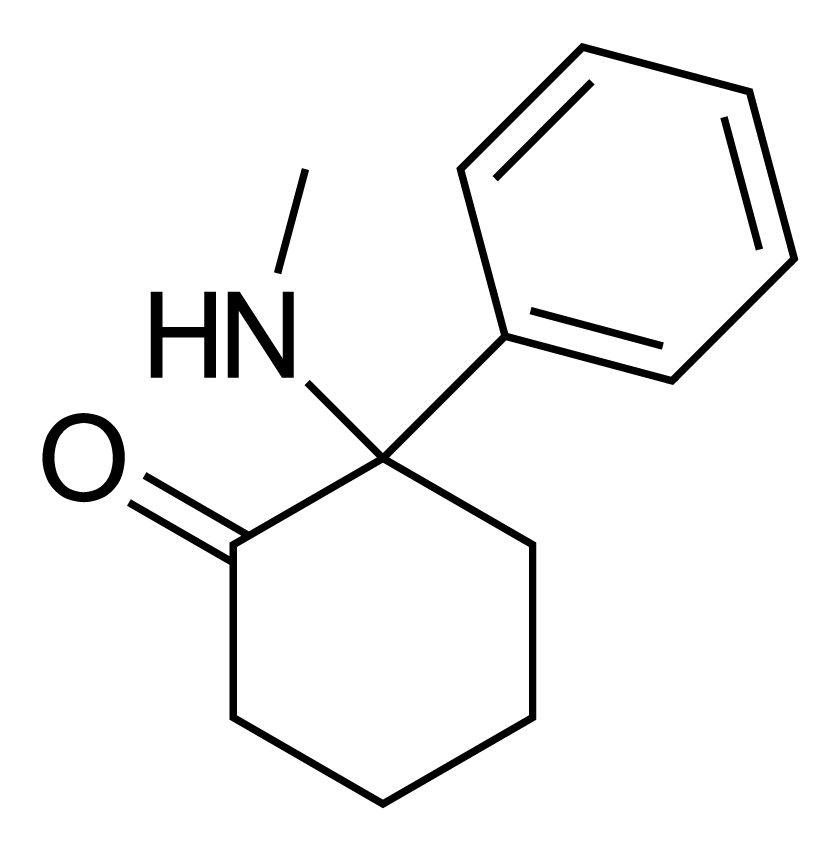
Deschloroketamine
From Wikipedia, the free encyclopedia
This is an old revision of this page, as edited by Narayansg (talk | contribs) at 22:15, 29 June 2018 (link to Direct Action Everywhere). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
"DXE" redirects here. For the animal rights group, see Direct Action Everywhere.
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H17NO |
| Molar mass | 203.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Deschloroketamine (DXE, DCK, 2'-Oxo-PCM) is a dissociative anesthetic[1][2] that has been sold online as a designer drug.[3][4][5][6] It has also been proposed for the treatment of bacterial, fungal, viral or protozoal infections and for immunomodulation at doses of 2 mg per day.[7]
Legal Status
Deschloroketamine is illegal in Latvia,[8] and is covered by blanket bans in Canada and the UK.
References
- ^ Edward G. Robins; Yongjun Zhao; Imtiaz Khan; Anthony Wilson; Sajinder K. Luthra; Erik Årstad (March 2010). "Synthesis and in vitro evaluation of 18F-labelled S-fluoroalkyl diarylguanidines: Novel high-affinity NMDA receptor antagonists for imaging with PET". Bioorganic & Medicinal Chemistry Letters. 20 (5): 1749–1751. doi:10.1016/j.bmcl.2010.01.052. PMID 20138515.
- ^ Calvin L Stevens (31 May 1966). "Patent US 3254124 - Aminoketones and methods for their production". Retrieved 2 October 2015.
- ^ Giampietro Frison; Luca Zamengo; Flavio Zancanaro; Francesco Tisato; Pietro Traldi (January 2016). "Characterization of the designer drug deschloroketamine (2-methylamino-2-phenylcyclohexanone) by gas chromatography/mass spectrometry, liquid chromatography/high-resolution mass spectrometry, multistage mass spectrometry, and nuclear magnetic resonance". Rapid Communications in Mass Spectrometry. 30 (1): 151–160. doi:10.1002/rcm.7425. PMID 26661982.
- ^ "Alerta: descloroketamina vendida como ketamina en Barcelona" (in Spanish). Energy Control. Retrieved 2 October 2015.
- ^ Juanjo Villalba (27 March 2015). "Los efectos desconocidos de la sequía de ketamina" (in Spanish). Vice. Retrieved 2 October 2015.
- ^ Jeremy Knibbs (22 October 2015). "Party-drug testing shows its worth". The Medical Republic.
- ^ Detlef Preiss; Akos Tatar (23 May 1995). "Patent DE4409671 - Use of 2-methylamino-2-phenylcyclohexanone for the treatment of bacterial, fungal, viral or protozoal infections and for immunomodulation". Retrieved 2 October 2015.
- ^ Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem
| |||||||||||||||||||||||||||||||||||||
This hallucinogen-related article is a stub. You can help Wikipedia by expanding it. |
Hidden categories:
