Cytoskeleton
| Cell biology | |
|---|---|
| Animal cell diagram | |
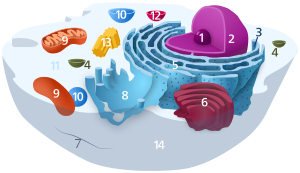 Components of a typical animal cell:
|

A cytoskeleton is present in all cells of all domains of life (archaea, bacteria, eukaryotes). It is a complex network of interlinking filaments and tubules that extend throughout the cytoplasm, from the nucleus to the plasma membrane[1] The cytoskeletal systems of different organisms are composed of similar proteins. In eukaryotes, the cytoskeletal matrix is a dynamic structure composed of three main proteins, which are capable of rapid growth or disassembly dependent on the cell's requirements at a certain period of time.[2]
The structure, function and dynamic behaviour of the cytoskeleton can be very different, depending on organism and cell type.[3][4] Even within one cell the cytoskeleton can change through association with other proteins and the previous history of the network.[5]
There is a multitude of functions that the cytoskeleton can perform. Primarily, it gives the cell shape and mechanical resistance to deformation,[3] and through association with extracellular connective tissue and other cells it stabilizes entire tissues.[3][6] The cytoskeleton can also actively contract, thereby deforming the cell and the cell's environment and allowing cells to migrate.[5] Moreover, it is involved in many cell signaling pathways, in the uptake of extracellular material (endocytosis),[7] segregates chromosomes during cellular division,[3] is involved in cytokinesis (the division of a mother cell into two daughter cells),[4] provides a scaffold to organize the contents of the cell in space [5] and for intracellular transport (for example, the movement of vesicles and organelles within the cell);[3] and can be a template for the construction of a cell wall.[3] Furthermore, it forms specialized structures, such as flagella, cilia, lamellipodia and podosomes.
A large-scale example of an action performed by the cytoskeleton is muscle contraction. During contraction of a muscle, within each muscle cell, myosin molecular motors collectively exert forces on parallel actin filaments. This action contracts the muscle cell, and through the synchronous process in many muscle cells, the entire muscle.
History
In 1903, Nikolai K. Koltsov proposed that the shape of cells was determined by a network of tubules that he termed the cytoskeleton. The concept of a protein mosaic that dynamically coordinated cytoplasmic biochemistry was proposed by Rudolph Peters in 1929 [8] while the term (cytosquelette, in French) was first introduced by French embryologist Paul Wintrebert in 1931.[9]
Eukaryotic cytoskeleton

Eukaryotic cells contain three main kinds of cytoskeletal filaments: microfilaments, microtubules, and intermediate filaments. Each cytoskeletal filament has a shape and intracellular distribution. Additionally, the filaments are formed by polymerization of different types of sub-units. The microfilament consist of the polymers of the protein actin which has a diameter of 7 nm. The microtubules are made up of the protein called tubulin which has a diameter of 25 nm. Intermediate filaments are made up of various proteins which varies depending on the cell type. These type of filament normally have diameters ranging from 8-12 nm.[1] The cytoskeleton provides the cell with structure and shape, and by excluding macromolecules from some of the cytosol, it adds to the level of macromolecular crowding in this compartment.[10] Cytoskeletal elements interact extensively and intimately with cellular membranes.[11] A number of small molecule cytoskeletal drugs have been discovered that interact with actin and microtubules. These compounds have proven useful in studying the cytoskeleton and several have clinical applications.
All filaments interact with accessory proteins that regulate and link the filaments to other cell compounds and each other. The accessory proteins are essential for controlled assembly of cytoskeletal filaments in particular locations, and they include motor proteins.[12]
Microfilaments (actin filaments)
Microfilaments are composed of linear polymers of G-actin proteins, and generate force when the growing (plus) end of the filament pushes against a barrier, such as the cell membrane. They also act as tracks for the movement of myosin molecules that attach to the microfilament and "walk" along them. Myosin motoring along F-actin filaments generates contractile forces in so-called actomyosin fibers, both in muscle as well as most non-muscle cell types.[13] Actin structures are controlled by the Rho family of small GTP-binding proteins such as Rho itself for contractile acto-myosin filaments ("stress fibers"), Rac for lamellipodia and Cdc42 for filopodia.
