Nicotine: Difference between revisions
Undid revision 549990750 by 178.94.224.67 (talk) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{About|the |
{{About|the chemical|other uses|Nicotine (disambiguation)}} |
||
{{Drugbox| Verifiedfields = changed |
{{Drugbox| Verifiedfields = changed |
||
| verifiedrevid = 420440849 |
| verifiedrevid = 420440849 |
||
Revision as of 16:50, 3 May 2013
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nicorette, Nicotrol |
| AHFS/Drugs.com | Monograph |
| Dependence liability | High |
| Routes of administration | smoked (as smoking tobacco, mapacho, etc.), insufflated (as tobacco snuff or nicotine nasal spray), chewed (as nicotine gum, tobacco gum or chewing tobacco), transdermal (as nicotine patch, nicogel or topical tobacco paste), intrabuccal (as dipping tobacco, snuffs, dissolvable tobacco or creamy snuff), vaporized (as electronic cigarette, etc.), directly inhaled (as nicotine inhaler), oral (as nicotini), buccal (as snus) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20 to 45% (oral) |
| Metabolism | hepatic |
| Elimination half-life | 2 hours; 20 hours active metabolite (cotinine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.177 |
| Chemical and physical data | |
| Formula | C10H14N2 |
| Molar mass | 162.12 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.01 g/cm3 |
| Melting point | −79 °C (−110 °F) |
| Boiling point | 247 °C (477 °F) |
| |
| |
| | |
Nicotine is a potent parasympathomimetic alkaloid found in the nightshade family of plants (Solanaceae). It acts as a nicotinic acetylcholine receptor agonist. It is made in the roots and accumulates in the leaves of the plants. It constitutes approximately 0.6–3.0% of the dry weight of tobacco[2] and is present in the range of 2–7 µg/kg of various edible plants.[3] It functions as an antiherbivore chemical; therefore, nicotine was widely used as an insecticide in the past[4][5][6] and nicotine analogs such as imidacloprid are currently widely used.
In smaller doses (an average cigarette yields about 1 mg of absorbed nicotine), the substance acts as a stimulant in mammals, while high amounts (30–60 mg[7]) can be fatal.[8] This stimulant effect is likely a major contributing factor to the dependence-forming properties of tobacco smoking.[citation needed] According to the American Heart Association, nicotine addiction has historically been one of the hardest addictions to break, while the pharmacological and behavioral characteristics that determine tobacco addiction are similar to those determining addiction to heroin and cocaine. The nicotine content of popular American-brand cigarettes has slowly increased over the years, and one study found that there was an average increase of 1.78% per year between the years of 1998 and 2005. This was found for all major market categories of cigarettes.[9]
Research in 2011 has found that nicotine inhibits chromatin-modifying enzymes (class I and II histone deacetylases) which increases the ability of cocaine to cause an addiction.[10]
History and name
Nicotine is named after the tobacco plant Nicotiana tabacum, which in turn is named after the French ambassador in Portugal, Jean Nicot de Villemain, who sent tobacco and seeds to Paris in 1560, and who promoted their medicinal use. The tobacco and seeds were brought to ambassador Nicot from Brazil by Luis de Gois, a Portuguese colonist in São Paulo. Nicotine was first isolated from the tobacco plant in 1828 by physician Wilhelm Heinrich Posselt and chemist Karl Ludwig Reimann of Germany, who considered it a poison.[11][12] Its chemical empirical formula was described by Melsens in 1843,[13] its structure was discovered by Adolf Pinner and Richard Wolffenstein in 1893,[14][clarification needed] and it was first synthesized by Amé Pictet and A. Rotschy in 1904.[15]
Historical use of nicotine as an insecticide
Tobacco was introduced to Europe in 1559, and by the late 17th century, it was used not only for smoking but also as an insecticide. After World War II, over 2,500 tons of nicotine insecticide (waste from the tobacco industry) were used worldwide, but by the 1980s the use of nicotine insecticide had declined below 200 tons. This was due to the availability of other insecticides that are cheaper and less harmful to mammals.[5]
Currently, nicotine is a permitted pesticide for organic farming because it is derived from a botanical source. Nicotine sulfate sold for use as a pesticide is labeled "DANGER," indicating that it is highly toxic.[6] However, in 2008, the EPA received a request to cancel the registration of the last nicotine pesticide registered in the United States.[16] This request was granted, and after 1 January 2014, this pesticide will not be available for sale.[17]
Chemistry
Nicotine is a hygroscopic, oily liquid that is miscible with water in its base form. As a nitrogenous base, nicotine forms salts with acids that are usually solid and water soluble, for example nicotine sulfate which, being a solid, is easier to handle in its use as an insecticide. (For retail use it is sold as solution in water ready for spraying.)[6] Its flash point is 95°C and its auto-ignition temperature is 244°C.[18]
Optical activity
Nicotine is optically active, having two enantiomeric forms. The naturally occurring form of nicotine is levorotatory with a specific rotation of [α]D = –166.4° ((−)-nicotine). The dextrorotatory form, (+)-nicotine is physiologically less active than (–)-nicotine. (−)-nicotine is more toxic than (+)-nicotine.[19] The salts of (+)-nicotine are usually dextrorotatory.
Biosynthesis

The biosynthetic pathway of nicotine involves a coupling reaction between the two cyclic structures that compose nicotine. Metabolic studies show that the pyridine ring of nicotine is derived from niacin (nicotinic acid) while the pyrrolidone is derived from N-methyl-Δ1-pyrrollidium cation.[20][21] Biosynthesis of the two component structures proceeds via two independent syntheses, the NAD pathway for niacin and the tropane pathway for N-methyl-Δ1-pyrrollidium cation.
The NAD pathway in the genus nicotiana begins with the oxidation of aspartic acid into α-imino succinate by aspartate oxidase (AO). This is followed by a condensation with glyceraldehyde-3-phosphate and a cyclization catalyzed by quinolinate synthase (QS) to give quinolinic acid. Quinolinic acid then reacts with phosphoriboxyl pyrophosphate catalyzed by quinolinic acid phosphoribosyl transferase (QPT) to form niacin mononucleotide (NaMN). The reaction now proceeds via the NAD salvage cycle to produce niacin via the conversion of nicotinamide by the enzyme nicotinamidase.
