Barbiturate: Difference between revisions
m Reverted edits by 204.11.186.62 (talk) to last revision by Tahney (HG) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[File:Kwas barbiturowy.svg|thumb|[[Barbituric acid]], the basic structure of all barbiturates]] |
[[File:Kwas barbiturowy.svg|thumb|[[Barbituric acid]], the basic structure of all barbiturates]] |
||
'''Barbiturates''' are [[Pharmaceutical drug|drug]]s that act as [[central nervous system]] [[depressant]]s, and can therefore produce a wide spectrum of effects, from mild [[sedation]] to total [[anesthesia]]. They are also effective as [[anxiolytic]]s, [[hypnotic]]s, and [[anticonvulsant]]s. Barbiturates also have [[analgesic]] effects; however, these effects are somewhat weak, preventing barbiturates from being used in [[surgery]] in the absence of other analgesics. They have addiction potential, both physical and psychological. Barbiturates have now largely been replaced by [[benzodiazepine]]s in routine medical practice – for example, in the treatment of anxiety and insomnia – mainly because benzodiazepines are significantly less dangerous in [[Drug overdose|overdose]]. However, barbiturates are still used in general anesthesia, for [[epilepsy]], and assisted suicide.<ref>{{cite web | url = http://www.dignitas.ch/index.php?option=com_content&view=article&id=22&Itemid=62&lang=de | title = DIGNITAS | accessdate =2011-06-14 | archiveurl= http://web.archive.org/web/20110721131617/http://www.dignitas.ch/index.php?option=com_content&view=article&id=22&Itemid=62&lang=de| archivedate= 21 July 2011 <!--DASHBot-->| deadurl= no}}</ref> Barbiturates are derivatives of [[barbituric acid]].<ref>{{Cite journal|doi=10.1002/jps.2600600807|title=Kinetics of hydrolysis of barbituric acid derivatives|url=http://onlinelibrary.wiley.com/doi/10.1002/jps.2600600807/abstract| author= Edward R. Garrett, Jacek T. Bojarski†, Gerald J. Yakatan|date=21 SEP 2006}}</ref> |
'''Barbiturates''' are bnity [[Pharmaceutical drug|drug]]s that act as [[central nervous system]] [[depressant]]s, and can therefore produce a wide spectrum of effects, from mild [[sedation]] to total [[anesthesia]]. They are also effective as [[anxiolytic]]s, [[hypnotic]]s, and [[anticonvulsant]]s. Barbiturates also have [[analgesic]] effects; however, these effects are somewhat weak, preventing barbiturates from being used in [[surgery]] in the absence of other analgesics. They have addiction potential, both physical and psychological. Barbiturates have now largely been replaced by [[benzodiazepine]]s in routine medical practice – for example, in the treatment of anxiety and insomnia – mainly because benzodiazepines are significantly less dangerous in [[Drug overdose|overdose]]. However, barbiturates are still used in general anesthesia, for [[epilepsy]], and assisted suicide.<ref>{{cite web | url = http://www.dignitas.ch/index.php?option=com_content&view=article&id=22&Itemid=62&lang=de | title = DIGNITAS | accessdate =2011-06-14 | archiveurl= http://web.archive.org/web/20110721131617/http://www.dignitas.ch/index.php?option=com_content&view=article&id=22&Itemid=62&lang=de| archivedate= 21 July 2011 <!--DASHBot-->| deadurl= no}}</ref> Barbiturates are derivatives of [[barbituric acid]].<ref>{{Cite journal|doi=10.1002/jps.2600600807|title=Kinetics of hydrolysis of barbituric acid derivatives|url=http://onlinelibrary.wiley.com/doi/10.1002/jps.2600600807/abstract| author= Edward R. Garrett, Jacek T. Bojarski†, Gerald J. Yakatan|date=21 SEP 2006}}</ref> |
||
== History == |
== History == |
||
Revision as of 16:36, 14 November 2013

Barbiturates are bnity drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, hypnotics, and anticonvulsants. Barbiturates also have analgesic effects; however, these effects are somewhat weak, preventing barbiturates from being used in surgery in the absence of other analgesics. They have addiction potential, both physical and psychological. Barbiturates have now largely been replaced by benzodiazepines in routine medical practice – for example, in the treatment of anxiety and insomnia – mainly because benzodiazepines are significantly less dangerous in overdose. However, barbiturates are still used in general anesthesia, for epilepsy, and assisted suicide.[1] Barbiturates are derivatives of barbituric acid.[2]
History
Barbituric acid was first synthesized December 6, 1864, by German researcher Adolf von Baeyer. This was done by condensing urea (an animal waste product) with diethyl malonate (an ester derived from the acid of apples). There are several stories about how the substance got its name. The most likely story is that Von Baeyer and his colleagues went to celebrate their discovery in a tavern where the town's artillery garrison were also celebrating the feast of Saint Barbara—the patron saint of artillerymen. An artillery officer is said to have christened the new substance by amalgamating Barbara with urea.[3] Another story holds that Von Baeyer synthesized the substance from the collected urine of a Munich waitress named Barbara.[4] No substance of medical value was discovered, however, until 1903 when two German scientists working at Bayer, Emil Fischer and Joseph von Mering, discovered that barbital was very effective in putting dogs to sleep. Barbital was then marketed by Bayer under the trade name Veronal. It is said that Von Mering proposed this name because the most peaceful place he knew was the Italian city of Verona.[3]
