Intermediate filament

| Intermediate filament tail domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structure of lamin a/c globular domain | |||||||||
| Identifiers | |||||||||
| Symbol | IF_tail | ||||||||
| Pfam | PF00932 | ||||||||
| InterPro | IPR001322 | ||||||||
| PROSITE | PDOC00198 | ||||||||
| SCOP2 | 1ivt / SCOPe / SUPFAM | ||||||||
| |||||||||
| Intermediate filament protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 human vimentin coil 2b fragment (cys2) | |||||||||
| Identifiers | |||||||||
| Symbol | Filament | ||||||||
| Pfam | PF00038 | ||||||||
| InterPro | IPR016044 | ||||||||
| PROSITE | PDOC00198 | ||||||||
| SCOP2 | 1gk7 / SCOPe / SUPFAM | ||||||||
| |||||||||
| Intermediate filament head (DNA binding) region | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Filament_head | ||||||||
| Pfam | PF04732 | ||||||||
| InterPro | IPR006821 | ||||||||
| SCOP2 | 1gk7 / SCOPe / SUPFAM | ||||||||
| |||||||||
Intermediate filaments (IFs) are cytoskeletal components found in the cells of vertebrate animal species,[1][2] and perhaps also in other animals, fungi, plants, and unicellular organisms.[3] They are composed of a family of related proteins sharing common structural and sequence features. Initially designated 'intermediate' because their average diameter (10 nm) is between those of narrower microfilaments (actin) and wider myosin filaments found in muscle cells, the diameter of Intermediate filaments is now commonly compared to actin microfilaments (7 nm) and microtubules (25 nm).[1][4] Most types of intermediate filaments are cytoplasmic, but one type, the lamins, are nuclear.
Structure
The structure of proteins that form IF was first predicted by computerized analysis of the amino acid sequence of a human epidermal keratin derived from cloned cDNAs.[5] Analysis of a second keratin sequence revealed that the two types of keratins share only about 30% amino acid sequence homology but share similar patterns of secondary structure domains.[6] As suggested by the first model, all IF proteins appear to have a central alpha-helical rod domain that is composed of four alpha-helical segments (named as 1A, 1B, 2A and 2B) separated by three linker regions.[6][7]
The N and C-termini of IF proteins are non-alpha-helical regions and show wide variation in their lengths and sequences across IF families. The basic building-block for IFs is a parallel and in-register dimer. The dimer is formed through the interaction of the rod domain to form a coiled coil.[8] Cytoplasmic IF assemble into non-polar unit-length filaments (ULF). Identical ULF associate laterally into staggered, antiparallel, soluble tetramers, which associate head-to-tail into protofilaments that pair up laterally into protofibrils, four of which wind together into an intermediate filament.[9]
Part of the assembly process includes a compaction step, in which ULF tighten and assume a smaller diameter. The reasons for this compaction are not well understood, and IF are routinely observed to have diameters ranging between 6 and 12 nm.
The N-terminal "head domain" binds DNA.[10] Vimentin heads are able to alter nuclear architecture and chromatin distribution, and the liberation of heads by HIV-1 protease may play an important role in HIV-1 associated cytopathogenesis and carcinogenesis.[11] Phosphorylation of the head region can affect filament stability.[12] The head has been shown to interact with the rod domain of the same protein.[13]
C-terminal "tail domain" shows extreme length variation between different IF proteins.[14]
The anti-parallel orientation of tetramers means that, unlike microtubules and microfilaments, which have a plus end and a minus end, IFs lack polarity and cannot serve as basis for cell motility and intracellular transport.
Also, unlike actin or tubulin, intermediate filaments do not contain a binding site for a nucleoside triphosphate.
Cytoplasmic IFs do not undergo treadmilling like microtubules and actin fibers, but are dynamic. For a review see: [1].
Biomechanical properties
IFs are rather deformable proteins that can be stretched several times their initial length.[15] The key to facilitate this large deformation is due to their hierarchical structure, which facilitates a cascaded activation of deformation mechanisms at different levels of strain.[8] Initially the coupled alpha-helices of unit-length filaments uncoil as they're strained, then as the strain increases they transition into beta-sheets, and finally at increased strain the hydrogen bonds between beta-sheets slip and the ULF monomers slide along each other.[8]
Types
There are about 70 different genes coding for various intermediate filament proteins. However, different kinds of IFs share basic characteristics: In general, they are all polymers that measure between 9-11 nm in diameter when fully assembled.
IF are subcategorized into six types based on similarities in amino acid sequence and protein structure.
Types I and II – acidic and basic keratins
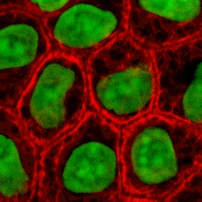
These proteins are the most diverse among IFs and constitute type I (acidic) and type II (basic) IF proteins. The many isoforms are divided in two groups:
- epithelial keratins (about 20) in epithelial cells (image to right)
- trichocytic keratins (about 13) (hair keratins), which make up hair, nails, horns and reptilian scales.