The N-methyl-Δ1-pyrrollidium cation used in the synthesis of nicotine is an intermediate in the synthesis of tropane-derived alkaloids. Biosynthesis begins with decarboxylation of ornithine by ornithine decarboxylase (ODC) to produce putrescine. Putrescine is then converted into N-methyl putrescine via methylation by SAM catalyzed by putrescine N-methyltransferase (PMT). N-methylputrescine then undergoes deamination into 4-methylaminobutanal by the N-methylputrescine oxidase (MPO) enzyme, 4-methylaminobutanal then spontaneously cyclize into N-methyl-Δ1-pyrrollidium cation.
The final step in the synthesis of nicotine is the coupling between N-methyl-Δ1-pyrrollidium cation and niacin. Although studies conclude some form of coupling between the two component structures, the definite process and mechanism remains undetermined. The current agreed theory involves the conversion of niacin into 2,5-dihydropyridine through 3,6-dihydronicotinic acid. The 2,5-dihydropyridine intermediate would then react with N-methyl-Δ1-pyrrollidium cation to form enantiomerically pure (–)-nicotine.[22]
Pharmacology
Pharmacokinetics
As nicotine enters the body, it is distributed quickly through the bloodstream and crosses the blood–brain barrier reaching the brain within 10–20 seconds after inhalation.[24] The elimination half-life of nicotine in the body is around two hours.[25]
The amount of nicotine absorbed by the body from smoking depends on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. For chewing tobacco, dipping tobacco, snus and snuff, which are held in the mouth between the lip and gum, or taken in the nose, the amount released into the body tends to be much greater than smoked tobacco.[clarification needed] Nicotine is metabolized in the liver by cytochrome P450 enzymes (mostly CYP2A6, and also by CYP2B6). A major metabolite is cotinine.
Other primary metabolites include nicotine N'-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide.[26] Under some conditions, other substances may be formed such as myosmine.[27]
Glucuronidation and oxidative metabolism of nicotine to cotinine are both inhibited by menthol, an additive to mentholated cigarettes, thus increasing the half-life of nicotine in vivo.[28]
Detection of use
Medical detection
Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids.[29][30] Nicotine use is not regulated in competitive sports programs, yet the drug has been shown to have a significant beneficial effect on athletic endurance in subjects who have not used nicotine before.[31]
Pharmacodynamics
Nicotine acts on the nicotinic acetylcholine receptors, specifically the ganglion type nicotinic receptor and one CNS nicotinic receptor. The former is present in the adrenal medulla and elsewhere, while the latter is present in the central nervous system (CNS). In small concentrations, nicotine increases the activity of these receptors. Nicotine also has effects on a variety of other neurotransmitters through less direct mechanisms.
In the central nervous system

By binding to nicotinic acetylcholine receptors, nicotine increases the levels of several neurotransmitters – acting as a sort of "volume control". It is thought that increased levels of dopamine in the reward circuits of the brain are responsible for the apparent euphoria and relaxation, and addiction caused by nicotine consumption. Nicotine has a higher affinity for acetylcholine receptors in the brain than those in skeletal muscle, though at toxic doses it can induce contractions and respiratory paralysis.[32] Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.[33]
Tobacco smoke contains anabasine, anatabine, and nornicotine. It also contains the monoamine oxidase inhibitors harman and norharman.[34] These beta-carboline compounds significantly decrease MAO activity in smokers.[34][35] MAO enzymes break down monoaminergic neurotransmitters such as dopamine, norepinephrine, and serotonin. It is thought that the powerful interaction between the MAOIs and the nicotine is responsible for most of the addictive properties of tobacco smoking.[36] The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats.[37]
Chronic nicotine exposure via tobacco smoking up-regulates alpha4beta2* nAChR in cerebellum and brainstem regions[38][39] but not habenulopeduncular structures.[40] Alpha4beta2 and alpha6beta2 receptors, present in the ventral tegmental area, play a crucial role in mediating the reinforcement effects of nicotine.[41]
In the sympathetic nervous system
Nicotine also activates the sympathetic nervous system,[42] acting via splanchnic nerves to the adrenal medulla, stimulates the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and noradrenaline) into the bloodstream. Nicotine also has an affinity for melanin-containing tissues due to its precursor function in melanin synthesis or due to the irreversible binding of melanin and nicotine. This has been suggested to underlie the increased nicotine dependence and lower smoking cessation rates in darker pigmented individuals. However, further research is warranted before a definite conclusive link can be inferred.[43]
In adrenal medulla
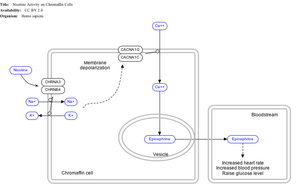
By binding to ganglion type nicotinic receptors in the adrenal medulla nicotine increases flow of adrenaline (epinephrine), a stimulating hormone and neurotransmitter. By binding to the receptors, it causes cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of epinephrine (and norepinephrine) into the bloodstream. The release of epinephrine (adrenaline) causes an increase in heart rate, blood pressure and respiration, as well as higher blood glucose levels.[44]
Nicotine is the natural product of tobacco, having a half-life of 1 to 2 hours. Cotinine is an active metabolite of nicotine that remains in the blood for 18 to 20 hours, making it easier to analyze due to its longer half-life.[45]
Psychoactive effects
Nicotine's mood-altering effects are different by report: in particular it is both a stimulant and a relaxant.[46] First causing a release of glucose from the liver and epinephrine (adrenaline) from the adrenal medulla, it causes stimulation. Users report feelings of relaxation, sharpness, calmness, and alertness.[47] Like any stimulant, it may very rarely cause the often uncomfortable neuropsychiatric effect of akathisia. By reducing the appetite and raising the metabolism, some smokers may lose weight as a consequence.[48][49]
When a cigarette is smoked, nicotine-rich blood passes from the lungs to the brain within seven seconds and immediately stimulates the release of many chemical messengers such as acetylcholine, norepinephrine, epinephrine, vasopressin, histamine, arginine, serotonin, dopamine, autocrine agents, and beta-endorphin.[50] This release of neurotransmitters and hormones is responsible for most of nicotine's effects. Nicotine appears to enhance concentration[51] and memory due to the increase of acetylcholine. It also appears to enhance alertness due to the increases of acetylcholine and norepinephrine. Arousal is increased by the increase of norepinephrine. Pain is reduced by the increases of acetylcholine and beta-endorphin. Anxiety is reduced by the increase of beta-endorphin. Nicotine also extends the duration of positive effects of dopamine[52] and increases sensitivity in brain reward systems.[53] Most cigarettes (in the smoke inhaled) contain 1 to 3 milligrams of nicotine.[54]
Research suggests that, when smokers wish to achieve a stimulating effect, they take short quick puffs, which produce a low level of blood nicotine.[55] This stimulates nerve transmission. When they wish to relax, they take deep puffs, which produce a high level of blood nicotine, which depresses the passage of nerve impulses, producing a mild sedative effect. At low doses, nicotine potently enhances the actions of norepinephrine and dopamine in the brain, causing a drug effect typical of those of psychostimulants. At higher doses, nicotine enhances the effect of serotonin and opiate activity, producing a calming, pain-killing effect. Nicotine is unique in comparison to most drugs, as its profile changes from stimulant to sedative/pain killer in increasing dosages and use.