It was not until the 1950s that the behavioural disturbances and physical dependence potential of barbiturates became recognized.[5]
Barbituric acid itself does not have any direct effect on the central nervous system and chemists have derived over 2,500 compounds from it that possess pharmacologically active qualities. The broad class of barbiturates is further broken down and classified according to speed of onset and duration of action. Ultrashort-acting barbiturates are commonly used for anesthesia because their extremely short duration of action allows for greater control. These properties allow doctors to rapidly put a patient "under" in emergency surgery situations. Doctors can also bring a patient out of anesthesia just as quickly, should complications arise during surgery. The middle two classes of barbiturates are often combined under the title "short/intermediate-acting." These barbiturates are also employed for anesthetic purposes, and are also sometimes prescribed for anxiety or insomnia. This is not a common practice anymore, however, owing to the dangers of long-term use of barbiturates; they have been replaced by the benzodiazepines for these purposes. The final class of barbiturates are known as long-acting barbiturates (the most notable one being phenobarbital, which has a half-life of roughly 92 hours). This class of barbiturates is used almost exclusively as anticonvulsants, although on rare occasions they are prescribed for daytime sedation. Barbiturates in this class are not used for insomnia, because, owing to their extremely long half-life, patients would awake with a residual "hang-over" effect and feel groggy.
Barbiturates can in most cases be used either as the free acid or as salts of sodium, calcium, potassium, magnesium, lithium, etc. Codeine- and Dionine-based salts of barbituric acid have been developed. In 1912, Bayer introduced another barbituric acid derivative, phenobarbital, under the trade name Luminal, as a sedative-hypnotic.[6]
Therapeutic uses
Barbiturates such as phenobarbital were long used as anxiolytics and hypnotics. Today, benzodiazepines have largely supplanted them for these purposes, because benzodiazepines have less potential for lethal overdoses.[7][8][9]
Other uses related to their physiological properties
Barbiturates in high doses are used for physician-assisted suicide (PAS), and in combination with a muscle relaxant for euthanasia and for capital punishment by lethal injection.[10][11] Thiopental is an ultra-short acting barbiturate that is marketed under the name sodium pentothal. It is often mistaken for "truth serum" or sodium amytal, an intermediate-acting barbiturate that is used for sedation and to treat insomnia, but was also used in so-called sodium amytal "interviews" where the person being questioned would be much more likely to provide the truth whilst under the influence of this drug. When dissolved in water, sodium amytal can be swallowed, or it can be administered by intravenous injection. The drug does not itself force people to tell the truth, but is thought to decrease inhibitions, making subjects more likely to be caught off guard when questioned.[12]
Mechanism of action
The principal mechanism of action of barbiturates is believed to be positive allosteric modulation of GABAA receptors.[13] GABA is the principal inhibitory neurotransmitter in the mammalian central nervous system (CNS). Barbiturates bind to the GABAA receptor at the beta subunit, which are binding sites distinct from GABA itself and also distinct from the benzodiazepine binding site. Like benzodiazepines, barbiturates potentiate the effect of GABA at this receptor. In addition to this GABA-ergic effect, barbiturates also block the AMPA receptor, a subtype of glutamate receptor. Glutamate is the principal excitatory neurotransmitter in the mammalian CNS. Taken together, the findings that barbiturates potentiate inhibitory GABAA receptors and inhibit excitatory AMPA receptors can explain the CNS-depressant effects of these agents. At higher concentration, they inhibit the Ca2+-dependent release of neurotransmitters.[14] Barbiturates produce their pharmacological effects by increasing the duration of chloride ion channel opening at the GABAA receptor (pharmacodynamics: This increases the efficacy of GABA), whereas benzodiazepines increase the frequency of the chloride ion channel opening at the GABAA receptor (pharmacodynamics: This increases the potency of GABA). The direct gating or opening of the chloride ion channel is the reason for the increased toxicity of barbiturates compared to benzodiazepines in overdose.[15][16]
