L-DOPA: Difference between revisions
VasKalCorp (talk | contribs) m Added two missing References Tags: nowiki added Visual edit |
|||
| Line 113: | Line 113: | ||
==History== |
==History== |
||
In work that earned him a [[Nobel Prize in Physiology or Medicine|Nobel Prize]] in 2000, [[Swedish people|Swedish]] scientist [[Arvid Carlsson]] first showed in the 1950s that administering <small>L</small>-DOPA to animals with with drug-induced (reserpine) Parkinsonian [[symptom]]s caused a reduction in the intensity of the animals' symptoms. In 1960/61 Oleh Hornykiewicz, after discovering greatly reduced levels of dopamine in autopsied brains of patients with Parkinson’s disease, published together with the neurologist Walther Birkmayer dramatic therapeutic antiparkinson effects of intravenously administered L-DOPA in patients. This treatment was later extended to manganese poisoning and later Parkinsonism by [[George Cotzias]] and his coworkers,<ref>{{cite journal | last1 = Cotzias | first1 = GC | last2 = Papavasiliou | first2 = PS | last3 = Gellene | first3 = R | title = L-dopa in parkinson's syndrome | journal = The New England Journal of Medicine | volume = 281 | issue = 5 | pages = 272 | year = 1969 | pmid = 5791298 |name-list-format=vanc | doi = 10.1056/NEJM196907312810518 }}</ref> who used greatly increased oral doses. The [[neurology|neurologist]] [[Oliver Sacks]] describes this treatment in human patients with [[encephalitis lethargica]] in his book ''[[Awakenings (book)|Awakenings]]'', upon which [[Awakenings|the movie of the same name]] is based. |
In work that earned him a [[Nobel Prize in Physiology or Medicine|Nobel Prize]] in 2000, [[Swedish people|Swedish]] scientist [[Arvid Carlsson]] first showed in the 1950s that administering <small>L</small>-DOPA to animals with with drug-induced (reserpine) Parkinsonian [[symptom]]s caused a reduction in the intensity of the animals' symptoms. In 1960/61 Oleh Hornykiewicz, after discovering greatly reduced levels of dopamine in autopsied brains of patients with Parkinson’s disease<ref><nowiki><ref name="pmid13726012">{{cite journal| author=EHRINGER H, HORNYKIEWICZ O| title=[Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. | journal=Klin Wochenschr | year= 1960 | volume= 38 | issue= | pages= 1236-9 | pmid=13726012 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=13726012 }} </ref></nowiki></ref>, published together with the neurologist Walther Birkmayer dramatic therapeutic antiparkinson effects of intravenously administered L-DOPA in patients<ref><nowiki><ref name="pmid13869404">{{cite journal| author=BIRKMAYER W, HORNYKIEWICZ O| title=[The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. | journal=Wien Klin Wochenschr | year= 1961 | volume= 73 | issue= | pages= 787-8 | pmid=13869404 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=13869404 }} </ref></nowiki></ref>. This treatment was later extended to manganese poisoning and later Parkinsonism by [[George Cotzias]] and his coworkers,<ref>{{cite journal | last1 = Cotzias | first1 = GC | last2 = Papavasiliou | first2 = PS | last3 = Gellene | first3 = R | title = L-dopa in parkinson's syndrome | journal = The New England Journal of Medicine | volume = 281 | issue = 5 | pages = 272 | year = 1969 | pmid = 5791298 |name-list-format=vanc | doi = 10.1056/NEJM196907312810518 }}</ref> who used greatly increased oral doses. The [[neurology|neurologist]] [[Oliver Sacks]] describes this treatment in human patients with [[encephalitis lethargica]] in his book ''[[Awakenings (book)|Awakenings]]'', upon which [[Awakenings|the movie of the same name]] is based. |
||
The 2001 [[Nobel Prize in Chemistry]] was also related to <small>L</small>-DOPA: the Nobel Committee awarded one-quarter of the prize to [[William S. Knowles]] for his work on chirally catalysed [[hydrogenation]] reactions, the most noted example of which was used for the synthesis of <small>L</small>-DOPA:<ref>{{cite journal | doi = 10.1021/ar00087a006 | title = Asymmetric hydrogenation | year = 1983 | last1 = Knowles | first1 = William S. | journal = Accounts of Chemical Research | volume = 16 | issue = 3 | pages = 106}}</ref><ref>{{cite web | url = http://www.chem.wisc.edu/areas/reich/syntheses/dopa-monsanto-knowles.htm | title = Synthetic scheme for total synthesis of DOPA, L- (Monsanto) | publisher = UW Madison, Department of Chemistry | accessdate = Sep 30, 2013}}</ref> |