Technically, nicotine is not significantly addictive, as nicotine administered alone does not produce significant reinforcing properties.[56] However, after coadministration with an MAOI, such as those found in tobacco, nicotine produces significant behavioral sensitization, a measure of addiction potential. This is similar in effect to amphetamine.[36]
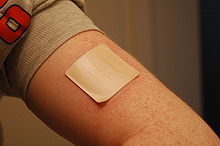
Nicotine gum, usually in 2-mg or 4-mg doses, and nicotine patches are available, as well as smokeless tobacco, nicotine lozenges and electronic cigarettes.
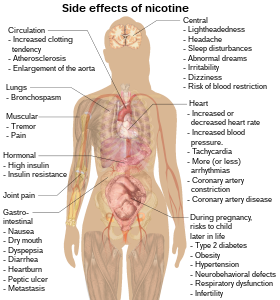
Side effects
Nicotine increases blood pressure and heart rate in humans.[59] Nicotine can stimulate abnormal proliferation of vascular endothelial cells, similar to that seen in atherosclerosis.[60] Nicotine induces potentially atherogenic genes in human coronary artery endothelial cells.[61] Nicotine could cause microvascular injury through its action on nicotinic acetylcholine receptors (nAChRs),[62] but other mechanisms are also likely at play.
A study on rats showed that nicotine exposure abolishes the beneficial and protective effects of estrogen on the hippocampus,[63] an estrogen-sensitive region of the brain involved in memory formation and retention.
Dependence and withdrawal
Modern research shows that nicotine acts on the brain to produce a number of effects. Specifically, research examining its addictive nature has been found to show that nicotine activates the mesolimbic pathway ("reward system") – the circuitry within the brain that regulates feelings of pleasure and euphoria.[64]
Dopamine is one of the key neurotransmitters actively involved in the brain. Research shows that by increasing the levels of dopamine within the reward circuits in the brain, nicotine acts as a chemical with intense addictive qualities. In many studies it has been shown to be more addictive than cocaine and heroin.[65][66][67] Like other physically addictive drugs, nicotine withdrawal causes downregulation of the production of dopamine and other stimulatory neurotransmitters as the brain attempts to compensate for artificial stimulation. As dopamine regulates the sensitivity of nicotinic acetylcholine receptors decreases. To compensate for this compensatory mechanism, the brain in turn upregulates the number of receptors, convoluting its regulatory effects with compensatory mechanisms meant to counteract other compensatory mechanisms. An example is the increase in norepinephrine, one of the successors to dopamine, which inhibit reuptake of the glutamate receptors,[68] in charge of memory and cognition. The net effect is an increase in reward pathway sensitivity, the opposite of other addictive drugs such as cocaine and heroin, which reduce reward pathway sensitivity.[53] This neuronal brain alteration can persist for months after administration ceases.
A study found that nicotine exposure in adolescent mice retards the growth of the dopamine system, thus increasing the risk of substance abuse during adolescence.[69]
Immunology prevention

Because of the severe addictions and the harmful effects of smoking, vaccination protocols have been developed. The principle operates under the premise that if an antibody is attached to a nicotine molecule, it will be prevented from diffusing through the capillaries, thus making it less likely that it ever affects the brain by binding to nicotinic acetylcholine receptors.
These include attaching the nicotine molecule as a hapten to a protein carrier such as Keyhole limpet hemocyanin or a safe modified bacterial toxin to elicit an active immune response. Often it is added with bovine serum albumin.