Further, barbiturates are relatively non-selective compounds that bind to an entire superfamily of ligand-gated ion channels, of which the GABAA receptor channel is only one of several representatives. This superfamily of ion channels includes the neuronal nAChR channel, the 5HT3R channel, the GlyR channel and others. However, while GABAA receptor currents are increased by barbiturates (and other general anaesthetics), ligand-gated ion channels that are predominantly permeable for cationic ions are blocked by these compounds. For example, neuronal nAChR channels are blocked by clinically relevant anaesthetic concentrations of both thiopental and pentobarbital.[17] Such findings implicate (non-GABA-ergic) ligand-gated ion channels, e.g. the neuronal nAChR channel, in mediating some of the (side) effects of barbiturates.[18]
Tolerance, dependence, overdose, and adverse reaction
There are special risks to consider for older adults, women who are pregnant, and babies. When a person ages, the body becomes less able to rid itself of barbiturates. As a result, people over the age of sixty-five are at higher risk of experiencing the harmful effects of barbiturates, including drug dependence and accidental overdose.[19] When barbiturates are taken during pregnancy, the drug passes through the mother's bloodstream to her fetus. After the baby is born, it may experience withdrawal symptoms and have trouble breathing. In addition, nursing mothers who take barbiturates may transmit the drug to their babies through breast milk.[20]
Tolerance and dependence
With regular use, tolerance to the effects of barbiturates develops.
Overdose
Symptoms of an overdose typically include sluggishness, incoordination, difficulty in thinking, slowness of speech, faulty judgement, drowsiness, shallow breathing, staggering, and in severe cases coma and death. The lethal dosage of barbiturates varies greatly with tolerance and from one individual to another. The amount of 1 g in dose orally can be highly poisonous with dosages from 2g to 10 g being generally fatal depending on the person's tolerance level. Even in inpatient settings, however, the development of tolerance is still a problem, as dangerous and unpleasant withdrawal symptoms can result when the drug is stopped after dependence has developed. Barbiturates in overdose with other CNS (central nervous system) depressants for example, alcohol, opiates or benzodiazepines is even more dangerous due to additive CNS and respiratory depressant effects. In the case of benzodiazepines not only do they have additive effects, barbiturates also increase the binding affinity of the benzodiazepine binding site thus leading to an exaggerated effect of benzodiazepines.
Marilyn Monroe, Ellen Wilkinson, Dalida, Judy Garland, Dorothy Kilgallen, Jean Seberg, Jimi Hendrix, Edie Sedgwick and Kenneth Williams died from barbiturate overdose. Ingeborg Bachmann may have died of the consequences of barbiturate withdrawal.
Adverse reaction
A rare adverse reaction to barbiturates is Stevens-Johnson syndrome, which primarily affects the mucous membranes.
Recreational use
Barbiturates produce effects similar to ethanol during intoxication. The symptoms of barbiturate intoxication include respiratory depression, lowered blood pressure, fatigue, fever, unusual excitement, irritability, dizziness, poor concentration, sedation, confusion, impaired coordination, impaired judgement, addiction, and respiratory arrest, which may lead to death.[21]
Recreational users report that a barbiturate high gives them feelings of relaxed contentment and euphoria. The main risk of acute barbiturate abuse is respiratory depression. Physical and psychological dependence may also develop with repeated use.[22] Other effects of barbiturate intoxication include drowsiness, lateral and vertical nystagmus, slurred speech and ataxia, decreased anxiety, a loss of inhibitions. Barbiturates are also used to alleviate the adverse or withdrawal effects of illicit drug misuse.[23][24]
Drug users tend to prefer short-acting and intermediate-acting barbiturates.[25] The most commonly abused are amobarbital (Amytal), pentobarbital (Nembutal), and secobarbital (Seconal). A combination of amobarbital and secobarbital (called Tuinal) is also highly abused. Short-acting and intermediate-acting barbiturates are usually prescribed as sedatives and sleeping pills. These pills begin acting fifteen to forty minutes after they are swallowed, and their effects last from five to six hours. Veterinarians use pentobarbital to anesthetise animals before surgery; in large doses, it can be used to euthanise animals.[26]
Slang terms for barbiturates include barbs, bluebirds, dolls, downers, goofballs, sleepers, 'reds & blues' and tooties.[27]
Legal status
In the 1940s, military personnel were given "Goofballs" during WWII in the South Pacific region to allow soldiers to tolerate the heat and humidity of daily working conditions. Goofballs were distributed to lower the respiratory system and blood pressure to combat the extreme conditions. Many soldiers returned with addictions that required several months of rehabilitation before discharge. This led to addiction problems through the 1950s and 1960s. [citation needed]
In the 1950s and 1960s, increasing reports began to be published about barbiturate overdoses and dependence problems, which eventually led to the scheduling of barbiturates as controlled drugs.
