2): 378–86. doi:10.1016/j.bbrc.2004.11.033. PMID 15582589.
- ^ Fowler JS, Volkow ND, Wang GJ; et al. (1998). "Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition". J Addict Dis. 17 (1): 23–34. doi:10.1300/J069v17n01_03. PMID 9549600.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Villégier AS, Blanc G, Glowinski J, Tassin JP (September 2003). "Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors". Pharmacol. Biochem. Behav. 76 (2): 267–74. doi:10.1016/S0091-3057(03)00223-5. PMID 14592678.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, Tassin JP (August 2006). "Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine". Neuropsychopharmacology. 31 (8): 1704–13. doi:10.1038/sj.npp.1300987. PMID 16395299.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schütz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J (January 2008). "Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain". Neurosci. Lett. 430 (1): 34–7. doi:10.1016/j.neulet.2007.10.011. PMID 17997038.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Walsh H, Govind AP, Mastro R; et al. (2008). "Up-regulation of nicotinic receptors by nicotine varies with receptor subtype". J. Biol. Chem. 283 (10): 6022–32. doi:10.1074/jbc.M703432200. PMID 18174175.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Nguyen HN, Rasmussen BA, Perry DC (2003). "Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography". J. Pharmacol. Exp. Ther. 307 (3): 1090–7. doi:10.1124/jpet.103.056408. PMID 14560040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pons S, Fattore L, Cossu G; et al. (November 2008). "Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration". J. Neurosci. 28 (47): 12318–27. doi:10.1523/JNEUROSCI.3918-08.2008. PMC 2819191. PMID 19020025.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Yoshida T, Sakane N, Umekawa T, Kondo M (Jan 1994). "Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress". Physiol. Behav. 55 (1): 53–7. doi:10.1016/0031-9384(94)90009-4. PMID 8140174.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET (June 2009). "Link between facultative melanin and tobacco use among African Americans". Pharmacol. Biochem. Behav. 92 (4): 589–96. doi:10.1016/j.pbb.2009.02.011. PMID 19268687.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Elaine N. Marieb and Katja Hoehn (2007). Human Anatomy & Physiology (7th Ed.). Pearson. pp. ?. ISBN 0-8053-5909-5.[page needed]
- ^ Bhalala, Oneil (Spring 2003). "Detection of Cotinine in Blood Plasma by HPLC MS/MS". MIT Undergraduate Research Journal. 8: 45–50.
- ^ Posselt, W.; Reimann, L. (1828). "Chemische Untersuchung des Tabaks und Darstellung eines eigenthümlich wirksamen Prinzips dieser Pflanze". Magazin für Pharmacie (in German). 6 (24): 138–161.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Henningfield JE, Zeller M (March 2006). "Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward". Psychopharmacology (Berl.). 184 (3–4): 286–91. doi:10.1007/s00213-006-0308-4. PMID 16463054.
- ^ Melsens, Louis-Henri-Frédéric (1843) "Note sur la nicotine," Annales de chimie et de physique, third series, vol. 9, pages 465-479; see especially page 470. [Note: The empirical formula that Melsens provides is incorrect because at that time, chemists used the wrong atomic mass for carbon (6 instead of 12).]
- ^ Pinner, A.; Wolffenstein, R. (1891). "Ueber Nicotin". Berichte der deutschen chemischen Gesellschaft. 24: 1373. doi:10.1002/cber.189102401242.
- ^ Pinner, A. (1893). "Ueber Nicotin. Die Constitution des Alkaloïds". Berichte der deutschen chemischen Gesellschaft. 26: 292. doi:10.1002/cber.18930260165.
- ^ Pinner, A. (1893). "Ueber Nicotin. I. Mitteilung". Archiv der Pharmazie. 231 (5–6): 378. doi:10.1002/ardp.18932310508.
- ^ Pictet, Amé; Rotschy, A. (1904). "Synthese des Nicotins". Berichte der deutschen chemischen Gesellschaft. 37 (2): 1225. doi:10.1002/cber.19040370206.
- ^ US Code of Federal Regulations. 7 CFR 205.602 - Nonsynthetic substances prohibited for use in organic crop production
- ^ Staff, IFOAM. Criticisms and Frequent Misconceptions about Organic Agriculture: The Counter-Arguments: Misconception Number 7
- ^ USEPA (29 October 2008). "Nicotine; Notice of Receipt of Request to Voluntarily Cancel a Pesticide Registration". Federal Register: 64320–64322. Retrieved 8 April 2012.
- ^ USEPA (3 June 2009). "Nicotine; Product Cancellation Order". Federal Register: 26695–26696. Retrieved 8 April 2012.
Further reading
- Bilkei-Gorzo A, Rácz I, Michel K, Darvas M, Rafael Maldonado López, Zimmer A. (2008). "A common genetic predisposition to stress sensitivity and stress-induced nicotine craving". Biol. Psychiatry. 63 (2): 164–71. doi:10.1016/j.biopsych.2007.02.010. PMID 17570348.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gorrod, John W.; Peyton, Jacob,III, eds. (November 16, 1999). Analytical Determination of Nicotine and Related Compounds and their Metabolites. Amsterdam: Elsevier. ISBN 978-0-08-052551-8.
{{cite book}}: CS1 maint: multiple names: editors list (link) - Willoughby JO, Pope KJ, Eaton V (Sep 2003). "Nicotine as an antiepileptic agent in ADNFLE: an N-of-one study". Epilepsia. 44 (9): 1238–40. doi:10.1046/j.1528-1157.2003.11903.x. PMID 12919397.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Minna JD (Jan 2003). "Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer". J Clin Invest. 111 (1): 31–3. doi:10.1172/JCI17492. PMC 151841. PMID 12511585.
- Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG (Mar 2005). "Gender: a major determinant of brain response to nicotine". Int J Neuropsychopharmacol. 8 (1): 17–26. doi:10.1017/S1461145704004730. PMID 15579215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - West KA, Brognard J, Clark AS; et al. (Jan 2003). "Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells". J Clin Invest. 111 (1): 81–90. doi:10.1172/JCI16147. PMC 151834. PMID 12511591.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - National Institute on Drug Abuse
- Erowid information on tobacco
External links
- Nicotine bound to proteins in the PDB
- Description of nicotine mechanisms
- Erowid Nicotine Vault : Nicotine Material Safety Data Sheet
- Thomas, Gareth AO; Rhodes, John; Ingram, John R (2005). "Mechanisms of Disease: Nicotine—a review of its actions in the context of gastrointestinal disease". Nature Clinical Practice Gastroenterology & Hepatology. 2 (11): 536. doi:10.1038/ncpgasthep0316.
- CDC - NIOSH Pocket Guide to Chemical Hazards