Additionally, because of concerns with the unique immune systems of individuals being liable to produce antibodies against endogenous hormones and over the counter drugs, monoclonal antibodies have been developed for short term passive immune protection. They have half-lives varying from hours to weeks. Their half-lives depend on their ability to resist degradation from pinocytosis by epithelial cells.[70]
Toxicology
| NFPA 704 | ||||
|---|---|---|---|---|
| ||||
The LD50 of nicotine is 50 mg/kg for rats and 3 mg/kg for mice. 30–60 mg (0.5–1.0 mg/kg) can be a lethal dosage for adult humans.[7][71] Nicotine therefore has a high toxicity in comparison to many other alkaloids such as cocaine, which has an LD50 of 95.1 mg/kg when administered to mice. It is unlikely that a person would overdose on nicotine through smoking alone, although overdose can occur through combined use of nicotine patches or nicotine gum and cigarettes at the same time.[8] Spilling a high concentration of nicotine onto the skin can cause intoxication or even death, since nicotine readily passes into the bloodstream following dermal contact.[72]
Historically, nicotine has not been regarded as a carcinogen and the IARC has not evaluated nicotine in its standalone form or assigned it to an official carcinogen group. While no epidemiological evidence supports that nicotine alone acts as a carcinogen in the formation of human cancer (on the contrary, a mechanism of urinary excretion of nicotine metabolites was identified as the link between smoking and bladder cancer [73]), research over the last decade has identified nicotine's carcinogenic potential in animal models and cell culture.[74][75] Nicotine has been noted to directly cause cancer through a number of different mechanisms such as the activation of MAP Kinases.[76] Indirectly, nicotine increases cholinergic signalling (and adrenergic signalling in the case of colon cancer[77]), thereby impeding apoptosis (programmed cell death), promoting tumor growth, and activating growth factors and cellular mitogenic factors such as 5-LOX, and EGF. Nicotine also promotes cancer growth by stimulating angiogenesis and neovascularization.[78][79] In one study, nicotine administered to mice with tumors caused increases in tumor size (twofold increase), metastasis (nine-fold increase), and tumor recurrence (threefold increase).[80]
The teratogenic properties of nicotine has been investigated. According to a study of ca. 77,000 pregnant women in Denmark[citation needed], women who used nicotine gum and patches during the early stages of pregnancy were found to face an increased risk of having babies with birth defects. The study showed that women who used nicotine-replacement therapy in the first 12 weeks of pregnancy had a 60% greater risk of having babies with birth defects compared to women who were non-smokers.
Tobacco use among pregnant women has also been correlated to increased frequency of ADHD. Children born to mothers who used tobacco were two and a half times more likely to be diagnosed with ADHD.[81] Froelich estimated that "exposure to higher levels of lead and prenatal tobacco each accounted for 500,000 additional cases of ADHD in U.S. children".[82]
Effective April 1, 1990, the Office of Environmental Health Hazard Assessment (OEHHA) of the California Environmental Protection Agency added nicotine to the list of chemicals known to cause developmental toxicity.[83]
Therapeutic uses
The primary therapeutic use of nicotine is in treating nicotine dependence in order to eliminate smoking with the damage it does to health. Controlled levels of nicotine are given to patients through gums, dermal patches, lozenges, electronic/substitute cigarettes or nasal sprays in an effort to wean them off their dependence.
However, in a few situations, smoking has been observed to be of therapeutic value. These are often referred to as "Smoker’s Paradoxes".[84] Although in most cases the actual mechanism is understood only poorly or not at all, it is generally believed that the principal beneficial action is due to the nicotine administered, and that administration of nicotine without smoking may be as beneficial as smoking, without the higher risk to health due to tar and other ingredients found in tobacco.
For instance, studies suggest that smokers require less frequent repeated revascularization after percutaneous coronary intervention (PCI).[84] Risk of ulcerative colitis has been frequently shown to be reduced by smokers on a dose-dependent basis; the effect is eliminated if the individual stops smoking.[85][86] Smoking also appears to interfere with development of Kaposi's sarcoma in patients with HIV.[87][88]
Nicotine reduces the chance of preeclampsia,[89] and atopic disorders such as allergic asthma.[90][dubious ] A plausible mechanism of action in these cases may be nicotine acting as an anti-inflammatory agent, and interfering with the inflammation-related disease process, as nicotine has vasoconstrictive effects.[91]
Tobacco smoke has been shown to contain compounds capable of inhibiting monoamine oxidase, which is responsible for the degradation of dopamine in the human brain. When dopamine is broken down by MAO-B, neurotoxic by-products are formed, possibly contributing to Parkinson's and Alzheimers disease.[92]
Many such papers regarding Alzheimer's disease[93] and Parkinson's Disease[94] have been published. While tobacco smoking is associated with an increased risk of Alzheimer's disease,[95] there is evidence that nicotine itself has the potential to prevent and treat Alzheimer's disease.[96] Nicotine has been shown to delay the onset of Parkinson's disease in studies involving monkeys and humans.[97][98][99] A study has shown a protective effect of nicotine itself on neurons due to nicotine activation of α7-nAChR and the PI3K/Akt pathway which inhibits apoptosis-inducing factor release and mitochondrial translocation, cytochrome c release and caspase 3 activation.[100]
Studies have indicated that nicotine can be used to help adults suffering from autosomal dominant nocturnal frontal lobe epilepsy. The same areas that cause seizures in that form of epilepsy are responsible for processing nicotine in the brain.[101]
Studies suggest a correlation between smoking and schizophrenia, with estimates near 75% for the proportion of schizophrenic patients who smoke. Although the nature of this association remains unclear, it has been argued that the increased level of smoking in schizophrenia may be due to a desire to self-medicate with nicotine.[102][103] Other research found that mildly dependent users got some benefit from nicotine, but not those who were highly dependent.[104]
Research at Duke University Medical Center found that nicotine may improve the symptoms of depression.[105] Nicotine appears to improve ADHD symptoms. Some studies have focused on benefits of nicotine therapy in adults with ADHD.[106]
While acute/initial nicotine intake causes activation of nicotine receptors, chronic low doses of nicotine use leads to desensitisation of nicotine receptors (due to the development of tolerance) and results in an antidepressant effect, with research showing low dose nicotine patches being an effective treatment of major depressive disorder in non-smokers.[107]
Nicotine (in the form of chewing gum or a transdermal patch) has been explored as an experimental treatment for OCD. Small studies show some success, even in otherwise treatment-refractory cases.[108][109][110]
The relationship between smoking and inflammatory bowel disease has been firmly established, but remains a source of confusion among both patients and doctors. It is negatively associated with ulcerative colitis but positively associated with Crohn's disease. In addition, it has opposite influences on the clinical course of the two conditions with benefit in ulcerative colitis but a detrimental effect in Crohn's disease.[111][112]
In media
In some anti-smoking literature, the harm that tobacco smoking and nicotine addiction does is personified as Nick O'Teen, represented as a humanoid with some aspect of a cigarette or cigarette butt about him or his clothes.[113]
See also
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Smoking and Tobacco Control Monograph No. 9" (PDF). Retrieved 2012-12-19.
