Testolactone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 59: | Line 59: | ||
==Pharmacology== |
==Pharmacology== |
||
The principal action of testolactone is reported to be inhibition of [[aromatase]] activity and the reduction in [[estrogen]] synthesis that follows. Androstenedione, a 19-carbon [[steroid hormone]] produced in the [[adrenal glands]] and the [[gonads]], is where estrone synthesis originates and is the source of estrogen in [[postmenopausal]] women. [[In vitro]] studies report that the aromatase inhibition may be noncompetitive and irreversible, and could possibly account for the persistence of this drug's effect on estrogen synthesis after drug withdrawal.<ref name=drugbank/> |
The principal action of testolactone is reported to be inhibition of [[aromatase]] activity and the reduction in [[estrogen]] synthesis that follows. Androstenedione, a 19-carbon [[steroid hormone]] produced in the [[adrenal glands]] and the [[gonads]], is where estrone synthesis originates and is the source of estrogen in [[postmenopausal]] women.<ref>{{Cite news|url=http://www.nicehair.org/jak-inhibitors-hair-loss-breakthrough-cure/|title=JAK Inhibitors for Hair Loss: A Breakthrough Cure?|last=|first=|date=2017-04-12|access-date=2017-04-19|archive-url=|archive-date=|dead-url=|language=en-US}}</ref> [[In vitro]] studies report that the aromatase inhibition may be noncompetitive and irreversible, and could possibly account for the persistence of this drug's effect on estrogen synthesis after drug withdrawal.<ref name=drugbank/> |
||
In addition to its activity as an aromatase inhibitor, testolactone also reportedly possesses some anabolic activity and weak androgenic activity via binding to and activation of the [[androgen receptor]].<ref name="LemkeWilliams2012">{{cite book|author1=Thomas L. Lemke|author2=David A. Williams|title=Foye's Principles of Medicinal Chemistry|url=https://books.google.com/books?id=Sd6ot9ul-bUC&pg=PA1362|date=24 January 2012|publisher=Lippincott Williams & Wilkins|isbn=978-1-60913-345-0|pages=1362–}}</ref> However, androgenic side effects such as [[hirsutism]], [[acne]], and [[voice change]]s have been reported in no women in clinical trials.<ref name="Lupulescu1990">{{cite book|author=Aurel Lupulescu|title=Hormones and Vitamins in Cancer Treatment|url=https://books.google.com/books?id=VddUa-2cp-YC&pg=PA64|date=24 October 1990|publisher=CRC Press|isbn=978-0-8493-5973-6|pages=64–}}</ref> |
In addition to its activity as an aromatase inhibitor, testolactone also reportedly possesses some anabolic activity and weak androgenic activity via binding to and activation of the [[androgen receptor]].<ref name="LemkeWilliams2012">{{cite book|author1=Thomas L. Lemke|author2=David A. Williams|title=Foye's Principles of Medicinal Chemistry|url=https://books.google.com/books?id=Sd6ot9ul-bUC&pg=PA1362|date=24 January 2012|publisher=Lippincott Williams & Wilkins|isbn=978-1-60913-345-0|pages=1362–}}</ref> However, androgenic side effects such as [[hirsutism]], [[acne]], and [[voice change]]s have been reported in no women in clinical trials.<ref name="Lupulescu1990">{{cite book|author=Aurel Lupulescu|title=Hormones and Vitamins in Cancer Treatment|url=https://books.google.com/books?id=VddUa-2cp-YC&pg=PA64|date=24 October 1990|publisher=CRC Press|isbn=978-0-8493-5973-6|pages=64–}}</ref> |
||
Revision as of 16:20, 19 April 2017
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Protein binding | ~85% |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.304 |
| Chemical and physical data | |
| Formula | C19H24O3 |
| Molar mass | 300.39 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| | |
Testolactone (INN, USAN) (brand name Teslac) is a non-selective, irreversible, steroidal aromatase inhibitor used as an antineoplastic drug to treat advanced-stage breast cancer.[1][2][3] The drug was discontinued in 2008 and is no longer available for medical use.[3]
Uses
This drug is mainly used for treating various types of breast cancer in women who have been through menopause or whose ovaries no longer function.[4] It works by blocking the production of estrogen, which helps prevent the growth of breast cancers that are activated by estrogen. It may also prevent tumor cells from being activated by other hormones.[4] It also has been used to postpone precocious puberty because of its ability to block estrogen production.[5]
Side effects
The most common side effects include:
- Abnormal skin sensations
- Aches of the legs and arms
- General body discomfort
- Hair loss
- Loss of appetite
- Nausea
- Redness of the tongue
- Vomiting
Pharmacology
The principal action of testolactone is reported to be inhibition of aromatase activity and the reduction in estrogen synthesis that follows. Androstenedione, a 19-carbon steroid hormone produced in the adrenal glands and the gonads, is where estrone synthesis originates and is the source of estrogen in postmenopausal women.[6] In vitro studies report that the aromatase inhibition may be noncompetitive and irreversible, and could possibly account for the persistence of this drug's effect on estrogen synthesis after drug withdrawal.[1]
In addition to its activity as an aromatase inhibitor, testolactone also reportedly possesses some anabolic activity and weak androgenic activity via binding to and activation of the androgen receptor.[3] However, androgenic side effects such as hirsutism, acne, and voice changes have been reported in no women in clinical trials.[7]
Chemistry
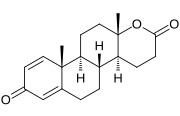
Testolactone is a synthetic 18-oxasteroid and a D-homo-18-oxo analogue of androstenedione (androst-4-en-3,17-dione), with a six-membered lactone ring in place of the five-membered carbocyclic D-ring.[3]
References
- ^ a b Testolactone at DrugBank.ca
- ^ Dunkel L (July 2006). "Use of aromatase inhibitors to increase final height". Mol. Cell. Endocrinol. 254–255: 207–16. doi:10.1016/j.mce.2006.04.031. PMID 16766117.
- ^ a b c d Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1362–. ISBN 978-1-60913-345-0.
- ^ a b Testolactone facts and comparisons at Drugs.com
- ^ Carel, J.-C.; Lahlou, N; Roger, M; Chaussain, JL (2004). "Precocious puberty and statural growth". Human Reproduction Update. 10 (2): 135–47. doi:10.1093/humupd/dmh012. PMID 15073143.
- ^ "JAK Inhibitors for Hair Loss: A Breakthrough Cure?". 2017-04-12. Retrieved 2017-04-19.
{{cite news}}: Cite has empty unknown parameter:|dead-url=(help) - ^ Aurel Lupulescu (24 October 1990). Hormones and Vitamins in Cancer Treatment. CRC Press. pp. 64–. ISBN 978-0-8493-5973-6.