Regardless of the group, keratins are either acidic or basic. Acidic and basic keratins bind each other to form acidic-basic heterodimers and these heterodimers then associate to make a keratin filament.
Type III
There are four proteins classed as type III IF proteins, which may form homo- or heteropolymeric proteins.
- Desmin IFs are structural components of the sarcomeres in muscle cells.
- GFAP (glial fibrillary acidic protein) is found in astrocytes and other glia.
- Peripherin found in peripheral neurons.
- Vimentin, the most widely distributed of all IF proteins, can be found in fibroblasts, leukocytes, and blood vessel endothelial cells. They support the cellular membranes, keep some organelles in a fixed place within the cytoplasm, and transmit membrane receptor signals to the nucleus.
Type IV
- α-Internexin
- Neurofilaments - the type IV family of intermediate filaments that is found in high concentrations along the axons of vertebrate neurons.
- Synemin
- Syncoilin
Type V - nuclear lamins
Lamins are fibrous proteins having structural function in the cell nucleus.
In metazoan cells, there are A and B type lamins, which differ in their length and pI. Human cells have three differentially regulated genes. B-type lamins are present in every cell. B type lamins, B1 and B2, are expressed from the LMNB1 and LMNB2 genes on 5q23 and 19q13, respectively. A-type lamins are only expressed following gastrulation. Lamin A and C are the most common A-type lamins and are splice variants of the LMNA gene found at 1q21.
These proteins localize to two regions of the nuclear compartment, the nuclear lamina—a proteinaceous structure layer subjacent to the inner surface of the nuclear envelope and throughout the nucleoplasm in the nucleoplasmic "veil".
Comparison of the lamins to vertebrate cytoskeletal IFs shows that lamins have an extra 42 residues (six heptads) within coil 1b. The c-terminal tail domain contains a nuclear localization signal (NLS), an Ig-fold-like domain, and in most cases a carboxy-terminal CaaX box that is isoprenylated and carboxymethylated (lamin C does not have a CAAX box). Lamin A is further processed to remove the last 15 amino acids and its farnesylated cysteine.
During mitosis, lamins are phosphorylated by MPF, which drives the disassembly of the lamina and the nuclear envelope.
Type VI
Unclassified
Beaded Filaments-- Filensin, Phakinin
Cell adhesion
At the plasma membrane, some keratins interact with desmosomes (cell-cell adhesion) and hemidesmosomes (cell-matrix adhesion) via adapter proteins.
Associated proteins
Filaggrin binds to keratin fibers in epidermal cells. Plectin links vimentin to other vimentin fibers, as well as to microfilaments, microtubules, and myosin II. Kinesin is being researched and is suggested to connect vimentin to tubulin via motor proteins.
Keratin filaments in epithelial cells link to desmosomes (desmosomes connect the cytoskeleton together) through plakoglobin, desmoplakin, desmogleins, and desmocollins; desmin filaments are connected in a similar way in heart muscle cells.
Diseases arising from mutations in IF genes
- Arrhythmogenic right ventricular cardiomyopathy (ARVC), mutations in the DES gene.[17][18]
- Epidermolysis bullosa simplex; K5 or K14 mutation
- Laminopathies are a family of diseases caused by mutations in nuclear lamins and include Hutchinson Gilford progeria syndrome and various lipodystrophies and cardiomyopathies among others.
- Human Intermediate Filament Database(HIFD), a comprehensive database of human intermediate filament proteins, their associated variations and diseases.
References
- ^ a b Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U (July 2007). "Intermediate filaments: from cell architecture to nanomechanics". Nat. Rev. Mol. Cell Biol. 8 (7): 562–73. doi:10.1038/nrm2197. PMID 17551517.
- ^ Karabinos, Anton, Dieter Riemer, Andreas Erber, and Klaus Weber. "Homologues of Vertebrate Type I, II and III Intermediate Filament (IF) Proteins in an Invertebrate: The IF Multigene Family of the Cephalochordate Branchiostoma." FEBS Letters 437.1-2 (1998): 15-18. Web.
- ^ Intermediate Filaments: A Review, Springer Berlin Heidelberg, 2012, p. 33, ISBN 9783642702303
{{citation}}: Unknown parameter|authors=ignored (help) - ^ Ishikawa H, Bischoff R, Holtzer H (September 1968). "Mitosis and intermediate-sized filaments in developing skeletal muscle". J. Cell Biol. 38 (3): 538–55. doi:10.1083/jcb.38.3.538. PMC 2108373. PMID 5664223.
- ^ Hanukoglu I, Fuchs E (November 1982). "The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins". Cell. 31 (1): 243–52. doi:10.1016/0092-8674(82)90424-X. PMID 6186381.
- ^ a b Hanukoglu I, Fuchs E (July 1983). "The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins". Cell. 33 (3): 915–24. doi:10.1016/0092-8674(83)90034-X. PMID 6191871.