- ^ "Determination of the Nicotine Content of Various Edible Nightshades (Solanaceae) and Their Products and Estimation of the Associated Dietary Nicotine Intake". Retrieved 2008-10-05.
- ^ Rodgman, Alan; Perfetti, Thomas A. (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 1-4200-7883-6.
- ^ a b Ujváry, István (1999). "Nicotine and Other Insecticidal Alkaloids". In Yamamoto, Izuru; Casida, John (eds.). Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 29–69.
- ^ a b c "Some Pesticides Permitted in Organic Gardening".
- ^ a b "Nicotine (PIM)". Inchem.org. Retrieved 2012-12-19.
- ^ a b Genetic Science Learning Center. "How Drugs Can Kill".
- ^ Connolly, G. N; Alpert, H. R; Wayne, G. F; Koh, H (2007). "Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997–2005". Tobacco Control. 16 (5): e5. doi:10.1136/tc.2006.019695. PMC 2598548. PMID 17897974.
- ^ Volkow ND (2011). "Epigenetics of nicotine: another nail in the coughing". Sci Transl Med. 3 (107): 107ps43. doi:10.1126/scitranslmed.3003278. PMID 22049068.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Posselt, W.; Reimann, L. (1828). "Chemische Untersuchung des Tabaks und Darstellung eines eigenthümlich wirksamen Prinzips dieser Pflanze". Magazin für Pharmacie (in German). 6 (24): 138–161.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Henningfield JE, Zeller M (2006). "Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward". Psychopharmacology (Berl.). 184 (3–4): 286–91. doi:10.1007/s00213-006-0308-4. PMID 16463054.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Melsens, Louis-Henri-Frédéric (1843) "Note sur la nicotine," Annales de chimie et de physique, third series, vol. 9, pages 465-479; see especially page 470. [Note: The empirical formula that Melsens provides is incorrect because at that time, chemists used the wrong atomic mass for carbon (6 instead of 12).]
- ^ See:
- A. Pinner and R. Wolffenstein (1891) "Ueber Nicotin," Berichte der deutschen chemischen Gesellschaft, vol. 24, no.1, pages 1373-1377.
- A. Pinner (1893) "Ueber Nicotin. Die Constitution des Alkaloïds," Berichte der deutschen chemischen Gesellschaft, vol. 26, pages 292-305.
- A. Pinner (1893) "Ueber Nicotin. I. Mitteilung," Archive für Pharmacie, vol. 231, pages 378-448.
- ^ Amé Pictet and A. Rotschy (1904) "Synthese des Nicotins," Berichte der Deutschen Chemischen Gesellschaft, vol. 37, pages 1225-1235.
- ^ USEPA (29 October 2008). "Nicotine; Notice of Receipt of Request to Voluntarily Cancel a Pesticide Registration". Federal Register: 64320–64322. Retrieved 8 April 2012.
- ^ USEPA (3 June 2009). "Nicotine; Product Cancellation Order". Federal Register: 26695–26696. Retrieved 8 April 2012.
- ^ www.sciencelab.com/msds.php?msdsId=9926222 Material Safety Data Sheet L-Nicotine MSDS
- ^ Gause, G. F. (1941). "Chapter V: Analysis of various biological processes by the study of the differential action of optical isomers". In Luyet, B. J. (ed.). Optical Activity and Living Matter. A series of monographs on general physiology. Vol. 2. Normandy, Missouri: Biodynamica.
- ^ "Biochimica et Biophysica Acta - Ornithine as a precursor for the pyrrolidine ring of nicotine". ScienceDirect.com. 2002-12-16. Retrieved 2012-12-19.
- ^ Dawson RF, Christman DR, D'Adamo A, Solt ML, Wolf AP (1960). "The Biosynthesis of Nicotine from Isotopically Labeled Nicotinic Acids". Journal of the American Chemical Society. 82 (10): 2628–2633. doi:10.1021/ja01495a059.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Komamine, edited by Hiroshi Ashihara, Alan Crozier, Atsushi. Plant metabolism and biotechnology. Cambridge: Wiley. ISBN 9780470747032.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ References and comments are found in image description in Commons.
- ^ Le Houezec J (2003). "Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review". Int. J. Tuberc. Lung Dis. 7 (9): 811–9. PMID 12971663.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Benowitz NL, Jacob P, Jones RT, Rosenberg J (1982). "Interindividual variability in the metabolism and cardiovascular effects of nicotine in man". J. Pharmacol. Exp. Ther. 221 (2): 368–72. PMID 7077531.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hukkanen J, Jacob P, Benowitz NL (2005). "Metabolism and disposition kinetics of nicotine". Pharmacol. Rev. 57 (1): 79–115. doi:10.1124/pr.57.1.3. PMID 15734728.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "The danger of third-hand smoke". Chromatography Online. 7 (3). 22 February 2011.
- ^ Benowitz NL, Herrera B, Jacob P 3rd., NL; Herrera, B; Jacob P, 3rd (2004). "Mentholated Cigarette Smoking Inhibits Nicotine Metabolism". J Pharmacol Exp Ther. 310 (3): 1208–15. doi:10.1124/jpet.104.066902. PMID 15084646.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Benowitz NL, Hukkanen J, Jacob P (2009). "Nicotine chemistry, metabolism, kinetics and biomarkers". Handbook of Experimental Pharmacology. 192: 29–60. doi:10.1007/978-3-540-69248-5_2. ISBN 978-3-540-69246-1. PMC 2953858. PMID 19184645.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Baselt, Randall Clint (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Biomedical Publications. pp. 1103–7. ISBN 978-0-9626523-7-0.
