-
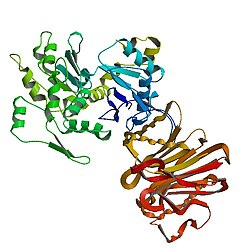
1atn: Atomic structure of the actin:DNASE I complex
-
1c0g: CRYSTAL STRUCTURE OF 1:1 COMPLEX BETWEEN GELSOLIN SEGMENT 1 AND A DICTYOSTELIUM/TETRAHYMENA CHIMERA ACTIN (MUTANT 228: Q228K/T229A/A230Y/E360H)
-
1d4x: Crystal Structure of Caenorhabditis Elegans Mg-ATP Actin Complexed with Human Gelsolin Segment 1 at 1.75 A resolution.
-
1eqy: COMPLEX BETWEEN RABBIT MUSCLE ALPHA-ACTIN: HUMAN GELSOLIN DOMAIN 1
-
1esv: COMPLEX BETWEEN LATRUNCULIN A:RABBIT MUSCLE ALPHA ACTIN:HUMAN GELSOLIN DOMAIN 1
-
1h1v: GELSOLIN G4-G6/ACTIN COMPLEX
-
1hlu: STRUCTURE OF BOVINE BETA-ACTIN-PROFILIN COMPLEX WITH ACTIN BOUND ATP PHOSPHATES SOLVENT ACCESSIBLE
-
1ijj: THE X-RAY CRYSTAL STRUCTURE OF THE COMPLEX BETWEEN RABBIT SKELETAL MUSCLE ACTIN AND LATRUNCULIN A AT 2.85 A RESOLUTION
-
1j6z: UNCOMPLEXED ACTIN
-
1kxp: CRYSTAL STRUCTURE OF HUMAN VITAMIN D-BINDING PROTEIN IN COMPLEX WITH SKELETAL ACTIN
-
1lcu: Polylysine Induces an Antiparallel Actin Dimer that Nucleates Filament Assembly: Crystal Structure at 3.5 A Resolution
-
1lot: CRYSTAL STRUCTURE OF THE COMPLEX OF ACTIN WITH VITAMIN D-BINDING PROTEIN
-
1m8q: Molecular Models of Averaged Rigor Crossbridges from Tomograms of Insect Flight Muscle
-
1ma9: Crystal structure of the complex of human vitamin D binding protein and rabbit muscle actin
-
1mdu: Crystal structure of the chicken actin trimer complexed with human gelsolin segment 1 (GS-1)
-
1mvw: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1nlv: Crystal Structure Of Dictyostelium Discoideum Actin Complexed With Ca ATP And Human Gelsolin Segment 1
-
1nm1: Crystal Structure of D. Dicsoideum Actin Complexed With Gelsolin Segment 1 and Mg ATP at 1.8 A Resolution
-
1nmd: Crystal Structure of D. Discoideum Actin-Gelsolin Segment 1 Complex Crystallized In Presence Of Lithium ATP
-
1nwk: CRYSTAL STRUCTURE OF MONOMERIC ACTIN IN THE ATP STATE
-
1o18: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o19: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o1a: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o1b: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o1c: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o1d: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o1e: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o1f: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1o1g: MOLECULAR MODELS OF AVERAGED RIGOR CROSSBRIDGES FROM TOMOGRAMS OF INSECT FLIGHT MUSCLE
-
1p8z: Complex Between Rabbit Muscle alpha-Actin: Human Gelsolin Residues Val26-Glu156
-
1qz5: Structure of rabbit actin in complex with kabiramide C
-
1qz6: Structure of rabbit actin in complex with jaspisamide A
-
1rdw: Actin Crystal Dynamics: Structural Implications for F-actin Nucleation, Polymerization and Branching Mediated by the Anti-parallel Dimer
-
1rfq: Actin Crystal Dynamics: Structural Implications for F-actin Nucleation, Polymerization and Branching Mediated by the Anti-parallel Dimer
-
1rgi: Crystal structure of gelsolin domains G1-G3 bound to actin
-
1s22: Absolute Stereochemistry of Ulapualide A
-
1sqk: CRYSTAL STRUCTURE OF CIBOULOT IN COMPLEX WITH SKELETAL ACTIN
-
1t44: Structural basis of actin sequestration by thymosin-B4: Implications for arp2/3 activation
-
1wua: The structure of Aplyronine A-actin complex
-
1y64: Bni1p Formin Homology 2 Domain complexed with ATP-actin
-
1yxq: Crystal structure of actin in complex with swinholide A
-
2a3z: Ternary complex of the WH2 domain of WASP with Actin-DNAse I
-
2a40: Ternary complex of the WH2 domain of WAVE with Actin-DNAse I
-
2a41: Ternary complex of the WH2 Domain of WIP with Actin-DNAse I
-
2a42: Actin-DNAse I Complex
-
2a5x: Crystal Structure of a Cross-linked Actin Dimer
-
2asm: Structure of Rabbit Actin In Complex With Reidispongiolide A
-
2aso: Structure of Rabbit Actin In Complex With Sphinxolide B
-
2asp: Structure of Rabbit Actin In Complex With Reidispongiolide C
-
2btf: THE STRUCTURE OF CRYSTALLINE PROFILIN-BETA-ACTIN
-
2d1k: Ternary complex of the WH2 domain of mim with actin-dnase I
-
2ff3: Crystal structure of Gelsolin domain 1:N-wasp V2 motif hybrid in complex with actin
-
2ff6: Crystal structure of Gelsolin domain 1:ciboulot domain 2 hybrid in complex with actin
-
2fxu: X-ray Structure of Bistramide A- Actin Complex at 1.35 A resolution.
-
2gwj: SpvB ADP-ribosylated actin: hexagonal crystal form
-
2gwk: SpvB ADP-ribosylated actin: orthorhombic crystal form
-
2hf3: Crystal structure of monomeric Actin in the ADP bound state
-
2hf4: Crystal structure of Monomeric Actin in its ATP-bound state
-
2hmp: Uncomplexed actin cleaved with protease ECP32
-
2oan: Structure of oxidized beta-actin
-
2q1n: Actin Dimer Cross-linked Between Residues 41 and 374
-
2q31: Actin Dimer Cross-linked Between Residues 41 and 374 and proteolytically cleaved by subtilisin between residues 47 and 48.
-
2q36: Actin Dimer Cross-linked between Residues 191 and 374 and complexed with Kabiramide C