- ^ Lee CH, Kim MS, Chung BM, Leahy DJ, Coulombe PA (July 2012). "Structural basis for heteromeric assembly and perinuclear organization of keratin filaments". Nat. Struct. Mol. Biol. 19 (7): 707–15. doi:10.1038/nsmb.2330. PMC 3864793. PMID 22705788.
- ^ a b c Qin Z, Kreplak L, Buehler MJ (2009). "Hierarchical structure controls nanomechanical properties of vimentin intermediate filaments". PLoS ONE. 4 (10): e7294. doi:10.1371/journal.pone.0007294. PMC 2752800. PMID 19806221.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lodish H; Berk A; Zipursky SL; et al. (2000). Molecular Cell Biology. New York: W. H. Freeman. p. Section 19.6, Intermediate Filaments. ISBN 0-07-243940-8.
- ^ Wang Q, Tolstonog GV, Shoeman R, Traub P (August 2001). "Sites of nucleic acid binding in type I-IV intermediate filament subunit proteins". Biochemistry. 40 (34): 10342–9. doi:10.1021/bi0108305. PMID 11513613.
- ^ Shoeman RL, Huttermann C, Hartig R, Traub P (January 2001). "Amino-terminal polypeptides of vimentin are responsible for the changes in nuclear architecture associated with human immunodeficiency virus type 1 protease activity in tissue culture cells". Mol. Biol. Cell. 12 (1): 143–54. doi:10.1091/mbc.12.1.143. PMC 30574. PMID 11160829.
- ^ Takemura M, Gomi H, Colucci-Guyon E, Itohara S (August 2002). "Protective role of phosphorylation in turnover of glial fibrillary acidic protein in mice". J. Neurosci. 22 (16): 6972–9. PMID 12177195.
- ^ Parry DA, Marekov LN, Steinert PM, Smith TA (2002). "A role for the 1A and L1 rod domain segments in head domain organization and function of intermediate filaments: structural analysis of trichocyte keratin". J. Struct. Biol. 137 (1–2): 97–108. doi:10.1006/jsbi.2002.4437. PMID 12064937.
- ^ Quinlan R, Hutchison C, Lane B (1995). "Intermediate filament proteins". Protein Profile. 2 (8): 795–952. PMID 8771189.
- ^ Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U (July 2007). "Intermediate filaments: from cell architecture to nanomechanics". Nat. Rev. Mol. Cell Biol. 8 (7): 562–73. doi:10.1038/nrm2197. PMID 17551517.Qin Z, Kreplak L, Buehler MJ (2009). "Hierarchical structure controls nanomechanical properties of vimentin intermediate filaments". PLoS ONE. 4 (10): e7294. doi:10.1371/journal.pone.0007294. PMC 2752800. PMID 19806221.
{{cite journal}}: CS1 maint: unflagged free DOI (link)Kreplak L, Fudge D (January 2007). "Biomechanical properties of intermediate filaments: from tissues to single filaments and back". BioEssays. 29 (1): 26–35. doi:10.1002/bies.20514. PMID 17187357.Qin Z, Buehler MJ, Kreplak L (January 2010). "A multi-scale approach to understand the mechanobiology of intermediate filaments". J Biomech. 43 (1): 15–22. doi:10.1016/j.jbiomech.2009.09.004. PMID 19811783.Qin Z, Kreplak L, Buehler MJ (October 2009). "Nanomechanical properties of vimentin intermediate filament dimers". Nanotechnology. 20 (42): 425101. doi:10.1088/0957-4484/20/42/425101. PMID 19779230. - ^ Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD (April 1999). "A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin". J. Biol. Chem. 274 (14): 9881–90. doi:10.1074/jbc.274.14.9881. PMID 10092680.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Klauke B, Kossmann S, Gaertner A, Brand K, Stork I, Brodehl A, Dieding M, Walhorn V, Anselmetti D, Gerdes D, Bohms B, Schulz U, Zu Knyphausen E, Vorgerd M, Gummert J, Milting H (December 2010). "De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy". Hum. Mol. Genet. 19 (23): 4595–607. doi:10.1093/hmg/ddq387. PMID 20829228.
- ^ Brodehl A, Hedde PN, Dieding M, Fatima A, Walhorn V, Gayda S, Šarić T, Klauke B, Gummert J, Anselmetti D, Heilemann M, Nienhaus GU, Milting H (May 2012). "Dual color photoactivation localization microscopy of cardiomyopathy-associated desmin mutants". J. Biol. Chem. 287 (19): 16047–57. doi:10.1074/jbc.M111.313841. PMC 3346104. PMID 22403400.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
Further reading
- Intermediate filaments. Springer. 1998. ISBN 978-0-306-45854-5.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - Intermediate filament cytoskeleton. Gulf Professional Publishing. 2004. ISBN 978-0-12-564173-9.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - Paramio JM, ed. (2006). Intermediate filaments. Springer. ISBN 978-0-387-33780-7.
External links
- Intermediate+Filament+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- http://www.interfil.org/browse_interfil.php