Intermediate filaments

Intermediate filaments are a part of the cytoskeleton of all animals. These filaments, averaging 10 nanometers in diameter, are more stable (strongly bound) than actin filaments, and heterogeneous constituents of the cytoskeleton. Like actin filaments, they function in the maintenance of cell-shape by bearing tension (microtubules, by contrast, resist compression but can also bear tension during mitosis and during the positioning of the centrosome). Intermediate filaments organize the internal tridimensional structure of the cell, anchoring organelles and serving as structural components of the nuclear lamina. They also participate in some cell-cell and cell-matrix junctions. Nuclear lamina exist in all animals and all tissues. Some animals like the fruit fly do not have any cytoplasmic intermediate filaments. In those animals that express cytoplasmic intermediate filaments, these are tissue specific.[6]
Different intermediate filaments are:
- made of vimentins. Vimentin intermediate filaments are in general present in mesenchymal cells.
- made of keratin. Keratin is present in general in epithelial cells.
- neurofilaments of neural cells.
- made of lamin, giving structural support to the nuclear envelope.
- made of desmin, play an important role in structural and mechanical support of muscle cells.[14]
Microtubules

Microtubules are hollow cylinders about 23 nm in diameter (lumen = approximately 15 nm in diameter), most commonly comprising 13 protofilaments that, in turn, are polymers of alpha and beta tubulin. They have a very dynamic behavior, binding GTP for polymerization. They are commonly organized by the centrosome.
In nine triplet sets (star-shaped), they form the centrioles, and in nine doublets oriented about two additional microtubules (wheel-shaped), they form cilia and flagella. The latter formation is commonly referred to as a "9+2" arrangement, wherein each doublet is connected to another by the protein dynein. As both flagella and cilia are structural components of the cell, and are maintained by microtubules, they can be considered part of the cytoskeleton.
They play key roles in:
- intracellular transport (associated with dyneins and kinesins, they transport organelles like mitochondria or vesicles).
- the axoneme of cilia and flagella.
- the mitotic spindle.
- synthesis of the cell wall in plants.
Comparison
| Cytoskeleton type[15] | Diameter (nm)[16] | Structure | Subunit examples[15] |
|---|---|---|---|
| Microfilaments | 6 | double helix | actin |
| Intermediate filaments | 10 | two anti-parallel helices/dimers, forming tetramers |
|
| Microtubules | 23 | protofilaments, in turn consisting of tubulin subunits in complex with stathmin[17] | α- and β-tubulin |
Septins
Septins are a group of the highly conserved GTP binding proteins found in eukaryotes. Different septins form protein complexes with each other. These can assemble to filaments and rings. Therefore, septins can be considered part of the cytoskeleton.[18] The function of septins in cells include serving as a localized attachment site for other proteins, and preventing the diffusion of certain molecules from one cell compartment to another.[18] In yeast cells, they build scaffolding to provide structural support during cell division and compartmentalize parts of the cell. Recent research in human cells suggests that septins build cages around bacterial pathogens, immobilizing the harmful microbes and preventing them from invading other cells.[19]
Spectrin
Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffolding and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure.[20]
Yeast cytoskeleton
In budding yeast (an important model organism), actin forms cortical patches, actin cables, and a cytokinetic ring and the cap. Cortical patches are discrete actin bodies on the membrane and are important for endocytosis, especially the recycling of glucan synthase which is important for cell wall synthesis. Actin cables are bundles of actin filaments and are involved in the transport of vesicles towards the cap (which contains a number of different proteins to polarize cell growth) and in the positioning of mitochondria. The cytokinetic ring forms and constricts around the site of cell division.[21]
Prokaryotic cytoskeleton
The cytoskeleton was once thought to be a feature only of eukaryotic cells, but homologues to all the major proteins of the eukaryotic cytoskeleton have been found in prokaryotes.[22] Although the evolutionary relationships are so distant that they are not obvious from protein sequence comparisons alone, the similarity of their three-dimensional structures and similar functions in maintaining cell shape and polarity provides strong evidence that the eukaryotic and prokaryotic cytoskeletons are truly homologous.[23] However, some structures in the bacterial cytoskeleton may not have been identified as of yet.[13][24]
FtsZ
FtsZ was the first protein of the prokaryotic cytoskeleton to be identified. Like tubulin, FtsZ forms filaments in the presence of guanosine triphosphate (GTP), but these filaments do not group into tubules. During cell division, FtsZ is the first protein to move to the division site, and is essential for recruiting other proteins that synthesize the new cell wall between the dividing cells.