- ^ Mündel, T. and Jones, D. A. (2006). "Effect of transdermal nicotine administration on exercise endurance in men". Exp Physiol. 91 (4): 705–713. doi:10.1113/expphysiol.2006.033373. PMID 16627574.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Katzung, Bertram G. (2006). Basic and Clinical Pharmacology. New York: McGraw-Hill Medical. pp. 99–105.
- ^ Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA (2009). "Nicotine binding to brain receptors requires a strong cation-pi interaction". Nature. 458 (7237): 534–7. doi:10.1038/nature07768. PMC 2755585. PMID 19252481.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Herraiz T, Chaparro C (2005). "Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors". Biochem. Biophys. Res. Commun. 326 (2): 378–86. doi:10.1016/j.bbrc.2004.11.033. PMID 15582589.
- ^ Fowler JS, Volkow ND, Wang GJ; et al. (1998). "Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition". J Addict Dis. 17 (1): 23–34. doi:10.1300/J069v17n01_03. PMID 9549600.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Villégier AS, Blanc G, Glowinski J, Tassin JP (2003). "Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors". Pharmacol. Biochem. Behav. 76 (2): 267–74. doi:10.1016/S0091-3057(03)00223-5. PMID 14592678.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, Tassin JP (2006). "Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine". Neuropsychopharmacology. 31 (8): 1704–13. doi:10.1038/sj.npp.1300987. PMID 16395299.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schütz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J (2008). "Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain". Neurosci. Lett. 430 (1): 34–7. doi:10.1016/j.neulet.2007.10.011. PMID 17997038.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Walsh H, Govind AP, Mastro R; et al. (2008). "Up-regulation of nicotinic receptors by nicotine varies with receptor subtype". J. Biol. Chem. 283 (10): 6022–32. doi:10.1074/jbc.M703432200. PMID 18174175.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Nguyen HN, Rasmussen BA, Perry DC (2003). "Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography". J. Pharmacol. Exp. Ther. 307 (3): 1090–7. doi:10.1124/jpet.103.056408. PMID 14560040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pons S, Fattore L, Cossu G; et al. (2008). "Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration". J. Neurosci. 28 (47): 12318–27. doi:10.1523/JNEUROSCI.3918-08.2008. PMC 2819191. PMID 19020025.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yoshida T, Sakane N, Umekawa T, Kondo M (1994). "Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress". Physiol Behav. 55 (1): 53–7. doi:10.1016/0031-9384(94)90009-4. PMID 8140174.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET (2009). "Link between facultative melanin and tobacco use among African Americans". Pharmacol. Biochem. Behav. 92 (4): 589–96. doi:10.1016/j.pbb.2009.02.011. PMID 19268687.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Elaine N. Marieb and Katja Hoehn (2007). Human Anatomy & Physiology (7th Ed.). Pearson. pp. ?. ISBN 0-8053-5909-5.
- ^ "Detection of Cotinine in Blood Plasma by HPLC MS/MS" (PDF). MIT Undergraduate Research Journal. 8. Massachusetts Institute of Technology. Spring 2003.
- ^ Effective Clinical Tobacco Intervention, Therapeutics Letter, issue 21, September–October 1997, University of British Columbia
- ^ Lagrue G, Lebargy F, Cormier A (2001). "From nicotinic receptors to smoking dependence: therapeutic prospects". Alcoologie et Addictologie. 23 (2S): 39S–42.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Orsini J- (2001). "Dependence on tobacco smoking and brain systems controlling glycemia and appetite". Alcoologie et Addictologie. 23 (2S): 28S–36S.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Smokers lose their appetite : Media Releases : News : The University of Melbourne
- ^ Chemically Correct: Nicotine, Andrew Novick
- ^ Rusted J, Graupner L, O'Connell N, Nicholls C (1994). "Does nicotine improve cognitive function?". Psychopharmacology (Berl.). 115 (4): 547–9. doi:10.1007/BF02245580. PMID 7871101.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Easton, John (March 28, 2002). "Nicotine extends duration of pleasant effects of dopamine". The University of Chicago Chronicle. 21 (12).
- ^ a b Kenny PJ, Markou A (2006). "Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity". Neuropsychopharmacology. 31 (6): 1203–11. doi:10.1038/sj.npp.1300905. PMID 16192981.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Erowid Nicotine Vault : Dosage". Erowid.org. 2011-10-14. Retrieved 2012-12-19.
- ^ Einstein, Stanley (1989). Drug and Alcohol Use: Issues and Factors. Springer. pp. 101–118. ISBN 0-306-41378-7.
- ^ Guillem K, Vouillac C, Azar MR; et al. (2005). "Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats". J. Neurosci. 25 (38): 8593–600. doi:10.1523/JNEUROSCI.2139-05.2005. PMID 16177026.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Stead LF, Perera R, Bullen C, Mant D, Lancaster T (2008). Stead, Lindsay F (ed.). "Nicotine replacement therapy for smoking cessation". Cochrane Database Syst Rev (1): CD000146. doi:10.1002/14651858.CD000146.pub3. PMID 18253970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Millstone, Ken (February 13, 2007). "Nixing the patch: Smokers quit cold turkey". Columbia.edu News Service. Retrieved May 23, 2010.
- ^ Sabha M, Tanus-Santos JE, Toledo JC, Cittadino M, Rocha JC, Moreno H (2000). "Transdermal nicotine mimics the smoking-induced endothelial dysfunction". Clin. Pharmacol. Ther. 68 (2): 167–74. doi:10.1067/mcp.2000.108851. PMID 10976548.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Glass CK, Witztum JL (2001). "Atherosclerosis. the road ahead". Cell. 104 (4): 503–16. PMID 11239408.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zhang S, Day I, Ye S (2001). "Nicotine induced changes in gene expression by human coronary artery endothelial cells". Atherosclerosis. 154 (2): 277–83. PMID 11166759.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hawkins BT, Brown RC, Davis TP (2002). "Smoking and ischemic stroke: a role for nicotine?". Trends Pharmacol. Sci. 23 (2): 78–82. doi:10.1016/S0165-6147(02)01893-X. PMID 11830264.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Raval AP, Bhatt A, Saul I (2009). "Chronic nicotine exposure inhibits 17beta-estradiol-mediated protection of the hippocampal CA1 region against cerebral ischemia in female rats". Neurosci. Lett. 458 (2): 65–9. doi:10.1016/j.neulet.2009.04.021. PMID 19442878.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ National Institute on Drug Abuse (June 2009). "Extent, Impact, Delivery, and Addictiveness". Tobacco Addiction. NIDA Report Research Series. Bethesda MA: National Institutes of Health. 09-4342.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Hilts, Philip J. (1994-08-02). "Is Nicotine Addictive? It Depends on Whose Criteria You Use". The New York Times.