The 2001 [[Nobel Prize in Chemistry]] was also related to <small>L</small>-DOPA: the Nobel Committee awarded one-quarter of the prize to [[William S. Knowles]] for his work on chirally catalysed [[hydrogenation]] reactions, the most noted example of which was used for the synthesis of <small>L</small>-DOPA:<ref>{{cite journal | doi = 10.1021/ar00087a006 | title = Asymmetric hydrogenation | year = 1983 | last1 = Knowles | first1 = William S. | journal = Accounts of Chemical Research | volume = 16 | issue = 3 | pages = 106}}</ref><ref>{{cite web | url = http://www.chem.wisc.edu/areas/reich/syntheses/dopa-monsanto-knowles.htm | title = Synthetic scheme for total synthesis of DOPA, L- (Monsanto) | publisher = UW Madison, Department of Chemistry | accessdate = Sep 30, 2013}}</ref> |
||
Revision as of 19:35, 22 October 2015
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Metabolism | Aromatic-L-amino-acid decarboxylase |
| Elimination half-life | 0.75–1.5 hours |
| Excretion | renal 70–80% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.405 |
| Chemical and physical data | |
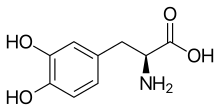
| Formula | C9H11NO4 |
| Molar mass | 197.19 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
L-DOPA (/ˌɛlˈdoʊpə/ or /ˌlɛvoʊˈdoʊpə/) (alt., L-3,4-dihydroxyphenylalanine) is a chemical that is made and used as part of the normal biology of humans, some animals and plants. Some animals and humans make it via biosynthesis from the amino acid L-tyrosine. L-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) collectively known as catecholamines. L-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Parcopa, Atamet, Stalevo, Madopar, and Prolopa. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.
L-DOPA has a counterpart with opposite chirality, D-DOPA. As is true for many molecules, the human body produces only one of these isomers (the L-DOPA form).
Therapeutic use
L-DOPA crosses the protective blood–brain barrier, whereas dopamine itself cannot. Thus, L-DOPA is used to increase dopamine concentrations in the treatment of Parkinson's disease and dopamine-responsive dystonia. This treatment was made practical and proven clinically by George Cotzias and his coworkers, for which they won the 1969 Lasker Prize.[1][2] Once L-DOPA has entered the central nervous system, it is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase. Pyridoxal phosphate (vitamin B6) is a required cofactor in this reaction, and may occasionally be administered along with L-DOPA, usually in the form of pyridoxine.
Besides the central nervous system, L-DOPA is also converted into dopamine from within the peripheral nervous system. Excessive peripheral dopamine signaling causes many of the adverse side effects seen with sole L-DOPA administration. To bypass these effects, it is standard clinical practice to coadminister (with L-DOPA) a peripheral DOPA decarboxylase inhibitor (DDCI) such as carbidopa (medicines containing carbidopa, either alone or in combination with L-DOPA, are branded as Lodosyn, Sinemet, Parcopa, Atamet, and Stalevo) or with a benserazide (combination medicines are branded Madopar or Prolopa), to prevent the peripheral synthesis of dopamine from L-DOPA. Coadministration of pyridoxine without a DDCI accelerates the peripheral decarboxylation of L-DOPA to such an extent that it negates the effects of L-DOPA administration, a phenomenon that historically caused great confusion.
In addition, L-DOPA, co-administered with a peripheral DDCI, has been investigated as a potential treatment for restless leg syndrome. However, studies have demonstrated "no clear picture of reduced symptoms".[3]
The two types of response seen with administration of L-DOPA are:
- The short-duration response is related to the half-life of the drug.
- The longer-duration response depends on the accumulation of effects over at least two weeks, during which ΔFosB accumulates in nigrostriatal neurons. In the treatment of Parkinson's disease, this response is evident only in early therapy, as the inability of the brain to store dopamine is not yet a concern.
Dietary supplements
Herbal extracts containing L-DOPA are available; high-yielding sources include Mucuna pruriens (velvet bean),[4] and Vicia faba (broad bean),[5] while other sources include the genera Phanera, Pileostigma, Cassia, Canavalia, and Dalbergia.[6]
Biological role
L-DOPA is produced from the amino acid L-tyrosine by the enzyme tyrosine hydroxylase. It is also the precursor for the monoamine or catecholamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline). Dopamine is formed by the decarboxylation of L-DOPA.