In 1970, several barbiturates were designated in the United States as controlled substances with the passage of the American Controlled Substances Act of 1970. Pentobarbital, secobarbital and amobarbital were designated schedule II drugs, butabarbital schedule III, and barbital and phenobarbital schedule IV. No barbiturates are schedule I or V. In 1971, the Convention on Psychotropic Substances was signed in Vienna. Designed to regulate amphetamines, barbiturates, and other synthetics, the treaty today regulates secobarbital, amobarbital, butalbital, cyclobarbital, and pentobarbital as schedule III, and allobarbital, methylphenobarbital, phenobarbital, and vinylbital as schedule IV scheduled substances.
Other uses in chemistry
In 1988, the synthesis and binding studies of an artificial receptor binding barbiturates by 6 complementary hydrogen bonds was published.[28] Since this first article, different kind of receptors were designed, as well as different barbiturates and cyanurates, not for their efficiencies as drugs but for applications in supramolecular chemistry, in the conception of materials and molecular devices.
Sodium barbital and barbital are the buffering components of the traditional Veronal buffer, which is widely used for serum electrophoresis in agarose gel.
Examples
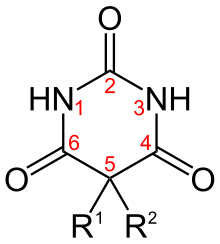
| Short Name | R1 | R2 | IUPAC Name |
|---|---|---|---|
| Allobarbital | CH2CHCH2 | CH2CHCH2 | 5,5-diallylbarbiturate |
| Amobarbital | CH2CH3 | CH2CH2CH(CH3)2 | 5-ethyl-5-isopentyl-barbiturate |
| Aprobarbital | CH2CHCH2 | CH(CH3)2 | 5-allyl-5-isopropyl-barbiturate |
| Alphenal | CH2CHCH2 | C6H5 | 5-allyl-5-phenyl-barbiturate |
| Barbital | CH2CH3 | CH2CH3 | 5,5-diethylbarbiturate |
| Brallobarbital | CH2CHCH2 | CH2CBrCH2 | 5-allyl-5-(2-bromo-allyl)-barbiturate |
| Phenobarbital | CH2CH3 | C6H5 | 5-ethyl-5-phenylbarbiturate |
See also
- Benzodiazepines
- Psycholeptic
- The Dille–Koppanyi reagent, used as a spot test for barbiturates.
- The Zwikker reagent, also used as a spot test for barbiturates.
References
- ^ "DIGNITAS". Archived from the original on 21 July 2011. Retrieved 2011-06-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Edward R. Garrett, Jacek T. Bojarski†, Gerald J. Yakatan (21 SEP 2006). "Kinetics of hydrolysis of barbituric acid derivatives". doi:10.1002/jps.2600600807.
{{cite journal}}: Check date values in:|date=(help); Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ a b "Barbiturates". Archived from the original on 7 November 2007. Retrieved 2007-10-31.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Medical Curiosities. Youngson, Robert M. London: Robinson Publishing, 1997. Page 276.
- ^ Galanter, Marc; Kleber, Herbert D. (1 July 2008). The American Psychiatric Publishing Textbook of Substance Abuse Treatment (4th ed.). United States of America: American Psychiatric Publishing Inc. p. 217. ISBN 978-1-58562-276-4.
{{cite book}}: Cite has empty unknown parameter:|chapterurl=(help) - ^ Sneader, Walter (2005-06-23). Drug Discovery. John Wiley and Sons. p. 369. ISBN 0-471-89979-8.