MreB and ParM
Prokaryotic actin-like proteins, such as MreB, are involved in the maintenance of cell shape. All non-spherical bacteria have genes encoding actin-like proteins, and these proteins form a helical network beneath the cell membrane that guides the proteins involved in cell wall biosynthesis.[25]
Some plasmids encode a partitioning system that involves an actin-like protein ParM. Filaments of ParM exhibit dynamic instability, and may partition plasmid DNA into the dividing daughter cells by a mechanism analogous to that used by microtubules during eukaryotic mitosis.[13][26]
Crescentin
The bacterium Caulobacter crescentus contains a third protein, crescentin, that is related to the intermediate filaments of eukaryotic cells. Crescentin is also involved in maintaining cell shape, such as helical and vibrioid forms of bacteria, but the mechanism by which it does this is currently unclear.[27]
Common features and differences between prokaryotes and eukaryotes
By definition, the cytoskeleton is composed of proteins that can form longitudinal arrays (fibres) in all organisms. These filament forming proteins have been classified into 4 classes. Tubulin-like, actin-like, Walker A cytoskeletal ATPases (WACA-proteins), and intermediate filaments.[4][13]
Tubulin-like proteins are tubulin in eukaryotes and FtsZ, TubZ, RepX in prokaryotes. Actin-like proteins are actin in eukaryotes and MreB, FtsA in prokaryotes. An example of a WACA-proteins, which are mostly found in prokaryotes, is MinD. Examples for intermediate filaments, which have almost exclusively been found in animals (i.e. eukaryotes) are the lamins, keratins, vimentin, neurofilaments, desmin.[4]
Although tubulin-like proteins share some amino acid sequence similarity, their similarity in protein-fold and the similarity in the GTP binding site is more striking. The same holds true for the actin-like proteins and their structure and ATP binding domain.[4][13]
Cytoskeletal proteins are usually associated with cell shape, DNA segregation and cell division in prokaryotes and eukaryotes. Which proteins fulfill which task is very different. For example, DNA segregation in all eukaryotes happens through use of tubulin, but in prokaryotes either WACA proteins, actin-like or tubulin-like proteins can be used. Cell division is mediated in eukaryotes by actin, but in prokaryotes usually by tubulin-like (often FtsZ-ring) proteins and sometimes (Crenarchaeota) ESCRT-III, which in eukaryotes still has a role in the last step of division.[4]
See also
References
- ^ a b Hardin, Jeff; Bertoni, Gregory; Kleinsmith, Lewis J. (2015). Becker's World of the Cell (8th ed.). New York: Pearson. pp. 422–446. ISBN 978013399939-6.
- ^ McKinley, Michael; Dean O'Loughlin, Valerie; Pennefather-O'Brien, Elizabeth; Harris, Ronald (2015). Human Anatomy (4th ed.). New York: McGraw Hill Education. p. 29. ISBN 0-07-352573-1.
- ^ a b c d e f Alberts, Bruce; et al. (2008). Molecular Biology of the Cell (5th ed.). New York: Garland Science. ISBN 978-0-8153-4105-5.
- ^ a b c d e f Wickstead B, Gull K (Aug 2011). "The evolution of the cytoskeleton". The Journal of Cell Biology. 194 (4): 513–25. doi:10.1083/jcb.201102065. PMC 3160578. PMID 21859859.
- ^ a b c Fletcher DA, Mullins RD (Jan 2010). "Cell mechanics and the cytoskeleton". Nature. 463 (7280): 485–92. Bibcode:2010Natur.463..485F. doi:10.1038/nature08908. PMC 2851742. PMID 20110992.
- ^ a b Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U (Jul 2007). "Intermediate filaments: from cell architecture to nanomechanics". Nature Reviews Molecular Cell Biology. 8 (7): 562–73. doi:10.1038/nrm2197. PMID 17551517.
- ^ Geli MI, Riezman H (Apr 1998). "Endocytic internalization in yeast and animal cells: similar and different". Journal of Cell Science. 111 (8): 1031–7. PMID 9512499.
- ^ Peters RA. "The Harben Lectures, 1929. Reprinted in: Peters, R. A. (1963) Biochemical lesions and lethal synthesis, p. 216. Pergamon Press, Oxford".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Frixione E (Jun 2000). "Recurring views on the structure and function of the cytoskeleton: a 300-year epic". Cell Motility and the Cytoskeleton. 46 (2): 73–94. doi:10.1002/1097-0169(200006)46:2<73::AID-CM1>3.0.CO;2-0. PMID 10891854.
- ^ Minton AP (Oct 1992). "Confinement as a determinant of macromolecular structure and reactivity". Biophysical Journal. 63 (4): 1090–100. Bibcode:1992BpJ....63.1090M. doi:10.1016/S0006-3495(92)81663-6. PMC 1262248. PMID 1420928.
- ^ Doherty GJ, McMahon HT (2008). "Mediation, modulation, and consequences of membrane-cytoskeleton interactions". Annual Review of Biophysics. 37: 65–95. doi:10.1146/annurev.biophys.37.032807.125912. PMID 18573073.