L-DOPA can be directly metabolized by catechol-O-methyl transferase to 3-O-methyldopa, and then further to vanillactic acid. This metabolic pathway is nonexistent in the healthy body, but becomes important after peripheral L-DOPA administration in patients with Parkinson's disease or in the rare cases of patients with aromatic L-amino acid decarboxylase enzyme deficiency.[10]
L-Phenylalanine, L-tyrosine, and L-DOPA are all precursors to the biological pigment melanin. The enzyme tyrosinase catalyzes the oxidation of L-DOPA to the reactive intermediate dopaquinone, which reacts further, eventually leading to melanin oligomers.
Side effects
The side effects of L-DOPA may include:
- Hypotension, especially if the dosage is too high
- Arrhythmias, although these are uncommon
- Nausea, which is often reduced by taking the drug with food, although protein interferes with drug absorption
- Gastrointestinal bleeding
- Disturbed respiration, which is not always harmful, and can actually benefit patients with upper airway obstruction
- Hair loss
- Disorientation and confusion
- Extreme emotional states, particularly anxiety, but also excessive libido
- Vivid dreams or insomnia
- Auditory or visual hallucinations
- Effects on learning; some evidence indicates it improves working memory, while impairing other complex functions
- Somnolence and narcolepsy
- A condition similar to stimulant psychosis
Although many adverse effects are associated with L-DOPA, in particular psychiatric ones, it has fewer than other antiparkinsonian agents, such as anticholinergics and dopamine receptor agonists.
More serious are the effects of chronic L-DOPA administration in the treatment of Parkinson's disease, which include:
- End-of-dose deterioration of function
- On/off oscillations
- Freezing during movement
- Dose failure (drug resistance)
- Dyskinesia at peak dose (levodopa-induced dyskinesia)
- Possible dopamine dysregulation: The long-term use of L-DOPA in Parkinson's disease has been linked to the so-called dopamine dysregulation syndrome.[11]
Clinicians try to avoid these side effects by limiting L-DOPA doses as much as possible until absolutely necessary.
Possible overdose symptoms
Some in vitro studies suggest a cytotoxic role in the promotion and occurrence of adverse effects associated with L-DOPA treatment.[12] Though the drug is generally safe in humans, some researchers have reported an increase in cytotoxicity markers in rat pheochromocytoma PC12 cell lines treated with L-DOPA.[13][14] Other authors have attributed the observed toxic effects of L-DOPA in neural dopamine cell lines to enhanced formation of quinones through increased auto-oxidation and subsequent cell death in mesencephalic cell cultures.[15][16] There is no evidence of neurotoxicity in patients with Parkinson's disease and it is generally considered safe, but some controversy surrounds its use in the treatment of Parkinson's disease, given some test tube data indicate a deleterious effect on intracellular and neuronal tissue involved in the pathogenesis of the disease.[17]
History
In work that earned him a Nobel Prize in 2000, Swedish scientist Arvid Carlsson first showed in the 1950s that administering L-DOPA to animals with with drug-induced (reserpine) Parkinsonian symptoms caused a reduction in the intensity of the animals' symptoms. In 1960/61 Oleh Hornykiewicz, after discovering greatly reduced levels of dopamine in autopsied brains of patients with Parkinson’s diseaseCite error: A <ref> tag is missing the closing </ref> (see the help page).</nowiki></ref>, published together with the neurologist Walther Birkmayer dramatic therapeutic antiparkinson effects of intravenously administered L-DOPA in patientsCite error: A <ref> tag is missing the closing </ref> (see the help page).</nowiki></ref>. This treatment was later extended to manganese poisoning and later Parkinsonism by George Cotzias and his coworkers,[18] who used greatly increased oral doses. The neurologist Oliver Sacks describes this treatment in human patients with encephalitis lethargica in his book Awakenings, upon which the movie of the same name is based.
The 2001 Nobel Prize in Chemistry was also related to L-DOPA: the Nobel Committee awarded one-quarter of the prize to William S. Knowles for his work on chirally catalysed hydrogenation reactions, the most noted example of which was used for the synthesis of L-DOPA:[19][20]
Marine adhesion
L-DOPA is a key compound in the formation of marine adhesive proteins, such as those found in mussels.[21][22] It is believed to be responsible for the water-resistance and rapid curing abilities of these proteins. L-DOPA may also be used to prevent surfaces from fouling by bonding antifouling polymers to a susceptible substrate.[23]
As a nootropic
One double-blind, placebo controlled study (n=40) found that L-DOPA enhances learning of pseudowords. The drug group showed better learning in all comparisons. Furthermore, a dose-response relationship was tested and found to be the case: lighter people from the drug group did better than the heavier people.[24]
See also
- D-DOPA (Dextrodopa)
- L-DOPS (Droxidopa)
- Methyldopa (Aldomet, Apo-Methyldopa, Dopamet, Novomedopa, etc.)
- Dopamine (Intropan, Inovan, Revivan, Rivimine, Dopastat, Dynatra, etc.)
