6-APB: Difference between revisions
fix ref |
aka |
||
| Line 39: | Line 39: | ||
| StdInChIKey = FQDAMYLMQQKPRX-UHFFFAOYSA-N |
| StdInChIKey = FQDAMYLMQQKPRX-UHFFFAOYSA-N |
||
}} |
}} |
||
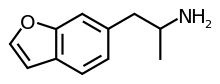
'''6-(2-aminopropyl)benzofuran''' or '''1-benzofuran-6-ylpropan-2-amine''' ('''6-APB''') is an entactogenic compound of the [[phenethylamine]] and [[substituted amphetamine|amphetamine]] classes. It is [[structural analog|similar]] in structure to [[3,4-methylenedioxyamphetamine|MDA]] but differs in that the 3,4-[[methylenedioxy]]phenyl [[functional group|ring]] system has been replaced with a [[benzofuran]] ring. 6-APB is also the unsaturated benzofuran derivative of [[6-APDB]]. |
'''6-(2-aminopropyl)benzofuran''' or '''1-benzofuran-6-ylpropan-2-amine''' ('''6-APB'''), also known as ''Benzofury'' is an entactogenic compound of the [[phenethylamine]] and [[substituted amphetamine|amphetamine]] classes. It is [[structural analog|similar]] in structure to [[3,4-methylenedioxyamphetamine|MDA]] but differs in that the 3,4-[[methylenedioxy]]phenyl [[functional group|ring]] system has been replaced with a [[benzofuran]] ring. 6-APB is also the unsaturated benzofuran derivative of [[6-APDB]]. |
||
==Pharmacology== |
==Pharmacology== |
||
Revision as of 22:24, 12 September 2013
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H13NO |
| Molar mass | 175.23 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
6-(2-aminopropyl)benzofuran or 1-benzofuran-6-ylpropan-2-amine (6-APB), also known as Benzofury is an entactogenic compound of the phenethylamine and amphetamine classes. It is similar in structure to MDA but differs in that the 3,4-methylenedioxyphenyl ring system has been replaced with a benzofuran ring. 6-APB is also the unsaturated benzofuran derivative of 6-APDB.
Pharmacology
6-APB is a triple monoamine reuptake inhibitor with Ki values of 117, 150 and 2698 for NET, DAT and SERT respectively as well as being a potent agonist for the 5-HT2B receptor (Ki 3.7nM).[1] The subjective effects and structure–activity relationship suggest that it is also a releasing agent.
The agonism for 5-HT2B makes it likely that 6-APB would be cardiotoxic with long term use, as seen in other 5-HT2B agonists such as fenfluramine and MDMA.[1] 6-APB has also been claimed to act as an agonist of the 5-HT2C receptor and therefore be useful as an appetite suppressant.[2] However while 6-APB is an agonist at all three 5-HT2 receptor subtypes, its potency at 5-HT2B is significantly higher, and it is actually both more potent and more selective over other serotonin receptors than the reference agonist BW723C86 which is commonly used for research into 5-HT2B receptors.
Law
6-APB is not listed under the Opium Law or the Medicine Act in the Netherlands, and thus currently legal.
6-APB is unscheduled in the United States, but not currently approved by the Food and Drug Administration for human consumption.
Certain countries contain a "substantially similar" catch-all clause in their drug law, such as New Zealand and Australia. This includes 6-APB as it is in some respects similar in chemical structure to the class A drug MDA[citation needed], meaning 6-APB may be viewed as a controlled substance analogue in these jurisdictions.[3]
6-APB is unscheduled under the Controlled Drugs and Substances Act (CDSA) in Canada, although there is a Schedule III amphetamine analogue clause. Due to 6-APB's structure, it may be considered an analogue of MDA, but these types of offenses are rarely prosecuted.[citation needed] Canada's CDSA defines an analogue as any substance that, in relation to a controlled substance, has a substantially similar chemical structure.
6-APB is unscheduled in France and Italy.
On June 10th 2013 6-APB and a number of analogues were classified as Temporary Class Drugs in the UK following an ACMD reccomendation.[4] This means that sale and import of the named substances are criminal offences and are treated as for class B drugs.[5]
6-APB is illegal in Germany since the 17th of July, 2013, when it was added to the Betäubungsmittelgesetz.[6]
6-APB is illegal in Sweden since the 4th of September, 2012, when it was added to the list of unhealthy substances.[7]
See also
References
- ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.ejphar.2012.12.006 , please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.ejphar.2012.12.006instead. - ^ US patent 7045545, Karin Briner et al, "Aminoalkylbenzofurans as serotonin (5-HT(2c)) agonists", published 2000-01-19, issued 2006-16-03
- ^ "Misuse of Drugs Act 1975 New Zealand". legislation.govt.nz. Archived from the original on 28 July 2010. Retrieved 2010-08-06.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Temporary class drug order report on 5-6APB and NBOMe compounds". UK Home Office. 04 Jun 2013. Retrieved 2013-06-13.
{{cite web}}: Check date values in:|date=(help) - ^ "'NBOMe' and 'Benzofury' banned". UK Home Office. 4 Jun 2013. Retrieved 2013-06-13.
- ^ Verordnung zur Änderung von betäubungsmittelrechtlichen Vorschriften, Art. 1 VO vom 9. Juli 2013(BGBl. I S. 2274)
- ^ Svensk författningssamling 1999:58 Förordning (1999:58) om förbud mot vissa hälsofarliga varor. riksdagen.se. Retrieved on 2012-11-12.