- ^ Blakeslee, Sandra (1987-03-29). "Nicotine: Harder to Kick...Than Heroin". The New York Times.
- ^ "Division of Periodontology: Tobacco Use Cessation Program". .umn.edu. Retrieved 2012-12-19.
- ^ Yoshida T, Nishioka H, Nakamura Y, Kondo M (1984). "Reduced norepinephrine turnover in mice with monosodium glutamate-induced obesity". Metab. Clin. Exp. 33 (11): 1060–3. doi:10.1016/0026-0495(84)90238-5. PMID 6493048.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nolley EP, Kelley BM (2007). "Adolescent reward system perseveration due to nicotine: studies with methylphenidate". Neurotoxicol Teratol. 29 (1): 47–56. doi:10.1016/j.ntt.2006.09.026. PMID 17129706.
- ^ Peterson EC, Owens SM (2009). "Designing immunotherapies to thwart drug abuse". Mol. Interv. 9 (3): 119–24. doi:10.1124/mi.9.3.5. PMC 2743871. PMID 19592672.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T (1994). "Effects of aging on acute toxicity of nicotine in rats". Pharmacol Toxicol. 75 (1): 1–6. doi:10.1111/j.1600-0773.1994.tb00316.x. PMID 7971729.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lockhart LP (1933). "Nicotine poisoning". Br Med J. 1 (3762): 246–7. doi:10.1136/bmj.1.3762.246-c.
- ^ http://cetp.fmed.ulaval.ca/Tabagisme/Medias/66722/PDF/Module6_8.pdf
- ^ Hecht SS (1999). "Tobacco smoke carcinogens and lung cancer". J. Natl. Cancer Inst. 91 (14): 1194–210. doi:10.1093/jnci/91.14.1194. PMID 10413421.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wu WK, Cho CH (2004). "The pharmacological actions of nicotine on the gastrointestinal tract". J. Pharmacol. Sci. 94 (4): 348–58. doi:10.1254/jphs.94.348. PMID 15107574.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chowdhury P, Udupa KB (2006). "Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation". World J. Gastroenterol. 12 (46): 7428–32. PMID 17167829.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH (2007). "Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation". Toxicol. Sci. 97 (2): 279–87. doi:10.1093/toxsci/kfm060. PMID 17369603.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M (2003). "Nicotine enhances neovascularization and promotes tumor growth". Mol. Cells. 16 (2): 143–6. PMID 14651253.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH (2004). "Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway". J. Pharmacol. Exp. Ther. 308 (1): 66–72. doi:10.1124/jpet.103.058321. PMID 14569062.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Davis R, Rizwani W, Banerjee S; et al. (2009). Pao, William (ed.). "Nicotine promotes tumor growth and metastasis in mouse models of lung cancer". PLoS ONE. 4 (10): e7524. Bibcode:2009PLoSO...4.7524D. doi:10.1371/journal.pone.0007524. PMC 2759510. PMID 19841737.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Rauch, Stephen and Lanphear, Bruce (2012). "Prevention of Disability in Children:Elevating the Role of Environment" (PDF). Future of Children. 22 (1).
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tanya Froelich; et al. (2009). "Association of Tobacco and Lead Exposures with Attention-Deficit/Hyperactivity Disorder". Pediatrics (124).
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ http://oehha.ca.gov/prop65/prop65_list/files/P65single121809.pdf
- ^ a b Cohen DJ, Doucet M, Cutlip DE, Ho KK, Popma JJ, Kuntz RE (2001). "Impact of smoking on clinical and angiographic restenosis after percutaneous coronary intervention: another smoker's paradox?". Circulation. 104 (7): 773–8. doi:10.1161/hc3201.094225. PMID 11502701.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Longmore, M., Wilkinson, I., Torok, E. Oxford Handbook of Clinical Medicine (5th ed.). p. 232.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Green JT, Richardson C, Marshall RW, Rhodes J, McKirdy HC, Thomas GA, Williams GT (2000). "Nitric oxide mediates a therapeutic effect of nicotine in ulcerative colitis". Aliment. Pharmacol. Ther. 14 (11): 1429–34. doi:10.1046/j.1365-2036.2000.00847.x. PMID 11069313.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Goedert JJ, Vitale F, Lauria C, Serraino D, Tamburini M, Montella M, Messina A, Brown EE, Rezza G, Gafà L, Romano N (2002). "Risk factors for classical Kaposi's sarcoma". J. Natl. Cancer Inst. 94 (22): 1712–8. doi:10.1093/jnci/94.22.1712. PMID 12441327.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Smoking Cuts Risk of Rare Cancer". UPI. March 29, 2001. Retrieved 2006-11-06.
- ^ Lain KY, Powers RW, Krohn MA, Ness RB, Crombleholme WR, Roberts JM (1999). "Urinary cotinine concentration confirms the reduced risk of preeclampsia with tobacco exposure". Am. J. Obstet. Gynecol. 181 (5 Pt 1): 1192–6. doi:10.1016/S0002-9378(99)70107-9. PMID 10561644.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hjern A, Hedberg A, Haglund B, Rosén M (2001). "Does tobacco smoke prevent atopic disorders? A study of two generations of Swedish residents". Clin. Exp. Allergy. 31 (6): 908–14. doi:10.1046/j.1365-2222.2001.01096.x. PMID 11422156.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Melton L (2006). "Body Blazes". Scientific American. 294 (6): 24. Bibcode:2006SciAm.294f..24M. doi:10.1038/scientificamerican0606-24. PMID 16711354.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Fratiglioni L, Wang HX (2000). "Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies". Behav. Brain Res. 113 (1–2): 117–20. doi:10.1016/S0166-4328(00)00206-0. PMID 10942038.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Thompson C. "Alzheimer's disease is associated with non-smoking". Retrieved 2006-11-06.