{{cite book}}:|access-date=requires|url=(help) - ^ Whitlock FA (June 14, 1975). "Suicide in Brisbane, 1956 to 1973: the drug-death epidemic". Med J Aust. 1 (24): 737–43. PMID 239307.
- ^ Johns MW (1975). "Sleep and hypnotic drugs". Drugs. 9 (6): 448–78. doi:10.2165/00003495-197509060-00004. PMID 238826.
- ^ Jufe GS (2007). "[New hypnotics: perspectives from sleep physiology]". Vertex. 18 (74): 294–9. PMID 18265473.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^
"Administration and Compounding Of Euthanasic Agents". Archived from the original on 7 June 2008. Retrieved 15 July 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Daniel Engber. "Why do lethal injections have three drugs?". Slate Magazine. Archived from the original on 25 July 2008. Retrieved 15 July 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Neuroscience for Kids - Barbiturates". Archived from the original on 16 June 2008. Retrieved 2008-06-02.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 23205959, please use {{cite journal}} with
|pmid= 23205959instead. - ^ Brunton, Laurence L.; Lazo, John S.; Parker, Keith L.; Goodman, Louis Sanford; Gilman, Alfred Goodman (2005). Goodman & Gilman's Pharmacological Basis of Therapeutics. McGraw-Hill. ISBN 0-07-142280-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Neil Harrison (2000). "Barbiturates". Neuropsychopharmacology. Retrieved 15 July 2008.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Society for Neurochemistry, American (1999) [1998]. "Part 2 Chapter 16". Basic Neurochemistry - Molecular, Cellular and Medical Aspects (Sixth ed.). Lippincott Williams and Wilkins. ISBN 0-397-51820-X. Retrieved July 2008.
{{cite book}}: Check date values in:|accessdate=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Weber, M; Motin, L; Gaul, S; Beker, F; Fink, RH; Adams, DJ (2005). "Intravenous anaesthetics inhibit nicotinic acetylcholine receptor-mediated currents and Ca2+ transients in rat intracardiac ganglion neurons". British Journal of Pharmacology. 144 (1): 98–107. doi:10.1038/sj.bjp.0705942. PMC 1575970. PMID 15644873.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Franks, NP; Lieb, WR (23 November 1998). "Which molecular targets are most relevant to general anaesthesia?". Toxicology Letters. 100–101: 1–8. doi:10.1016/S0378-4274(98)00158-1. PMID 10049127.
- ^ WebMD. "Toxicity, Barbiturate". eMedicine. Archived from the original on 20 July 2008. Retrieved 15 July 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Nau H (1982). "Anticonvulsants during pregnancy and lactation. Transplacental, maternal and neonatal pharmacokinetics". Clin Pharmacokinet. 7 (6): 508–43. doi:10.2165/00003088-198207060-00003. PMID 6819105.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ National Institute on Drug Abuse. "Commonly Abused Drugs". p. 1. Archived from the original on 22 July 2008. Retrieved 15 July 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Schlatter J (February 17, 2001). "[Drugs and drug abusers]". Presse Med. 30 (6): 282–7. PMID 11252979.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Emedicine Health. "Barbiturate Abuse". p. 1. Archived from the original on 2 August 2008. Retrieved 15 July 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Faulkner TP (1979). "Dose-response studies on tolerance to multiple doses of secobarbital and methaqualone in a polydrug abuse population". Clin Toxicol. 15 (1): 23–37. doi:10.3109/15563657908992476. PMID 498734.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Coupey SM (1997). "Barbiturates". Pediatr Rev. 18 (8): 260–4. doi:10.1542/pir.18-8-260. PMID 9255991.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ DEA Brief on Barbiturates
- ^ Hamid H. (2005). "Substance Abuse: Medical and Slang Terminology". South Med J. 98 (3). Medscape: 350–362. doi:10.1097/01.SMJ.0000153639.23135.6A. PMID 15813163.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Chang, Suk Kyu.; Hamilton, Andrew D. (1988). "Molecular recognition of biologically interesting substrates: Synthesis of an artificial receptor for barbiturates employing six hydrogen bonds". Journal of the American Chemical Society. 110 (4): 1318–1319. doi:10.1021/ja00212a065.
External links
- U.S. Drug Enforcement Administration Source for some public domain text used on this page.
- History of Barbiturates