- Ciladopa
References
- ^ Lasker Award 1969 Description, accessed April 1, 2013
- ^ Tanya Simuni and Howard Hurtig. "Levadopa: A Pharmacologic Miracle Four Decades Later", in Parkinson's Disease: Diagnosis and Clinical Management (Google eBook). Eds. Stewart A Factor and William J Weiner. Demos Medical Publishing, 2008
- ^ "L-dopa for RLS". Bandolier. 1 April 2007. Retrieved 2008-10-16.
- ^ Pankaj Oudhia. "Kapikachu or Cowhage". Retrieved Nov 3, 2013.
- ^ Singh, AK; Bharati, RC; Manibhushan, NC; Pedpati, A (December 2013). "An assessment of faba bean (Vicia faba L.) current status and future prospect" (PDF). African Journal of Agricultural Research. 8 (50): 6634–6641. doi:10.5897/AJAR2013.7335.
- ^ Ingle, PK (May–June 2003). "L-DOPA bearing plants". Natural Product Radiance. 2 (3): 126–133.
{{cite journal}}:|access-date=requires|url=(help) - ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ Hyland K, Clayton PT (December 1992). "Aromatic L-amino acid decarboxylase deficiency: diagnostic methodology" (PDF). Clinical chemistry. 38 (12): 2405–10. PMID 1281049.
- ^ Merims D, Giladi N (2008). "Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson's disease". Parkinsonism Relat Disord. 14 (4): 273–280. doi:10.1016/j.parkreldis.2007.09.007. PMID 17988927.
- ^ Cheng N; Maeda T; Kume T; et al. (December 1996). "Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons". Brain Research. 743 (1–2): 278–83. doi:10.1016/S0006-8993(96)01056-6. PMID 9017256.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sadigh-Eteghad, Saeed; Talebi, Mahnaz; Farhoudi, Mehdi; Mahmoudi, Javad; Reyhani, Bahram (2013). "Effects of Levodopa loaded chitosan nanoparticles on cell viability and caspase-3 expression in PC12 neural like cells". Neurosciences (Riyadh). 18 (3): 281–283. PMID 23887222.
- ^ Basma AN, Morris EJ, Nicklas WJ, Geller HM (February 1995). "L-dopa cytotoxicity to PC12 cells in culture is via its autoxidation". Journal of Neurochemistry. 64 (2): 825–32. doi:10.1046/j.1471-4159.1995.64020825.x. PMID 7830076.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pardo B, Mena MA, Casarejos MJ, Paíno CL, De Yébenes JG (June 1995). "Toxic effects of L-DOPA on mesencephalic cell cultures: protection with antioxidants". Brain Research. 682 (1–2): 133–43. doi:10.1016/0006-8993(95)00341-M. PMID 7552304.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mytilineou C, Han SK, Cohen G (October 1993). "Toxic and protective effects of L-dopa on mesencephalic cell cultures". Journal of Neurochemistry. 61 (4): 1470–8. doi:10.1111/j.1471-4159.1993.tb13642.x. PMID 8376999.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Simuni T, Stern MB (June 1999). "Does levodopa accelerate Parkinson's disease?". Drugs & aging. 14 (6): 399–408. doi:10.2165/00002512-199914060-00001. PMID 10408739.
- ^ Cotzias, GC; Papavasiliou, PS; Gellene, R (1969). "L-dopa in parkinson's syndrome". The New England Journal of Medicine. 281 (5): 272. doi:10.1056/NEJM196907312810518. PMID 5791298.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Knowles, William S. (1983). "Asymmetric hydrogenation". Accounts of Chemical Research. 16 (3): 106. doi:10.1021/ar00087a006.
- ^ "Synthetic scheme for total synthesis of DOPA, L- (Monsanto)". UW Madison, Department of Chemistry. Retrieved Sep 30, 2013.
- ^ Waite, J. Herbert; Andersen, Niels Holten; et al. (2005). "Mussel Adhesion: Finding the Tricks Worth Mimicking". J Adhesion. 81 (3–4): 1–21. doi:10.1080/00218460590944602.
- ^ "Study Reveals Details Of Mussels' Tenacious Bonds". Science Daily. Aug 16, 2006. Retrieved Sep 30, 2013.
- ^ Mussel Adhesive Protein Mimetics
- ^ Knecht, S; Breitenstein, C; Bushuven, S; Wailke, S; Kamping, S; Flöel, A; Zwitserlood, P; Ringelstein, EB (July 2004). "Levodopa: faster and better word learning in normal humans". Annals of Neurology. 56 (1): 20–6. doi:10.1002/ana.20125. PMID 15236398.