- ^ Thompson C. "Parkinson's disease is associated with non-smoking". Retrieved 2006-11-06.
- ^ Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C (2008). "Smoking, dementia and cognitive decline in the elderly, a systematic review". BMC Geriatr. 8: 36. doi:10.1186/1471-2318-8-36. PMC 2642819. PMID 19105840.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Henningfield JE, Zeller M (2009). "Nicotine psychopharmacology: policy and regulatory". Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 192: 511–34. doi:10.1007/978-3-540-69248-5_18. ISBN 978-3-540-69246-1. PMID 19184661.
- ^ DeNoon D (2006-08-11). "Nicotine Slows Parkinson's Disease". Retrieved 2009-12-27.
- ^ Peck P (2002-07-25). "Smoking Significantly Increases Risk of Alzheimer's Disease Among Those Who Have No Genetic Predisposition". Retrieved 2009-12-27.
- ^ Fox M (2007-10-24). "Nicotine may ease Parkinson's symptoms: U.S. study". Reuters. Retrieved 2009-12-27.
- ^ Yu W, Mechawar N, Krantic S, Quirion R (2011). "α7 Nicotinic receptor activation reduces β-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling". J. Neurochem. 119 (4): 848–58. doi:10.1111/j.1471-4159.2011.07466.x. PMID 21884524.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Nicotine as an antiepileptic agent in ADNFLE: An n-of-one study".
- ^ de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ (2002). "Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital". Schizophr Res. 56 (1–2): 55–65. doi:10.1016/S0920-9964(01)00192-X. PMID 12084420.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (1995). "Schizophrenia and smoking: an epidemiological survey in a state hospital". Am J Psychiatry. 152 (3): 453–5. PMID 7864277.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Aguilar MC, Gurpegui M, Diaz FJ, de Leon J (2005). "Nicotine dependence and symptoms in schizophrenia: naturalistic study of complex interactions". Br J Psychiatry. 186 (3): 215–21. doi:10.1192/bjp.186.3.215. PMID 15738502.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Nicotine shows potential medical benefits". News Medical. 12 September 2006. Retrieved 29 August 2012.
- ^ "Attention-Deficit Hyperactivity Disorder". Retrieved 21 September 2009.
- ^ Mineur YS, Picciotto MR (2010). "Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis". Trends Pharmacol. Sci. 31 (12): 580–6. doi:10.1016/j.tips.2010.09.004. PMC 2991594. PMID 20965579.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Pasquini M, Garavini A, Biondi M (2005). "Nicotine augmentation for refractory obsessive-compulsive disorder. A case report". Prog. Neuropsychopharmacol. Biol. Psychiatry. 29 (1): 157–9. doi:10.1016/j.pnpbp.2004.08.011. PMID 15610960.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lundberg S, Carlsson A, Norfeldt P, Carlsson ML (2004). "Nicotine treatment of obsessive-compulsive disorder". Prog. Neuropsychopharmacol. Biol. Psychiatry. 28 (7): 1195–9. doi:10.1016/j.pnpbp.2004.06.014. PMID 15610934.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tizabi Y, Louis VA, Taylor CT, Waxman D, Culver KE, Szechtman H (2002). "Effect of nicotine on quinpirole-induced checking behavior in rats: implications for obsessive-compulsive disorder". Biol. Psychiatry. 51 (2): 164–71. doi:10.1016/S0006-3223(01)01207-0. PMID 11822995.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thomas GA, Rhodes J, Green JT, Richardson C (2000). "Role of smoking in inflammatory bowel disease: implications for therapy". Postgrad Med J. 76 (895): 273–9. doi:10.1136/pmj.76.895.273. PMC 1741576. PMID 10775279.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rubin DT, Hanauer SB (2000). "Smoking and inflammatory bowel disease". Eur J Gastroenterol Hepatol. 12 (8): 855–62. doi:10.1097/00042737-200012080-00004. PMID 10958212.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Nick O'Teen
Further reading
- Bilkei-Gorzo A, Rácz I, Michel K, Darvas M, Rafael Maldonado López, Zimmer A. (2008). "A common genetic predisposition to stress sensitivity and stress-induced nicotine craving". Biol. Psychiatry. 63 (2): 164–71. doi:10.1016/j.biopsych.2007.02.010. PMID 17570348.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gorrod, John W.; Peyton, Jacob,III, eds. (1999). Analytical Determination of Nicotine and Related Compounds and their Metabolites. Amsterdam: Elsevier. p. 772. ISBN 0444500952.
{{cite book}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: editors list (link) - Willoughby JO, Pope KJ, Eaton V (2003). "Nicotine as an antiepileptic agent in ADNFLE: an N-of-one study". Epilepsia. 44 (9): 1238–40. doi:10.1046/j.1528-1157.2003.11903.x. PMID 12919397.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Minna JD (2003). "Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer". J Clin Invest. 111 (1): 31–3. doi:10.1172/JCI17492. PMC 151841. PMID 12511585.
{{cite journal}}: Unknown parameter|month=ignored (help) - Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG (2005). "Gender: a major determinant of brain response to nicotine". Int J Neuropsychopharmacol. 8 (1): 17–26. doi:10.1017/S1461145704004730. PMID 15579215.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - West KA, Brognard J, Clark AS; et al. (2003). "Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells". J Clin Invest. 111 (1): 81–90. doi:10.1172/JCI16147. PMC 151834. PMID 12511591.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - National Institute on Drug Abuse
- Erowid information on tobacco