- ^ Alberts, Bruce (2015). Molecular Biology of the Cell. Garland Science. p. 889. ISBN 978-0-8153-4464-3.
- ^ a b c d e Gunning PW, Ghoshdastider U, Whitaker S, Popp D, Robinson RC (Jun 2015). "The evolution of compositionally and functionally distinct actin filaments". Journal of Cell Science. 128 (11): 2009–2019. doi:10.1242/jcs.165563. PMID 25788699.
- ^ Paulin D, Li Z (Nov 2004). "Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle". Experimental Cell Research. 301 (1): 1–7. doi:10.1016/j.yexcr.2004.08.004. PMID 15501438.
- ^ a b Unless else specified in boxes, then ref is:Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1300. ISBN 1-4160-2328-3. Page 25
- ^ Fuchs E, Cleveland DW (Jan 1998). "A structural scaffolding of intermediate filaments in health and disease". Science. 279 (5350): 514–9. Bibcode:1998Sci...279..514F. doi:10.1126/science.279.5350.514. PMID 9438837.
- ^ Steinmetz MO (May 2007). "Structure and thermodynamics of the tubulin-stathmin interaction". Journal of Structural Biology. 158 (2): 137–47. doi:10.1016/j.jsb.2006.07.018. PMID 17029844.
- ^ a b Mostowy S, Cossart P (Mar 2012). "Septins: the fourth component of the cytoskeleton". Nature Reviews Molecular Cell Biology. 13 (3): 183–94. doi:10.1038/nrm3284. PMID 22314400.
- ^ Mascarelli A (December 2011). "Septin proteins take bacterial prisoners: A cellular defence against microbial pathogens holds therapeutic potential". Nature. doi:10.1038/nature.2011.9540.
- ^ "Calpain proteolysis of alpha-II-spectrin in the normal adult human brain". Neurosci. Lett. 316 (1): 41–4. December 2001. doi:10.1016/S0304-3940(01)02371-0. PMID 11720774.
- ^ Pruyne D, Bretscher A (Feb 2000). "Polarization of cell growth in yeast". Journal of Cell Science. 113 (4): 571–85. PMID 10652251.
- ^ Shih YL, Rothfield L (Sep 2006). "The bacterial cytoskeleton". Microbiology and Molecular Biology Reviews. 70 (3): 729–54. doi:10.1128/MMBR.00017-06. PMC 1594594. PMID 16959967.
- ^ Michie KA, Löwe J (2006). "Dynamic filaments of the bacterial cytoskeleton" (PDF). Annual Review of Biochemistry. 75: 467–92. doi:10.1146/annurev.biochem.75.103004.142452. PMID 16756499.
- ^ Briegel A, Dias DP, Li Z, Jensen RB, Frangakis AS, Jensen GJ (Oct 2006). "Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography". Molecular Microbiology. 62 (1): 5–14. doi:10.1111/j.1365-2958.2006.05355.x. PMID 16987173.
- ^ Popp D, Narita A, Maeda K, Fujisawa T, Ghoshdastider U, Iwasa M, Maéda Y, Robinson RC (2010). "Filament structure, organization, and dynamics in MreB sheets". The Journal of Biological Chemistry. 285 (21): 15858–65. doi:10.1074/jbc.M109.095901. PMC 2871453. PMID 20223832.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Popp D, Narita A, Lee LJ, Ghoshdastider U, Xue B, Srinivasan R, Balasubramanian MK, Tanaka T, Robinson RC (2012). "Novel actin-like filament structure from Clostridium tetani". The Journal of Biological Chemistry. 287 (25): 21121–9. doi:10.1074/jbc.M112.341016. PMC 3375535. PMID 22514279.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ausmees N, Kuhn JR, Jacobs-Wagner C (Dec 2003). "The bacterial cytoskeleton: an intermediate filament-like function in cell shape". Cell. 115 (6): 705–13. doi:10.1016/S0092-8674(03)00935-8. PMID 14675535.
External links
- - Cytoskeleton Monthly News and Blog
- - MBInfo - Cytoskeleton Dynamics
- Cytoskeleton, Cell Motility and Motors - The Virtual Library of Biochemistry and Cell Biology
- Cytoskeleton database, clinical trials, recent literature, lab registry ...
- Animation of leukocyte adhesion (Animation with some images of actin and microtubule assembly and dynamics.)
- http://cellix.imba.oeaw.ac.at/ Cytoskeleton and cell motility including videos
- Open access review article on the emergent complexity of the cytoskeleton (appeared in Advances in Physics, 2013)
